Introduction
Hepatitis B virus (HBV) infection is a challenging
disease, and has a high incidence in regions around the world.
China is one of the regions with highest incidences of HBV
infection, and the disease prevention is still urgent. It was
reported that there were about 93 million people in China who were
chronic carriers of HBV in 2006, and ~30,000 people became new
patients with chronic hepatitis B. Each year the mortality from
cirrhosis, liver cancer and other diseases associated with
hepatitis B was as many as 300,000 (1,2). As
early as 1992, China started to include hepatitis B vaccination in
the national planned immunization program, requesting all newborn
infants be inoculated with the hepatitis B vaccine (3). Although remarkable achievements have
been made, a study found that the rate of non- and hypo-response to
hepatitis B vaccine was higher for newborn infants born to mothers
who were positive for HBV surface antigen (HBsAg) than that for
newborn infants born to normal mothers of general population. These
infants had a higher risk to be HBV carriers (4), which caused great physiological and
psychological stress to infants themselves and their parents as
well. Hepatitis B immune globulin (HBIG) belongs to the category of
passive antibodies. HBIG can produce anti-HBs antibodies within a
few hours after injection, which neutralize and remove toxins from
the body. HBIG provides passive immunization for patients who are
already exposed to HBV (5). Studies
have shown that combination of the above two immunization
approaches had a certain advantage in blocking the mother to infant
transmission of HBV (6).
We further studied the effect of HBIG combined with
hepatitis B vaccine on blocking HBV transmission between mother and
infant, with the aim to confirm and promote this combined
immunization remedy from the aspect of immune function, by
observing the effect of HBIG on immune cells.
Subjects and methods
Subjects
Ninety newborn infants (single birth) born to
maternity patients in Linyi Hospital who were confirmed to be
HBsAg-positive in prenatal examination from June 2014 to August
2016 were recruited as research subjects. All maternity patients
who met the selection criteria in this study or their families
signed the informed consent. The research protocol was approved by
the Hospital Ethics Committee. Based on the principle of voluntary
participation, the subjects were divided into three groups. Thirty
subjects in group A received the hepatitis B vaccine at 0, 1 and 6
months after birth at a dose of 10 µg each time. Thirty subjects in
group B received an intramuscular injection of 100 IU HBIG 2 h
after birth before getting the same treatment as group A. Mothers
of 30 subjects in group C received a total of three gluteus maxinus
injections of 200 IU HBIG each time at 28 weeks of gestation, 4
weeks and 8 weeks later. The subjects in group C got the same
treatment as group B. In Table I are
given the general medical data of the maternity patients and the
newborn infants in the three groups, including maternal average
age, pregnancy cycle, HBsAb-positive cases, HBsAg/HBeAg double
positive cases, mode of delivery, average infant birth weight, and
infant sex. The data were comparable between groups, and the
differences were not statistically significant (p>0.05).
 | Table I.General data of subjects in the three
groups. |
Table I.
General data of subjects in the three
groups.
|
|
|
|
| Mode of delivery |
|
| Infant sex |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Group | HBsAb positive
cases | HBsAg/HBeAg positive
cases | Maternal age
(years) | Natural |
| Caesarean | Pregnancy cycle
(week) | Infant birth weight
(kg) | Male |
| Female |
|---|
| A | 30 | 11 | 26.23±6.35 | 19 |
| 11 | 38.23±2.07 | 3.43±0.76 | 16 |
| 14 |
| B | 30 | 10 | 27.09±6.87 | 18 |
| 12 | 38.12±2.11 | 3.36±0.68 | 17 |
| 13 |
| C | 30 | 12 | 26.67±6.33 | 20 |
| 10 | 38.43±2.43 | 3.49±0.43 | 16 |
| 14 |
| P-value | >0.05 | >0.05 | >0.05 |
| >0.05 |
| >0.05 | >0.05 |
| >0.05 |
|
Methods
Maternity patients in group A and B underwent no
pretreatment for HBV infection before delivery, but were provided
with normal supply of nutrients and adequate psychological
counseling. Maternity patients in group C received a total of three
gluteus maxinus injections of 200 IU HBIG each time at 28 weeks of
gestation, 4 weeks and 8 weeks later. Infants in groups A-C were
all vaccinated with hepatitis B vaccine on an immunization schedule
of 0, 1, and 6 months with a dose of 10 µg. In addition to
receiving the required hepatitis B vaccination, infants in group B
and C were required to receive an intramuscular injection of 100 IU
HBIG within 2 h after birth. All newborn infants were followed-up
until the age of 12 months. Venous blood samples were collected
before treatment and at the age of 12 months.
