Introduction
The incidence rate of thyroid tumor has increased
over the past 30 years and tends to affect the young (1–3).
Although thyroid carcinoma is rare and accounts for approximately
1% among human malignancies, it is the most common malignancy of
the endocrine system (4,5). Among thyroid malignancies,
differentiated thyroid cancer (DTC) comprises the vast majority,
~90%, and includes papillary and follicular histological types
(6). DTC cells have the ability to
concentrate iodine and to synthesize and release thyroid hormones.
This characteristic enables the administration of radioactive
iodine-131 (131I) to treat metastatic diseases and to
ablate remnant thyroid tissues following thyroidectomy (7). In accordance with the European
Association of Nuclear Medicine guidelines, certain patients with
DTC are required to receive 131I therapy after surgery
(8).
Immunological mechanisms are vital in the
surveillance of malignancy and the control of cancer progression.
Immune responses against DTC have long been recognized. T cells are
crucial participants in the immune response. Previous studies have
revealed that the imbalance between T helper (Th)1 and Th2 cells is
a major immunological mechanism in the pathogenesis of DTC
(9–11). Three novel T cell subgroups,
including Th17 cells [CD4+/interleukin
(IL)17+ T] cells, cytotoxic T (Tc17;
CD8+IL17+ T) cells and regulatory T (Treg;
CD4+CD25+Foxp3+ T) cells have
gained increasing attention, as they may serve key roles in the
development of infections, allergic reactions, autoimmune diseases
and tumorigenesis (12,13). Th17 cells exhibit the characteristics
of CD4+ T cells and secrete ILs, including IL-17, IL-21
and IL-22. Differentiation of Th17 cells occurs in the presence of
IL-6, IL-21 and tumor growth factor (TGF)-β, and their stability is
sustained by IL-1β and IL-23 (14,15).
Similar to Th17 cells, Tc17 cells are characterized by the
production of IL-17. Tc17 cells have been implicated in
immune-associated diseases and carcinomas (16,17).
Treg cells secrete TGF-β and IL-10. The release of IL-10 aids Treg
cells to greatly repress host immune responses, mainly by
contributing to impaired antitumorimmunity in cancer-bearing
subjects (18). In previous reports,
the reciprocal relationships between Th17 and either Tc17 or Treg
cell numbers in tumors have been emphasized. The imbalance between
Th17 and Tc17 or Treg cells has been observed in several types of
tumor, such as gastric (19),
ovarian (20) and prostate cancer
(21). In particular, recent studies
have revealed that Thl7, Tc17 and Treg cell levels are also
abnormal in patients with DTC (11,15,19,22);
however, the specific relationships between Th17 cells and Tc17 or
Treg cells, as well as the related cytokine secretion, remain
unclear. 131I treatment may have an effect on the
hematopoietic system of patients with DTC, particularly on the
lymphocytes; however, there little is known about T cells in
patients with DTC patients following 131I treatment.
Therefore, the present study aimed to investigate the effects of
131I on the immune cell subsets, Th17, Tc17 and Treg
cells, and the related cytokine production in patients with
DTC.
Materials and methods
Patients
The study population included 40 patients with DTC
(26 female; 14 male; age, 24–72 years); patient characteristics are
provided in Table I. Among them, 29
(72.5%) had papillary carcinomas and 11 (27.5%) had follicular
carcinomas. None of the patients had autoimmune diseases, chronic
inflammation, allergic disease or other conditions that may affect
the tested parameters. All patients with DTC underwent total
thyroidectomy. At 4–6 weeks following surgery, patients received a
low-iodine diet (<50 µg/day) for 1–2 weeks and subsequently were
treated with a single dose of 3.7 GBq (100 mCi) of 131I
(Chengdu Gaotong Isotope Cooperation, Chengdu, China) administered
orally according to the EANM guidelines (8,12). The
Control group comprised 13 age- and sex-matched healthy subjects.
This study protocol was approved by the Ethics Committee of The
First Affiliated Hospital of Zhejiang Chinese Medical University
(Hangzhou, China). All patients and Control subjects provided
informed written consent prior to enrollment in the study.
 | Table I.Clinicopathological characteristics
of the 40 patients with differentiated thyroid cancer. |
Table I.
Clinicopathological characteristics
of the 40 patients with differentiated thyroid cancer.
| Characteristic | Category | n (%) |
|---|
| Age (years) | ≥45 | 32 (80.0) |
|
| <45 | 8
(20.0) |
| Sex | Male | 14 (35.0) |
|
| Female | 26 (65.0) |
| Histology type | Papillary thyroid
carcinoma | 29 (72.5) |
|
| Follicular thyroid
carcinoma | 11 (27.5) |
| Lymph node
metastases | Positive | 32 (80.0) |
|
| Negative | 8
(20.0) |
Peripheral blood mononuclear cells
(PBMCs) and serum preparation
Peripheral blood samples (5 ml) from the study
subjects were collected in EDTA tubes following venipuncture. Blood
(5 ml) was collected from Control subjects only once, whereas
samples were collected from patients with DTC at day 0, as well as
7, 30 and 90 days following 131I treatment. The blood
(0.5 ml per subject) was combined with RPMI-1640 medium (5 ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 10% fetal
bovine serum (FBS, Sigma-Aldrich; Merck KGaA), 2 mM glutamine, 100
U/ml penicillin and 100 U/ml streptomycin and incubated at 37°C for
72 h. The supernatant was harvested by centrifugation at 1,400 × g
for 12 min at room temperature and stored at −20°C until required
(11). Serum for the detection of
cytokine levels was obtained by centrifugation at 2,000 × g for 15
min at 4°C and stored at −80°C.
