Introduction
Hepatitis C virus (HCV) was discovered by Choo et
al in the United States in 1989 (1). It revealed that over 90% of cases
diagnosed previously as non-A non-B hepatitis is caused by HCV.
There are an estimated 170 million HCV-infected patients worldwide
(2–4). It is estimated that 15–30% of such
patients will develop serious complications, including liver
cirrhosis, end-stage liver disease and hepatocellular carcinoma
(5). HCV-infected patients have
mortality rate of 5.0 deaths/100,000 population in 2013 (6).
Recently, direct acting antiviral agents (DAAs) were
developed and advanced interferon (IFN)-free treatment. As a
result, a high rate of sustained virologic response (SVR) has
shown, and a reduction of side effects during treatment is also
anticipated. DAAs selectively inhibit HCV proteins such as
nonstructural protein NS 3/4A protease, NS5A, NS5B polymerase
(7,8). New DAA combination therapies such as
sofosbuvir plus ledipasvir and ombitasvir/paritaprevir/ritonavir
have also recently been approved in Japan (9–11).
Previous studies have shown that HCV-infected patients treated with
IFN-containing DAAs experience a significant health-related quality
of life (HRQOL) impairment. IFN-containing DAAs often cause
side-effects, such as flu-like-symptoms and fatigue,
gastrointestinal disorders. Thereby reducing HRQOL during treatment
(12,13). On the other hand, IFN-free DAAs have
been reported to have little impact on HRQOL and side effects
during treatment (14–17). However, very few studies have
examined HRQOL during daclatasvir/asunaprevir therapy (DCV/ASV
therapy).
The purpose of this study was to evaluate HRQOL on
the clinical course of patients with chronic hepatitis C (CHC)
receiving DCV/ASV therapy using the Short Form-36 (SF-36) and
comparison between younger (<70) and elderly (≥70) patients.
Subjects and methods
A prospective observational design was used to
conduct this study. A total 30 CHC and cirrhotic patients underwent
DCV/ASV therapy were invited to participate in the study from
September 2014 to February 2017 at Saiseikai Niigata Daini Hospital
(Niigata, Japan). Written informed consent was obtained from all
patients, and the Ethics Committee of Saiseikai Niigata Daini
Hospital (Niigata, Japan) approved this study, which was conducted
in accordance with the Declaration of Helsinki.
All patients received fixed dose of DCV (60 mg once
daily) and ASV (100 mg twice daily) for 24 weeks. Two of these 30
patients were excluded from analysis because they required
treatments for hepatocellular cancer and other malignant tumors
during therapy. As a result, 28 patients were analyzed. HCV-RNA
were measured using the RealTime HCV assay (Abbott Laboratories,
Abott Park, IL, USA) with a lower limit of qualification of 12
IU/ml at baseline, every 2 weeks during treatment and every 2 weeks
until 24 weeks after completion or cession of the dual oral
therapy. SVR was defined as negative for serum HCV RNA at 24 weeks
after end of treatment (EOT). HRQOL was measured by the SF-36. The
SF-36 comprised 36 questions, with 8 subscales related to physical
and mental health: Physical functioning (PF), physical role
functioning (RP), bodily pain (BP), general health (GH), vitality
(VT), social role functioning (SF), emotional role functioning
(RE), and mental health (MH). Each subscale is scored from 0 to
100, and higher scores indicate greater HRQOL. In this study,
patients were asked to fill out the SF-36 before DCV/ASV therapy
(baseline), after 12 weeks of DCV/ASV therapy (12 weeks), at the
EOT, and at SVR week 24 (SVR24) to evaluate HRQOL. Blood
biochemistry was also investigated during the same period, and
associations between these results and SF-36 were investigated.
Statistical analysis
Patient characteristics were summarized with means
and standard deviations. SF-36 scores were summarized as
proportions with median and interquartile range. Continuous
variables were compared by Student's t-test or Mann-Whitney U test.
Categorical variables were compared by Fisher's exact test. The
changes in SF-36 and blood biochemistry measurements from baseline
were compared using Student's t-test. The Friedman test was used
for comparison of repeated measures over time, and Bonferroni's
multiple comparison correction was used for post hoc analysis.