The blood samples were tested for HBV infection
(two-and-half pair test) including hepatitis B surface antigen and
antibody (HbsAg and HBsAb), hepatitis B e-antigen and e-antibody
(HBeAg and HBeAb), and hepatitis B core antibody (HBcAb). Blocking
effects of different treatments on HBV transmission were analyzed
from these data. Changes of immune cells or related cytokines
before and after interventions were also analyzed.
Observed indicators
The blocking effect on HBV mother-to-infant
transmission was assessed using the following criteria (7). Among the five above-mentioned tested
items, a positive HBsAb only indicated a successful blockade;
positive HBsAb, HBeAb and HBcAb together indicated an effective
blockade; either positive HBsAg or positive HBeAg indicated an
invalid blockade.
Cellular immune functions were evaluated based on
following indicators (8). Levels of
CD3+, CD4+ and CD8+, and the ratio
of CD4+/CD8+ in serum were measured by
streptavidin alkaline phosphatase (SAP) method. Levels of IFN-γ and
IL-2 were measured by ELISA before and after immunization.
Statistical analysis
The SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA) package was used for statistical analysis. Measurement data
are expressed as mean ± SD, and t-test was used. Enumeration data
are expressed as %, and χ2 test was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
Blocking effects on HBV transmission
between mother and infant
In Table II are
listed the results of blocking effects on HBV transmission between
mother and infant, which showed that the three different treatments
were all effective. The numbers of infants who were HbsAb-positive
were 24, 27 and 29, respectively, in groups A-C, corresponding to
blocking success rates of 80, 90 and 97%, respectively. The
differences of blocking success rates between group A and B, and
between group A and C as well, were statistically significantly
(p<0.05), but there was no significant difference between group
B and C (p>0.05), though the overall blocking effect in group C
was better than in group B.
 | Table II.Blocking effects on HBV transmission
between mother and infant. |
Table II.
Blocking effects on HBV transmission
between mother and infant.
| Group | Cases | Cases of successful
blockade | Cases of effective
blockade | Cases of invalid
blockade | Successful blocking
rate (%) |
|---|
| A | 30 | 24 | 3 | 3 | 80 |
| B | 30 | 27 | 1 | 2 | 90 |
| C | 30 | 29 | 0 | 1 | 97 |
| Total | 90 | 82 | 4 | 5 | 91 |
Blocking effects on HBV transmission
between mother and infant when mothers were postitive for both
HBsAg and HBeAg
The blocking results are shown in Table III when mothers were postitive for
both HBsAg and HBeAg. The successful blocking rate in group A was
lower than those in both group B and C, and the differences were
statistically significant (p<0.05). In addition, the successful
blocking rate in group B was lower than that in group C
(p<0.05).
 | Table III.Blocking effects on HBV transmission
between mother and infant when mothers were postitive for both
HBsAg and HBeAg. |
Table III.
Blocking effects on HBV transmission
between mother and infant when mothers were postitive for both
HBsAg and HBeAg.
| Cases | Cases of successful
blockade | Cases of effective
blockade | Cases of invalid
blockade | Successful blocking
rate (%) |
|---|
| 11 | 5 | 3 | 3 | 45 |
| 10 | 7 | 1 | 2 | 70 |
| 12 | 11 | 0 | 1 | 92 |
| 22 | 18 | 1 | 3 | 82 |
Effects of three blocking approaches
on immune cells
The effects of different immunological approaches on
immune cells were analyzed by comparing the changes of T lymphocyte
subsets from the first immunological intervention to the end of the
12 months follow-up. The results are given in Table IV, showing levels of T lymphocyte
subsets CD3+, CD4+ and CD8+ in
peripheral blood of the newborn infants in group C were lower than
those in group A and B at 24 h after birth, and the differences
were significant (p<0.05). The ratios of
CD4+/CD8+ in both group B and C were lower
than that in group A (p<0.05).
 | Table IV.Changes of T lymphocyte subsets in the
three groups. |
Table IV.
