Introduction
Systemic lupus erythematosus (SLE) is a type of
autoimmune disease characterized by multisystem damage combined
with the formation of a variety of autoantibodies, though has
unknown etiology (1,2). The kidneys are the primary affected
organ, and once affected, the condition is classified as lupus
nephritis (LN) (3). LN is an
important feature of SLE in the clinic, as ~30–50% of patients with
SLE present with renal damage (4).
The clinical manifestations of renal damage include proteinuria,
erythrocyturia, leucocyturia, cylindruria, glomerular filtration
dysfunction and renal tubular hypofunction (5). The manifestation of LN typically
alternates between active and inactive stages. Additionally, the
severity of renal damage has been associated with the prognosis of
SLE (6). A progressive decrease in
renal function in active stage LN is among the leading causes of
mortality in SLE patients (7). Renal
puncture biopsy is considered the gold standard for LN diagnosis,
assessment of LN activity status and determination of renal damage
severity (8). However, renal
puncture biopsies are invasive with poor reproducibility.
Identification of LN activity-related clinical
indicators is of importance for improving diagnosis and treatment.
Previous studies have investigated novel potential biomarkers for
the assessment of LN activity (8,9). In
addition, a variety of studies have demonstrated the value of TWEAK
in assessing renal damage severity (10–12).
Notably, it has been reported that urine TWEAK content in patients
with active LN was significantly higher than in those with inactive
LN (13), and that urine TWEAK level
was correlated with the degree of renal damage (14). Previous results also indicated that
TWEAK was highly expressed in the renal tissue of an LN animal
model (15). Furthermore, blockade
of TWEAK expression or knockout of its cognate receptor fibroblast
growth factor inducer 14 (Fn14) in LN mice relieved renal tissue
damage and alleviated inflammatory cell infiltration, inflammatory
cytokine production and immunoglobulin deposition (16).
Nuclear factor (NF)-κB is a transcription factor
that forms a p65-p50 heterodimer in the cytoplasm and participates
in the immune inflammatory response, cell differentiation, cell
growth and apoptosis regulation (17). TWEAK may activate the NF-κB signaling
pathway through binding with the Fn14 receptor, which may represent
an underlying mechanism regarding LN activity status (18). A previous study has indicated that
TWEAK is associated with LN activity (19); however, the specific mechanism
involved remains unclear. Thus, the present study aimed to
investigate the roles of TWEAK and the NF-κB signaling pathway in
LN.
Materials and methods
Case collection
A total of 65 patients with SLE with LN (6 males and
59 females; age, 36.7±14.5 years old) were enrolled from Yantaishan
Hospital (Yantai, China) between January 2014 and August 2015. The
diagnosis of SLE was determined according to the 1999 SLE
classification standards revised by the American rheumatism
association (20). The following LN
diagnostic criteria were used: Patients with SLE presenting with
persistent proteinuria (24-h urine protein, >0.5 g),
erythrocyturia, leucocyturia, cylindruria and confirmation by renal
biopsy. A total of 45 patients with SLE without LN (5 males and 40
females; age, 37.2±15.1 years old) were enrolled as a non-LN SLE
group over the same time period. Patients with malignant tumors,
acute or chronic infection, or other autoimmune diseases were
excluded. All the enrolled patients had received no
immunosuppressant therapy (including cyclophosphamide, methyl
prednisone and cyclosporine A), immunomodulators or hormone
therapy. Additionally, 50 subjects (5 males and 45 females; age,
35.5±13.9 years old) receiving physical health examinations were
enrolled as the normal controls. The subjects in the normal control
group did not suffer from diabetes or hypertension, had a normal
biochemical index (serum creatine kinase, triglycerides, C3/4,
C-reactive protein and high density lipoprotein) and tested
negative for autoantibodies. No statistically significant
differences in age or gender were observed among the groups
(Table I). In addition, another 30
patients with renal tumors (3 males and 27 females; age, 36.2±12.1)
were also recruited between January 2014 and August 2015 (Table I), these patients had no primary
glomerular nephritis or other diseases that may affected renal
function, such as diabetes or hypertension.
 | Table I.Characteristics of patients and
controls. |
Table I.