P<0.05 was considered to indicate a statistically significant
difference.
All statistical analyses were performed with EZR
(Saitama Medical Center, Jichi Medical University, Saitama, Japan),
which is a graphical user interface for R (The R Foundation for
Statistical Computing, Vienna, Austria). More precisely, it is a
modified version of R commander designed to add statistical
functions frequently used in biostatistics (18).
Results
Twenty-eight patients who underwent DCV/ASV therapy
and SVR assessment between September 2014 and February 2017 were
analyzed. Subjects comprised 12 men and 16 women, with a mean age
of 70.46 years. Among 28 patients, SVR24 was achieved by 26 (93%).
Gender, mean age, and blood biochemistry measurements before
therapy are shown in Table I, and
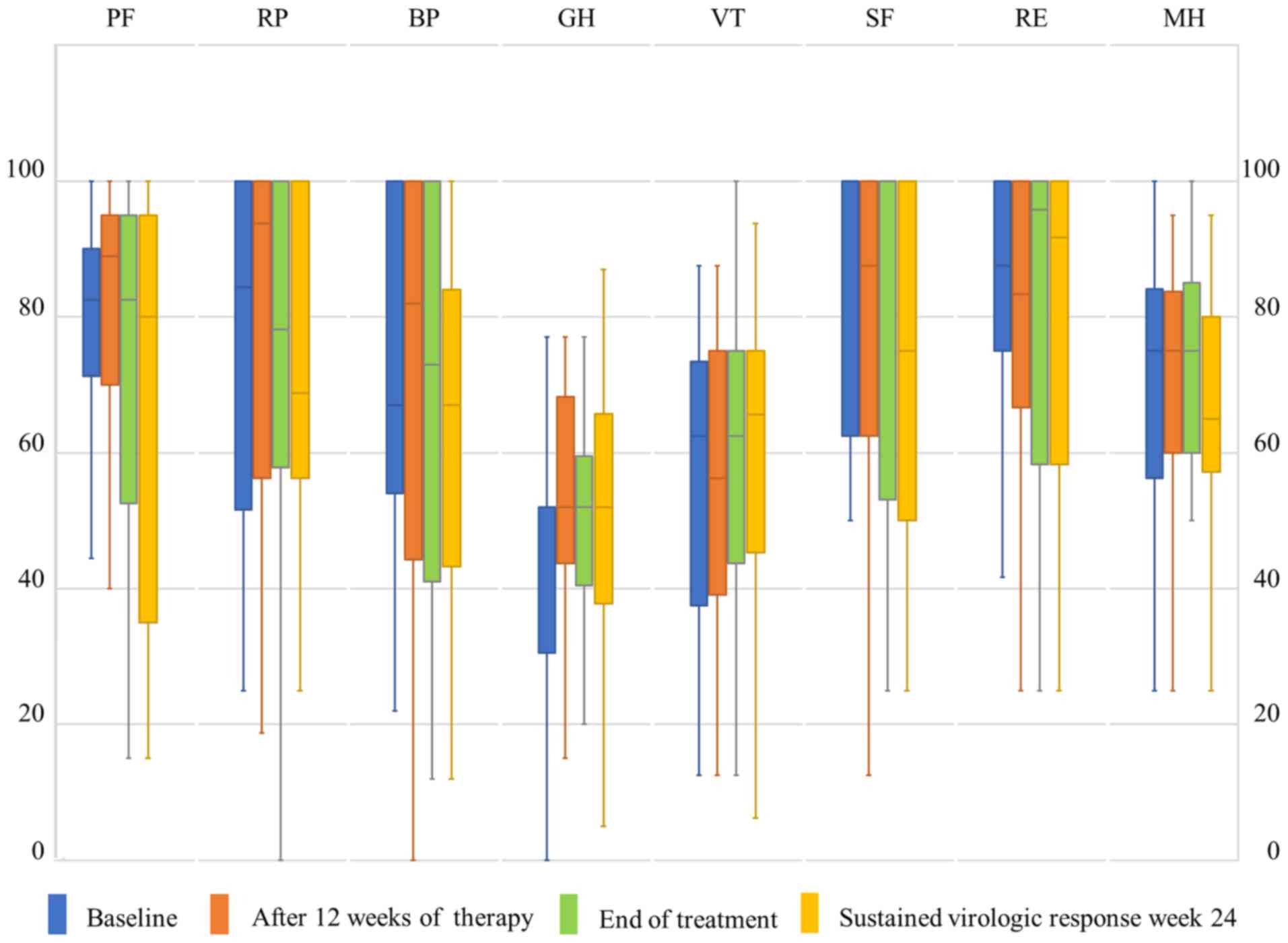
SF-36 scores at baseline are shown in Table II. GH was low in patients undergoing
this therapy (Table II). No
significant changes in the courses of any SF-36 subscale were
identified during therapy (Fig. 1).
