Introduction
Degenerative lumbar spinal stenosis is a common
lumbar disease and one of the main reasons for the high incidence
of intermittent claudication (1).
The clinical manifestation of degenerative lumbar spinal stenosis
is chronic pain in the waist and lower limbs, which limits activity
and alters normal work and daily life activities (2). The traditional therapeutic method to
treat degenerative lumbar spinal stenosis are laminectomy,
decompression, and bone graft fusion. However, these therapies have
significant disadvantages, resulting in movement disturbances of
the fused segments, which impacts the adjacent segments and
promotes degeneration (3).
The Coflex system, which has been applied in the
clinic since the 1990s, can effectively resolve intermittent
claudication, relieve lumbar spinal stenosis, and is especially
suitable for elderly patients (4).
The aim of the present study was to demonstrate a
satisfactory curative effect by conducting dynamic fixation Coflex
treatment for patients with degenerative lumbar spinal
stenosis.
Patients and methods
Clinical data
We recruited 78 patients with degenerative lumbar
spinal stenosis who were admitted to our hospital from July 2014 to
June 2015. Inclusion criteria for the study were: degenerative
lumbar spinal stenosis identified by imagological examination such
as MRI and X-ray. Patients had obvious intermittent claudication
combined with backache or lower limb radiating pain. Patients
signed informed consent. Exclusion criteria for the study were:
olisthy or instability of adjacent segments; and history of lumbar
surgery, serious osteoporosis, or lumbar vertebral wound. Patients
were divided equally into the control and observation groups
(n=39). The control group received traditional decompression
fusion, whereas the observation group was treated with dynamic
fixation Coflex system. The general biometric data for the patients
in the two groups showed no statistical differences (Table I).
 | Table I.Baseline patient information. |
Table I.
Baseline patient information.
| Items | Control (n=39) | Observation
(n=39) | t/χ2 | P-value |
|---|
| Sex
(male/female) | 20/19 | 22/17 | 0.051 | 0.820 |
| Age (years) | 45–75 | 45–80 |
|
|
| Average age
(years) | 58.56±6.47 | 58.85±6.38 | 0.199 | 0.842 |
| Intermittent
claudication type (n, %) |
|
|
|
|
| Gesture
claudication | 21 (53.84) | 19 (48.72) | 0.051 | 0.820 |
| Ischemic
claudication | 18 (46.16) | 20 (51.28) |
|
|
| Pain type (n, %) |
|
|
|
|
| Backache
combined with leg pain | 26 (66.67) | 29 (74.35) | 0.247 | 0.619 |
| Backache
or leg pain | 13 (33.33) | 10 (35.65) |
|
|
Preoperative preparation
Before surgery, the clinical history of patients was
evaluated and surgical contraindications were excluded to decrease
the surgical risk. Functional scores were collected, including the
Japanese Orthopaedic Association (JOA) score, Visual analogue scale
(VAS) pain score, and the Oswestry disability index (ODI) score.
Appropriate surgical timing was selected and methylprednisolone was
provided 30 min before surgery followed by general anesthesia.
Traditional fusion for the control
group
After general anesthesia, the patients were placed
in the prone position and X-ray machine perspective was used to
locate the surgical segments. An 8–10 cm incision was carried out
along the waist posterior median. The deep fascia and the
paravertebral muscle were peeled to expose the spinous process of
the vertebral plate. A guide needle was used to puncture the
bilateral locating point of L4 and L5, and a pedicle screw was
implanted according to the depth. X-ray perspective was used to
check the position, then fixed with preloaded nut. Rongeur was
applied for L4 and L5 spinous process to eliminate incrassated
(ossified) ligamentum flavum and small zygopophysis as well as the
vertebral plate that induced bony stenosis, with a full
decompression. Bilateral rod was shaped to assemble the segments,
nuts were screwed down, and washed with normal saline. The cut
spinous process and vertebral plate were trimmed to proper size,
which were implanted between the intervertebral spaces. A drainage
tube was indwelled and the incision was sutured layer by layer to
conclude the surgery.
