Introduction
Hepatocellular carcinoma (HCC) is the most prevalent
form of primary liver cancer, which exhibits a high recurrence and
the second highest cancer-associated mortality rates following
radiotherapy, chemotherapy and surgery in Chinese patients
(1). HCC not only exhibits a high
incidence among human cancers, but potential therapeutic strategies
remain limited, especially for patients with advanced late-stage
HCC (2). At present, the recommended
clinical therapies of surgery, chemotherapy and radiotherapy
present only modest efficacy due to tumor progression and side
effects, including myelosuppression and digestive system toxicity
during and following the treatment period (3–5).
Therefore, novel clinical strategies are required in order to
enhance curative effects, minimize adverse response and potentially
prolong survival of patients with HCC (6,7).
Various oncolytic protocols for targeting different
signal pathways have been characterized (8,9). These
cellular signal pathways presented key signal transduction for
various extracellular growth factors and receptors of HCC cells.
However, the high occurrence (62–82%) of drug resistance is a major
issue in clinical HCC therapy (10,11).
Tumors have been demonstrated to acquire apoptotic-resistance
during early stage apoptosis or incomplete apoptotic responses to
oncolytic treatments and anticancer drugs, and the consequent
requirement for long-term medical therapy presented a major
clinical problem in HCC (12).
Therefore, novel effective therapeutic agents for HCC therapy are
required to inhibit metastasis for patients with HCC.
Human telomerase reverse transcriptase (hTERT) is a
target of tumor therapy which is strongly expressed in the majority
of tumor cells and is rarely expressed in normal cells (13). A previous study has reported that
hTERT is overexpressed in 80% HCC cells, and suggest that telomere
elongation is a potential target of gene therapy for HCC (14). In the present study, it was
demonstrated that the anti-tumor efficacy of hTERT receptor
(hTERTR) was mediated by altering endogenous telomerase activity;
thereby significantly inhibiting HCC growth in vitro and
strongly suppressing tumor cell proliferation and cancer cell
metastasis in anti-telomerase therapy mice.
Family with sequence similarity 96 member A (FAM96A)
is identified as a member of the cytosolic Fe/S protein in charge
of regulator of cellular iron homeostasis and assembly machinery,
which exhibits apoptosome-activating activities and pro-apoptotic
tumor suppressor potential in HCC (15–17). The
results of the present study demonstrated that FAM96A induced no
adverse side effects for normal, non-cancerous human cells, which
indicated that FAM96A protein may be able to enhance apoptosis
sensitivity via the mitochondrial apoptosis pathway in vitro
and promote immunologic cytotoxicity for apoptotic fragments in
vivo (18).
In the present study, combination therapy of hTERTR
and FAM96A for HCC through apoptosis was explored and their
antitumor efficacies were confirmed in a murine model of HCC.
Notably, compared with sole administration of FAM96A and hTERTR,
combination therapy demonstrated markedly improved therapeutic
effects for HCC in vitro and in vivo. These findings
support the use of multi-target fusion protein drugs for treatment
of HCC cells and suggest that hTERTR and FAM96A may be efficient
anti-cancer agents for HCC treatment.
Materials and methods
Ethical approval
The present study was performed in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals (19) of Weifang
City People's Hospital. All experimental protocols and animal
maintenance were approved by the Committee on the Ethics of Weifang
City People's Hospital (Shandong, China). All surgery and
euthanasia were performed to minimize suffering.
Cells culture and reagents
HepG2 and H22 cells were obtained from Frederick
Cancer Research Facility, Division of Cancer Treatment Tumor
Repository (Frederick, MD, USA) and normal mice liver cells
(NCTC-1496 cells) were obtained from American Type Culture
Collection (Manassas, VA, USA). All cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
heat-inactivated fetal bovine serum, 3 mM L-glutamine, 50 µg/ml
gentamicin (all from Biowhittaker; Lonza Group, Ltd., Basel,
Switzerland) and 1% penicillin/streptomycin under standard culture
conditions (5% CO2, 37°C). Cells were treated with 0.10,
0.18, 0.25, 0.32 mg/ml hTERTR and/or FAM96A for 72 h at 37°C.
Construction of recombinant hTERTR and
FAM96A
The hTERTR and FAM96A genes were obtained from lung
tissue from the Microbiological Laboratory of Shandong University
(Shandong, China). A pET-27b expression system (cat. no. addgene
0020; Shanghai North Connaught Biological Technology Co., Ltd,
Shanghai, China) was used to construct the recombinant hTERTR and
FAM96A proteins. DNA sequence encoding 130 bp of FAM96A (forward,
5′-ATGTTATCGTTCTTCCGCAAG-3′ and reverse,
5′-CCAGCTGAGAGAGGGATGGC-3′) was amplified by polymerase chain
reaction (PCR) from pMD-18-FAM96A (Weifang Medical University,
Weifang, China) using 1 µl PCR clone product mixed with 10 µl of
TaqMan Universal PCR MasterMix (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). PCR was performed under the
following conditions: 95°C for 30 sec, 30 cycles at 95°C for 15 sec
and 55°C for 1 min and then 72°C for 1 min. FAM96A (50 ng) was
subcloned into the rPET-27b plasmids and named rPET-27-FAM96A using
electrotransformation (cat. no. 1359; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) following a previously described protocol
(20). Gene edited cell-penetrating
peptide (CPP; Weifang Medical University) was inserted into
plasmids with proteins following 25-µl volume PCR to induce cells
to take the plasmids up. The same method was used to clone
recombinant hTERTR (forward, 5′-AAGGAATTTGTAACAAAGGT-3′ and
reverse, 5′-AGACCTGTGAGATGACCTCC-3′) with the CPP into PET-27b
plasmids, which were then named rPET-27-hTERTR. PCR, as performed
previously and gene sequencing (Invitrogen; Thermo Fisher
Scientific, Inc.) were used to select the correct monoclonal
sequence identified by Invitrogen (Invitrogen; Thermo Fisher
Scientific, Inc.) (21). Recombinant
rPET-27-hTERTR (4 µg) or rPET-27-FAM96A (4 µg) was expressed in E.
coli in LB medium at 37°C for 12 h. hTERTR and FAM96A protein was
extracted and purified as described previously (17).