Changes of T lymphocyte subsets in the
three groups.
|
| Group A (%) | Group B (%) | Group C (%) |
|---|
|
|
|
|
|
|---|
| Item | 24 h after birth | 12 months | 24 h after birth | 12 months | 24 h after birth | 12 months |
|---|
| CD3+ | 62.31±9.61 | 63.96±10.01 | 60.46±10.65 | 67.48±9.87 | 58.73±5.76 | 62.87±6.82 |
| CD4+ | 43.52±11.34 | 45.63±12.45 | 42.89±10.67 | 46.03±11.09 | 40.12±6.18 | 47.67±5.91 |
|
CD8+ | 16.37±5.91 | 17.02±4.89 | 14.65±3.12 | 15.57±5.12 | 14.16±4.92 | 15.36±6.64 |
|
CD4+/CD8+ | 2.65 | 2.68 | 2.86 | 2.95 | 2.83 | 3.1 |
At the end of the 12 months follow-up, the level of
T lymphocyte subset CD3+ in group B was significantly
higher than those in group A and C (p<0.05). The CD4+
level and the CD4+/CD8+ ratio in group C were
all higher than those in group A and B (p<0.05), but the
differences of these two items between group A and B, and between
group B and C, were not significant (p>0.05). The
CD8+ level in group C was lower than those in group A
and B. The difference with group A was significant (p<0.05), but
the difference with group B was not significant (p>0.05).
For all the three immunological approaches, levels
of T lymphocyte CD3+, CD4+ and
CD8+, and CD4+/CD8+ ratio were all
elevated at the end of follow-up, compared with those at 24 h after
birth. The differences of CD4+ levels before and after
treatment were significant in group B and C (p<0.05).
Effects of three blocking approaches