Dynamic fixation Coflex for the
observation group
After general anesthesia, the patients were placed
in prone position and C-arm X-ray perspective was used to locate
the surgical spot and the median incision (approximately 5 cm long)
was selected. The supraspinous ligament was separated till the
upper edge of the L4 spinous process and the lower edge of the L5
spinous process appeared. Lumbodorsal fascia was stripped and the
paravertebral muscle was bluntly stripped to completely expose the
spinous process and bilateral vertebral plate. Bilateral facet
joints were protected. Incrassated ligamentum flavum and
proliferous zygopophysis were cut, paying attention to lateral
recess decompression. If an obvious protrusion of intervertebral
disc was identified, the nucleus pulposus of protrusion was picked
out. Proper Coflex fixator was selected and placed on L4-L5 that
pressed close to the spinous process root and vertebral plate
(approximately 2 mm away from the dural sac), and X-ray perspective
was used to check the position. After the appropriate position was
confirmed and tight, the two ends of the fixator were clamped
moderately clinging to spinous process. The prosthesis bye hole and
supraspinous ligament were sutured together, placing drainage tube,
and suturing the incision.
Postoperative care
The patients were sent back to the ward and kept
under electrocardiograph monitoring. Patients were required to
remain prostrate on the bed for rest. After surgery, conventional
rehydration, antibiotics, and neurotrophy drugs were administered
as well as pain management. The drainage tube was removed 48 h
after surgery, and the patients were provided with a waist belt for
more than 3 months to avoid excessive contortion and extension. Day
three after surgery, bed exercise of waist function was initiated.
Day four after surgery, patients could start early off-bed activity
and anteroposterior movement. Lateral X-ray of the lumbar vertebra
was taken for observation of the implantated materials. The
patients were evaluated again 12 months later.
Evaluation methods
Clinical data were: age of patients ≤60 years
marking 1 score, and BMI ≤25 marking 1 score. MRI iconography was
used to determine the average area of the spinal canal of patients.
We compared the surgery time, intraoperative blood loss, volume of
drainage after surgery, and hospitalization days to determine the
effects of the surgeries. During follow-up 12 months later, the
evaluation was conducted according to the MacNab standard as
follows: Excellent, straight leg raise >70°, normal muscle force
of lower limbs and movement, no pain in the waist and lower
extremities. Good, straight leg raise <70°, but >30°, Grade 4
for muscle force, normal work and daily life with slight pain in
waist and lower extremities sometimes. Medium, straight leg raise
<30°, but >15°, Grade 3 for muscle force, alleviated pain in
waist and lower extremities, with some use of drugs. Poor, no
change, even aggravated, which required drugs to relieve pain. The
total effective rate was calculated as: (excellent + good + medium)
× 100.
VAS was used to evaluate pain level, with 0
indicating no pain and 10 indicating unbearable and acute pain. The
JOA score was applied to evaluate low back pain level before and
after surgery (5), with total score
of 29. The evaluation standards were: poor, <10 score; medium,
10–15; good, 16–24; excellent, ≥25.
ODI was applied to score for dysfunction, with 0
indicating no dysfunction and 5 indicating obvious dysfunction. ODI
was applied in three dimensions, including single capacity, pain,
and personal comprehensive abilities and 9 total items (sexual life
was excluded due to age), with a total maximum score of 45. ODI was
calculated as accumulating scores/100×100.
The Pfirrmann grading method (6) was used and combined with the clinical
data to produce a quantitative score from 0–10: Grade I, 4 points;
Grade II, 3 points; Grade III, 2 points; Grade IV, 1 point; and
Grade V, 0 points.
Imagological examination: sagittal plane angle of
adjacent segment ≥10°, 1 point; lateral displacement ≤3 mm, 1
point; intercalated disc wedging ≤5°, 1 point; sagittal plane
shifting ≤4 mm, 1 point.