Flow cytometric analysis of Th17, Tc17
and Treg cells
In the present study
CD3+CD8−IL17+ T cells were
identified as Th17 cells,
CD3+CD8+IL17+ T cells as Tc17
cells and CD4+CD25+Foxp3+ T cells
as Treg cells. The intracellular cytokine levels were assessed by
flow cytometry to identify the cytokine-producing cells, as
previously described (13). Briefly,
PBMCs (2×107) were stimulated with phorbol myristate
acetate (25 ng/ml), monensin (1.7 µg/ml) and ionomycin (1 µg/ml;
all were purchased from Alexis Biochemicals; Enzo Life Sciences,
Inc., Farmingdale, NY, USA). The cells were incubated with
anti-CD3-phycoerythrin (PE)-Cy5 (0.6 µg/ml, 15-0038-42,
eBioscience; Thermo Fisher Scientific, Inc., Waltham,, MA, USA) and
anti-CD8-fluoresciene isothiocyanate (FITC) (5 µg/ml, MHCD0801,
eBioscience; Thermo Fisher Scientific, Inc.) monoclonal antibodies
at room temperature in the dark for 15 min. Following fixation with
4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) and
permeabilization with 1% saponin (Sigma-Aldrich; Merck KGaA) for 15
min at room temperature, cells were stained anti-IL17-PE monoclonal
antibody (2.5 µg/ml, 12-7517-42, eBioscience; Thermo Fisher
Scientific, Inc.) at room temperature in the dark for 15 min.
Similarly, the culture supernatant was incubated with a mixture of
anti-CD4-FITC monoclonal antibody (2.5 µg/ml, 11-0048-42,
eBioscience; Thermo Fisher Scientific, Inc.) and
anti-CD25-allophycocyanin (APC) monoclonal antibody (0.6 µg/ml,
17-0257-42, eBioscience; Thermo Fisher Scientific, Inc.) at 4°C in
the dark for 30 min. Following fixation and permeabilization, the
cells were stained with anti-Foxp3-PE monoclonal antibody (2.5
µg/ml, 12-4776-42 eBioscience; Thermo Fisher Scientific, Inc.) at
4°C in the dark for 45 min. Flow cytometric analysis was performed
using a FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA). Data
were analyzed with the BD FACSDiva software (version 6.0, BD
Biosciences) (13).
ELISA
Serum was used to determine cytokine levels,
including IL-10 (F01360), IL-17 (F01450), IL-23 (F01534) and TGF-β
(F02750), with a quantitative sandwich-enzyme immunoassay
technique, according to the manufacturer's instructions (Westang;
Biotech Co., Ltd., Shanghai, China). All samples were measured in
duplicate.
Statistical analysis
Data are expressed as the mean ± standard deviation
and median values. Differences between groups were determined using
one-way analysis of the variance, followed by the Tukey's test.
Correlation analysis was evaluated by Pearson's correlation test.
All tests were performed using SPSS 13.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Frequencies of Th17 and Tc17 cells in
DTC patients pre- and post-131I therapy
The frequency of Th17 and Tc17 cells in the
peripheral blood samples from 13 Control subjects and 40 patients
with DTC who had total thyroidectomy were analyzed by flow
cytometry prior to and following 131I therapy. The
percentage of Th17 and Tc17 cells was significantly increased in
patients with DTC at day 0 compared with those in the Control group
(Fig. 1A-C). At days 7, 30 and 90
following 131I therapy, the percentage of Th17 and Tc17
cells significantly decreased compared with the levels at day 0; in
addition, at 90 days following 131I therapy, no
significant difference in the percentage of Th17 and Tc17 cells was
detected between patients with DTC and healthy subjects (Fig. 1A-C).
