Introduction
Human glioma is derived from the neural ectoderm and
is the most common type of primary malignant tumor in human brains
(1). Also in China, human gliomas
are the most common type of intracranial tumor, accounting for
40–50% (2). In recent years, the
risk for gliomas has been gradually increasing in young adults
(3). On the World Health
Organization tumor grading scale, primary gliomas are normally
divided into low-grade glioma, such as fibrillary astrocytomas and
pilocytic astrocytomas, and high-grade glioma, including
glioblastoma and anaplastic astrocytoma (4,5).
Clinical therapy of glioma depends on the size,
type, grade and location of the tumor, as well as the age and
overall health of the patient, and mainly consists of surgical
resection followed by radiotherapy, chemotherapy, Chinese medicine
treatment, gene therapy, immunotherapy and psychotherapy (6,7).
However, the overall mortality rate remains high in glioma patients
(8,9). It is therefore important and urgent to
clarify the mechanisms of the genesis of human gliomas, which may
be helpful for providing approaches for the therapy of human
gliomas.
MicroRNAs (miRNAs/miRs) are a class of small
non-coding RNA molecules of approximately 21–25 nucleotides in
length, which are conserved in plants, animals and certain types of
virus (10). miRNAs are involved in
the regulation of gene expression through RNA silencing and
post-transcriptional gene silencing via binding to the
3′-untranslated region (3′-UTR) of specific mRNAs (11,12).
miRNAs regulate diverse aspects of development and physiology, such
as metabolic diseases (13),
cardiovascular disease (14,15), immune dysfunction (16,17) and
cancer (18,19). A large number of studies have
reported that miRNAs are key factors in regulating cell
differentiation and growth, migration, apoptosis and necrosis.
miR-370 is one of the endogenous non-coding RNAs that has a
critical role in carcinogenesis. However, contradictory effects of
miR-370 on malignancies have been identified among various human
cancer types. miR-370 was reported to function as a tumor
suppressor by targeting forkhead box protein (FOX)M1 to inhibit the
development and progression of acute myeloid leukemia (20). By contrast, Mollainezhad et al
(21) identified miR-370 as an
onco-miR and observed six-fold up-regulation of miR-370 in breast
cancer tissue compared with that in normal adjacent tissue.
Considering that β-catenin was previously reported to be a target
of miR-370 (22), and miR-370a is a
key oncogene in the progression of numerous human cancers, it was
thus assessed in the present study whether there was a regulatory
mechanism between miR-370 and FOXO3a in human gliomas. Thus, the
present study explored the role of miR-370 in the progression and
proliferation of human gliomas and investigated the underlying
mechanisms.
Materials and methods
Patients
A total of 16 clinical specimens were obtained by
surgical resection of the glioma tissues and collected from
Shandong Cancer Hospital affiliated to Shandong University (Jinan,
China) between February 2014 and October 2015. The patients were
diagnosed by pathological identification. The glioma and
peritumoral tissues 2 cm adjacent from the tumors were collected by
surgical resection. The tissues were immediately frozen in liquid
nitrogen. In compliance with the Helsinki Declaration, the subjects
or their families were well informed of the details and signed
relevant consent forms prior to the study. The experiment was
approved by the Ethics Committee of Shandong Cancer Hospital
affiliated to Shandong University (Jinan, China).
Cell lines and agents
Human astrocytes isolated from a human brain
(cerebral cortex) were purchased from ScienCell Research
Laboratories (cat. no. 1800; San Diego, CA, USA) and cultured in
astrocyte medium (cat. no. 1801; ScienCell Research Laboratories).
The U-251MG human astrocytoma (grade III glioma) cell line was
purchased from Jining Shiye Corp. (cat. no. JN-B1757; Shanghai,
China) and the U-87MG glioblastoma cell line was from the American
Type Culture Collection (ATCC; Manassas, VA, USA; ATCC®
HTB-14™). Recently, the U-87MG ATCC cell line was reported to be
misidentified; it is not the original glioblastoma cell line
established in 1968 at the University of Uppsala, but is most
probably also a glioblastoma cell line whose origin is unknown.
Although it is a contaminated cell line, the contamination is
unlikely to affect the interpretation of the results or conclusions
of the present study (23). The
cells were cultured in Dulbecco's modified Eagle's medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
calf serum (Thermo Fisher Scientific, Inc.) with penicillin and
streptomycin in a humidified atmosphere containing 5%
CO2 at 37°C. MTT agent was obtained from Sigma-Aldrich
(Merck KGaA). Lipofectamine 2000 was from Invitrogen (Thermo Fisher
Scientific, Inc.). micrON™ Homo sapiens (hsa)-miR-370 mimics
(cat. no. miR10000722-1-5) and micrON™ agomir Negative Control #24
(cat. no. miR04201-1-2) were obtained from Ribobio Corp.
(Guangzhou, China).