Statistical analysis
SPSS 19.0 software (IBM SPSS, Armonk, NY, USA) was
used to conduct statistical analysis. Measurement data are shown as
mean ± standard deviation and tested by t-test. Enumeration data
was expressed by rate (%) and tested by χ2. Ranked data
were analyzed by rank sum test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Surgery and postoperative condition of
patients
Surgery time and hospitalization days of the
patients in the observation group were significantly shorter than
in the control group (Table II).
Intraoperative blood loss and volume of drainage after surgery were
significantly smaller in the observation group than in the control
group (Table II).
 | Table II.Surgical and post-surgical data. |
Table II.
Surgical and post-surgical data.
| Groups | Cases | Surgery time
(min) | Intraoperative blood
loss (ml) | Volume of drainage
after surgery (ml) | Hospitalization
(days) |
|---|
| Observation | 39 | 56.83±3.62 | 75.47±6.63 | 77.86±7.45 | 5.23±1.53 |
| Control | 39 | 98.14±3.57 | 162.57±9.38 | 131.97±8.37 | 8.56±1.47 |
| t-test |
| 50.740 | 47.354 | 30.157 | 9.801 |
| P-value |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 |
MacNab curative effect
The effective rate for the observation and control
groups were 97.44 and 76.93%, respectively, and the difference was
statistically significant (Table
III).
 | Table III.Macnab curative effect (n, %). |
Table III.
Macnab curative effect (n, %).
| Groups | Cases | Excellent | Good | Medium | Poor |
|---|
| Observation | 39 | 18 (46.15) | 16 (41.02) | 4 (10.26) | 1 (2.56) |
| Control | 39 | 14 (35.89) | 11 (28.21) | 5 (12.82) | 9
(23.07) |
