Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the most aggressive and lethal malignancies worldwide (1). Nutritional deficiencies,
nitrosamine-rich or mycotoxin-contaminated foods and low
socioeconomic status are likely to contribute to ESCC (1,2). It has
been reported that heavy smoking and alcohol consumption are key
environmental risk factors for ESCC (3,4).
Dysphagia is the most common symptom of ESCC. Although surgery is
considered to be the most effective treatment for ESCC in terms of
locoregional control and long-term survival, recurrence and
metastases to the liver occur in the majority of cases following
complete resection, resulting in a 15–25% five-year survival rate
in patients with ESCC (5).
Therefore, the identification of molecular mechanisms that pinpoint
biologically aggressive tumors is critical for the effective
management of ESCC.
Homeodomain-interacting protein kinase-2 (HIPK2) is
a member of an evolutionary conserved family of serine/threonine
kinases and is considered to be a tumor suppressor that modulates a
number of basic cellular processes, including apoptosis,
proliferation, differentiation and metastasis (6–9). It has
previously been demonstrated that HIPK2 mediates apoptosis and
epithelial-mesenchymal transition (EMT) of renal tubular epithelial
cells contributing to fibrosis, implying that it may be a potential
target for anti-fibrosis therapy (10). HIPK2 has also been demonstrated to
activate the p53 protein via direct binding and phosphorylation at
serine 46 following severe DNA damage (11). Puca et al (12) demonstrated that HIPK2 is an important
regulator of p53 activity in response to chemotherapeutic agent
cisplatin and Lazzari et al (13) indicated that HIPK2 knockdown induces
resistance to multiple anticancer agents, including doxorubicin and
cisplatin. HIPK2-mediated vimentin downregulation may contribute to
the inhibition of breast cancer cell invasion (14). In bladder cancer, HIPK2 inhibition
promotes EMT and subsequent cell invasion, at least in part by
activating Wnt signaling (15).
However, the biological role and clinical significance of HIPK2 in
ESCC remain largely unknown.
The present study aimed to investigate whether HIPK2
regulates metastasis and chemosensitivity in ESCC. It was
identified that upregulation of HIPK2 inhibits cell metastasis and
suppresses cell viability during cisplatin treatment, implicating a
potential application of HIPK2 in ESCC therapy.
Materials and methods
ESCC specimens
A total of 56 paired ESCC specimens (34 males and 22
females) and adjacent non-cancerous tissues were collected from the
Department Of Thoracic Surgery of the First People's Hospital of
Nanyang (Nanyang, China) between March 2015 and February 2016. The
mean age was 63.22 years (range, 44–84 years). According to the
AJCC tumor stage (16), 30 patients
had stage 1–2 and 26 had stage 3–4. Samples were immediately frozen
in liquid nitrogen and stored at −80°C. Written informed consent
was obtained from all patients prior to their involvement in the
current study. None of the patients had undergone preoperative
anticancer therapies. The study was approved by the Ethics
Committee of the First People's Hospital of Nanyang.
Cell lines and transfection
Human ESCC cell lines EC109 and EC9706 clone EC1
(EC1) and the human epithelial cell line Het-1a were maintained in
RPMI-1640 (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in a humidified atmosphere with
5% CO2 at 37°C. The cisplatin-resistant sub-line
(EC109/cis) was established by continuous exposure to increasing
concentrations (0.1, 0.2, 0.4, 0.6 and 1 µg/ml) cisplatin over 12
months (17). After continuous
exposure to cisplatin for 2 days, the medium was replaced with a
fresh cisplatin free medium until the surviving cells recovered
favorably. When cells grew to the 60–70% confluency, cisplatin was
added to the medium again. Each concentration was repeated six
times.
The pEGFP-N1 and pEGFP-N1-HIPK2 plasmids were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China) and
verified by sequencing using an ABI 3730xl automated sequencer
(Applied Biosystems; Thermo Fisher Scientific, Inc.). For plasmid
transfection, 3×105 cells (EC109, EC1, EC109/cis) were
seeded in 6-well plates 24 h prior to transfection with 4 µg
plasmid DNA using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Transfected cells were selected using G418 (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) 2 days after transfection to generate
stably transfected monoclonal cell lines. After 14 days of
screening, stable transfectants were selected for further
amplification, and were then tested by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) for
overexpression of HIPK2.