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted with an RNApure kit (cat.
no. RP1201; Bioteke, Beijing, China) according to the
manufacturer's protocols for human glioma, peritumoral tissues, as
well as the glioblastoma and astrocytoma cell lines and human
normal astrocytes. RT was performed using the PrimeScript-RT
reagent kit (cat. no. RR037A; Takara Co., Lt d., Tokyo, Japan) in a
20-ml final reaction volume according to the protocols provided by
the manufacturer. The miR-370 levels were determined by a qPCR
assay performed using SYBR Premix ExTaq™ II (Takara Co., Ltd.) on
ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Thermal cycling conditions were 30 cycles of 10 sec at 94°C
and 1 min at 56°C Each sample was tested in triplicate. Templates
and RT were omitted for the negative controls. U6 small nuclear RNA
was used for normalization. The sequences of the primers were as
follows: hsa-mir-370 forward, 5′-GCCUGCUGGGGUGGAACCUGGU-3′ and
reverse, 5′-CAGGUUCCACCCCAGCAGGCUU-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Expression levels were quantified using the 2−∆∆Cq
method (24).
MTT assay
The cell viability was determined by an MTT assay.
The U-251MG and U-87MG cell lines were seeded in 48-well plates and
following 8 h of incubation, they were transfected with micrON™
hsa-miR-370 mimics and micrON™ agomir Negative Control #24 for 1–5
days. Each sample was set up as two replicates. At the end of the
incubation, 5 mg/ml MTT agent was added to each well, followed by
culture for 4 h. The purple crystals were dissolved in 100 µl
dimethyl sulfoxide. The optical density was determined at a
wavelength of 490 nm.
Western blot analysis
The levels of β-catenin and FOXO3a in clinical
specimens or astrocytoma and glioblastoma cell lines were
determined by western blot analysis as previously described
(25,26). The antibodies used in the present
study were as follows: mouse anti-β-catenin monoclonal
immunoglobulin (Ig)G1 (1:1,000 dilution; cat. no. sc-133239; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit
anti-phosphorylated (p)-FOXO3a (S253) monoclonal IgG (1:1,000
dilution; cat. no. ab154786; Abcam, Cambridge, MA, USA), rabbit
FOXO3a monoclonal antibody (1:1,000 dilution; cat. no. 2497; Cell
Signaling Technology, Inc., Danvers, MA, USA), mouse lamin B1
monoclonal IgG1 (1:1,000 dilution; cat. no. sc-374015; Santa Cruz
Biotechnology, Inc.) and mouse anti-β-actin monoclonal antibody
(1:1,000 dilution; cat. no. sc-47778; Santa Cruz Biotechnology,
Inc.). The secondary antibodies included horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (cat. no. sc-2004) and
HRP-conjugated goat anti-mouse IgG (cat. no. sc-2005; both from
Santa Cruz Biotechnology, Inc.).
Statistical analysis
All of the results were analyzed by SPSS software
version 20.0 (SPSS, Inc., IBM Corp., Armonk, NY, USA). Two
independent groups of samples were performed by t-test. The
multiple comparisons were analyzed by analysis of variance and
Dunnett's post hoc test. Values are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-370 is downregulated in glioma
tissues
miRs are 21–25 nucleotides in length and are
involved in post-transcriptional gene silencing. In order to
clarify the role of miR-370 in human glioma, RT-qPCR was used to
determine the levels of miR-370 in human glioblastoma and paired
peritumoral tissue samples. U6 small nuclear RNA was used as an
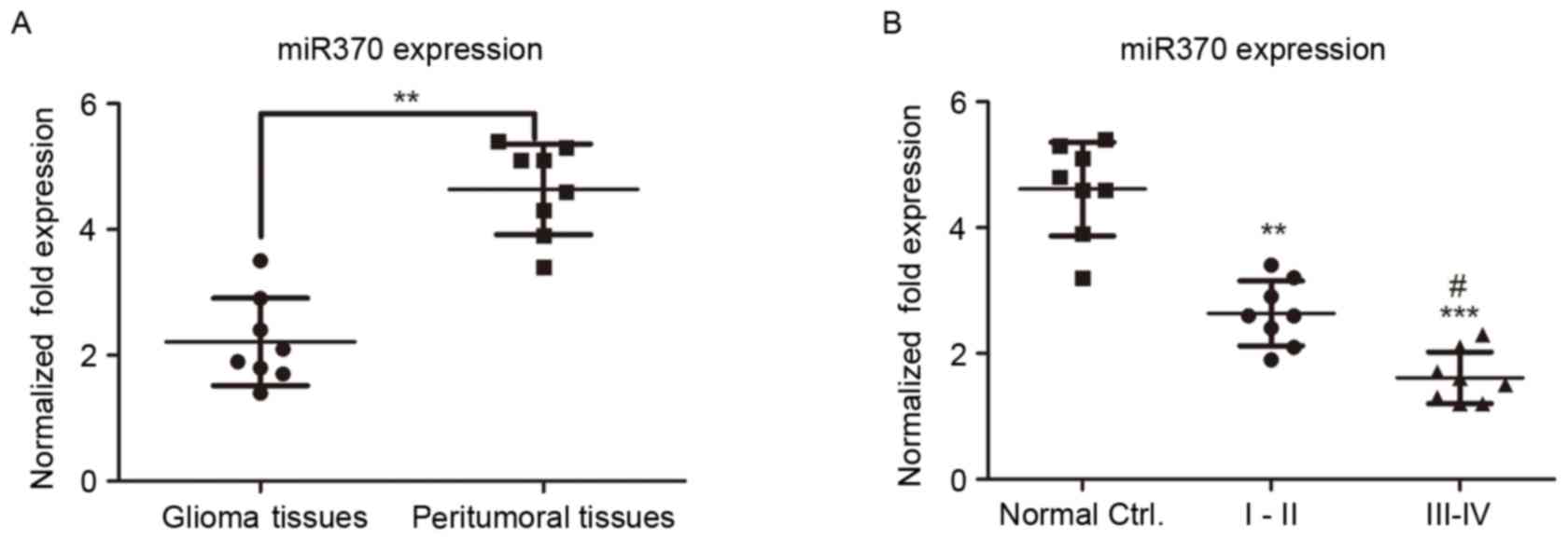
internal control in the experiment. As presented in Fig. 1A, miR-370 levels were significantly
decreased in human glioma tissues compared with those in
peritumoral control tissues (P<0.01), suggesting that miR-370
has a tumor suppressor function in gliomas.