Characteristics of patients and
controls.
| Characteristic | SLE with LN | SLE without LN | Normal
controls | Renal tumor
patients |
|---|
| Group size | 65 | 45 | 50 | 30 |
| Male/female | 6/59 | 5/40 | 5/45 | 3/27 |
| Age (mean ±
SD) | 36.7±14.5 | 37.2±15.1 | 35.5±13.9 | 36.2±12.1 |
The study protocol was approved by the Research
Ethics Committee of Yantaishan Hospital and all patients provided
their informed written consent prior to study commencement.
Reagents and materials
Recombinant human TWEAK cytokine (cat. no.
1090-TW-025) and goat anti-human TWEAK antibody (cat. no.
AF1199-SP) were purchased from R&D Systems, Inc. (Minneapolis,
MN, USA). NF-κB p65 (cat. no. sc-372), NF-κB p65 [phospho (p)-S536;
p-p65; cat. no. sc-136548] and β-actin antibodies (cat. no.
sc-47778) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). p-NF-κ light polypeptide gene enhancer in
B-cells inhibitor-α (IκBα; p-S32; cat. no. 9246) and histone H3.1
antibodies (cat. no. 9715) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Rabbit antihuman cluster of
differentiation (CD)68 (cat. no. ab125212) and Ki-67 antibodies
(cat. no. ab92742) were obtained from Abcam (Cambridge, MA, USA).
The allophycocyanin (APC)-tagged mouse antihuman Ki-67 antibody
(cat. no. 17-5699-42) for flow cytometry and TWEAK ELISA kit (cat.
no. 15215014) were purchased from eBioscience; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). A Total Protein Extraction kit
was purchased from in Phygene Life Sciences Co., Ltd. (Fuzhou,
China). The NF-κB inhibitor BAY 11-7082, cytoplasm and
nucleoprotein separation kits were obtained from Beyotime Institute
of Biotechnology (Haimen, China). Human normal mesangial cells
(cat. no. T4086) were purchased from Applied Biological Materials
Inc. (Richmond, BC, Canada).
Sample collection and indicator
detection
Blood (5 ml) and urine (3 ml) specimens were
collected from all subjects. Blood samples were clotted and
centrifuged at 1,500 × g for 10 min at room temperature to separate
the upper serum. The contents of TWEAK in the blood and urine were
determined by ELISA according to the manufacturer's protocol. SLE
disease activity index (SLEDAI) in SLE patients with or without LN
was evaluated according to clinical manifestation and laboratory
examination as previously described (21). SLEDAI scores ≤4 were considered to
represent inactive stage LN, whereas SLEDAI scores ≥5 were
considered to represent active LN (22). Among the total 65 patients with LN,
40 cases were assigned to the non-active LN group) and 25 cases
were assigned to the active LN group (Table II). No significant age or gender
differences were identified between these groups. Renal tissue
pathological samples from patients with LN were collected during a
biopsy. Other renal tissues from age- and gender-matched renal
tumor patients were selected as controls. The samples were
collected from a site at least 3 cm from the tumor and confirmed to
be normal renal tissue by pathology. The collected samples were
used for frozen sectioning (5-µm-thick) at −80°C and renal cortex
protein extraction.
 | Table II.Characteristics of SLE patients with
LN. |
Table II.
Characteristics of SLE patients with
LN.
| Characteristic | Non-actin LN | Active LN |
|---|
| Group size (n) | 40 | 25 |
| Gender (m/f) | 4/36 | 2/23 |
| Age | 35.9±15.1 | 37.1±14.8 |
Western blotting
Total protein and nucleoprotein were extracted using
a Total Protein Extraction, and Nuclear and Cytoplasmic Protein
Extraction kits, respectively, according to the manufacturer's
protocol. Proteins were quantified using a BCA assay kit and 20 µg
of protein was loaded per lane were loaded and separated by 12%
SDS-PAGE. Proteins were subsequently transferred to polyvinylidene
difluoride membranes and the membranes were blocked using 5%
skimmed milk at room temperature for 1 h. The membranes were
subsequently incubated with primary antibodies directed against
TWEAK (1:1,000), p-p65 (1:500), p-IκBα (1:1,000) and β-actin
(1:1,000) at 4°C overnight, and then HRP-conjugated goat-anti
rabbit (cat. no. 65-6120; 1:5,000) or rabbit anti-mouse (cat. no.