For blood biochemistry measurements, aspartate aminotransferase
(AST), alanine aminotransferase (ALT), serum albumin (ALB),
α-fetoprotein (AFP), Platelets count (PLT), and FIB-4 index were
all significantly improved at each time point compared to baseline
(Table III).
 | Table I.Baseline characteristics of the
patients recruited to the present study (n=28). |
Table I.
Baseline characteristics of the
patients recruited to the present study (n=28).
| Characteristic | Mean ± standard
deviation, or number |
|---|
| Age (years) |
70.46±12.00 |
| Gender
(male/female) | 12/16 |
| AST (IU/l) |
41.43±19.40 |
| ALT (IU/l) |
36.14±20.48 |
| Hb (g/dl) | 12.54±2.18 |
| PLT
(×104) | 15.26±8.55 |
| Fib-4 |
5.28±5.19 |
| ALB (mg/dl) |
3.80±0.42 |
| AFP (ng/ml) | 4.90 (3.08,
8.93) |
| eGFR (ml/m/1.73
m2) |
70.54±23.95 |
| Hepatocellular
carcinoma (yes/no) | 6/22 |
 | Table II.Baseline Short Form-36 survey score of
participants. |
Table II.
Baseline Short Form-36 survey score of
participants.
| SF-36 subscale | Median (interquartile
range) |
|---|
| Physical
functioning | 82.50 (73.75,
90.00) |
| Physical role
functioning | 84.38
(54.69, 100.00) |
| Bodily pain | 67.00
(58.00, 100.00) |
| General health | 52.00 (31.50,
52.00) |
| Vitality | 62.50 (37.50,
70.31) |
| Social role
functioning | 100.00 (62.50,
100.00) |
| Emotional role
functioning | 87.50
(75.00, 100.00) |
| Mental health | 75.00 (58.75,
82.19) |
 | Table III.Differences in blood biochemistry
during and following daclatasvir/asunaprevir therapy. |
Table III.
Differences in blood biochemistry
during and following daclatasvir/asunaprevir therapy.
| Factor | Baseline | 12 weeks | EOT | SVR24 | P-value |
|---|
| AST (IU/l) | 41.43±19.40 |
26.14±9.48a |
24.46±7.71a |
22.57±7.05a | <0.01 |
| ALT (IU/l) | 36.14±20.48 |
20.07±11.69a |
17.61±7.41a |
15.32±7.30a | <0.01 |
| Hb (g/dl) | 12.54±2.18 | 12.56±2.21 | 12.53±2.38 | 12.39±2.67 |
0.72 |
| PLT
(×104/µl) | 15.26±8.55 | 15.47±7.70 | 15.49±7.67 |
17.14±9.25a | <0.01 |
| Fib-4 | 5.28±5.19 |
3.79±2.77a |
3.63±2.62a |
3.40±2.33a | <0.01 |
| ALB (mg/dl) | 3.80±0.42 |
3.87±0.40 |
3.92±0.30 |
3.99±0.41a | <0.01 |
| AFP (ng/ml) | 4.90 (3.08,
8.93) | 2.80 (2.38,
5.92)a | 2.85 (2.30,
5.43)a | 3.05 (2.40,
5.45)a | <0.01 |
| eGFR (ml/m/1.73
m2) | 70.54±23.95 |
66.12±22.22 |
66.35±21.98 |
66.43±21.77 | <0.05 |
The healthy life expectancy of the Japanese is 70.66
years for males and 75.55 years for females (19). We assumed that about 70 years old is
a branch point of HRQOL and divided it into two groups of under 70
and over 70 years, and blood biochemistry measurements (Table IV) and SF-36 scores (Table V) at baseline were compared between
groups. Regarding changes in HRQOL during therapy, the ≥70-year-old
group displayed a significantly greater improvement in PF during
the period between baseline and 12 weeks compared to the
<70-year-old group (Table VI).