miR-370 expression is negatively
associated with the glioma grade
Gliomas are classified into four grades (I–IV);
tumor growth is slow in low-grade gliomas (grades I and II) and
progresses rapidly in high-grade gliomas (grades III and IV). In
order to investigate whether the miR-370 levels were associated
with the glioma grade, the miR-370 levels were detected by RT-qPCR
in patients with low- and high-grade glioma. As presented in
Fig. 1B, the expression of miR-370
in high-grade glioma was decreased compared with that in low-grade
glioma tissues, suggesting that miR-370 expression was negatively
associated with the glioma grade.
miR-370 expression is decreased in
human astrocytoma and glioblastoma cell lines
Next, miR-370 expression in astrocytoma and
glioblastoma cell lines was determined by RT-qPCR. Normal human
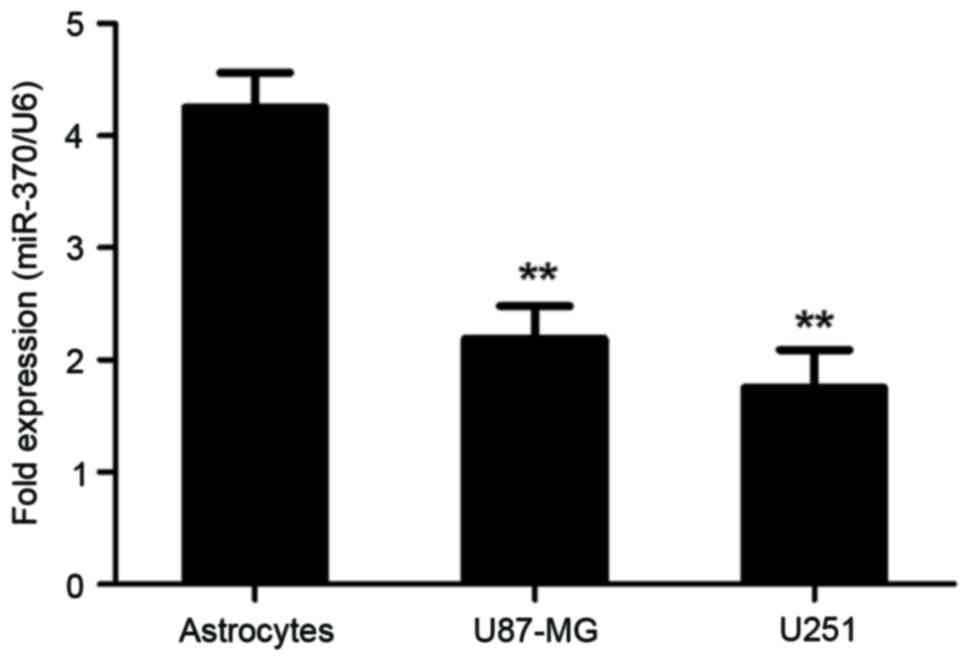
astrocytes were used as normal controls. As presented in Fig. 2, miR-370 was significantly decreased
in U-87MG cells and U-251MG cells, compared with that in normal
human astrocytes (P<0.01). These results demonstrated that
decreased levels of miR-370 were associated with the malignant
transformation of astrocytes into glioblastoma and astrocytoma
(grade III glioma).
β-catenin is upregulated in human
glioma tissues
It has been reported that the canonical
Wingless-type MMTV integration site family (Wnt)/beta-catenin
signaling pathway is aberrantly activated in human gliomas.