on IFN-γ and IL-2
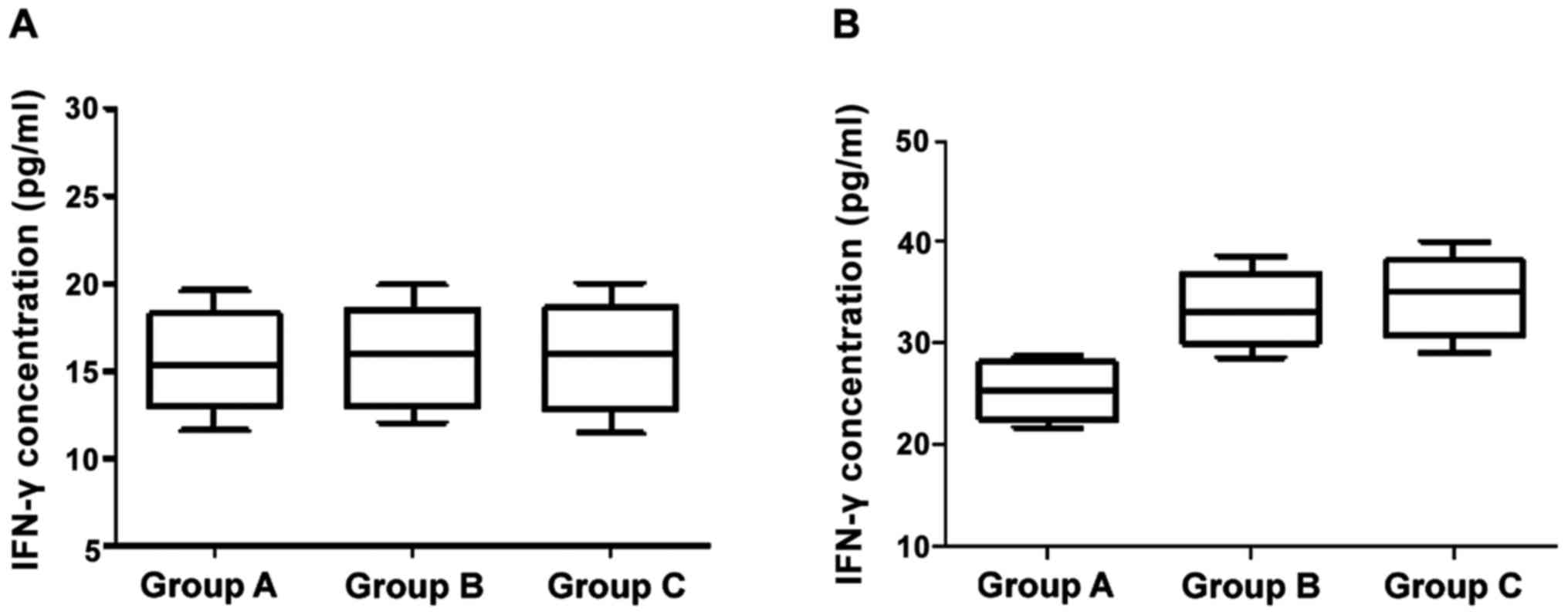
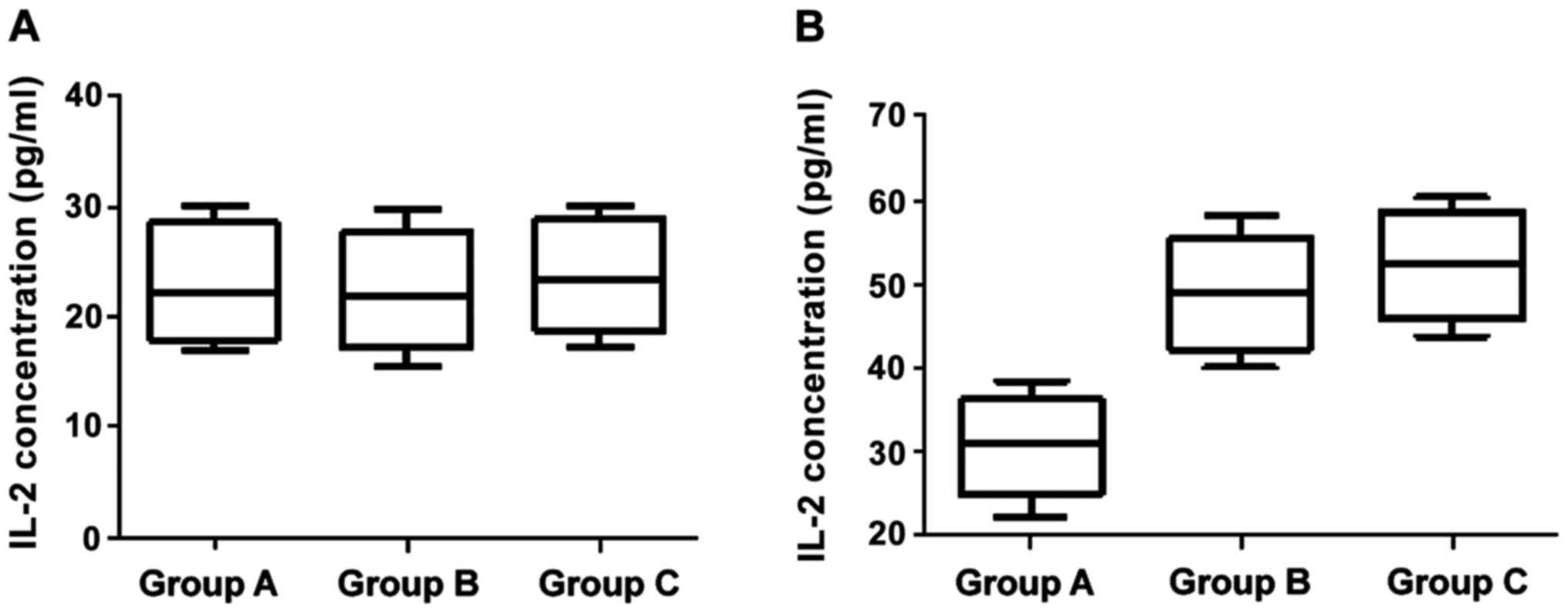
Changes of levels of IFN-γ and IL-2 at 24 h and 12
months after birth with corresponding immunological intervention
are shown in Figs. 1 and 2. The differences of IFN-γ and IL-2 levels
across the three groups were not statistically significant at 24 h
after birth (p>0.05). At the age of 12 months, however, the
differences between groups B/C who were treated with HBIG combined
with hepatitis B vaccine and group A were significant (p<0.05),
but the difference between group B and C was not statistically
significant (p>0.05).
Discussion
Hepatitis B is a highly contagious disease caused by
HBV. The disease can cause damage to a variety of organs at its
advanced stage (9). Hepatitis B is
widely distributed and hard to cure, thus is one of the major
diseases that threaten human life. Its prevention and control
measures should be implemented without delay. In the early 21st
century, China began to include hepatitis B vaccine in the
prevention plan for newborns. A total of three injections of 5
g/dose of recombinant yeast hepatitis B vaccine or 10 g/dose of CHO
hepatitis B vaccine were required for neonatal vaccination
(10). However, because
mother-to-infant transmission ranks first in all HBV transmission
routes, there are still many chronic HBV infection cases due to
perinatal mother-to-infant transmission (11).