Western blotting
The purified hTERTR or FAM96A was homogenized and
separated by SDS-PAGE, and subsequently transferred to
nitrocellulose membranes. For western blot analysis, hTERT and
apoptotic protease activating factor 1 (APAF1) were prepared. The
detection of protein was carried out by incubating the membranes
with hTERT and APAF1 with IL-1 as a negative control. The
procedures were performed as previously described (17). All experiments were performed in
triplicate and repeated at least three times.
Animal experiments
A total of 80 specific pathogen-free female BALB/c
nude mice (age, 6-week old; weight, 30–35 g) were purchased from
Harbin Veterinary Research Institute (Harbin, China). Mice were
feed under pathogen-free conditions and maintained at a controlled
environment (temperature, 23±1°C; humidity, 50–60%) with an
artificial simulation of 12 h light/dark cycles. H22 cells
(1×105) in 200 µl PBS were injected into the right flank
of female BALB/c nude mice to establish HCC. Therapy for
tumor-bearing mice with hTERTR and/or FAM96A, or PBS was initiated
when tumor diameters reached 6–8 mm at 7 days in the HCC mouse
model following tumor inoculation. Mice with HCC were randomly
divided into 4 groups (n=20 each) and injected intratumorally with
0.25 mg hTERTR and/or 0.32 mg FAM96A in PBS buffer (200 µl), or the
same volume of PBS (200 µl), respectively. Each treatment was
subsequently administered a further 7 times at two-day intervals,
giving a total of 15 administrations. Tumor diameters were recorded
once every two days and tumor volume was calculated using the
following formula: 0.52× smallest diameter2 × largest
diameter.
Lactate dehydrogenase (LDH) and
interferon (IFN)-γ release assays
HepG2 (1×106), H22 (1×106) and
NCTC-1496 cells (1×106) were incubated with hTERTR (0.20
µg/ml) and/or FAM96A (0.20 µg/ml) in DMEM for 96 h at 37°C. Cells
were washed with PBS three times for 2 min at room temperature.
Cells were subsequently incubated with 1% Triton-X-100 at 37°C for
30 min. LDH assay was determined using the CytoTox 96 assay kit
(Promega Corporation, Madison, WI, USA) and recorded at 490 nm
according to manufacturer's protocol. For IFN-γ release assays,
splenocytes were harvested from euthanized animals. Splenocytes
(1×106) were subsequently incubated with UV-inactivated
H22 cells (1×108) in DMEM at 37°C for 72 h. Cells were
centrifuged at 8,000 × g for 10 min at 37°C and IFN-γ release in
supernatants of the DMEM medium was subsequently measured by ELISA
(cat. no. DY485; Bio-Rad Laboratories, Inc.) according to
manufacturer's protocol. Then, T cells (1×106) from the
splenocyte sample were purified, as described previously (22), and co-cultured with fresh H22 cells
for 4 h at 37°C with target ratios of 5:1, 15:1 and 40:1. Specific
cytotoxic T lymphocyte (CTL) response to the target cells was
determined using a Pierce™ LDH Cytotoxicity assay kit (cat. no.
88953; Thermo Fisher Scientific, Inc.) cytotoxicity assays as
described previously (23).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted from H22 cells with
an RNeasy Mini kit (Qiagen Sciences, Inc., Gaithersburg, MD, USA)
and 1 µg RNA was subjected to a cDNA synthesis kit (Bio-Rad
Laboratories, Inc.). A total of 10% of the cDNA sample was
subjected to a 25-µl PCR (cDNA, 10 ng, 5 µl, PCR buffer, 2 µl, DNA
polymerase, 0.5 µl, primers, 2 µl, water, 15.5 µl) carried out in
an iCycler thermal cycler (Bio-Rad Laboratories, Inc.) using iQ
SYBR-Green Supermix (Bio-Rad Laboratories, Inc.). The reaction
conditions were performed as follows: 95°C for 10 min, 35 cycles of
95°C for 20 sec and 58°C for 1 min. The forward and reverse primers
for B-cell lymphoma 2 (Bcl-2) and c-Myc were synthesized by
Invitrogen (Thermo Fisher Scientific, Inc.). Primers were as
follows: Bcl-2 forward, 5′-CTGGTGGACAACATCGCTCTG-3′ and reverse,
5′-GGTCTGACCTCACTTGTG-3′; c-Myc forward, 5′-TTCATCCAGGATCGAGCAGA-3′
and reverse, 5′-GCAAAGTAGAAGGCAACG-3′; GAPDH forward,
5′-GGCCAAGATCATCCATGACAACT-3′ and reverse,
5′-ACCAGGACATGAGCTTGACAAAGT-3′. The amplified PCR products were
quantified by measuring the calculated quantification cycles (Cq)
of sample and GAPDH mRNA. Relative changes in mRNA expression were
calculated by the 2−ΔΔCq method (24). The results are expressed as the
n-fold difference relative to GAPDH (relative expression
levels).
Tumor cell migration and invasion
assays
HepG2 cells were treated with hTERTR and/or FAM96A
and non-treated cells were used as control. For migration assay,
HepG2 cells (1×105) were incubated with hTERTR (0.20
µg/ml) and/or FAM96A (0.20 µg/ml) in DMEM for 96 h at 37°C using
control culture inserts (BD Biosciences, Franklin Lakes, NJ, USA).
For invasion assay, hTERTR and/or FAM96A-treated cells were
suspended at a density of 1×105 cells in 500 µl
serum-free DMEM. HepG2 cells were then placed in the upper chambers
of BD BioCoat Matrigel Invasion Chambers (BD Biosciences) for 96 h
at 37°C according to the manufacturer's instructions. 0.1% crystal
violet was used to stain the membrane for 30 min at 37°C. After
washing with PBS, tumor cell invasion and migration were counted in
at least three random stained fields of view via microscopy
(Olympus BX51; Olympus Corporation, Tokyo, Japan).
Flow cytometry
HepG2 and H22 cells were cultured in DMEM medium
supplied 10% fetal calf serum. HepG2 and H22 (1×106)
cells were incubated with PBS, hTERTR and/or FAM96A at 37°C for 72
h. Subsequently, apoptosis of suspended cells were analyzed by flow
cytometry. Cells were collected and suspended with Annexin V-FITC
and PI for 30 min at 4°C according to the manufacturer protocol.