Pain VAS scores before and after
surgery
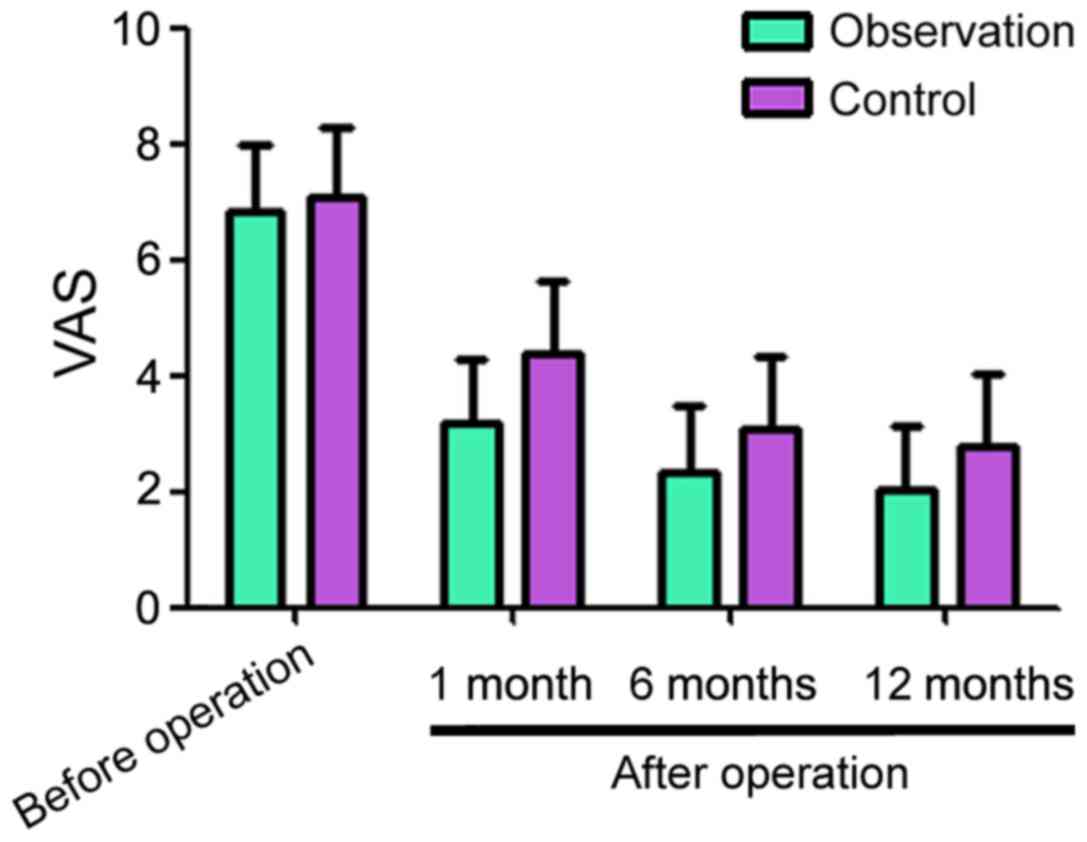
Before surgery, the pain VAS scores for the
observation and control groups were 6.83±1.14 and 7.06±1.23,
respectively, the difference had no statistical significance
(Fig. 1). One month after surgery,
the pain VAS scores for the observation and control groups were
3.16±1.13 and 4.36±1.25, respectively. A total of six months after
surgery, the VAS scores for the observation and control groups were
2.35±1.14 and 3.08±1.26, respectively. A total of 12 months after
surgery, the VAS scores for the observation and control groups were
2.03±1.12 and 2.76±1.17, respectively. The VAS scores decreased in
the two groups compared with before operation, but the observation
group showed lower values at each time point compared to the
respective control group (Fig.
1).
The JOA scores prior to surgery were similar between
the two groups (Table IV). The
scores increased in the two groups after surgery, but the values
were consistently higher in the observation group at 1, 6 and 12
months follow-up (Table IV).
 | Table IV.JOA scores before and after
surgery. |
Table IV.
JOA scores before and after
surgery.
| Groups | Cases | Pre-surgery | 1 month after
surgery | 6 months after
surgery | 12 months after
surgery |
|---|
| Observation | 39 | 12.81±2.24 | 17.62±2.63 | 20.78±2.24 | 23.13±2.23 |
| Control | 39 | 12.29±2.34 | 15.53±2.37 | 17.93±2.36 | 20.76±2.37 |
| t-test |
| 1.002 | 3.678 | 5.470 | 4.548 |
| P-value |
| 0.319 |
0.0004 | <0.0001 | <0.0001 |
ODI scores before and after
surgery
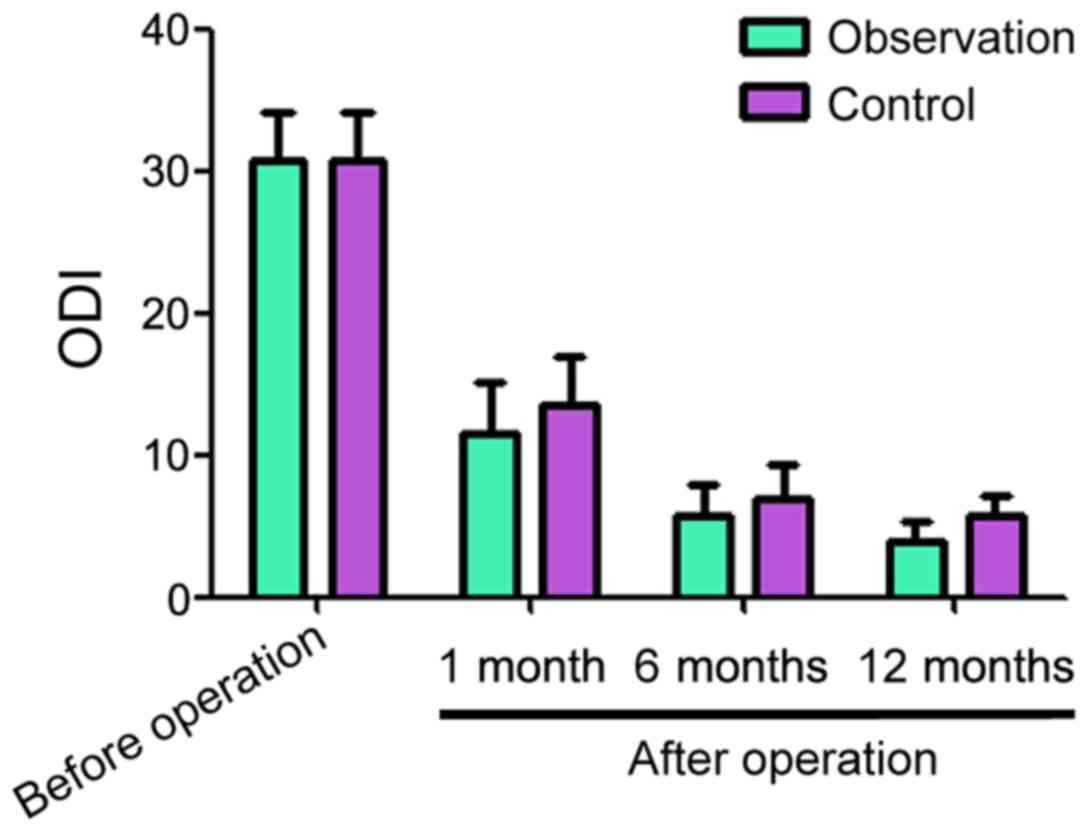
The ODI scores before surgery for the observation