β-catenin was reported to be significantly associated with the
histological malignancy grade and with an unfavorable prognosis for
patients with glioma (27). The
levels of β-catenin in the glioblastoma and peritumoral control
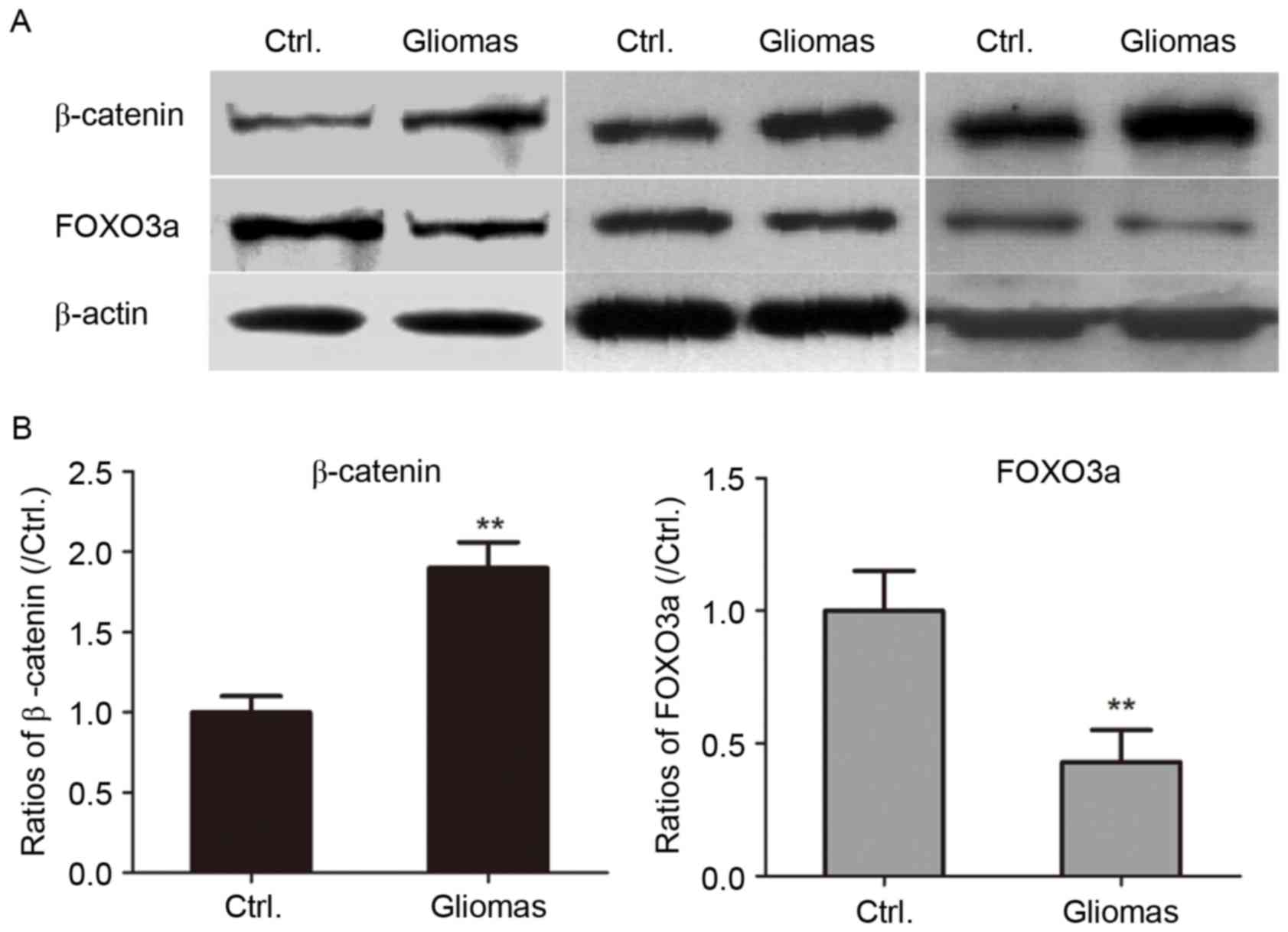
tissue specimens were determined by western blot analysis. As
presented in Fig. 3, the expression
of β-catenin was significantly upregulated in glioma tissues
compared with that in peritumoral tissues (P<0.01).
FOXO3a expression is significantly
decreased in human glioma specimens
The Akt/FOXO3a/Bim pathway was reported to be
critical in cancer progression in terms of elevation of Akt
activity and inactivation of FOXO3a through phosphorylation, which
led to downregulation of Bim (28).
Therefore, the present study assessed the levels of FOXO3a in
glioma and paired peritumoral tissues. As presented in Fig. 3, the levels of FOXO3a in human glioma
tissues were significantly decreased compared with those in control
specimens (P<0.01).