Fluorescence was measured with a fluorescence-activated cell
sorting flow cytometer (FCS Express™ 4 IVD; BD Biosciences) and
analyzed using Quantity One software (version 3.0; Bio-Rad
Laboratories, Inc.).
Immunohistochemical staining
Tumor tissues were fixed using 10% formalin solution
for 12 h at 4°C. Immunohistochemical staining was performed using
an avidin-biotin-peroxidase technique on tumor tissues obtained
from the BALB/c mice on day 30. Paraffin-embedded tissue sections 4
µm thick were prepared and epitope retrieval was performed for
further analysis. The paraffin sections were incubated with
hydrogen peroxide (3%) for 10–15 min at 37°C and were subsequently
blocked with a regular blocking solution (5% skim milk powder) for
10~15 minutes at 37°C. Sections were incubated with anti-Annexin
antibody (1:2,000, cat. no. ab14196; Abcam, Cambridge, UK) at 4°C
for 12 h following blocking. All sections were washed three times
with PBS at 37°C for 5 min and incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG mAb (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for 1 h at 37°C and were
counterstained with hematoxylin or DAPI for 1 h at 37°C. Images
were captured with a ZEISS LSM 510 confocal microscope (Zeiss AG,
Oberkochen, Germany) at 488 nm.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Comparisons of data between multiple groups were
performed using one-way analysis of variance followed by a Tukey's
multiple comparison post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Construction of recombinant hTERTR and
FAM96A, and in vitro activity
In order to construct the recombinant protein, two
recombinant plasmids were structured, rPET-27b-hTERTR and
rPET-27b-FAM96A. The structure of rPET-27b-hTERTR and
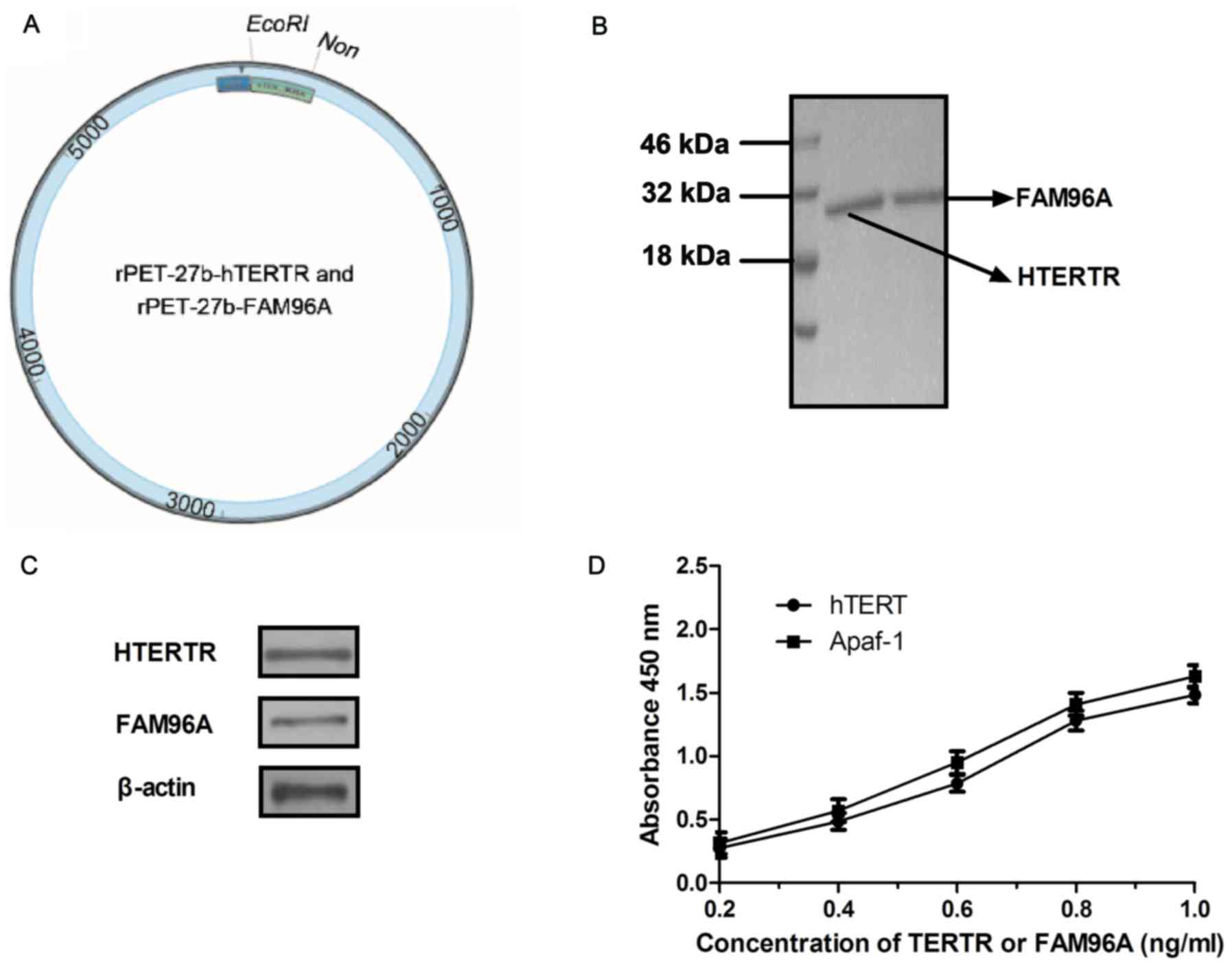
rPET-27b-FAM96A are presented in Fig.
1A. The recombinant plasmids of rPET-27b-hTERTR and
rPET-27b-FAM96A were respectively expressed by E. coli. The
molecular weight of hTERTR was ~28 kDa and FAM96A was ~30 kDa
(Fig. 1B). Western blotting revealed
a band of ~27 kDa (hTERTR) and 30 kDa (FAM96A) and identified the
purified protein of hTERTR or FAM96A specifically bound to hTERT
and APAF1, respectively (Fig. 1C).