and control groups were 30.83±3.24 and 30.77±3.34, respectively
(Fig. 2). One, 6 and 12 months after
surgery, the ODI scores for the observation and control groups
decreased compared with before the surgery. However, the scores for
the observation group were lower than for the control group at each
time point (Fig. 2).
Vertebral canal before and after
surgery
In terms of vertebral canal area, the preoperative
average vertebral canal area for both groups was comparable. The
postoperative average vertebral canal area for the observation
group was significantly larger than that for the control group 6
and 12 months later (Table V).
 | Table V.Vertebral canal area before and after
surgery (mm3). |
Table V.
Vertebral canal area before and after
surgery (mm3).
| Groups | Cases | Pre-surgery | 6 months after
surgery | 12 months after
surgery | F | P-value |
|---|
| Observation | 39 | 225.81±12.24 | 263.78±19.24 | 262.13±12.23 | 80.458 | <0.0001 |
| Control | 39 | 227.29±12.34 | 246.93±15.36 | 231.76±12.37 | 22.909 | <0.0001 |
| t-test |
| 0.532 | 4.271 | 10.903 |
|
|
| P-value |
| 0.596 |
0.0001 |
<0.0001 |
|
|
Adjacent segment quantitative
scores
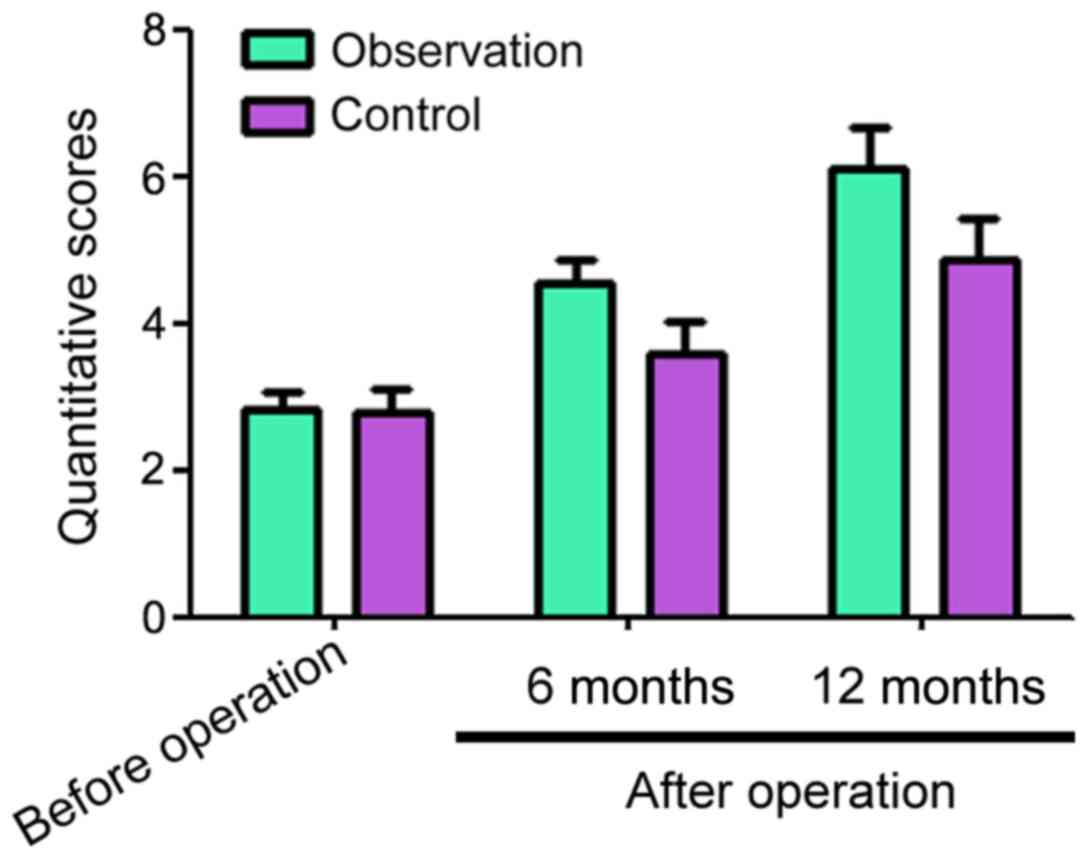
Before surgery, the adjacent segment quantitative
scores for the observation and control groups were 2.83±0.24 and
2.77±0.34, respectively (Fig. 3).
Six and 12 months after surgery, the adjacent segment quantitative
scores for the observation and control groups decreased compared
with before surgery, but the observation group had significantly
higher scores than the control group at both time points (Fig. 3).
Discussion
Degenerative lumbar spinal stenosis can be caused by