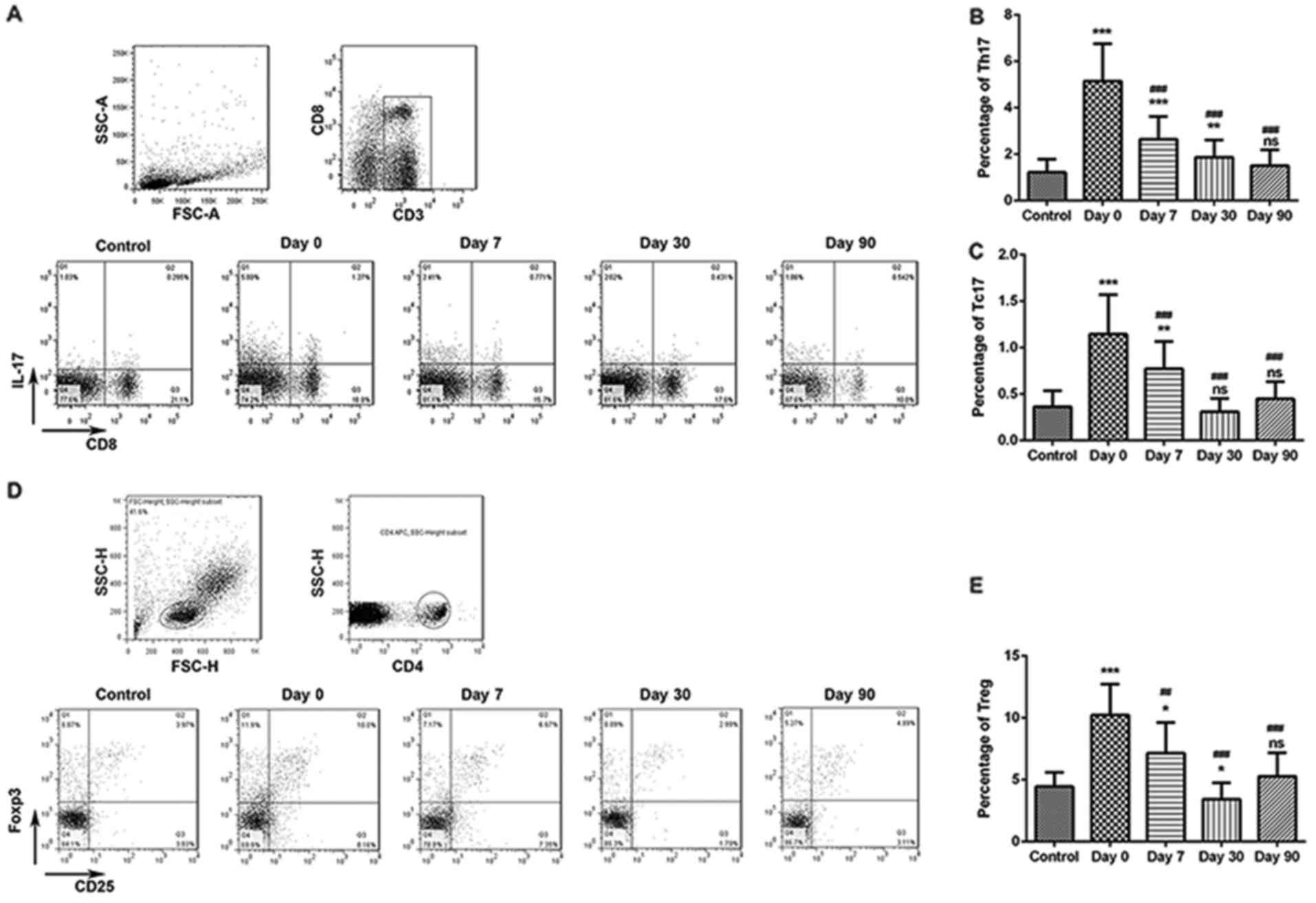 | Figure 1.Percentages of Th17, Tc17 and Treg
cells in healthy Control subjects and in patients with DTC. Levels
of Th17, Tc17 and Treg cells were examined prior to 131I therapy
(day 0) and at 7, 30 and 90 days post-therapy. (A) Frequency of
Th17 and Tc17 cells were detected by flow cytometry. (B) The
proportion of Th17 cells in Control patients and in patients with
DTC following 131I therapy. (C) Proportion of Tc17
cells. (D) Frequency of Treg cells. (E) Proportion of Treg cells.
*P<0.05, **P<0.01 and ***P<0.001 vs. Control group;
##P<0.01 and ###P<0.001 vs. Day 0.
131I, radioactive iodine-131; DTC, differentiated
thyroid cancer; Tc, T cytotoxic cell; Th, T helper cell; Treg,
regulatory T cell; ns, not significant. |
Treg cell frequency in patients with
DTC pre- and post-131-I therapy
At day 0, the percentage of Treg cells was
significantly increased in patients with DTC compared with Control
group (Fig. 1D and E). At 7, 30 and
90 days following therapy, the percentage of Treg cells was
significantly decreased compared with treated patients at day 0; at
90 days post-therapy, no significant difference in the percentage
of Treg cells was indicated between patients with DTC and healthy
Control subjects (Fig. 1D and
E).