ELISA also revealed that hTERTR-FAM96A was able to cross-bind both
hTERT and APAF1 (Fig. 1D). These
results indicated that the recombinant proteins hTERTR and FAM96A
were purified successfully and possessed the expected binding
potentials.
Cytolytic effects of hTERTR and/or
FAM96A on normal cells and HCC cells
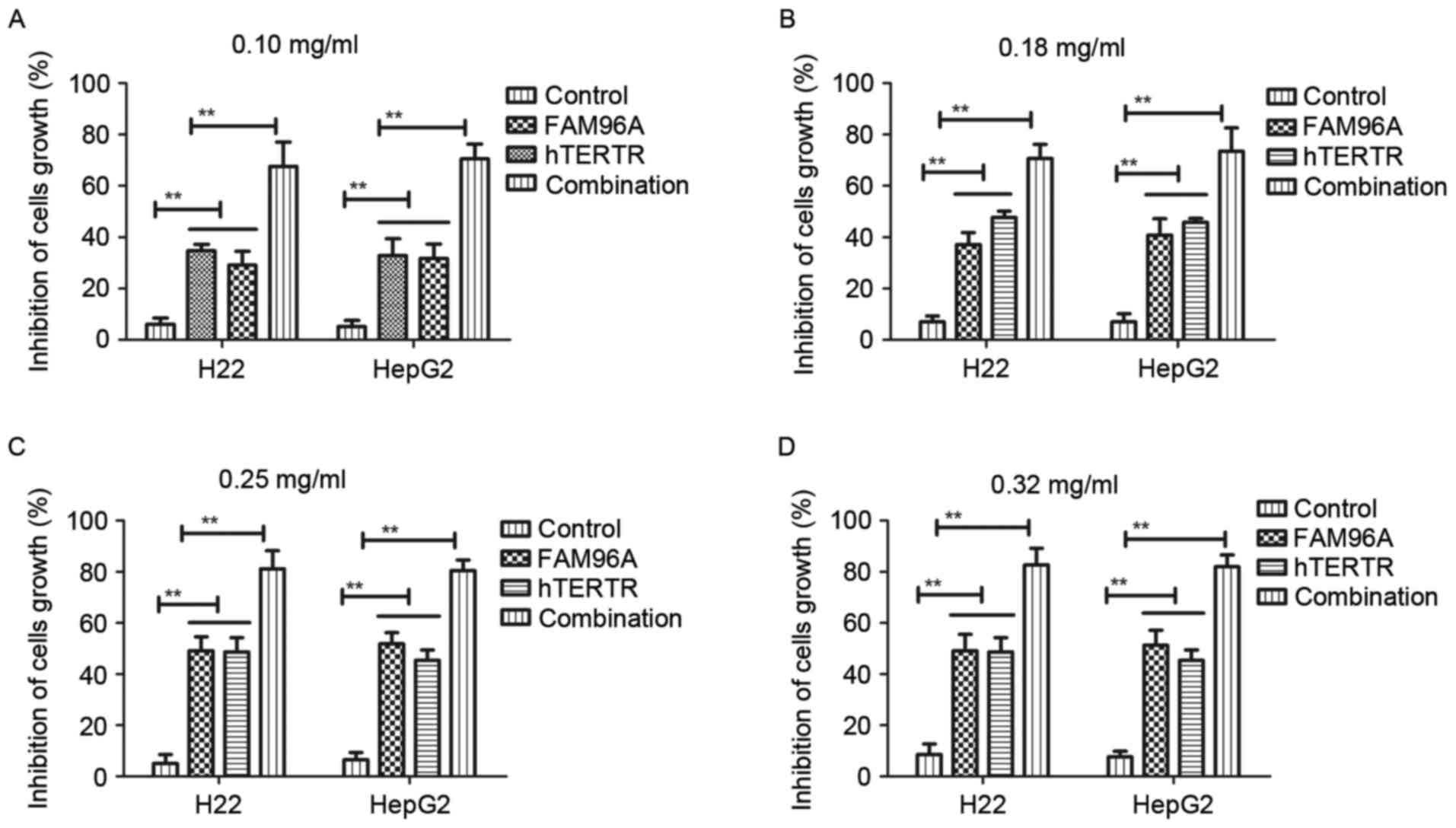
In the present study, the cytolytic effects of
hTERTR and/or FAM96A on H22 and HepG2 tumor cells were investigated
to determine the cytotoxic effects of hTERTR and/or FAM96A via LDH
assay, using untreated cells as a control. Different dosages of
hTERTR and/or FAM96A (0.10, 0.18, 0.25, 0.32 mg/ml) induced
different cytotoxic effects on tumor cell lines in a time-dependent
manner, but were not toxic for normal hepatocytes (Fig. 2A-D). The different dosages of hTERTR
or FAM96A (0.10–0.32 mg/ml) also demonstrated inhibiting effects
for tumor cell growth in a dose-dependent manner at 72 h
post-treatment. The present results indicated that hTERTR (0.18
mg/ml) and FAM96A (0.25 mg/ml) was enough to inhibit growth of
hepatic tumor cells. Nevertheless, combination treatment with
hTERTR and FAM96A resulted in great inhibition compared with either
agent administered alone. It was observed that there was no
significant difference in the growth-inhibiting rate at the maximum
concentration levels between hTERTR and FAM96A at 72 h
post-treatment. These data suggested that combination treatment of
hTERTR and FAM96A significantly inhibited HCC cells.