RT-qPCR
Total RNA was extracted from cells (Het-1a, EC109,
EC1, EC109/cis) and ESCC specimens using TRIzol according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.), and RT reactions were performed using a PrimeScript™ II 1st
Strand cDNA Synthesis kit (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's protocol. PCR analysis was
performed using SYBR® Premix Ex Taq™ II reagent (Takara
Biotechnology Co., Ltd.) on the ABI 7500 Fast System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The reaction protocol
involved heating for 10 sec at 95°C, followed by 40 cycles of
amplification (5 sec at 95°C and 30 sec at 60°C). The primer
sequences (HIPK2, GAPDH, E-cadherin and N-cadherin) used are
presented in Table I. GAPDH was used
as the internal standard. Data analysis was performed using the
2−ΔΔCq method (18). Each
sample was analyzed in triplicate.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Product size
(bp) |
|---|
| GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA | 138 |
| HIPK2 |
CCCGTGTACGAAGGTATGGC |
AGTTGGAACTCGGCTCTATTTTC | 109 |
| N-cadherin |
AGCCAACCTTAACTGAGGAGT |
GGCAAGTTGATTGGAGGGATG | 136 |
| E-cadherin |
ATTTTTCCCTCGACACCCGAT |
TCCCAGGCGTAGACCAAGA | 109 |
Transwell migration and invasion
assay
Transwell cell migration and Matrigel invasion
assays (8 µm pore size) were utilized to estimate the migration and
invasion ability of cells (EC109 and EC1) in vitro. For the
invasion assay, Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
was added to the upper surface of the membrane and allowed to gel
at 37°C for 30 min. A total of 600 µl of RPMI-1640 medium with 10%
FBS was placed in the bottom compartment of the chamber. Harvested
EC109 and EC1 cells (2×104) in 200 µl of serum-free RPMI
1640 medium were added into the upper compartment of the chamber.
Following 24 h of incubation at 37°C in an atmosphere containing 5%