Serum concentrations of IL-17, IL-23,
IL-10 and TGF-β1 in patients with DTC pre- and post-131I
therapy
Serum levels of IL-17, IL-23, IL-10 and TGF-β1 were
measured by ELISA. IL-17 and IL-23 levels were significantly
increased in patients with DTC at day 0 compared with the levels in
the healthy Control patients (Fig. 2A
and B), which was consistent with the elevated prevalence of
Th17 and Tc17 cells in these patients. Subsequently, at 7, 30 and
90 days following 131I therapy, IL-17 and IL-23 levels
were significantly decreased; no significant difference was
indicated for the expression levels of IL-17 and IL-23 in patients
with DTC 90 days post-therapy compared with healthy subjects
(Fig. 2A and B). Similarly, the
serum levels of IL-10 and TGF-β1 were significantly increased in
patients with DTC at day 0 compared with the Control patients
(Fig. 2C and D), which was
consistent with the elevated prevalence of Treg cells in these
patients. At days 7, 30 and 90 post-therapy, IL-10 and TGF-β1
levels were significantly decreased, and no significant difference
was indicated for the levels of IL-10 and TGF-β1 in patients with
DTC at 90 days compared with healthy patients (Fig. 2C and D).
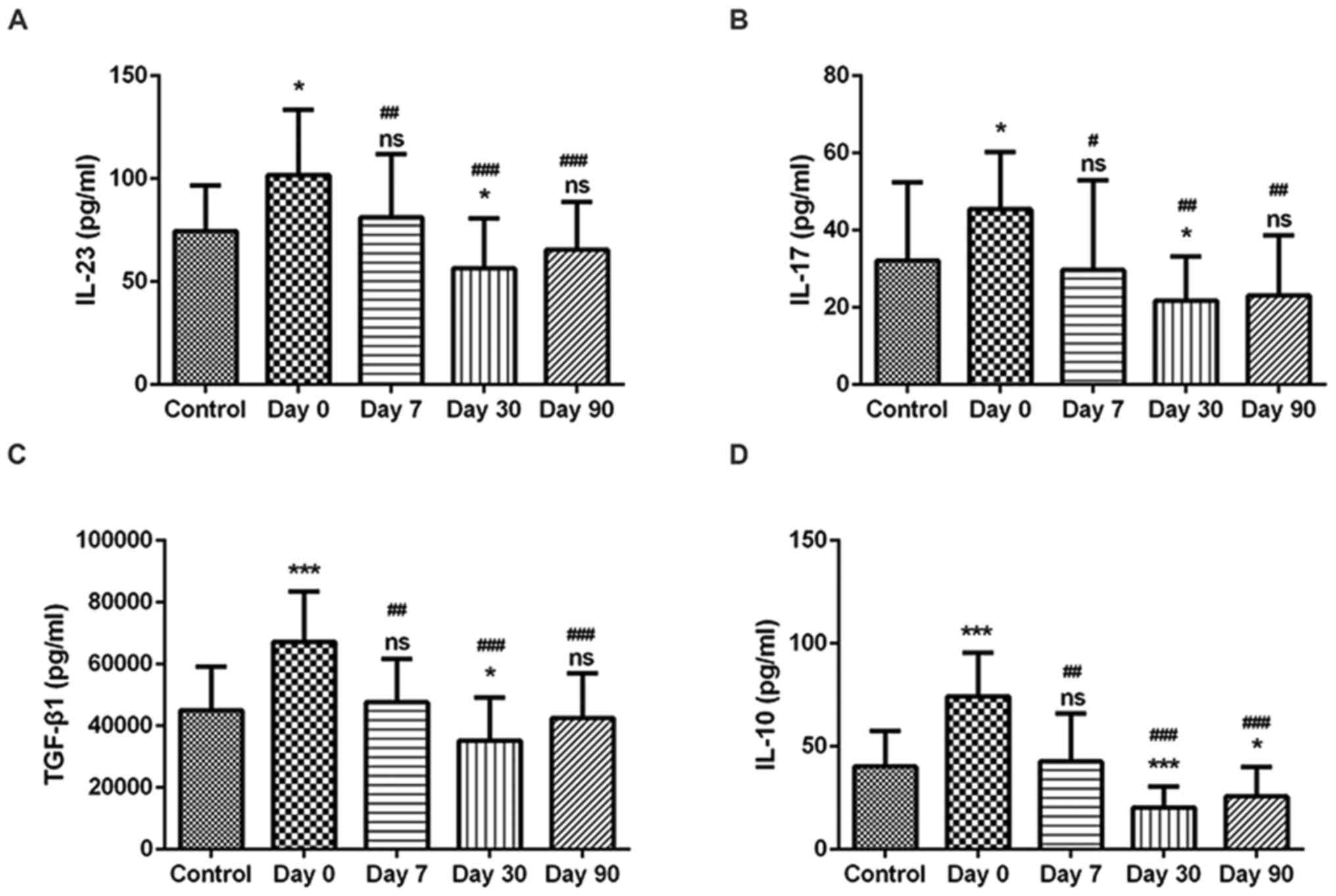 | Figure 2.Concentration levels of IL-23, IL-17,
TGF-β1 and IL-10 in healthy patients and in patients with DTC. The
concentration levels of (A) IL-23, (B) IL-17, (C) TGF-β1 and (D)
IL-10 were measured by ELISA in serum from Control subjects and in
patients with DTC prior to and 7, 30 and 90 days following
131I therapy. *P<0.05 and ***P<0.001 vs. Control
group; #P<0.05, ##P<0.01 and
###P<0.001 vs. Day 0. 131I, radioactive
iodine-131; DTC, differentiated thyroid cancer; IL, interleukin,
TGF-β1, transforming growth factor-β1; ns, not significant. |
Balances between Th17/Tc17 and
Treg/Th17 cells pre- and post-131I therapy
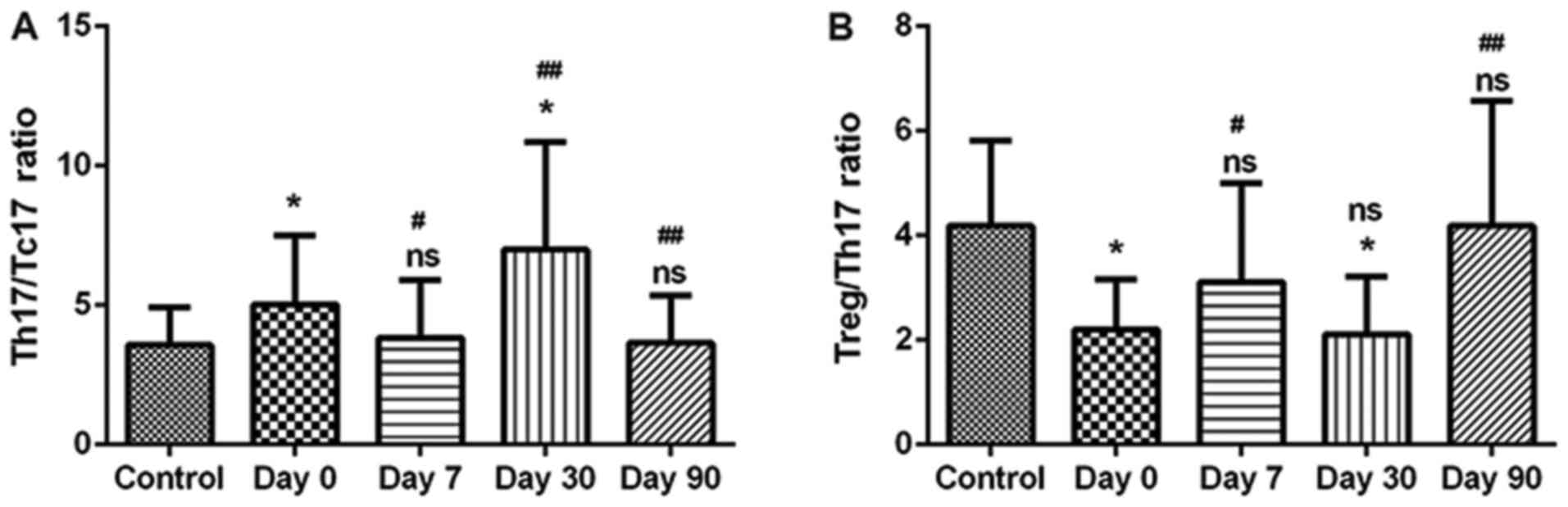
Compared with the Controls, the Th17/Tc17 ratio was
significantly elevated in patients with DTC at day 0 (Fig. 3A). Furthermore, this ratio in