HBIG is a highly effective immunoglobulin collected
from plasma of healthy people who are inoculated with hepatitis B
vaccine. Treatment of HBIG can reduce the virus level in the blood
system, so that less normal cells will be infected due to decreased
virus replication, thus enhancing humoral immune function (12). Studies showed that HBV
mother-to-infant transmission can be blocked by ~90% if a newborn
infant was injected with HBIG after birth and at the same time
inoculated with hepatitis B vaccine according to the vaccination
schedule (13). In another study, it
was reported that HBV infection of newborn infants can be
effectively prevented and blocked if maternity patients were
treated with HBIG before delivery and additionally newborn infants
were treated with HBIG combined with hepatitis B vaccination
(14). In the present study, the
effect of treatment approaches for both mothers and infants on
blocking HBV transmission was explored, in which mothers were
treated with HBIG before delivery and infants were treated with
HBIG combined with hepatitis B vaccination. The theoretical base of
this study is stated below (15,16).
Twenty weeks after gestation, the placenta possesses the function
of transferring maternal IgG antibodies to the fetus by active
transport. This is even more obvious in the late stage of
pregnancy. In addition, various fetal organs generally reach their
complete development at this stage, allowing drug administration to
be safer to the fetus. Thus prenatal intervention measures at this
time can be effective in blocking the transmission of HBV in the
uterus. The results of this study are consistent with this
theory.
The origin and progression of hepatitis B is
directly related to the immune response, which is mediated and
regulated by immune cells and their secreted cytokines. Therefore,
the study of the impact of immune intervention measures on immune
cells is important to further understand the disease. It was
reported that the main immunological indicators of physical
immunity were T lymphocyte levels and the ratios of T cell subsets
(17). T lymphocytes are important
immunologically active cells, whose subsets, the CD4+
cells and CD8+ cells, can modulate the activity of other
immune cells (18). Lower than
normal T lymphocyte level and its subset ratios indicate an HBV
infection and immune modulation disorder. Measures of immunization
intervention are implemented in order to improve physical immune
function. IFN-γ is a cytokine that is secreted by helper T cells,
specifically by Th1 cells. It has been shown to be a crucial player
in the immune response (19).
Immunization intervention increased IFN-γ levels in serum, which
inhibited replication of HBV and improved immune function of the
body (20).
In this study, the effects of three different
immunization approaches on blocking HBV mother-to-infant
transmission were compared. The results showed that the blocking
effect of HBIG combined with hepatitis B vaccination was better
than the blocking effect of hepatitis B vaccination alone for
newborn infants born to mothers who were HBsAg-positive. For those
newborn infants born to mothers who were positive for both HBsAg
and HBeAg, HBIG intervention for mothers during late pregnancy,
together with combined treatment of HBIG and hepatitis B
vaccination for infants, gave better blocking result of HBV
transmission, than the same treatment for infants only and not
affecting the mothers. In addition, in the study of effects of
different immunization approaches on immune cells, it was found
that the treatment approaches for both mother and infant increased
the levels of CD4+ and other T cell subsets, the subset
ratios, and the IFN-γ cytokine in infants. These changes made a
great impact on immune cells and thus improved the immune
function.
References
|
1
|
Alavian SM, Fallahian F and Lankarani KB:
The changing epidemiology of viral hepatitis B in Iran. J
Gastrointestin Liver Dis. 16:403–406. 2007.PubMed/NCBI
|
|
2
|
Rehermann B and Nascimbeni M: Immunology
of hepatitis B virus and hepatitis C virus infection. Nat Rev
Immunol. 5:215–229. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng YL, Wang SP, Wei JN, Shi XH, Zhang
JB, Guo Q, Wu XB, Fan H and Wang XF: Comprehensive study on the
risk factors of hepatitis B virus intrauterine infection. Zhonghua
Liu Xing Bing Xue Za Zhi. 29:132–135. 2008.(In Chinese). PubMed/NCBI
|
|
4
|
Komatsu H, Inui A, Sogo T, Hiejima E,
Tateno A, Klenerman P and Fujisawa T: Cellular immunity in children
with successful immunoprophylactic treatment for mother-to-child
transmission of hepatitis B virus. BMC Infect Dis. 10:1032010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beasley RP, Hwang LY, Lee GC, Lan CC, Roan
CH, Huang FY and Chen CL: Prevention of perinatally transmitted
hepatitis B virus infections with hepatitis B immune globulin and
hepatitis B vaccine. Lancet. 2:1099–1102. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang YL and Fu SM: Immunity mechanism of
vertical transmission blocking for HBV immunoglobulin injection to
pregnant women. Chin J Immunol. 31:818–821. 2015.