No significant differences in changes to blood biochemistry
measurements were seen between groups.
 | Table IV.Baseline characteristics of
participants and treatment outcome according to age. |
Table IV.
Baseline characteristics of
participants and treatment outcome according to age.
| Baseline | Age <70 | Age ≥70 | P-value |
|---|
| Age (years) | 58.09±7.84 | 78.47±5.58 | <0.01 |
| Gender
(male/female) | 7/4 | 5/12 | 0.12 |
| AST (IU/l) |
36.82±15.20 |
44.41±21.60 | 0.32 |
| ALT (IU/l) |
37.09±19.05 |
35.53±21.91 | 0.84 |
| Hb (g/dl) | 13.86±1.83 | 11.68±1.99 | <0.01 |
| PLT
(×104/dl) |
18.49±10.27 | 13.18±6.77 | 0.11 |
| Fib-4 |
3.46±3.85 |
6.45±5.70 | 0.14 |
| ALB (mg/dl) |
3.97±0.33 |
3.69±0.44 | 0.08 |
| AFP (ng/ml) | 4.70 (2.90,
10.05) | 5.00 (4.30,
6.50) | 0.69 |
| eGFR (ml/m/1.73
m2) |
75.65±29.89 |
67.23±19.50 | 0.37 |
| Hepatocellular
carcinoma (yes/no) | 0/11 | 6/11 | 0.06 |
| Treatment
outcome |
|
Sustained virologic response
week 24 (yes/no) | 11/0 | 15/2 | 0.51 |
 | Table V.Comparison of baseline Short Form-36
score according to age. |
Table V.
Comparison of baseline Short Form-36
score according to age.
| Measure | Age <70 | Age ≥70 | P-value |
|---|
| PF | 85.00 (80.00,
95.00) | 75.00 (60.00,
88.89) | 0.05 |
| RP | 100.00 (78.12,
100.00) | 75.00
(50.00, 100.00) | 0.26 |
| BP | 62.00 (57.00,
84.00) | 72.00
(60.00, 100.00) | 0.83 |
| GH | 52.00 (25.00,
64.50) | 52.00 (37.50,
52.00) | 0.55 |
| VT | 62.50 (18.75,
68.75) | 62.50 (43.75,
75.00) | 0.37 |
| SF | 100.00 (56.25,
100.00) | 87.50
(62.50, 100.00) | 0.89 |
| RE | 91.67
(70.83, 100.00) | 83.33
(75.00, 100.00) | 0.74 |
| MH | 80.00 (57.50,
87.50) | 75.00 (40.00,
95.00) | 0.60 |
 | Table VI.Comparison of differences in Short
Form-36 scores during and folowing daclatasvir/asunaprevir therapy
according to age. |
Table VI.
Comparison of differences in Short
Form-36 scores during and folowing daclatasvir/asunaprevir therapy
according to age.