Improvement of apoptotic sensitivity
in hTERTR and/or FAM96A-treated HCC cells
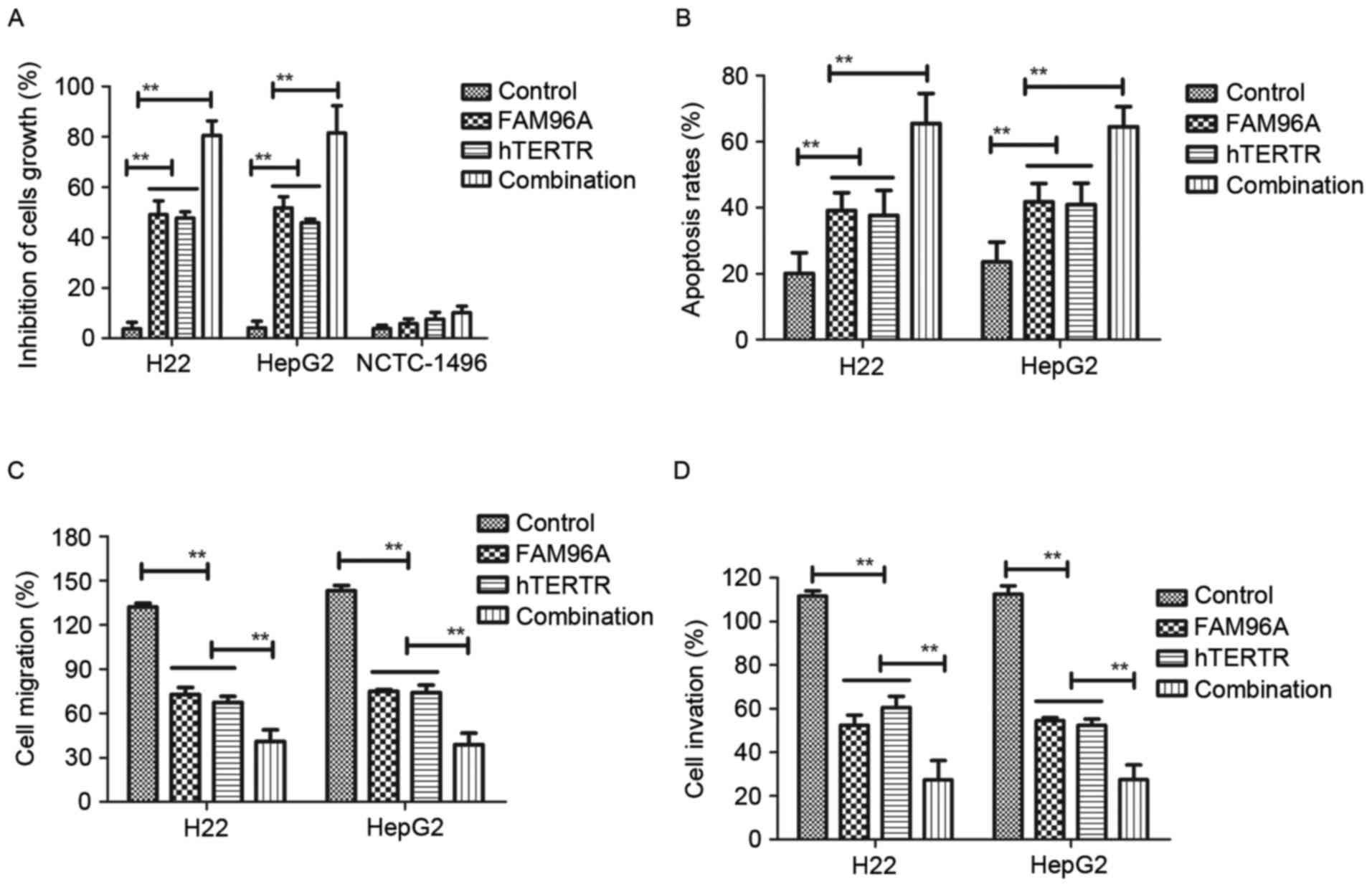
The cytotoxic effects on human or mouse normal liver
cell lines at the highest dose of hTERTR (0.18 mg/ml) or FAM96A
(0.25 mg/ml) were also investigated. The present results indicated
that hTERTR (0.18 mg/ml), FAM96A (0.25 mg/ml) or combination
treatment [hTERTR (0.18 mg/ml) and FAM96A (0.25 mg/ml)] had no
significant cytotoxic effect on NCTC-1496 cells (Fig. 3A). In addition, treatment with hTERTR
and/or FAM96A induced apoptosis of tumor cells (Fig. 3B). Furthermore, the inhibitory
effects of hTERTR and/or FAM96A on migration and invasion in HCC
cells were investigated. The present results indicated that hTERTR
and/or FAM96A administration significantly inhibited migration
(Fig. 3C) and invasion (Fig. 3D) of HCC cells. Notably, these
findings suggest that combination treatment with hTERTR and FAM96A
markedly promoted apoptosis and inhibited migration and invasion of
hepatocellular tumor cells. These results suggested that hTERTR
and/or FAM96A significantly inhibited hepatocellular tumor cells
growth, migration and invasion.