CO2, cells that had migrated or invaded to the basal
side of the membrane were stained with crystal violet (0.005%,
Sigma-Aldrich; Merck KGaA) for 20 min at room temperature and
counted as the number of cells per field of view under a light
microscope at ×200 magnification in 10 random fields.
Cell Counting Kit (CCK)-8 assay
Cells (EC109-Vector/HIPK2, EC109/cis-Vector/HIPK2)
were seeded in triplicate on a 96-well plate (Corning Incorporated,
Corning, NY, USA) at a density of 5×103 cells/well. Cell
proliferation was assessed after 24, 48 and 72 h using the CCK-8
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) method
according to the manufacturer's protocol.
Statistical analysis
All data are expressed as the mean ± standard
deviation. The differences between two groups were analyzed using
Student's t-test. The difference between three groups was assessed
by one-way analysis of variance followed by Tukey's post hoc test.
All statistical analyses were performed using SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
HIPK2 expression is decreased in ESCC
cells
The expression of HIPK2 in 56 paired ESCC tissues
was determined using RT-qPCR. The expression of HIPK2 mRNA was
significantly decreased in ESCC tissues compared with their
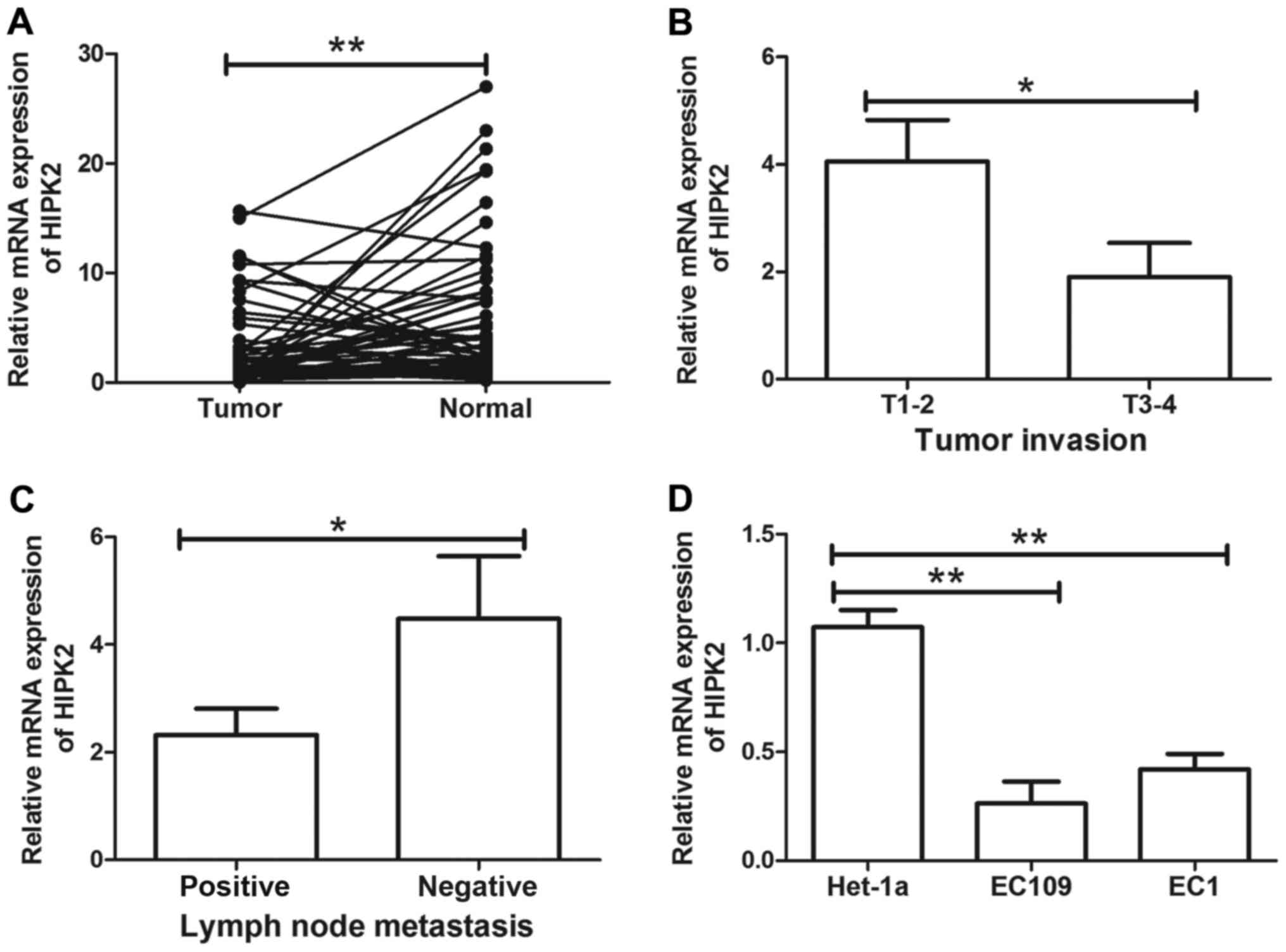
adjacent non-cancerous tissues (P<0.01; Fig. 1A). To further evaluate the role of
HIPK2 in human ESCC, the association between HIPK2 and clinical
parameters, including age, sex, TNM and cancer grade, was
evaluated. Decreased HIPK2 expression was significantly associated
with tumor invasion (P<0.05; Fig.
1B) and lymph node metastasis (P<0.05; Fig. 1C). HIPK2 was not significantly
associated with other clinical characteristics, including age,
gender, differentiation and tumor stage (data not shown). The
expression of HIPK2 in ESCC cell lines EC109 and EC1 and esophageal