| Group | Measure | Age <70 | Age ≥70 | P-value |
|---|
| Baseline-12
weeks | PF |
−5.00±17.75 |
8.79±15.22 | <0.05 |
|
| RP |
−5.00±39.06 |
3.31±15.80 | 0.44 |
|
| BP |
−3.09±31.45 |
3.64±23.31 | 0.58 |
|
| GH |
−1.00±15.97 |
7.66±12.90 | 0.14 |
|
| VT |
0.57±23.79 |
1.59±12.00 | 0.88 |
|
| SF |
−4.55±32.73 |
−5.15±22.56 | 0.95 |
|
| RE |
−5.83±39.49 |
−1.47±13.89 | 0.68 |
|
| MH |
−5.57±25.90 |
4.49±9.75 | 0.16 |
| Baseline-EOT | PF |
−6.82±23.16 |
1.27±21.42 | 0.35 |
|
| RP |
−11.93±43.79 |
2.94±16.55 | 0.21 |
|
| BP |
−14.27±37.50 |
4.06±21.79 | 0.11 |
|
| GH |
−1.09±12.80 |
9.44±13.47 | 0.05 |
|
| VT |
7.95±23.06 |
1.56±15.73 | 0.40 |
|
| SF |
−5.68±33.71 |
−1.47±21.14 | 0.69 |
|
| RE |
−3.03±39.66 |
1.96±9.56 | 0.62 |
|
| MH |
−0.11±24.22 |
2.89±12.13 | 0.67 |
| Baseline-SVR24 | PF |
−9.00±29.61 |
−4.05±24.39 | 0.64 |
|
| RP |
−10.62±36.09 |
−1.84±21.74 | 0.44 |
|
| BP |
1.00±29.58 |
−8.65±21.84 | 0.33 |
|
| GH |
−0.73±12.15 |
9.91±16.93 | 0.08 |
|
| VT |
6.82±25.69 |
0.74±14.13 | 0.43 |
|
| SF |
−4.55±28.65 |
−12.50±18.75 | 0.38 |
|
| RE |
2.27±40.84 |
−2.45±21.60 | 0.69 |
|
| MH |
−3.30±20.87 |
−2.21±17.67 | 0.88 |
In the analysis of SF-36 changes within each group,
GH changed significantly in the ≥70-year-old group. Many of the
blood biochemistry measurements improved in both groups, but ALB
improved only in the ≥70-year-old group. In addition, Fib4-index
significantly improved at all time points (12 weeks, EOT, and 48
weeks) compared to baseline in the ≥70-year-old group (Table VII).
 | Table VII.Differences in the blood chemistry
during and following daclatasvir/asunaprevir therapy according to
age. |
Table VII.
Differences in the blood chemistry
during and following daclatasvir/asunaprevir therapy according to
age.
| Measure | Baseline | 12 weeks | EOT | SVR24 | P-value |
|---|
| Age <70 |
| AST
(IU/l) |
36.82±15.20 | 21.00±6.78 |
21.18±6.42a |
20.55±6.31a | <0.01 |
| ALT
(IU/l) |
37.09±19.05 |
19.00±13.28 |
16.91±6.74a |
16.18±8.02a | <0.01 |
| Hb
(g/dl) | 13.86±1.83 | 13.93±1.64 | 14.14±1.69 | 14.16±1.77 | 0.74 |
| PLT
(×104/µl) |
18.49±10.27 | 17.84±8.35 | 18.46±8.96 |
20.35±10.16 | 0.01 |
|
Fib-4 |
3.46±3.85 |
2.30±1.81 |
2.30±1.78 |
2.16±1.74 | 0.01 |
| ALB
(mg/dl) |
3.97±0.33 |
3.97±0.38 |
4.03±0.25 |
4.05±0.39 | 0.71 |
| AFP
(ng/ml) | 4.70 (2.90,
10.05) | 2.80 (2.10,
5.95)a | 3.40 (2.25,
5.50) | 3.20 (2.40,
5.90) | <0.05 |
| eGFR
(ml/m/1.73 m2) |
75.65±29.89 |
71.70±27.12 |
72.68±28.93 |
70.43±29.03 | 0.14 |
| Age ≥70 |
| AST
(IU/l) |
44.41±21.60 | 29.47±9.64 |
26.59±7.91a |
23.88±7.37a | <0.01 |
| ALT
(IU/l) |
35.53±21.91 |
20.76±10.92 |
18.06±7.97a |
14.76±7.00a | <0.01 |
| Hb
(g/dl) | 11.68±1.99 | 11.68±2.12 | 11.48±2.19 | 11.24±2.54 | 0.38 |
| PLT
(×104/µl) | 13.18±6.77 | 13.95±7.07 | 13.57±6.26 | 15.06±8.25 | <0.05 |
|
Fib-4 |
6.45±5.70 |
4.75±2.90a |
4.49±2.75a |
4.21±2.35a | <0.01 |
| ALB
(mg/dl) |
3.69±0.44 |
3.81±0.40 |
3.85±0.32 |
3.95±0.43a | <0.01 |
| AFP
(ng/ml) | 5.00 (4.30,
6.50) | 2.80 (2.40,
5.60)a | 2.70 (2.30,
5.40) | 2.90 (2.40,
5.10) | <0.05 |
| eGFR
(ml/m/1.73 m2) |
67.23±19.50 |
62.51±18.39 |
62.25±15.71 |
63.84±15.97 | 0.07 |
Discussion
HCV patients have previously been reported to
experience chronically decreased HRQOL due to fatigue,
influenza-like symptoms, itchiness, and depression compared to
non-infected individuals (20,21). In