patients with DTC was reduced following 131I therapy for
7 days. Subsequently, this ratio was unexpectedly increased after
131I therapy for 30 days. Additionally, the Th17/Tc17
ratio was not significantly different from the Control group at 90
days following 131I therapy (Fig. 3A). It is possible that
131I also resulted in radioactive damage to DTC
patients. Conversely, the Treg/Th17 ratio was significantly
decreased in patients with DTC compared with the Controls, and this
ratio in patients with DTC was elevated following 131I
therapy for 7 days. Subsequently, at 30 days post-therapy, this
ratio was decreased. Furthermore, no significant difference was
indicated in the Treg/Th17 ratio between patients with DTC and the
healthy Controls following 131I therapy for 90 days
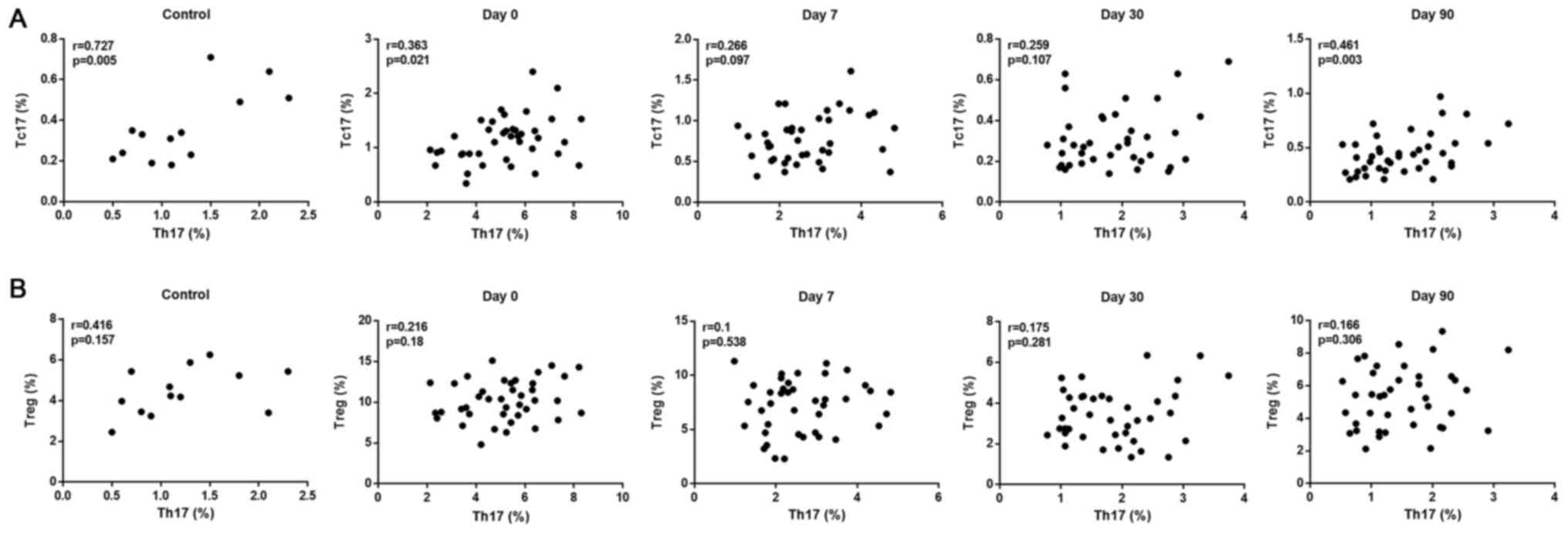
(Fig. 3B). In addition, a strong
positive correlation between Th17 and Tc17 cell levels was observed
in the healthy Controls and in the DTC patients that received
131I treatment for 90 days (Fig. 4A); while a weak positive correlation
between Th17 and Treg cell levels was identified in the healthy
Controls and no obvious correlation between Th17 and Treg cells was
observed in patients with DTC pre- and post-131I therapy
during the entire treatment period (Fig.
4B).
Discussion
A number of previous studies have demonstrated that
inflammation orchestrates the tumor microenvironment. For example,
inflammatory reactions in the tumor microenvironment were reported
to be key components of tumor-associated immunity (23), in which immune cells serve crucial
roles. Th17, Tc17 and Treg cells are three representative types of
immune cells that are important in antitumor immunity and
inflammation (19,22,24).
Additionally, it was reported that higher levels of Treg cells are
associated with poor survival (25).
These findings suggested the probable involvement of these three
cells in the development of DTC. In the present study, a
significant increase in the levels of Th17, Tc17 and Treg cells was
observed in patients with DTC; these results were similar to those