epithelial cell line Het-1a was then determined. HIPK2 was
significantly downregulated in EC109 and EC1 cells compared with
Het-1a cells (both P<0.01; Fig.
1D). These results indicated that HIPK2 downregulation may be
associated with ESCC metastasis and serve a tumor suppressive role
in ESCC.
HIPK2 overexpression inhibits cell
migration and invasion in vitro
To further investigate the role of HIPK2 in cell
invasion, ESCC cells stably transfected with a control vector or
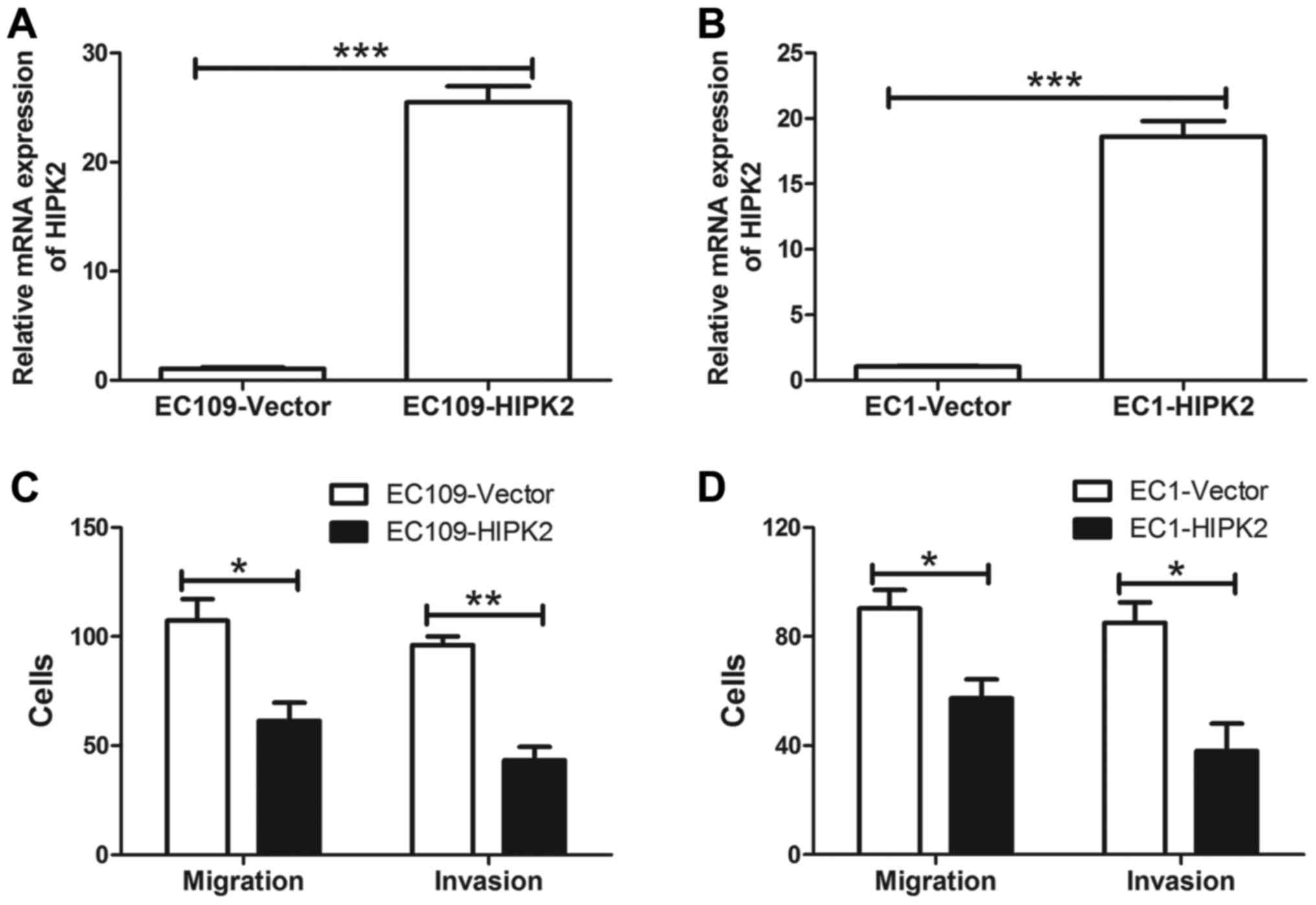
HIPK2 were analyzed. The expression of HIPK2 was confirmed in EC109
(Fig. 2A) and EC1 cells (Fig. 2B) transfected with HIPK2 or the
control vector. HIPK2 mRNA was significantly increased in EC109 and
EC1 cells transfected with HIPK2 compared with cells transfected
with the control vector (both P<0.001). In addition, HIPK2
overexpression significantly inhibited ESCC cell migration and
invasion in vitro (P<0.05; Fig. 2C and D). These results suggest that
HIPK2 negatively regulates ESCC cell migration and invasion.
HIPK2 overexpression suppresses
EMT
The initial stage of metastasis is dependent on the
prominent biological event referred to as EMT, which is
characterized by specific morphogenetic changes, loss of cell
adhesion and increased cell movement (19). The mechanism by which HIPK2 regulates
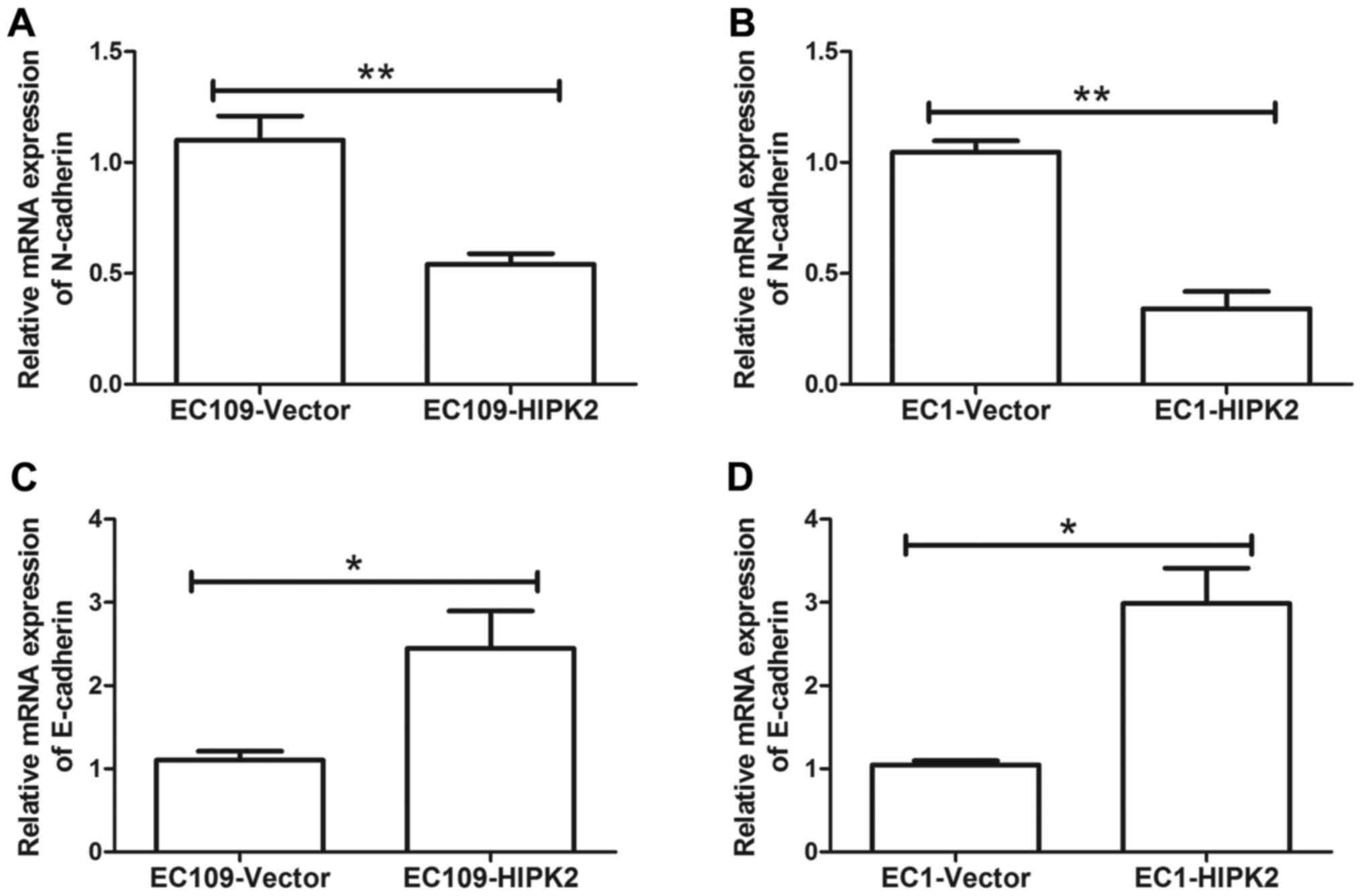
ESCC cell metastasis was investigated. HIPK2 overexpression
decreased the expression of the mesenchymal marker neural
(N)-cadherin mRNA in EC109 (P<0.01; Fig. 3A) and EC1 cells (P<0.01; Fig. 3B). HIPK2 overexpression significantly
increased the expression of epithelial (E)-cadherin mRNA in EC109
and EC1 cells (both P<0.05; Fig. 3C
and D). These results indicate that EMT was suppressed by HIPK2
upregulation.
HIPK2 overexpression inhibits cell
viability during cisplatin treatment
Cisplatin-based chemotherapy is a common regimen