Therapeutic effects of hTERTR-FAM96A
in HCC tumor-bearing mice
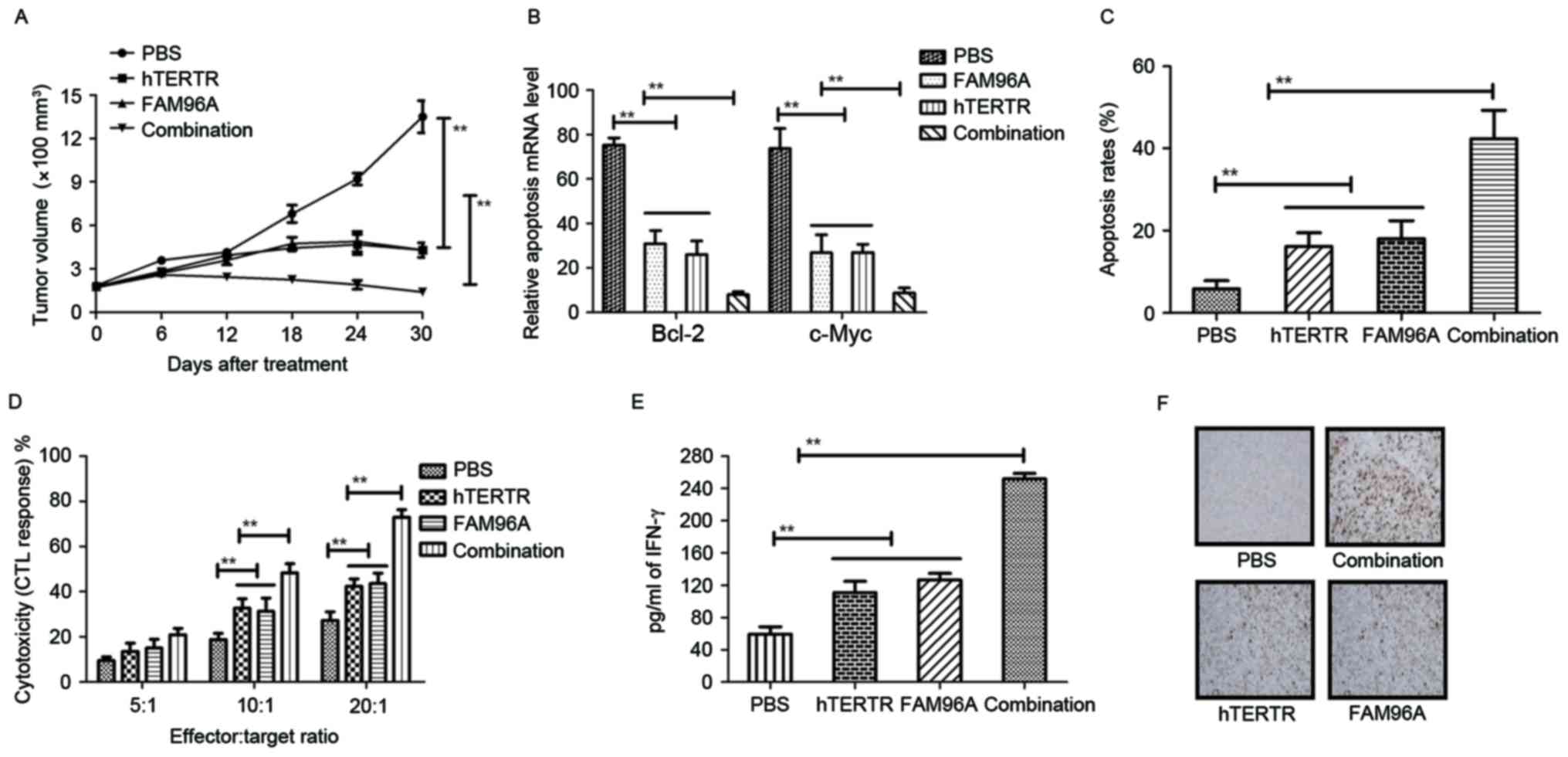
Therapeutic effects of hTERTR and/or FAM96A in HCC
tumor-bearing mice were evaluated by measuring tumor volume. Also,
tumor cellular immunity was analyzed at 10 days following
treatment. The results in Fig. 4A
showed that treatment with hTERTR and FAM96A exhibited
significantly increased inhibitory effects for HCC tumor growth
compared with hTERTR, FAM96A and PBS groups in a 30-day
observation. The result in Fig. 4B
showed that treatment with hTERTR and FAM96A markedly suppressed
expressions of apoptosis-suppressing genes (Bcl-2 and C-myc) in
tumors compared with hTERTR or FAM96A-treated tumors. In addition,
the results in Fig. 4C showed that
apoptosis rate was increased on tumor surface following combination
treatment with hTERTR and FAM96A. In addition, CTL responses and
IFN-γ release were also assessed in H22-bearing mice. Treatment
with hTERTR and FAM96A resulted in significantly higher CTL
activity and IFN-γ release when compared with control groups
(Fig. 4D and E). Furthermore, the
date in Fig. 4F indicated that
hTERTR and FAM96A-treated tumors generated more apoptotic bodies
analyzed by immunofluorescence compared with single agent-treated
tumors. These data suggest that hTERTR and FAM96A improved
apoptosis of HCC cells and enhanced CTL responses, more apoptotic
bodies and lymphocytes for HCC tumors.
Discussion
The majority of patients with HCC have exhibited
drug resistance and reduced apoptosis-induced immunologic
cytotoxicity in clinical trials (25). The ability to escape apoptosis
induced by immunologic cytotoxicity is a distinguishing feature of
tumorigenesis and an important factor in resistance to anti-cancer
treatments (26,27). A recent study has indicated that HCC
cells that developed resistance to the telomerase-activated prodrug
acycloguanosyl 5′-thymidyltriphosphate may undergo spontaneous
apoptosis (28). In addition, a
previous report demonstrated that dominant negative p63-α induced
drug resistance in HCC by interference with apoptosis signaling
pathways (29). Furthermore,
down-regulation of aquaporin expression has been demonstrated to
induce an increasing resistance to apoptosis in HCC (30). These findings suggested that
resistance to apoptosis was a major obstacle in clinical treatment
of HCC. Therefore, it was hypothesized that inhibition of
resistance to apoptosis may enhance the therapeutic efficacy for
patients with HCC.
In previous studies, the therapeutic effects of
targeting hTERT in patients with HCC were reported (31,32). The
hTERT protein is identified as a component of the cytosolic Fe/S
protein assembly machinery, and it is evolutionarily conserved and
critical for cellular iron homeostasis (33). In the present study, hTERTR was
identified as a novel target-regulating protein by binding with
hTERT in human and mouse hepatic carcinoma cells. Apoptosis of
tumor cells was also examined to confirm their association with
hTERTR; however, administration of a single anticancer agent of
hTERTR was not able to achieve the desired therapeutic effects in
HCC tumor model.
FAM96A is a 21 kDa (including the cell-penetrating
peptide) protein that is expressed at low levels in tumor cells
(34). Stehling et al
(15) previously demonstrated that
FAM96A is associated with metabolism, DNA maintenance, protein
translation and facilitating the effective induction of cell death
via the mitochondrial apoptosis pathway by binding to pro-apoptotic
APAF1 protein; however, it is poorly expressed in most tumor cells.
FAM96A, as a pro-apoptotic tumor suppressor, presented potential
therapeutic effects for tumor cells (15). Previous studies reported that
reestablishment of FAM96A expression enhanced apoptosis sensitivity
and inhibited tumor growth, leading to the hypothesis that the
limited apoptosis sensitivity of cancer cells was associated with
FAM96A loss (16,18). In the present study, it was observed
that FAM96A with cell penetrating peptide efficiently inhibited
tumor cells growth and induced apoptosis. However, in the HCC mouse
model, tumor cell were not eliminated completely.