Transfection with miR-370 mimics
suppresses the proliferation of human astrocytoma and glioblastoma
cells in vitro
U-251MG and U-87MG cells were used as cell models of
astrocytoma and glioblastoma, respectively. The miR-370 mimics and
negative controls were transfected into the cells for 1–5 days and
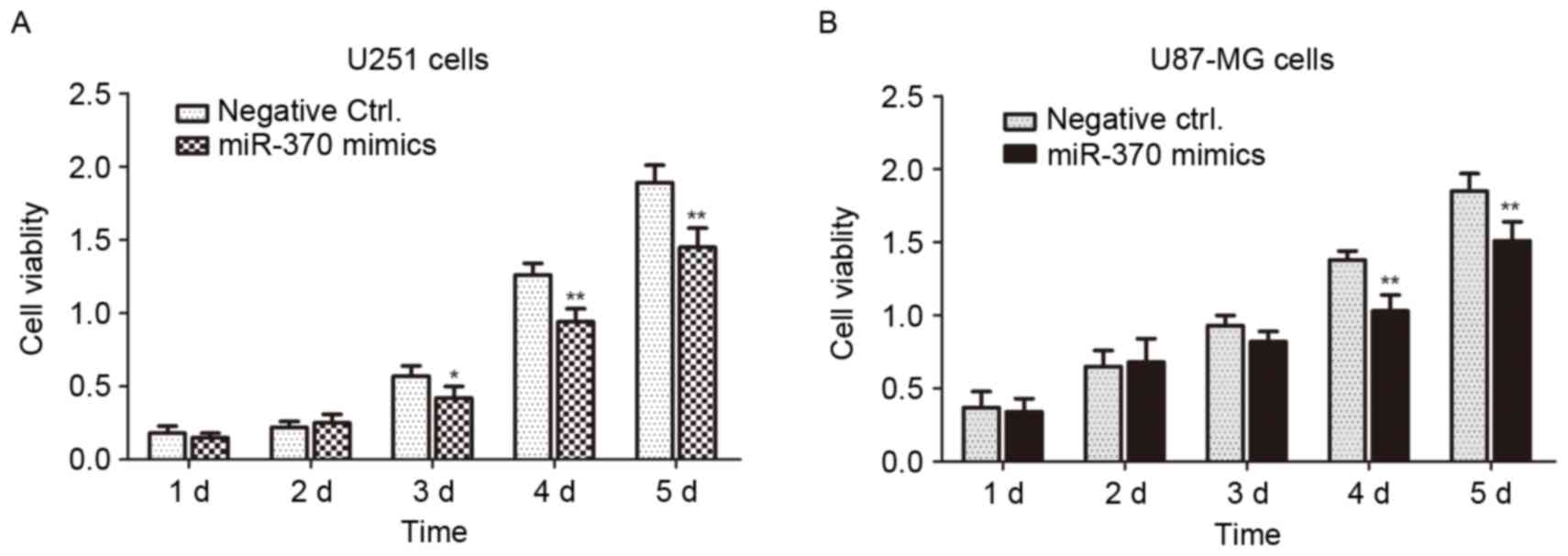
the cell viability was determined by an MTT assay. As presented in
Fig. 4, the cell viability of
miR-370-transfected cells was significantly decreased compared with
that of negative control-transfected cells (P<0.01 or
P<0.05). Overexpression of miR-370 led to an obvious inhibition
of the proliferation of human astrocytoma and glioblastoma
cells.
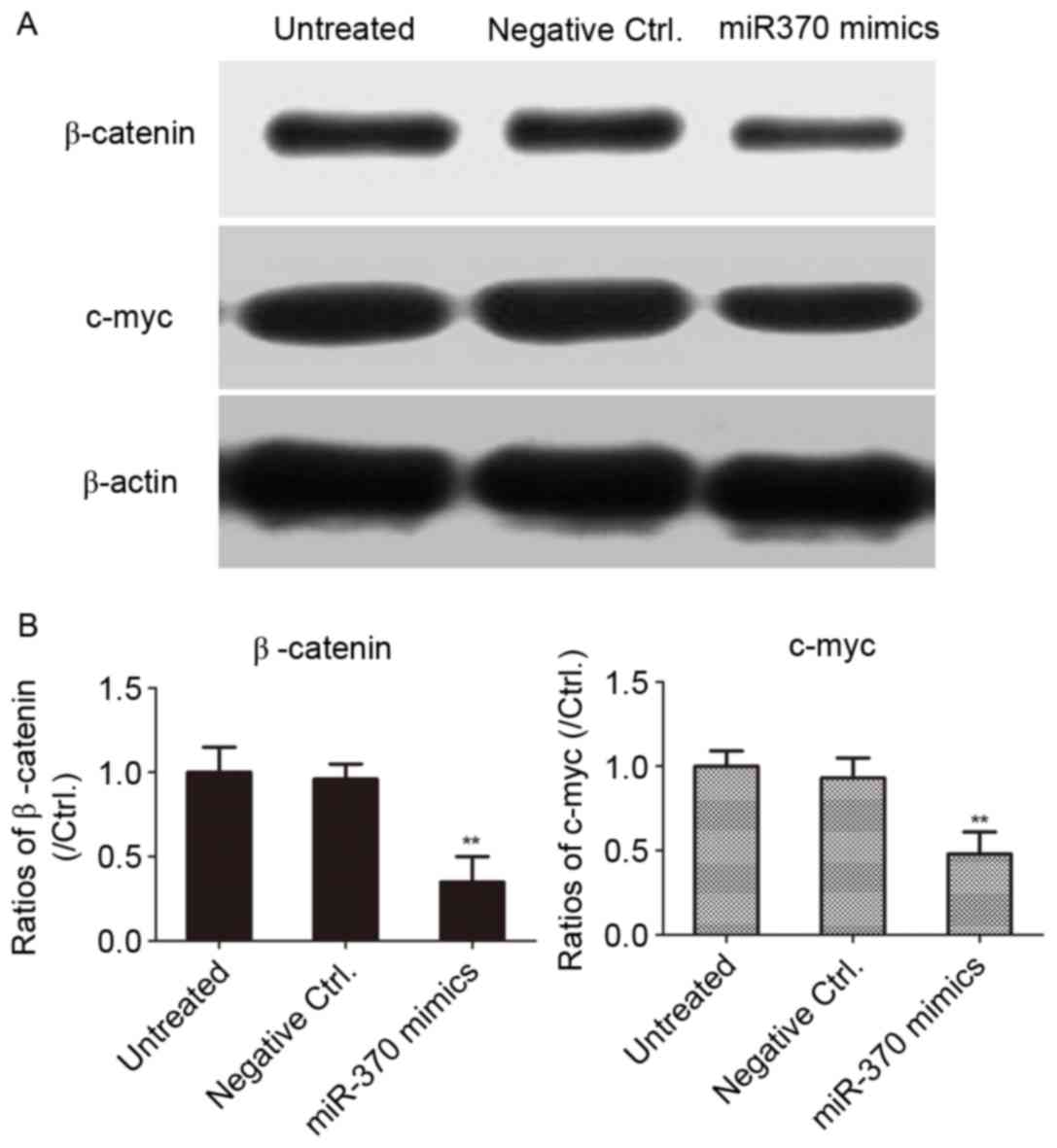
Transfection of miR-370 mimics
inhibits β-catenin expression in U251 cells
In order to clarify whether miR-370 regulates the
Wnt/β-catenin signaling pathway in human astrocytoma cells, the
U-251MG cell line was transfected with miR-370 mimics or negative
controls for 48 h. As presented in Fig.