relaxation and hypertrophy of the ligamentum flavum, proliferation
of the superior articular process, protrusion of the intervertebral
disc, hyperostosis of the vertebral posterior border, and other
reasons. Stenosis is divided into bony, which is caused by bony
channel stenosis, such as lateral recess, nerve root canal, or
central spinal canal, and non-bony, which is caused by spinal canal
volume lessening due to hypertrophy of the ligamentum flavum,
protrusion of the intervertebral disc, and calcification (7,8). The
development of this condition causes continuous oppression of the
spinal cord and spinal nerve root, leading to dysneuria and
long-term low back pain and leg pain (9). The course of degenerative lumbar spinal
stenosis is insidious and develops slowly. The initial pain is not
serious and is often combined with muscular soreness, which can be
improved by changing position or rest (10). The nucleus pulposus of the normal
intervertebral disc is even and the architectural features in each
direction of the intervertebral disc are uniform. When degeneration
occurs, the colloid in the nucleus pulposus becomes splintery and
heterogeneous. Occasionally, end plate fragments or fiber rings
enter into the intervertebral disc, leading to stress concentration
and maldistribution of end plate load. Normal load sharing and
conduction change, resulting in low back pain that gradually moves
down to the legs. It is often combined with regional low muscle
contraction and local numbness, which may oppress the cauda equina
and result in intermittent claudication, leading to serious
inconvenience to normal work and life activities (11–13).