recent years, treatment of HCV has been revolution with the
development of highly effective all-oral direct-acting antiviral
agents. These regimens such as ledipasvir/sofosbuvir and
ombitasvir/paritaprevir/ritonavir with high efficacy as well as
shorter and safer, with little side effects (9–11,22,23).
In the future, the significant factors in selecting treatment will
include not only the efficacy of treatments that target a viremia
or amelioration of Fibrosis, but also the improvement of patient
QOL during and after treatment.
In Japan, IFN-free, ribavirin-free all-oral therapy
with DCV and ASV for 24 weeks is approved as well tolerated and can
achieve a high rate of SVR in patients with HCV genotype 1b who
were ineligible, intolerant, or had not responded to prior
IFN-based therapy (24).
In the present study, GH and ALB were improved in
the ≥70-year-old group. In older patients, it may have led to an
amelioration of liver function as well as improvements in
nutritional status such as ALB. As a result, GH scores in the SF-36
improved. Previous reports on DAA treatment containing IFN have
indicated decreased HRQOL during treatment as well as an
association between hemoglobin and HRQOL (13). On the other hand, because side
effects such as loss of appetite and nausea are less likely to
occur during DCV/ASV therapy, patients were unaffected by such side
effects even during therapy (24).
This may have led to the amelioration of HRQOL and ALB through
improved liver function. Previous studies have also reported an
association between ALB and HRQOL in patients with hemodialysis
patients or liver cirrhosis (25,26).
Similar results were shown in this study. ALB was postulated to
have remained unimproved in the <70-year-old group because,
while no significant difference was seen at baseline compared to
the ≥70-year-old group (3.97 vs. 3.69, respectively; P=0.08), most
patients in the <70-year-old group did not have hypoalbuminemia
and had limited room for improvement.
Regarding changes in liver Fibrosis, Fib4-index, a
liver Fibrosis marker, (27)
improved over time in the ≥70-year-old group with indices of
6.45±5.70 at Baseline, 4.75±2.9 at 12 weeks, 4.49±2.75 at EOT, and
4.21±2.35 at SVR24. These results suggested that Fibrosis improved
with hepatitis C treatment in older individuals. Furthermore, liver
Fibrosis is a significant carcinogenic factor of hepatocellular
carcinoma (28), and the ultimate
improvement in liver Fibrosis through DCV/ASV therapy may lead to
the suppression of carcinogenesis in elderly individuals.
DCV/ASV therapy improves HRQOL, hepatic functional
reserve, nutritional status, and liver Fibrosis during therapy, and
could therefore prompt long-term improvements in HRQOL in
especially older HCV-infected patients. Recently, DAA with
treatment period of 12 weeks has appeared (9–11). For
example, ledipasvir/sofosbuvir are one of the most common
treatments in Japan, the achievement rate of SVR 12 is reported as
98.8%. The discontinuation of treatment due to serious side effects
is 0.6% (29). In addition, the
achievement rate of SVR 12 of ombitasvir/paritaprevir/ritonavir has
been reported 93.5–100% in Japan (30). In the future, further studies are
needed in order to understand the influence of DAAs with treatment
period of 12 weeks on HRQOL.