Several challenges remain in reducing HCC-associated
resistance to apoptosis by targeted anti-cancer treatments
(35–37). Combinations of anti-cancer agents
have previously demonstrated a strong therapeutic effect (38–40).
Therefore, combination treatment of hTERTR and FAM96A was
investigated in HCC cells and a xenograft mouse model. The present
study indicated that combination treatment with hTERTR and FAM96A
was efficient for HCC inhibition, which elucidated a reference for
protein drugs for HCC therapy (41,42). In
addition, combination treatment with hTERTR and FAM96A presented a
better outcome by inducing tumor cell apoptosis compared with
single hTERTR or FAM96A treatments in murine HCC models.
Furthermore, the efficacy of combination treatment hTERTR and
FAM96A also generated tumor-specific CTL responses, which indicated
that an increase in apoptotic bodies and debris was considered as
potential cytotoxic toxicity mediated by cellular immunity.
In conclusion, combination treatment hTERTR and
FAM96A was identified as a hTERT-targeting and
FAM96A-reestablishing molecular therapy, which has the potential to
inhibit hepatic carcinoma tumor cells growth by prompting
apoptosis, which suggest the beneficial effects of targeted
therapy.
References
|
1
|
Fung SK and Lok AS: Management of patients
with hepatitis B virus-induced cirrhosis. J Hepatol. 42
Suppl:S54–S64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang YH, Wu JC, Chen SC, Chen CH, Chiang
JH, Huo TI, Lee PC, Chang FY and Lee SD: Survival benefit of
transcatheter arterial chemoembolization in patients with
hepatocellular carcinoma larger than 10 cm in diameter. Aliment
Pharmacol Ther. 23:129–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Devlin EJ, Denson LA and Whitford HS:
Cancer treatment side effects: A Meta-analysis of the relationship
between response expectancies and experience. J Pain Symptom
Manage. 54:245–258.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo K and Nakagawa K: Management of side
effects with platinum doublet chemotherapy used for treatment of
non-small cell lung cancer. Nihon Rinsho. 73 Suppl 2:S542–S547.
2015.(In Japanese).
|
|
5
|
Vincent J, de Boer M, Lobbezoo DJ, Smeets
RE and Tjan-Heijnen VC: Combination of exemestane and everolimus
may produce toxic side effects: A new treatment option for
metastatic hormone-sensitive breast cancer. Ned Tijdschr Geneeskd.
158:A75232014.(In Dutch). PubMed/NCBI
|
|
6
|
Lubienski A, Bitsch RG, Schemmer P,
Grenacher L, Düx M and Kauffmann GW: Long-term results of
interventional treatment of large unresectable hepatocellular
carcinoma (HCC): Significant survival benefit from combined
transcatheter arterial chemoembolization (TACE) and percutaneous
ethanol injection (PEI) compared to TACE monotherapy. Rofo.
176:1794–1802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeh ML, Huang CI, Huang CF, Hsieh MY,
Huang JF, Dai CY, Lin ZY, Chen SC, Yu ML and Chuang WL: Neoadjuvant
transcatheter arterial chemoembolization does not provide survival
benefit compared to curative therapy alone in single hepatocellular
carcinoma. Kaohsiung J Med Sci. 31:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo Z, Yu H, Liu C, Si T, Yang X, Zhang W,
Xu Y and Li Y: Advances in endovascular therapy to treat primary
hepatocellular carcinoma. Drug Discov Ther. 9:342–351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang J, Yu C, Chen M, Tian S and Sun C:
Over-expression of TRIM37 promotes cell migration and metastasis in
hepatocellular carcinoma by activating Wnt/β-catenin signaling.
Biochem Biophys Res Commun. 464:1120–1127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizukoshi E, Nakagawa H, Kitahara M,
Yamashita T, Arai K, Sunagozaka H, Iida N, Fushimi K and Kaneko S:
Phase I trial of multidrug resistance-associated protein 3-derived
peptide in patients with hepatocellular carcinoma. Cancer Lett.
369:242–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ibrahim AA, Abdel Aleem MH, Abdella HM and
Helmy A: Study of the role of insulin resistance as a risk factor
in HCV related hepatocellular carcinoma. J Egypt Soc Parasitol.
45:107–113. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang TS, Shyu YC, Chen HY, Yuan SS, Shih
JN and Chen PJ: A systematic review and meta-analysis of adjuvant
interferon therapy after curative treatment for patients with viral
hepatitis-related hepatocellular carcinoma. J Viral Hepat.
20:729–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park YJ, Kim EK, Bae JY, Moon S and Kim J:
Human telomerase reverse transcriptase (hTERT) promotes cancer
invasion by modulating cathepsin D via early growth response
(EGR)-1. Cancer Lett. 370:222–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abdul-Ghani R, Ohana P, Matouk I, Ayesh S,
Ayesh B, Laster M, Bibi O, Giladi H, Molnar-Kimber K, Sughayer MA,
et al: Use of transcriptional regulatory sequences of telomerase
(hTER and hTERT) for selective killing of cancer cells. Mol Ther.
2:539–544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stehling O, Mascarenhas J, Vashisht AA,
Sheftel AD, Niggemeyer B, Rösser R, Pierik AJ, Wohlschlegel JA and
Lill R: Human CIA2A-FAM96A and CIA2B-FAM96B integrate iron
homeostasis and maturation of different subsets of
cytosolic-nuclear iron-sulfur proteins. Cell Metab. 18:187–198.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mas C, Chen KE, Brereton IM, Martin JL and
Hill JM: Backbone resonance assignments of the monomeric DUF59
domain of human Fam96a. Biomol NMR Assign. 7:117–120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen KE, Richards AA, Ariffin JK, Ross IL,
Sweet MJ, Kellie S, Kobe B and Martin JL: The mammalian DUF59
protein Fam96a forms two distinct types of domain-swapped dimer.
Acta Crystallogr D Biol Crystallogr. 68:637–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ouyang B, Wang L, Wan S, Luo Y, Lin J and
Xia B: Solution structure of monomeric human FAM96A. J Biomol NMR.