applied for the treatment of ESCC (20). Chemoresistance is a primary cause of
treatment failure in patients with cancer (21). To clarify the molecular mechanisms
underlying cisplatin resistance in ESCC cells, a cisplatin
resistant subline was established. The EC109 ESCC cell line was
treated with cisplatin in gradually increasing concentrations to
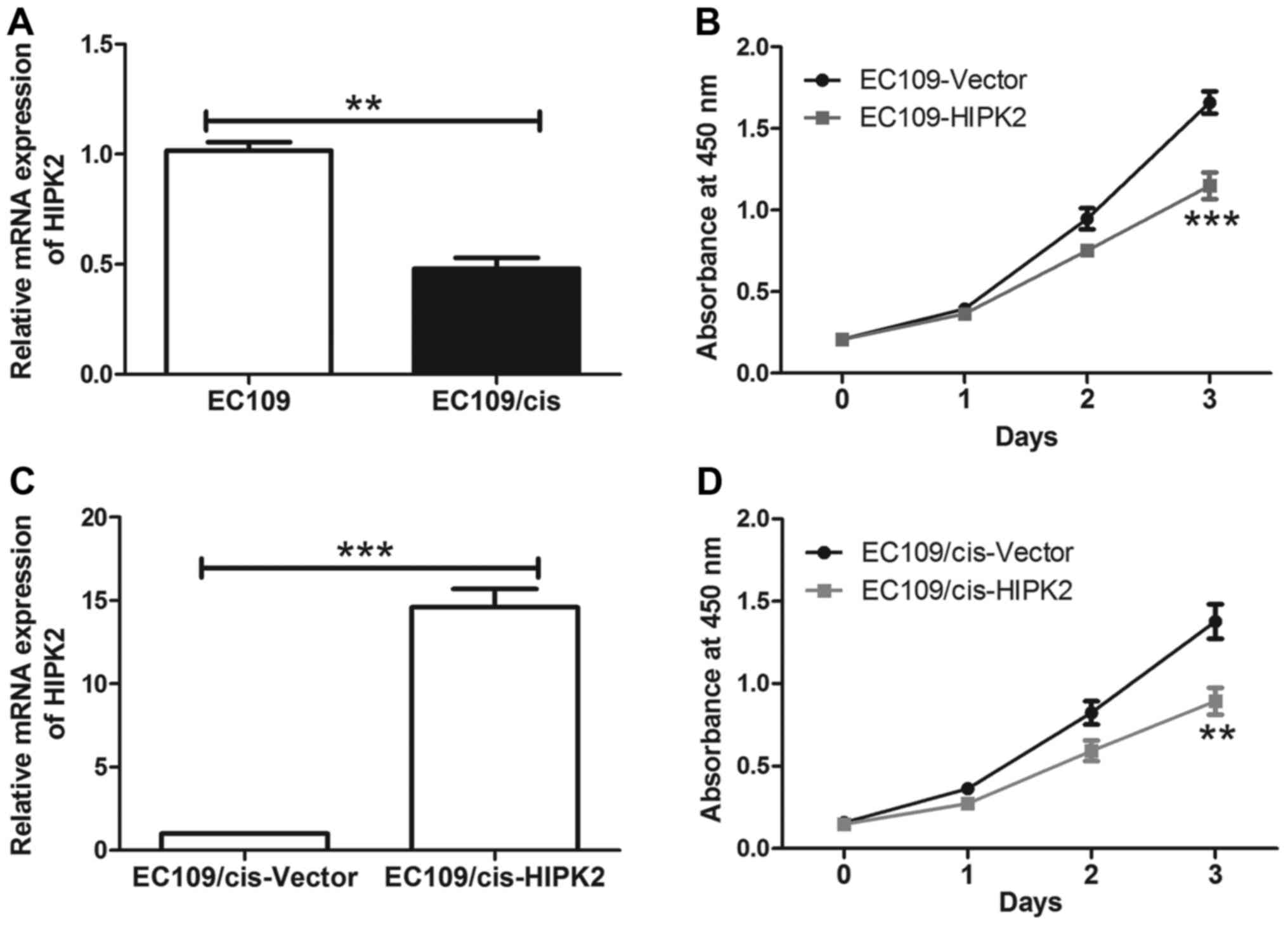
establish a cisplatin resistant cell line (EC109/cis). HIPK2 was
significantly downregulated in chemoresistant EC109/cis cells
(P<0.01; Fig. 4A), indicating
that HIPK2 downregulation may be associated with cisplatin
resistance in ESCC cells. The effect of HIPK2 on cisplatin
sensitivity in ESCC cells was also investigated. HIPK2
overexpression significantly decreased EC109 cell viability
compared with EC109 cells transfected with the control vector
during cisplatin treatment (P<0.001; Fig. 4B). In EC109/cis cells, cisplatin
treatment caused a modest decrease in cell viability compared with
EC109 cells, whereas overexpression of HIPK2 in EC109/cis cells
restored the sensitivity of chemoresistant cells to cisplatin
(P<0.01; Fig. 4C and D). The
overexpression of HIPK2 had no significant effect on the
proliferation of EC109 cells compared with EC109/cis cells. These
results suggest that HIPK2 increased the cisplatin sensitivity of
ESCC cells.
Discussion
The results of the present study indicate that HIPK2
is downregulated in ESCC tissues compared with their adjacent
non-cancerous tissues. This result is consistent with previous
studies, in which HIPK2 downregulation has been reported in bladder
cancer, breast cancer and ovarian cancer (12,14,22).
HIPK2 overexpression has also been demonstrated in patients with
cervical and colorectal cancer with familial adenomatous polyposis
(23,24). The current study demonstrated that
the low expression of HIPK2 was associated with lymph node