observed in endometrial carcinoma (13), which suggested that these three
effector T cells may serve important roles in the progression of
DTC.
Th17 and Tc17 cells have obtained an increasing
amount of attention owing to their roles in tumorigenesis; their
levels, as well as IL-17 production, are upregulated in the
peripheral blood of patients with cancer (19,26,27).
Similarly, results from the present study revealed significant
increases in the number of Th17 and Tc17 cells in the peripheral
blood of patients with DTC. In addition, a strong positive
correlation was identified between Th17 and Tc17 cells, and the
Th17/Tc17 ratio was significantly elevated in patients with DTC,
which suggests that the increase in Th17 cells may exceed that of
Tc17 cells. In addition, an elevated proportion of Treg cells, and
an imbalance between Treg and Th17 cells in patients with DTC were
also observed. Treg cells are known to inhibit host immunity
(28); it was reported that higher
levels of Treg cells is associated with poor survival (25). Increased Treg frequency may result in
tolerance of tumor cells from the host and may aid DTC cell
survival and migration. In the present study, the Treg/Th17 ratio
was significantly decreased in patients with DTC, and the positive
correlation between Th17 and Treg cells observed in healthy
Controls disappeared in patients with DTC. Similar results have
been demonstrated in patients with cervical cancer and prostate
cancer (21,26). The present study hypothesized that
Th17 and Treg cell levels increased owing to immune reactions in
the tumor microenvironment. Results suggested that the number of
Th17 cells produced was greater compared with the number of Treg
cells. Although cytokine concentrations have been previously
analyzed in patients with different cancers (29–31),
they have seldom been examined in patients with DTCs. Results from
the present study also demonstrated that levels of cytokines IL-17,
IL-23, IL-10 and TGF-β1 levels were significantly elevated in
patients with DTC.