5, overexpression of miR-370 mimics led to a downregulation of
the levels of β-catenin in human astrocytoma cells, as well as
c-myc, the downstream target gene of β-catenin. The results
revealed that overexpression of miR-370 obviously inhibited the
activity of the Wnt/β-catenin signaling pathway in human
astrocytoma cells.
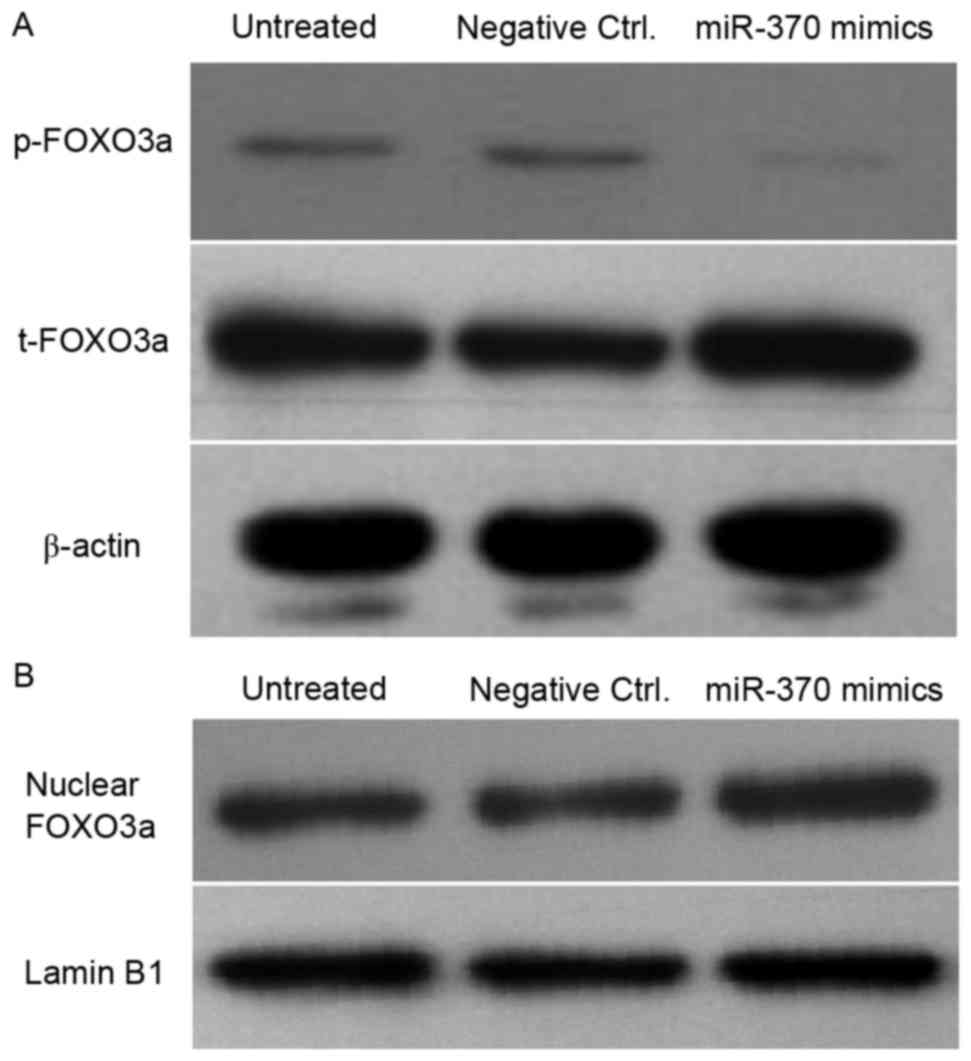
Transfection of miR-370 mimics leads
to a decrease of p-FOXO3a and accumulation of FOXO3a in the nuclei
of U251 cells
In order to investigate whether miR-370 was involved
in the Akt/FOXO3a signaling pathway, western blot analysis was
performed to detect the levels of p-FOXO3a, total FOXO3a and
nuclear FOXO3a in human astrocytoma cells. As presented in Fig. 6, the p-FOXO3a levels were obviously
decreased in miR-370 mimics-transfected cells; however,
overexpression of miR-370 significantly promoted the accumulation
of nuclear FOXO3a in U251 cells. These results may suggest that
miR-370 inhibited the proliferation of human astrocytoma cells
partly by regulating the phosphorylation and cellular localization
of FOXO3a.