|
|
7
|
Wang GL, Liu Y, Qiu P, Zhou SF, Xu LF, Wen
P, Wen JB and Xiao XZ: Cost-effectiveness of Lamivudine,
Telbivudine, Adefovir Dipivoxil and Entecavir on decompensated
hepatitis B virus-related cirrhosis. Eur Rev Med Pharmacol Sci.
20:866–872. 2016.PubMed/NCBI
|
|
8
|
Han GR, Jiang HX, Yue X, Ding Y, Wang CM,
Wang GJ and Yang YF: Efficacy and safety of telbivudine treatment:
An open-label, prospective study in pregnant women for the
prevention of perinatal transmission of hepatitis B virus
infection. J Viral Hepat. 22:754–762. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lavanchy D: Hepatitis B virus
epidemiology, disease burden, treatment, and current and emerging
prevention and control measures. J Viral Hepat. 11:97–107. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luna EJ, Moraes JC, Silveira L and Salinas
HS: Efficacy and safety of the Brazilian vaccine against hepatitis
B in newborns. Rev Saude Publica. 43:1014–1020. 2009.(In
Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko SC, Schillie SF, Walker T, Veselsky SL,
Nelson NP, Lazaroff J, Crowley S, Dusek C, Loggins K, Onye K, et
al: Hepatitis B vaccine response among infants born to hepatitis B
surface antigen-positive women. Vaccine. 32:2127–2133. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Féray C, Gigou M, Samuel D, Ducot B,
Maisonneuve P, Reynès M, Bismuth A and Bismuth H: Incidence of
hepatitis C in patients receiving different preparations of
hepatitis B immunoglobulins after liver transplantation. Ann Intern
Med. 128:810–816. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan HL and Jia J: Chronic hepatitis B in
Asia-new insights from the past decade. J Gastroenterol Hepatol. 26
Suppl 1:131–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raghuraman S, Park H, Osburn WO,
Winkelstein E, Edlin BR and Rehermann B: Spontaneous clearance of
chronic hepatitis C virus infection is associated with appearance
of neutralizing antibodies and reversal of T-cell exhaustion. J
Infect Dis. 205:763–771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ellinger I, Rothe A, Grill M and Fuchs R:
Apical to basolateral transcytosis and apical recycling of
immunoglobulin G in trophoblast-derived BeWo cells: Effects of low
temperature, nocodazole, and cytochalasin D. Exp Cell Res.
269:322–331. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ellinger I, Schwab M, Stefanescu A,
Hunziker W and Fuchs R: IgG transport across trophoblast-derived
BeWo cells: A model system to study IgG transport in the placenta.
Eur J Immunol. 29:733–744. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng Y, Huang Z, Chen X, Tian Y, Tang J,
Zhang Y, Zhang X, Zhou J, Mao Q, Ni B, et al: Effects of
telbivudine treatment on the circulating CD4+ T-cell
subpopulations in chronic hepatitis B patients. Mediators Inflamm.
2012:7898592012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
von Boehmer H: Mechanisms of suppression
by suppressor T cells. Nat Immunol. 6:338–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jones SW, Roberts RA, Robbins GR, Perry
JL, Kai MP, Chen K, Bo T, Napier ME, Ting JP, Desimone JM, et al:
Nanoparticle clearance is governed by Th1/Th2 immunity and strain
background. J Clin Invest. 123:3061–3073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang K, Fan X, Fan Y, Wang B, Han L and
Hou Y: Study on the function of circulating plasmacytoid dendritic
cells in the immunoactive phase of patients with chronic genotype B
and C HBV infection. J Viral Hepat. 14:276–282. 2007. View Article : Google Scholar : PubMed/NCBI
|