However, alterations of Th17, Tc17, Treg cell levels
and related cytokine secretion in patients with DTC that were
treated with 131I are not distinct. It has been reported
previously that iodine-125 stimulates the antitumor immune response
by facilitating Th2/Th1 deviation in patients with hepatocellular
carcinoma (32). In addition, the
proportions of Treg, IL-10 and TGF-β levels were significantly
reduced in patients with Graves disease, some of which recovered
following 131I treatment (33). It has also been reported that Th2
cytokine levels, including IL-4, IL-6 and IL-10, were reduced in
patients with DTC following 131I therapy (11). Another previous study reported that
the IL-23/Th17 axis may serve a key role in the pathogenesis of
Graves disease, and the levels of serum IL-17 and IL-23 were
demonstrated to gradually decrease following treatment with
131I (34). Results from
the present study indicated a significant decrease in the number of
Th17, Tc17, Treg cells as well as in the production of related
cytokines, including IL-17, IL-23, IL-10 and TGF-β1, in patients
with DTC following 131I therapy; these results differed
from those reported by Jing et al (33), perhaps owing to the differences in
the number and type of inflammatory cells associated with DTC and
Graves disease. In addition, the ratio of Th17/Tc17 was
significantly elevated in patients with DTC at day 0 and this ratio
fluctuated significantly following 131I therapy, but it
returned to the similar levels detected in healthy Controls 90 days
post-131I therapy. Meanwhile, Treg/Th17 ratio was
significantly reduced in patients with DTC at day 0 and this ratio
also fluctuated significantly following 131I therapy,
but it finally returned to the similar levels detected in healthy
Controls 90 days post-131I therapy. These results
indicated that 131I may influence the balance of
Th17/Tc17 and Treg/Th17 in patients with DTC.
In conclusion, the numbers of Th17, Tc17 and Treg
cells, as well as the levels of related cytokines (IL-17, IL-23,
IL-10 and TGF-β1) were significantly increased in patients with
DTCs; however, at 90 days following 131I therapy, the
levels decreased and returned to the similar values as detected in
healthy Control patients. These data suggested that 131I
may influence the balance of Th17/Tc17 and Treg/Th17 in patients
with DTC. Further studies are required to determine the
significance of these findings.
References
|
1
|
Burgess JR and Tucker P: Incidence trends
for papillary thyroid carcinoma and their correlation with thyroid
surgery and thyroid fine-needle aspirate cytology. Thyroid.
16:47–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu X, Groves FD, Mclaughlin CC, Jemal A,
Martin J and Chen VW: Cancer incidence patterns among adolescents
and young adults in the United States. Cancer Causes Control.
16:309–320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sherman SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman MP, Babb P, Damiecki P, Grosclaude
P, Honjo S, Jones J, Knerer G, Pitard A, Quinn MJ, Sloggett A and
De Stavola BL: Cancer survival trends in England and Wales,
1971–1995: deprivation and NHS RegionStudies in Medical and
Population Subjects no. 61. The Stationery Office; London: 1999
|
|
6
|
Schlumberger M and Sherman SI: Approach to
the patient with advanced differentiated thyroid cancer. Eur J
Endocrinol. 166:5–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer, ; Cooper DS, Doherty GM, Haugen BR, Hauger BR, Kloos RT,
Lee SL, Mandel SJ, Mazzaferri EL, McIver B, et al: Revised American
Thyroid Association management guidelines for patients with thyroid
nodules and differentiated thyroid cancer. Thyroid. 9:1167–1214.
2009.
|
|
8
|
Luster M, Clarke SE, Dietlein M, Lassmann
M, Lind P, Oyen WJ, Tennvall J and Bombardieri E; European
Association of Nuclear Medicine (EANM), : Guidelines for
radioiodine therapy of differentiated thyroid cancer. Eur J Nucl
Med Mol Imaging. 35:1941–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bodelon C, Polley MY, Kemp TJ, Pesatori
AC, Mcshane LM, Caporaso NE, Hildesheim A, Pinto LA and Landi MT:
Circulating levels of immune and inflammatory markers and long
versus short survival in early-stage lung cancer. Ann Oncol.