metastasis. These results suggest that HIPK2 may be a candidate
tumor suppressor associated with metastasis.
The role of lymphatic metastasis in aggressive
malignancies has been demonstrated in multiple studies and the
occurrence of regional lymph node metastasis at an early stage is a
crucial step in cancer progression (25,26). To
investigate the effect of HIPK2 on cell metastasis, stable HIPK2
overexpression and empty vector cell lines were established.
Upregulation of HIPK2 inhibited the migration and invasion of ESCC
cells. HIPK2 decreased the level of mesenchymal marker N-cadherin
mRNA, which is one of the most important markers of EMT (27). By contrast, HIPK2 increased the
expression of epithelial marker E-cadherin. The current study
therefore demonstrates that HIPK2 suppresses EMT, which is an
important step in cancer metastasis. Cell characteristics are
altered during EMT, resulting in altered cell-cell and cell-matrix
interactions, cell motility and invasiveness (28). Chung et al (29) previously reported that N-cadherin
suppresses RAC-γ serine/threonine-protein kinase to promote cell
motility in mammary tumor cells. Furthermore, Qian et al
(30) demonstrated that N-cadherin
served a pivotal role in promoting metastasis through the
regulation of extracellular-regulated kinase and protein kinase B.
Nodale et al (14)
demonstrated that MDA-MB-231 cells transfected with HIPK2 had
decreased levels of vimentin mRNA that strongly correlated with
re-expression of E-cadherin, which is indicative of a reversion of
the EMT phenotype. Tan et al (15) also demonstrated that HIPK2 knockdown
increased the levels of the mesenchymal markers N-cadherin and
fibronectin and decreased the level of E-cadherin, indicating that
EMT is induced by HIPK2 downregulation. The results of the current
study are consistent with previous studies.