Discussion
Human glioma is one of the most malignant primary
tumor type in the central nervous system (29). The present study assessed the levels
of miR-370 in human glioma and peritumoral tissue. First, clinical
specimens from the brains of 16 patients were obtained to detect
the levels of miR-370 by RT-qPCR. The results obviously
demonstrated that the miR-370 levels in human glioma tissues were
significantly decreased compared with those in paired peritumoral
tissues. In addition, the levels of miR-370 in clinical specimens
of low-grade and high-grade gliomas were detected. Of note, miR-370
levels in grade III/IV glioma tissues were lower than those in in
grade I/II specimens. All of these results obviously demonstrated
that miR-370 has an important role as a tumor suppressor gene that
is associated with the genesis and progression of human
gliomas.
Next, the biological role of miR-370 in human
astrocytoma and glioblastoma cell line was explored and the
molecular mechanism was investigated. RT-qPCR analysis revealed
that the levels of miR-370 were obviously decreased in the U-251MG
human astrocytoma and the U-87MG glioblastoma cell line cell line
compared with that in normal control astrocytes. This was
consistent with the results obtained for the clinical specimens,
suggesting that miR-370 was decreased in human astrocytoma and
glioblastoma cells. Furthermore, oligonucleotide mimics of miR-370
were transfected into U-251MG and U-87MG cells, which inhibited the
cell proliferation as demonstrated by an MTT assay. This was
consistent with the results of a study by Peng et al
(22), which reported that
miR-370-3p inhibited human astrocytoma and glioblastoma cell
proliferation and induced cell cycle arrest by directly targeting
β-catenin. While this abovementioned and the present study were on
miR-370 in human astrocytoma and glioblastoma cell proliferation,
the present study also detected the expression of β-catenin and
FOXO3a, which were two key factors in the progression of gliomas,
apart from detecting miR-370 levels in clinical specimens. However,
Peng et al (22) used a
luciferase reporter assay to test the direct binding of miR-370-3p
to a sequence from the 3′-UTR of β-catenin mRNA and also focused on
cell cycle analysis, revealing that miR-370-3p induced cell cycle
arrest at G0/G1 phase. The present study revealed that miR-370
mimics inhibited β-catenin/c-myc signaling. Furthermore, the
phosphorylated and total FOXO3a levels as well as the nuclear
localization of FOXO3a were assessed in miR-370 mimics-transfected
cells, revealing that miR-370 mimics decreased the phosphorylation
of FOXO3a promoted the accumulation of nuclear FOXO3a in U-251MG
cells.
The Wnt/β-catenin and the Akt/FOXO3a signaling
pathway are two important regulating pathways in human astrocytoma
and glioblastoma cells. Aberrant expression of β-catenin and FOXO3a
was found in astrocytoma and glioblastoma cells by western blot
analysis. A reciprocal association between miR-370 and β-catenin
and a positive association between miR-370 and FOXO3a were detected
by western blot analysis. Of note, transfection of miR-370 mimics
inhibited β-catenin expression in U-251MG cells. However,
transfection of miR-370 mimics contributed to a decrease of
p-FOXO3a and nuclear accumulation of FOXO3a in U251 cells. Thus, it
was speculated that miR-370 inhibited the proliferation of human
astrocytoma and glioblastoma cells by regulating β-catenin as well
as FOXO3a-associated signaling pathways. All of the results of the
present study demonstrated that miR-370 may be a tumor suppressor
whose downregulation has a role in the genesis and progression of
human astrocytoma and glioblastoma. miR-370 may represent a novel
target for the molecular therapy of human astrocytoma and
glioblastoma.
References
|
1
|
Hirst TC, Vesterinen HM, Conlin S, Egan
KJ, Antonic A, Lawson McLean A, Macleod MR, Grant R, Brennan PM,
Sena ES and Whittle IR: A systematic review and meta-analysis of
gene therapy in animal models of cerebral glioma: Why did promise
not translate to human therapy? Evid Based Preclin Med.
1:e000062014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Chen JX, Zhou Q, Liu YH, Mao Q,
You C, Chen N, Xiong L, Duan J and Liu L: Statistical report of
central nervous system tumors histologically diagnosed in the
sichuan province of China from 2008 to 2013: A west China glioma
center report. Ann Surg Oncol. 23 Suppl 5:S946–S953. 2016.
View Article : Google Scholar
|
|
3
|
Bauer R, Kaiser M and Stoll E: A
computational model incorporating neural stem cell dynamics
reproduces glioma incidence across the lifespan in the human
population. PLoS One. 9:e1112192014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matyja E, Grajkowska W, Stepien K and
Naganska E: Heterogeneity of histopathological presentation of
pilocytic astrocytoma-diagnostic pitfalls. A review. Folia
Neuropathol. 54:197–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borghei-Razavi H, Shibao S and Schick U:
Prechiasmatic transection of the optic nerve in optic nerve glioma:
Technical description and surgical outcome. Neurosurg Rev.