61-6520; 1:5,000) (both Thermo Fisher Scientific, Inc.) secondary
antibodies at room temperature for 1 h. Following enhanced
chemiluminescence development using the Pierce™ ECL
Western Blotting Substrate (cat. no. 32106; Thermo Fisher
Scientific, Inc.), the X-ray film was scanned to detect the
expression of TWEAK, p-p65, p-IκBα and β-actin in the renal
cortex.
Immunofluorescence
The frozen sections were dried at room temperature
for 15 min and washed three times with PBS to remove optimal
cutting temperature compound. Subsequently, the sections were
blocked in PBS containing 10% goat serum (cat. no. 16210064; Thermo
Fisher Scientific, Inc.) at room temperature for 60 min and
incubated with the rabbit anti-human CD68 (1 µg/ml) and Ki-67 (1
µg/ml) antibodies at 4°C overnight. After washing, the sections
were incubated with IgG Alexa Fluor 594 (cat. no. R37117) and Alexa
Fluor 488 (cat. no. R37116) fluorescence secondary antibodies (both
2 µg/ml; Thermo Fisher Scientific, Inc.) at room temperature for 1
h. Following staining with 4′,6-diamidino-2-phenylindole at room
temperature for 1 h, the sections were sealed and observed under a
fluorescence microscope (magnification, ×40).
Cell culture and grouping
HMCs were routinely cultured in RPMI-1640 medium
supplemented with 10% FBS, 10 mg/l insulin, 5.5 mg/l transferrin,
and 6.7 µg/l sodium selenite (all Thermo Fisher Scientific, Inc.)
at 37°C with 5% CO2. The cells were divided into five
groups: Normal control without any treatment; stimulus group (50
ng/ml recombinant TWEAK); neutralizing antibody group [50 ng/ml
TWEAK + 200 ng/ml anti-TWEAK antibody (blocking antibody)]; NF-κB
inhibitor group (50 ng/ml TWEAK + 2 µg/ml BAY 11–7082); and
combined inhibition group (50 ng/ml TWEAK + 200 ng/ml blocking
antibody + 2 µg/ml BAY 11–7082). BAY 11–7082 was added 30 min prior
to TWEAK treatment. The cells (1×106/ml seeding density)
were collected following incubation with the various treatments
(the anti-TWEAK blocking antibody was added 10 min prior to the
administration of TWEAK) for 48 h at 37°C and used in subsequent
assays. Western blot analysis was performed as above to detect p65
protein nuclear transport and IκBα phosphorylation in the HMCs with
the primary antibodies against NF-κB p65 (1:1,000) and p-IκBα
(1:1,000), using histone H3.1 (1:1,000) and β-actin (1:1,000) as
internal controls, respectively followed by the addition of
secondary antibodies as described above. Additionally, the cells
were subjected to reverse transcription quantitative-polymerase
chain reaction (RT-qPCR) for further marker detection.
RT-qPCR
Total RNA was extracted from cells using TRIzol
(Thermo Fisher Scientific, Inc.) and reverse transcribed to
complementary (c)DNA using random primers and oligdT primers from
the High-Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific Inc.). cDNA was used as a template for PCR
amplification. The primers used were as follows: Interleukin
(IL)-8, forward, 5′-TTTTGCCAAGGAGTGCTAAAGA-3′ and reverse,
5′-AACCCTCTGCACCCAGTTTTC-3′; IL-6, forward,
5′-ACTCACCTCTTCAGAACGAATTG-3′ and reverse,
5′-CCATCTTTGGAAGGTTCAGGTTG-3′; chemokine ligand 5 (CCL5), forward
5′-CCAGCAGTCGTCTTTGTCAC-3′ and reverse 5′-CTCTGGGTTGGCACACACTT-3′;
monocyte chemoattractant protein (MCP)-1, forward,
5′-CAGCCAGATGCAATCAATGCC-3′ and reverse,
5′-TGGAATCCTGAACCCACTTCT-3′; IL-10, forward
5′-GACTTTAAGGGTTACCTGGGTTG-3′ and reverse,
5′-TCACATGCGCCTTGATGTCTG-3′; and β-actin, forward
5′-GCACTCTTCCAGCCTTCC-3′ and reverse,
5′-AGAAAGGGTGTAACGCAACTAAG-3′. The reaction system contained 4.5 µl
2X SYBR Green PCR Master mix (Thermo Fisher Scientific, Inc.), 0.5
µl 5 µm/l primers, 1 µl cDNA, and 3.5 µl ddH2O. The
following thermocycling conditions were used: 95°C for 5 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. PCR
was performed using an ABI ViiA7 PCR amplifier, and the products
were quantified using the 2−ΔΔCq method (23) with β-actin as an internal reference.