The limitations of this study were that the sample
size was small, at only 28 patients, and that the data were
representative of only a single institution covering a limited
region. Future directions include expanding the study by increasing
the number of patients from other institutions and regions.
In conclusion, improvements in hepatic functional
reserve and nutritional status can be anticipated even during
DCV/ASV therapy. Furthermore, this therapy improves HRQOL,
especially in elderly patients.
References
|
1
|
Choo QL, Kuo G, Weiner AJ, Overby LR,
Bradley DW and Houghton M: Isolation of a cDNA clone derived from a
blood-borne non-A, non-B viral hepatitis genome. Science.
244:359–362. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hajarizadeh B, Grebely J and Dore GJ:
Epidemiology and natural history of HCV infection. Nat Rev
Gastroenterol Hepatol. 10:553–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gower E, Estes C, Hindman S,
Razavi-Shearer K and Razavi H: Global epidemiology and genotype
distribution of the hepatitis C virus infection. J Hepatol. 61 1
Suppl:S45–S57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lavanchy D: Evolving epidemiology of
hepatitis C virus. Clin Microbiol Infect. 17:107–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thein HH, Yi Q, Dore GJ and Krahn MD:
Estimation of stage-specific fibrosis progression rates in chronic
hepatitis C virus infection: A meta-analysis and meta-regression.
Hepatology. 48:418–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tyagi I and Koirala J: Hepatitis C.
StatPearls Publishing; Treasure Island, FL: 2017
|
|
7
|
Zhu GQ, Zou ZL, Zheng JN, Chen DZ, Zou TT,
Shi KQ and Zheng MH: Systematic review and network meta-analysis of
randomized controlled trials: Comparative effectiveness and safety
of direct-acting antiviral agents for treatment-naive hepatitis C
Genotype 1. Medicine (Baltimore). 95:e30042016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lawitz E, Matusow G, DeJesus E, Yoshida
EM, Felizarta F, Ghalib R, Godofsky E, Herring RW, Poleynard G,
Sheikh A, et al: Simeprevir plus sofosbuvir in patients with
chronic hepatitis C virus genotype 1 infection and cirrhosis: A
Phase 3 study (OPTIMIST-2). Hepatology. 64:360–369. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizokami M, Yokosuka O, Takehara T,
Sakamoto N, Korenaga M, Mochizuki H, Nakane K, Enomoto H, Ikeda F,
Yanase M, et al: Ledipasvir and sofosbuvir fixed-dose combination
with and without ribavirin for 12 weeks in treatment-naive and
previously treated Japanese patients with genotype 1 hepatitis C:
An open-label, randomised, phase 3 trial. Lancet Infect Dis.
15:645–653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumada H, Chayama K, Rodrigues L Jr,
Suzuki F, Ikeda K, Toyoda H, Sato K, Karino Y, Matsuzaki Y, Kioka
K, et al: Randomized phase 3 trial of
ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype
1b-infected japanese patients with or without cirrhosis.
Hepatology. 62:1037–1046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chayama K, Notsumata K, Kurosaki M, Sato
K, Rodrigues L Jr, Setze C, Badri P, Pilot-Matias T, Vilchez RA and
Kumada H: Randomized trial of interferon- and ribavirin-free
ombitasvir/paritaprevir/ritonavir in treatment-experienced
hepatitis c virus-infected patients. Hepatology. 61:1523–1532.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Younossi ZM, Stepanova M, Nader F, Lam B
and Hunt S: The patient's journey with chronic hepatitis C from
interferon plus ribavirin to interferon- and ribavirin-free
regimens: A study of health-related quality of life. Aliment
Pharmacol Ther. 42:286–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki M, Ishikawa T, Sakuma A, Abe S, Abe
H, Koyama F, Nakano T, Ueki A, Noguchi H, Hasegawa E, et al:
Evaluation of the health-related quality of life using the 36-item
short form health survey in patients with chronic hepatitisis C
receiving pegylated interferon/ribavirin/telaprevir triple
treatment. Exp Ther Med. 12:3353–3358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sulkowski MS, Vargas HE, Di Bisceglie AM,
Kuo A, Reddy KR, Lim JK, Morelli G, Darling JM, Feld JJ, Brown RS,
et al: Effectiveness of simeprevir plus sofosbuvir, with or without
ribavirin, in real-world patients with HCV genotype 1 infection.