24:2073–2079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Budhu A and Xin WW: The role of cytokines
in hepatocellular carcinoma. J Leukoc Biol. 80:1197–1213. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simonovic SZ, Mihaljevic O, Majstorovic I,
Djurdjevic P, Kostic I, Djordjevic OM and Teodorovic LM: Cytokine
production in peripheral blood cells of patients with
differentiated thyroid cancer: Elevated Th2/Th9 cytokine production
before and reduced Th2 cytokine production after radioactive iodine
therapy. Cancer Immunol Immunother. 64:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pluijmen MJ, Eustatia-Rutten C, Goslings
BM, Stokkel MP, Arias AM, Diamant M, Romijn JA and Smit JW: Effects
of low-iodide diet on postsurgical radioiodide ablation therapy in
patients with differentiated thyroid carcinoma. Clin Endocrinol
(Oxf). 58:428–435. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Hou F, Zhang Y, Tian Y, Jiao J,
Ma D, Kong B and Cui B: Changes of Th17/Tc17 and Treg/Th17 cells in
endometrial carcinoma. Gynecol Oncol. 132:599–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bailey SR, Nelson MH, Himes RA, Li Z,
Mehrotra S and Paulos CM: Th17 cells in cancer: The ultimate
identity crisis. Front Immunol. 5:2762014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carvalho DFG, Zanetti BR, Miranda L,
Hassumi-Fukasawa MK, Miranda-Camargo F, Crispim JCO and Soares EG:
High IL-17 expression is associated with an unfavorable prognosis
in thyroid cancer. Oncol Lett. 13:1925–1931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Henriques A, Inês L, Couto M, Pedreiro S,
Santos C, Magalhães M, Santos P, Velada I, Almeida A, Carvalheiro
T, et al: Frequency and functional activity of Th17, Tc17 and other
T-cell subsets in Systemic Lupus Erythematosus. Cell Immunol.
264:97–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garcia-Hernandez Mde L, Hamada H, Reome
JB, Misra SK, Tighe MP and Dutton RW: Adoptive transfer of
tumor-specific Tc17 effector T cells controls the growth of B16
melanoma in mice. J Immunol. 184:4215–4227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maruyama T, Kono K, Mizukami Y, Kawaguchi
Y, Mimura K, Watanabe M, Izawa S and Fujii H: Distribution of Th17
cells and FoxP3(+) regulatory T cells in tumor-infiltrating
lymphocytes, tumor-draining lymph nodes and peripheral blood
lymphocytes in patients with gastric cancer. Cancer Sci.
101:1947–1954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gerloni M and Zanetti M: CD4 T cells in
tumor immunity. Springer Seminars Immunopathol. 27:37–48. 2005.
View Article : Google Scholar
|
|
21
|
Sfanos KS, Bruno TC, Maris CH, Xu L,
Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB and Drake CG:
Phenotypic analysis of prostate-infiltrating lymphocytes reveals
Th17 and treg skewing. Clin Cancer Res. 14:3254–3261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou W and Restifo NP: T(H)17 cells in
tumour immunity and immunotherapy. Nat Rev Immunol. 10:248–256.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dubin PJ and Kolls JK: Th17 cytokines and
mucosal immunity. Immunol Rev. 226:160–171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fattorossi A, Battaglia A, Ferrandina G,
Buzzonetti A, Legge F, Salutari V and Scambia G: Lymphocyte
composition of tumor draining lymph nodes from cervical and
endometrial cancer patients. Gynecol Oncol. 92:106–115. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Ma D, Tian Y, Wang X, Qiao Y and
Cui B: The imbalance of Treg/Th17 in patients with uterine cervical
cancer. Clin Chim Acta. 412:894–900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rogala E, Nowicka A, Bednarek W,
Barczyński B, Wertel I, Zakrzewski M and Kotarski J: Evaluation of
the intracellular expression of interleukin 17 in patients with
ovarian cancer. Ginekol Pol. 83:424–428. 2012.(In Polish).
PubMed/NCBI
|
|
28
|
Wang RF: Regulatory T cells and innate
immune regulation in tumor immunity. Springer Semin Immunopathol.
28:17–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baier PK, Wolff-Vorbeck G, Eggstein S,
Baumgartner U and Hopt UT: Cytokine expression in colon carcinoma.
Anticancer Res. 25:2135–2139. 2005.PubMed/NCBI
|
|
30
|
Montero AJ, Diaz-Montero CM, Millikan RE,
Liu J, Do KA, Hodges S, Jonasch E, McIntyre BW, Hwu P and Tannir N:
Cytokines and angiogenic factors in patients with metastatic renal
cell carcinoma treated with interferon-alpha: Association of
pretreatment serum levels with survival. Ann Oncol. 20:1682–1687.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mocellin S, Provenzano M, Rossi CR, Pilati
P, Nitti D and Lise M: Use of quantitative real-time PCR to
determine immune cell density and cytokine gene profile in the
tumor microenvironment. J Immunol Methods. 280:1–11. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiang GA, Chen KY, Wang HN and Xiao JF:
Immunological influence of iodine-125 implantation in patients with
hepatocellular carcinoma resection. Nan Fang Yi Ke Da Xue Xue Bao.
30:292–294. 2010.PubMed/NCBI
|
|
33
|
Yang J, Pan T, Du YJ and Zhong X:
Endocrinology Do. Change of CD4+ CD25+ CD127 low regulatory T cells
in peripheral blood of patients with Graves disease treated by 131
I or antithyroid drugs therapy. J Anhui Medicinalis. 5:691–695.
2016.
|
|
34
|
Xue-Qin MA and Shi-Peng YU: Effects of
131I treatment on the IL-23/Th17 axis relevant factor
levels in graves disease patients. Chin J Immunol. 29:R3922013.
|