Accumulating evidence also suggests that EMT is
associated with the onset of drug resistance and tumor relapses,
representing an escape mechanism from apoptosis (31). Systematic chemotherapy is an
important clinical strategy used in the treatment of ESCC, which
targets tumor cells and reduces tumor recurrence (32). It serves an important role in ESCC
treatment, especially for patients with advanced and metastatic
ESCC cancer (33). Acquired
chemoresistance of a tumor leads to regrowth during anticancer
agent treatment, even if the tumor initially responds to the
anticancer agents (34). Lin et
al (22) indicated that HIPK2
sensitized chemoresistant bladder cancer cells to cisplatin. In the
present study, it was demonstrated that HIPK2 expression levels
were significantly downregulated in EC109/cis cells compared with
EC109 cells. The forced expression of HIPK2 reduced cell viability
during cisplatin treatment in EC109 cells and HIPK2 overexpression
ameliorated cisplatin resistance in EC109/cis cells. HIPK2 has been
demonstrated to be an important regulator of p53 activity in
response to chemotherapeutic agents (35). In addition, HIPK2 overexpression in
resensitized chemoresistant p53 wild type ovarian cancer cells to
chemotherapy by mediating p53 phosphorylation (12). Consistent with previous studies, the
present study demonstrated that HIPK2 ameliorated chemoresistance,
and this may be via regulation of p53 function, which will be
investigated in the future.
In conclusion, the results of the current study
demonstrate that HIPK2 is downregulated in ESCC specimens and is
associated with metastasis. The results suggest that HIPK2
overexpression suppresses EMT and subsequent cell metastasis.
Furthermore, HIPK2 overexpression partially ameliorates cisplatin
resistance in EC109/cis cells. These results suggest that HIPK2
serves an important role in regulating the metastasis and
chemoresistance of ESCC cells, suggesting a potential application
of HIPK2 in ESCC therapy.
References
|
1
|
Wang LD, Zhou FY, Li XM, Sun LD, Song X,
Jin Y, Li JM, Kong GQ, Qi H, Cui J, et al: Genome-wide association
study of esophageal squamous cell carcinoma in Chinese subjects
identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet.
42:759–763. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hongo M, Nagasaki Y and Shoji T:
Epidemiology of esophageal cancer: Orient to Occident. Effects of
chronology, geography and ethnicity. J Gastroenterol Hepatol.
24:729–735. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morita M, Kumashiro R, Kubo N, Nakashima
Y, Yoshida R, Yoshinaga K, Saeki H, Emi Y, Kakeji Y, Sakaguchi Y,
et al: Alcohol drinking, cigarette smoking, and the development of
squamous cell carcinoma of the esophagus: Epidemiology, clinical
findings, and prevention. Int J Clin Oncol. 15:126–134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown LM, Hoover R, Silverman D, Baris D,
Hayes R, Swanson GM, Schoenberg J, Greenberg R, Liff J, Schwartz A,
et al: Excess incidence of squamous cell esophageal cancer among US
Black men: Role of social class and other risk factors. Am J
Epidemiol. 153:114–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nardinocchi L, Puca R, Sacchi A, Rechavi
G, Givol D and D'Orazi G: Targeting hypoxia in cancer cells by
restoring homeodomain interacting protein-kinase 2 and p53 activity
and suppressing HIF-1alpha. PLoS One. 4:e68192009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Stefano V, Blandino G, Sacchi A, Soddu
S and D'Orazi G: HIPK2 neutralizes MDM2 inhibition rescuing p53
transcriptional activity and apoptotic function. Oncogene.
23:5185–5192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bon G, Di Carlo SE, Folgiero V, Avetrani
P, Lazzari C, D'Orazi G, Brizzi MF, Sacchi A, Soddu S, Blandino G,
et al: Negative regulation of beta4 integrin transcription by
homeodomain-interacting protein kinase 2 and p53 impairs tumor
progression. Cancer Res. 69:5978–5986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ann EJ, Kim MY, Yoon JH, Ahn JS, Jo EH,
Lee HJ, Lee HW, Kang HG, Choi DW, Chun KH, et al: Tumor suppressor
HIPK2 regulates malignant growth via phosphorylation of Notch1.
Cancer Res. 76:4728–4740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin Y, Ratnam K, Chuang PY, Fan Y, Zhong
Y, Dai Y, Mazloom AR, Chen EY, D'Agati V, Xiong H, et al: A systems
approach identifies HIPK2 as a key regulator of kidney fibrosis.
Nat Med. 18:580–588. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pistritto G, Puca R, Nardinocchi L, Sacchi
A and D'Orazi G: HIPK2-induced p53Ser46 phosphorylation activates
the KILLER/DR5-mediated caspase-8 extrinsic apoptotic pathway. Cell
Death Differ. 14:1837–1839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Puca R, Nardinocchi L, Pistritto G and
D'Orazi G: Overexpression of HIPK2 circumvents the blockade of
apoptosis in chemoresistant ovarian cancer cells. Gynecol Oncol.