56:387–392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hawkins P, Morton DB, Burman O, Dennison
N, Honess P, Jennings M, Lane S, Middleton V, Roughan JV, Wells S
and Westwood K; UK Joint Working Group on Refinement
BVAAWF/FRAME/RSPCA/UFAW, : A guide to defining and implementing
protocols for the welfare assessment of laboratory animals:
Eleventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group
on Refinement. Lab Anim. 45:1–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morotomi N, Fukuda K, Nakano M, Ichihara
S, Oono T, Yamazaki T, Kobayashi N, Suzuki T, Tanaka Y and
Taniguchi H: Evaluation of intestinal microbiotas of healthy
Japanese adults and effect of antibiotics using the 16S ribosomal
RNA gene based clone library method. Biol Pharm Bull. 34:1011–1020.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang X, Zhang H, Zhang E, Wei J, Li W,
Wang B, Dong S and Zhu J: Identification of the pXO1 plasmid in
attenuated Bacillus anthracis vaccine strains. Virulence.
7:578–586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greaves MF and Brown G: Purification of
human T and B lymphocytes. J Immunol. 112:420–423. 1974.PubMed/NCBI
|
|
23
|
Zamarin D, Vigil A, Kelly K, Garcia-Sastre
A and Fong Y: Genetically engineered Newcastle disease virus for
malignant melanoma therapy. Gene Ther. 16:796–804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang
PY, Zhang JB, Wang L, Wu WZ, Qin LX and Tang ZY: Human
hepatocellular carcinoma tumor-derived endothelial cells manifest
increased angiogenesis capability and drug resistance compared with
normal endothelial cells. Clin Cancer Res. 15:4838–4846. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hons JM: New insights into the
immunomodulatory effects of exercise and potential Impact on
tumorigenesis. Oncology (Williston Park). 29:921–922.
2015.PubMed/NCBI
|
|
27
|
Aldinucci D, Celegato M and Casagrande N:
Microenvironmental interactions in classical Hodgkin lymphoma and
their role in promoting tumor growth, immune escape and drug
resistance. Cancer Lett. 380:243–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Webb TE and Galli A: Hepatocellular
carcinoma cells that develop resistance to the telomerase-activated
prodrug ACV-TP-T may undergo spontaneous apoptosis. Med Hypotheses.
85:3832015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mundt HM, Stremmel W, Melino G, Krammer
PH, Schilling T and Müller M: Dominant negative (DeltaN) p63alpha
induces drug resistance in hepatocellular carcinoma by interference
with apoptosis signaling pathways. Biochem Biophys Res Commun.
396:335–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jablonski EM, Mattocks MA, Sokolov E,
Koniaris LG, Hughes FM Jr, Fausto N, Pierce RH and McKillop IH:
Decreased aquaporin expression leads to increased resistance to
apoptosis in hepatocellular carcinoma. Cancer Lett. 250:36–46.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizuno H, Honda M, Shirasaki T, Yamashita
T, Mizukoshi E and Kaneko S: Heterogeneous nuclear
ribonucleoprotein A2/B1 in association with hTERT is a potential
biomarker for hepatocellular carcinoma. Liver Int. 32:1146–1155.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Masutomi K, Kaneko S, Yasukawa M, Arai K,
Murakami S and Kobayashi K: Identification of serum anti-human
telomerase reverse transcriptase (hTERT) auto-antibodies during
progression to hepatocellular carcinoma. Oncogene. 21:5946–5950.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim YH, Kim KT, Lee SJ, Hong SH, Moon JY,
Yoon EK, Kim S, Kim EO, Kang SH, Kim SK, et al: Image-aided suicide
gene therapy utilizing multifunctional hTERT-targeting adenovirus
for clinical translation in hepatocellular carcinoma. Theranostics.
6:357–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwamb B, Pick R, Fernández SB, Völp K,
Heering J, Dötsch V, Bösser S, Jung J, Beinoraviciute-Kellner R,
Wesely J, et al: FAM96A is a novel pro-apoptotic tumor suppressor
in gastrointestinal stromal tumors. Int J Cancer. 137:1318–1329.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sacco PC, Maione P, Rossi A, Bareschino
MA, Schettino C, Guida C, Elmo M, Ambrosio R, Barbato V, Zeppa R,
et al: Combination of radiotherapy and targeted therapies in the
treatment of locally advanced non-small cell lung cancer. Target
Oncol. 6:171–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Batist G, Wu JH, Spatz A, Miller WH Jr,
Cocolakis E, Rousseau C, Diaz Z, Ferrario C and Basik M: Resistance
to cancer treatment: The role of somatic genetic events and the
challenges for targeted therapies. Front Pharmacol. 2:592011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aggarwal R and Ryan CJ:
Castration-resistant prostate cancer: Targeted therapies and
individualized treatment. Oncologist. 16:264–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rivera F, Lopez-Tarruella S, Vega-Villegas
ME and Salcedo M: Treatment of advanced pancreatic cancer: From
gemcitabine single agent to combinations and targeted therapy.
Cancer Treat Rev. 35:335–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bunn PA Jr and Kelly K: Combinations of
three chemotherapeutic agents and two chemotherapeutic agents plus
a targeted biologic agent in the treatment of advanced non
small-cell lung cancer. Clin Lung Cancer. 2 Suppl 1:S23–S28. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cabrespine A, Bay JO, Barthomeuf C, Curé
H, Chollet P and Debiton E: In vitro assessment of cytotoxic agent
combinations for hormone-refractory prostate cancer treatment.
Anticancer Drugs. 16:417–422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee BS, Kim HJ, Hwang JW, Cheong KH, Kim
KA, Cha HY, Lee JM and Kim CH: The dual inhibition of Met and EGFR
by ME22S, a novel Met/EGFR bispecific monoclonal antibody,
suppresses the proliferation and invasion of laryngeal cancer. Ann
Surg Oncol. 23:2046–2053. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Taki S, Kamada H, Inoue M, Nagano K, Mukai
Y, Higashisaka K, Yoshioka Y, Tsutsumi Y and Tsunoda S: A novel
bispecific antibody against human CD3 and ephrin receptor A10 for
breast cancer therapy. PLoS One. 10:e01447122015. View Article : Google Scholar : PubMed/NCBI
|