The treatment of degenerative lumbar spinal stenosis
can be divided into conservative and surgical approaches. The
conservative treatments include general treatment (physiotherapy,
waist protection, exercise of lumbodorsal muscles, and bed rest),
drug therapy (dehydration drugs, analgesia drugs, and neurotrophy
drugs), acupuncture, and block therapy (14). When the conservative treatment fails,
it is necessary to resort to the surgical treatment, which includes
decompression (wide laminectomy and ablation of the facet joint),
bone graft fusion internal fixation, and non-fusion (15). The short-term efficacy of traditional
fusion is excellent in relieving pain. However, the original
biomechanics environment is damaged after surgery, resulting in
accelerated degeneration of adjacent segments, with poor long-term
curative effect (16). Dynamic
fixation Coflex treatment belongs to the non-fusion category. This
method is combined with intraoperative decompression, which can
control the range of activity among vertebral bodies, and
selectively and effectively increases the cross sectional area of
the spinal canal (17). The results
of the present study showed that VAS, ODI, and JOA scores improved
after surgery in the observation group compared to the control
group. The vertebral canal area of the observation group was larger
than that in the control group. Compared with traditional fusion,
the holding time of the vertebral canal area improvement was
longer, because the Coflex system can reduce the burden on the
intervertebral disc and zygapophyseal joint, relieving low back
pain. It also improves stability and keeps the disc in place when
the spine takes activities such as anteflexion, rear protraction,
and rotation (18). Our results
showed that several intra and postoperative parameters improved in
the observation group. This is due to the advantages of the Coflex
system, like simple surgery and small wound compared with
traditional fusion. Therefore, surgery is shorter, reduce blood
loss, and accelerate recovery.
When the conservative treatment fails, the patients
receive traditional spinal fusion treatment, which often changes
the spine biomechanics and leads to segment movement dysfunction.
Under these circumstances, the compensatory mobility and
displacement of the adjacent segments increase and the stress in
the adjacent segments also become bigger, resulting in degeneration
(19). Coflex system implantation
has no remarkable influence on activity in different directions for
adjacent segments, and it also can change the burden distribution
of operative segments and make their mobility similar to the normal
spinal activity. This provides stability and changes the way
adjacent segments bear the burden to reduce the pressure, as well
as the influence on adjacent segments, therefore it can relieve the
degeneration of adjacent segments after fusion (20). The results of the present study
showed that postoperative quantitative scores for adjacent segments
in the observation group were superior to the control group,
suggesting that dynamic fixation Coflex can be regarded as the
connector between spine fusion segment and non-fusion segment, as a
transition area with less influence on adjacent segments compared
with traditional fusion.
In conclusion, dynamic fixation Coflex treatment for
patients with degenerative lumbar spinal stenosis features
remarkable effects. It can reduce the influence on adjacent
segments and delay degeneration, which improves spine stability.
The sample size of this study is small and the follow-up time is
short, which requires a further study of large sample size and
long-term follow-up.
References
|
1
|
Fay LY, Wu JC, Tsai TY, Wu CL, Huang WC
and Cheng H: Dynamic stabilization for degenerative
spondylolisthesis: Evaluation of radiographic and clinical
outcomes. Clin Neurol Neurosurg. 115:535–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choudhri TF, Mummaneni PV, Dhall SS, Eck
JC, Groff MW, Ghogawala Z, Watters WC III, Dailey AT, Resnick DK,
Sharan A, et al: Guideline update for the performance of fusion
procedures for degenerative disease of the lumbar spine Part 4
Radiographic assessment of fusion status. J Neurosurg Spine.
21:23–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin JS, Oh SH and Cho PG: Surgical
outcome of a zero-profile device comparing with stand-alone cage
and anterior cervical plate with iliac bone graft in the anterior
cervical discectomy and fusion. Korean J Spine. 11:169–177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu X-L, Wu L-J, Zheng RM and Ni WF: Finite
element model testing on Coflex dynamic fixation for L4/5 and L5/S1
segment of lower lumbar. Yiyong Shengwu Lixue. 28:477–483. 2013.(In
Chinese).
|
|
5
|
Resnick DK, Choudhri TF, Dailey AT, Groff
MW, Khoo L, Matz PG, Mummaneni P, Watters WC III, Wang J, Walters
BC, et al: Guidelines for the performance of fusion procedures for
degenerative disease of the lumbar spine. Part. 8:Lumbar fusion for
disc herniation and radiculopathy. J Neurosurg Spine 21: 37–41.