Gastroenterology. 150:419–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Younossi ZM, Stepanova M, Chan HL, Lee MH,
Yu ML, Dan YY, Choi MS and Henry L: Patient-reported outcomes in
Asian patients with chronic hepatitis C treated with ledipasvir and
sofosbuvir. Medicine (Baltimore). 95:e27022016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Younossi Z, Stepanova M, Omata M, Mizokami
M, Walters M and Hunt S: Health utilities using SF-6D scores in
Japanese patients with chronic hepatitis C treated with
sofosbuvir-based regimens in clinical trials. Health Qual Life
Outcomes. 15:252017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Youssef NF, El Kassas M, Farag A and
Shepherd A: Health-related quality of life in patients with chronic
hepatitis C receiving Sofosbuvir-based treatment, with and without
Interferon: A prospective observational study in Egypt. BMC
Gastroenterol. 17:182017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salomon JA, Wang H, Freeman MK, Vos T,
Flaxman AD, Lopez AD and Murray CJ: Healthy life expectancy for 187
countries, 1990–2010: A systematic analysis for the Global Burden
Disease Study 2010. Lancet. 380:2144–2162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spiegel BM, Younossi ZM, Hays RD, Revicki
D, Robbins S and Kanwal F: Impact of hepatitis C on health related
quality of life: A systematic review and quantitative assessment.
Hepatology. 41:790–800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Svirtlih N, Pavic S, Terzic D, Delic D,
Simonovic J, Gvazdenovic E and Boricic I: Reduced quality of life
in patients with chronic viral liver disease as assessed by SF12
questionnaire. J Gastrointest Liver Dis. 17:405–409. 2008.
|
|
22
|
Tao T, Jiang X, Chen Y and Song Y:
Efficacy and safety of ledipasvir/sofosbuvir with and without
ribavirin in patients with chronic hepatitis C virus genotype 1
infection: A meta-analysis. Int J Infect Dis. 55:56–71. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bunchorntavakul C and Reddy KR: Review
article: The efficacy and safety of daclatasvir in the treatment of
chronic hepatitis C virus infection. Aliment Pharmacol Ther.
42:258–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumada H, Suzuki Y, Ikeda K, Toyota J,
Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, et
al: Daclatasvir plus asunaprevir for chronic HCV genotype 1b
infection. Hepatology. 59:2083–2091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Santos PR and Franco Sansigolo Kerr LR:
Clinical and laboratory variables associated with quality of life
in Brazilian haemodialysis patients: A single-centre study. Rev Med
Chil. 136:1264–1271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parkash O, Iqbal R, Jafri F, Azam I and
Jafri W: Frequency of poor quality of life and predictors of health
related quality of life in cirrhosis at a tertiary care hospital
Pakistan. BMC Res Notes. 5:4462012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang DY and Friedman SL:
Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology.
56:769–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogawa E, Furusyo N, Nomura H, Dohmen K,
Higashi N, Takahashi K, Kawano A, Azuma K, Satoh T, Nakamuta M, et
al: NS5A resistance-associated variants undermine the effectiveness
of ledipasvir and sofosbuvir for cirrhotic patients infected with
HCV genotype 1b. J Gastroenterol. 52:845–854. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Atsukawa M, Tsubota A, Koushima Y, Ikegami
T, Watanabe K, Shimada N, Sato S, Kato K, Abe H, Okubo T, et al:
Efficacy and safety of ombitasvir/paritaprevir/ritonavir in
dialysis patients with genotype 1b chronic hepatitis C. Hepatol
Res. Apr 29–2017.(Epub ahead of print). View Article : Google Scholar
|