109:403–410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lazzari C, Prodosmo A, Siepi F, Rinaldo C,
Galli F, Gentileschi M, Bartolazzi A, Costanzo A, Sacchi A,
Guerrini L and Soddu S: HIPK2 phosphorylates ΔNp63α and promotes
its degradation in response to DNA damage. Oncogene. 30:4802–4813.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nodale C, Sheffer M, Jacob-Hirsch J,
Folgiero V, Falcioni R, Aiello A, Garufi A, Rechavi G, Givol D and
D'Orazi G: HIPK2 downregulates vimentin and inhibits breast cancer
cell invasion. Cancer Biol Ther. 13:198–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan M, Gong H, Zeng Y, Tao L, Wang J,
Jiang J, Xu D, Bao E, Qiu J and Liu Z: Downregulation of
homeodomain-interacting protein kinase-2 contributes to bladder
cancer metastasis by regulating Wnt signaling. J Cell Biochem.
115:1762–1767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rice TW, Blackstone EH and Rusch VW: 7th
edition of the AJCC cancer staging Manual: Esophagus and
esophagogastric junction. Ann Surg Oncol. 17:1721–1724. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han T, Zhu X, Wang J, Zhao H, Ma Q, Zhao
J, Qiu X and Fan Q: Establishment and characterization of a
cisplatin-resistant human osteosarcoma cell line. Oncol Rep.
32:1133–1139. 2014.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akita H, Doki Y, Miyata H, Hirao T, Yano
M, Takachi K, Miyashiro I, Sasaki Y, Ishikawa O, Ohigashi H and
Imaoka S: Clinical significance of the second cycle response to
cisplatin-based chemotherapy as preoperative treatment for
esophageal squamous cell carcinoma. J Surg Oncol. 93:401–409. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu A, Zhu J, Wu G, Cao L, Tan Z, Zhang S,
Jiang L, Wu J, Li M, Song L and Li J: Antagonizing miR-455-3p
inhibits chemoresistance and aggressiveness in esophageal squamous
cell carcinoma. Mol Cancer. 16:1062017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J, Zhang Q, Lu Y, Xue W, Xu Y, Zhu Y
and Hu X: Downregulation of HIPK2 increases resistance of bladder
cancer cell to cisplatin by regulating Wip1. PLoS One.
9:e984182014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng Y, Al-Beiti MA, Wang J, Wei G, Li J,
Liang S and Lu X: Correlation between homeodomain-interacting
protein kinase 2 and apoptosis in cervical cancer. Mol Med Rep.
5:1251–1255. 2012.PubMed/NCBI
|
|
24
|
D'Orazi G, Sciulli MG, Di Stefano V,
Riccioni S, Frattini M, Falcioni R, Bertario L, Sacchi A and
Patrignani P: Homeodomain-interacting protein kinase-2 restrains
cytosolic phospholipase A2-dependent prostaglandin E2 generation in
human colorectal cancer cells. Clin Cancer Res. 12:735–741. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu WH, Hsu PK, Hsieh CC, Huang CS and Wu
YC: The metastatic lymph node number and ratio are independent
prognostic factors in esophageal cancer. J Gastrointest Surg.
13:1913–1920. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hosch SB, Stoecklein NH, Pichlmeier U,
Rehders A, Scheunemann P, Niendorf A, Knoefel WT and Izbicki JR:
Esophageal cancer: The mode of lymphatic tumor cell spread and its
prognostic significance. J Clin Oncol. 19:1970–1975. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
29
|
Chung S, Yao J, Suyama K, Bajaj S, Qian X,
Loudig OD, Eugenin EA, Phillips GR and Hazan RB: N-cadherin
regulates mammary tumor cell migration through Akt3 suppression.
Oncogene. 32:422–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian X, Anzovino A, Kim S, Suyama K, Yao
J, Hulit J, Agiostratidou G, Chandiramani N, McDaid HM, Nagi C, et
al: N-cadherin/FGFR promotes metastasis through
epithelial-to-mesenchymal transition and stem/progenitor cell-like
properties. Oncogene. 33:3411–3421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mitra A, Mishra L and Li S: EMT, CTCs and
CSCs in tumor relapse and drug-resistance. Oncotarget.
6:10697–10711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin YY, Chen QJ, Xu K, Ren HT, Bao X, Ma
YN, Wei Y and Ma HB: Involvement of microRNA-141-3p in
5-fluorouracil and oxaliplatin chemo-resistance in esophageal
cancer cells via regulation of PTEN. Mol Cell Biochem. 422:161–170.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lagergren J, Smyth E, Cunningham D and
Lagergren P: Oesophageal cancer. Lancet. Jun 22–2017.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshida T, Miyoshi T, Seike J, Yamai H,
Takechi H, Yuasa Y and Tangoku A: Gene expression changes in a
chemoresistant model with human esophageal cancer xenografts using
cDNA microarray. Anticancer Res. 29:1163–1168. 2009.PubMed/NCBI
|
|
35
|
Puca R, Nardinocchi L, Givol D and D'Orazi
G: Regulation of p53 activity by HIPK2: MOlecular mechanisms and
therapeutical implications in human cancer cells. Oncogene.
29:4378–4387. 2010. View Article : Google Scholar : PubMed/NCBI
|