40:135–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luderer MJ, Muz B, de la Puente P,
Chavalmane S, Kapoor V, Marcelo R, Biswas P, Thotala D, Rogers B
and Azab AK: A hypoxia-targeted boron neutron capture therapy agent
for the treatment of glioma. Pharm Res. 33:2530–2539. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bouffet E and Ramaswamy V: Old
chemotherapy makes a comeback: Dual alkylator therapy for pediatric
high-grade glioma. Neuro Oncol. 18:1333–1334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cordier D, Krolicki L, Morgenstern A and
Merlo A: Targeted radiolabeled compounds in glioma therapy. Semin
Nucl Med. 46:243–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barajas RF Jr, Butowski NA, Phillips JJ,
Aghi MK, Berger MS, Chang SM and Cha S: The development of reduced
diffusion following bevacizumab therapy identifies regions of
recurrent disease in patients with high-grade glioma. Acad Radiol.
23:1073–1082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu X, Yang X, Xing C, Zhang S and Cao J:
miRNA: The nemesis of gastric cancer (Review). Oncol Lett.
6:631–641. 2013.PubMed/NCBI
|
|
12
|
Ma H, Wu Y, Yang H, Liu J, Dan H, Zeng X,
Zhou Y, Jiang L and Chen Q: MicroRNAs in oral lichen planus and
potential miRNA-mRNA pathogenesis with essential cytokines: A
review. Oral Surg Oral Med Oral Pathol Oral Radiol. 122:164–173.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mehta R, Otgonsuren M, Younoszai Z, Allawi
H, Raybuck B and Younossi Z: Circulating miRNA in patients with
non-alcoholic fatty liver disease and coronary artery disease. BMJ
Open Gastroenterol. 3:e0000962016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srinivasan H and Das S: Mitochondrial
miRNA (MitomiR): A new player in cardiovascular health. Can J
Physiol Pharmacol. 93:855–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Daimiel-Ruiz L, Klett-Mingo M,
Konstantinidou V, Micó V, Aranda JF, García B, Martínez-Botas J,
Dávalos A, Fernández-Hernando C and Ordovás JM: Dietary lipids
modulate the expression of miR-107, a miRNA that regulates the
circadian system. Mol Nutr Food Res. 59:1865–1878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Jeker LT, Fife BT, Zhu S, Anderson
MS, McManus MT and Bluestone JA: Selective miRNA disruption in T
reg cells leads to uncontrolled autoimmunity. J Exp Med.
205:1983–1991. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaked A, Chang BL, Barnes MR, Sayre P, Li
YR, Asare S, DesMarais M, Holmes MV, Guettouche T and Keating BJ:
An ectopically expressed serum miRNA signature is prognostic,
diagnostic, and biologically related to liver allograft rejection.
Hepatology. 65:269–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cramer DW and Elias KM: A prognostically
relevant miRNA signature for epithelial ovarian cancer. Lancet
Oncol. 17:1032–1033. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin YC, Lin JF, Tsai TF, Chou KY, Chen HE
and Hwang TI: Tumor suppressor miRNA-204-5p promotes apoptosis by
targeting BCL2 in prostate cancer cells. Asian J Surg. 40:396–406.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Zeng J, Zhou M, Li B, Zhang Y,
Huang T, Wang L, Jia J and Chen C: The tumor suppressive role of
miRNA-370 by targeting FoxM1 in acute myeloid leukemia. Mol Cancer.
11:562012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mollainezhad H, Eskandari N, Pourazar A,
Salehi M and Andalib A: Expression of microRNA-370 in human breast
cancer compare with normal samples. Adv Biomed Res. 5:1292016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng Z, Wu T, Li Y, Xu Z, Zhang S, Liu B,
Chen Q and Tian D: MicroRNA-370-3p inhibits human glioma cell
proliferation and induces cell cycle arrest by directly targeting
β-catenin. Brain Res. 1644:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang M, Pi H, Li M, Xu S, Zhang L, Xie J,
Tian L, Tu M, He M, Lu Y, et al: From the Cover: Autophagy
induction contributes to cadmium toxicity in Mesenchymal stem cells
via AMPK/FOXO3a/BECN1 signaling. Toxicol Sci. 154:101–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun G, Hou YB, Jia HY, Bi XH, Yu L and
Chen DJ: MiR-370 promotes cell death of liver cancer cells by
Akt/FoxO3a signalling pathway. Eur Rev Med Pharmacol Sci.
20:2011–2019. 2016.PubMed/NCBI
|
|
27
|
Denysenko T, Annovazzi L, Cassoni P,
Melcarne A, Mellai M and Schiffer D: WNT/β-catenin signaling
pathway and downstream modulators in low- and high-grade glioma.
Cancer Genomics Proteomics. 13:31–45. 2016.PubMed/NCBI
|
|
28
|
Guan H, Song L, Cai J, Huang Y, Wu J, Yuan
J, Li J and Li M: Sphingosine kinase 1 regulates the Akt/FOXO3a/Bim
pathway and contributes to apoptosis resistance in glioma cells.
PLoS One. 6:e199462011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amberger VR, Hensel T, Ogata N and Schwab
ME: Spreading and migration of human glioma and rat C6 cells on
central nervous system myelin in vitro is correlated with tumor
malignancy and involves a metalloproteolytic activity. Cancer Res.
58:149–158. 1998.PubMed/NCBI
|