2014.
|
|
6
|
Urrutia J, Besa P, Campos M, Cikutovic P,
Cabezon M, Molina M and Cruz JP: The Pfirrmann classification of
lumbar intervertebral disc degeneration: An independent inter- and
intra-observer agreement assessment. Eur Spine J. 25:2728–2733.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Li F, Yu S, Ma H, Chen Z, Zhang H
and Fu Q: Two-year follow-up results of the Isobar TTL Semi-Rigid
Rod System for the treatment of lumbar degenerative disease. J Clin
Neurosci. 20:394–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kreiner DS, Shaffer WO, Baisden JL,
Gilbert TJ, Summers JT, Toton JF, Hwang SW, Mendel RC and Reitman
CA: An evidence-based clinical guideline for the diagnosis and
treatment of degenerative lumbar spinal stenosis (update). Spine J.
13:734–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shamji MF, Mroz T, Hsu W and Chutkan N:
Management of degenerative lumbar spinal stenosis in the elderly.
Neurosurgery. 77:S68–S74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Passmore SR, Johnson MG, Kriellaars DJ,
Pelleck V, Enright A and Glazebrook CM: Fitts's Law using lower
extremity movement: Performance driven outcomes for degenerative
lumbar spinal stenosis. Hum Mov Sci. 44:277–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yi H, Wei X, Zhang W, Chen Z, Wang X, Ji
X, Zhu X, Wang F, Xu X, Li Z, et al: Reliability and validity of
simplified Chinese version of Swiss Spinal Stenosis Questionnaire
for patients with degenerative lumbar spinal stenosis. Spine.
39:820–825. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Puzzilli F, Gazzeri R, Galarza M, Neroni
M, Panagiotopoulos K, Bolognini A, Callovini G, Agrillo U and
Alfieri A: Interspinous spacer decompression (X-STOP) for lumbar
spinal stenosis and degenerative disk disease: A multicenter study
with a minimum 3-year follow-up. Clin Neurol Neurosurg.
124:166–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galarza M, Fabrizi AP, Maina R, Gazzeri R
and Martínez-Lage JF: Degenerative lumbar spinal stenosis with
neurogenic intermittent claudication and treatment with the Aperius
PercLID System: A preliminary report. Neurosurg Focus. 28:E32010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu SW, Yang SC, Ma CH, Wu CH, Yen CY and
Tu YK: Comparison of Dynesys posterior stabilization and posterior
lumbar interbody fusion for spinal stenosis L4L5. Acta Orthop Belg.
78:230–239. 2012.PubMed/NCBI
|
|
15
|
Yang M, Li C, Chen Z, Bai Y and Li M:
Short term outcome of posterior dynamic stabilization system in
degenerative lumbar diseases. Indian J Orthop. 48:574–581. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haddad B, Makki D, Konan S, Park D, Khan W
and Okafor B: Dynesys dynamic stabilization: Less good outcome than
lumbar fusion at 4-year follow-up. Acta Orthop Belg. 79:97–103.
2013.PubMed/NCBI
|
|
17
|
Wu X-L, Wu L-J, Zheng RM, Wang JS, Xu HZ,
Zhou Y and Wu AM: Biomechanical characteristics analysis on discs
with Coflex fixation on the different segments of lower lumbar
spine. Zhongguo gu shang. China J Orthop Traumatol. 27:938–942.
2014.(In Chinese).
|
|
18
|
Heuer F, Schmidt H, Käfer W, Graf N and
Wilke HJ: Posterior motion preserving implants evaluated by means
of intervertebral disc bulging and annular fiber strains. Clin
Biomech (Bristol, Avon). 27:218–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hartmann F, Dietz SO, Kuhn S, Hely H,
Rommens PM and Gercek E: Biomechanical comparison of an
interspinous device and a rigid stabilization on lumbar adjacent
segment range of motion. Acta Chir Orthop Traumatol Cech.
78:404–409. 2011.PubMed/NCBI
|
|
20
|
Lo CC, Tsai KJ, Chen SH, Zhong ZC and Hung
C: Biomechanical effect after Coflex and Coflex rivet implantation
for segmental instability at surgical and adjacent segments: A
finite element analysis. Comput Methods Biomech Biomed Engin.
14:969–978. 2011. View Article : Google Scholar : PubMed/NCBI
|