Each reaction was performed in triplicate.
Analysis of cell cycle progression and
Ki-67 expression by flow cytometry
Cells were digested using collagenase (1 mg/ml) and
washed with PBS. Following fixation with 70% ethanol overnight at
4°C, the cells were resuspended in 500 µl PBS containing 50 µg/ml
RNaseA at 37°C for 30 min. Subsequently, the cells were treated
with 0.1% Triton X-100 for 30 min at room temperature, then stained
with 50 µg/ml propidium iodide at 4°C for 30 min prior to cell
cycle analysis using a Propidium Iodide Flow Cytometry kit for cell
cycle analysis (cat. no. ab139418; Abcam). The data was analyzed
using BD CellQuest Pro Software, version 5.1 (BD Biosciences, San
Jose, CA, USA).
For measurement of Ki-67 expression, cells were
digested by using collagenase (1 µg/ml) and washed with PBS.
Subsequently, the cells were fixed with 4% paraformaldehyde at 4°C
for 30 min, and then with 0.1% saponin (Thermo Fisher Scientific,
Inc.) for 20 min at room temperature. Following incubation with the
APC-tagged Ki-67 antibody at 4°C for 60 min in the dark, the cells
were analyzed by flow cytometry and data were analyzed by BD
CellQuest Pro Software, version 5.1.
Transwell assay
Collagen type IV was used to coat Transwell plates
(pore size, 8 µm) and incubated for 24 h at 4°C. THP-1 macrophages
obtained from the American Type Culture Collection were seeded
(1×106/ml) in the upper chamber containing RPMI-1640
medium, while HMCs from each of the five groups were seeded
(1×106/ml) in the lower chamber containing RPMI-1640
medium. Following 24 h incubation at 37°C, the Transwell chamber
was fixed with methanol for 30 min at room temperature and stained
with 0.1% crystal violet for 20 min at room temperature. The cells
were observed under a light microscope (magnification, ×400) and
counted under five random fields of vision.
Statistical analysis
SPSS 18.0 software (IBM Corp., Armonk, NY, USA) was
used for data analysis. Data were presented as mean ± standard
deviation as indicated with a minimum of three independent
experiments performed for each assay. A Student's t-test was
performed for the comparison of differences between two groups.
One-way analysis of variance with a Newman-Keuls multiple
comparison post-hoc analysis was performed for significance
comparisons among multiple treatment groups, while the
χ2 test was used for enumeration data comparisons
(patient gender and age). P<0.05 was considered to indicate a
statistically significant difference.
Results
TWEAK levels in different groups of
patients and the association between TWEAK and LN activity
The ELISA results indicated that TWEAK levels in the
urine of patients in the LN group were significantly increased
compared with that in patients in the non-LN SLE and healthy
control groups (P<0.05; Table
III). Meanwhile, the urine levels of TWEAK between non-LN SLE
patients and healthy controls did not differ significantly
(P>0.05). Serum TWEAK levels were similar and not significantly
different among all three groups (P>0.05). Patients in the
active LN group exhibited significantly increased urine levels of
TWEAK compared with those in the LN inactive group (P<0.05),
while the serum TWEAK levels did not differ significantly between
the groups (P>0.05; Table
III).
 | Table III.TWEAK content in different patient
groups and the association between 19 TWEAK and LN activity. |
Table III.
TWEAK content in different patient
groups and the association between 19 TWEAK and LN activity.
| Grouping | n | Serum (nmol/l) | Urine (nmol/l) |
|---|
| Patients
groups |
|
|
|
| Healthy
control | 50 |
24.8±5.6 |
9.4±3.1 |
| Non-LN
SLE | 45 |
23.4±6.2 |
10.5±2.8 |
| LN | 65 |
25.2±6.7 |
15.4±3.1a,b |
| LN activity
groups |
|
|
|
| LN
inactive | 40 |
24.9±7.1 |
13.4±2.7 |
| LN
active | 25 |
26.4±5.8 |
19.2±3.9c |
TWEAK protein expression, inflammatory
cell infiltration and cell proliferation in LN renal tissue
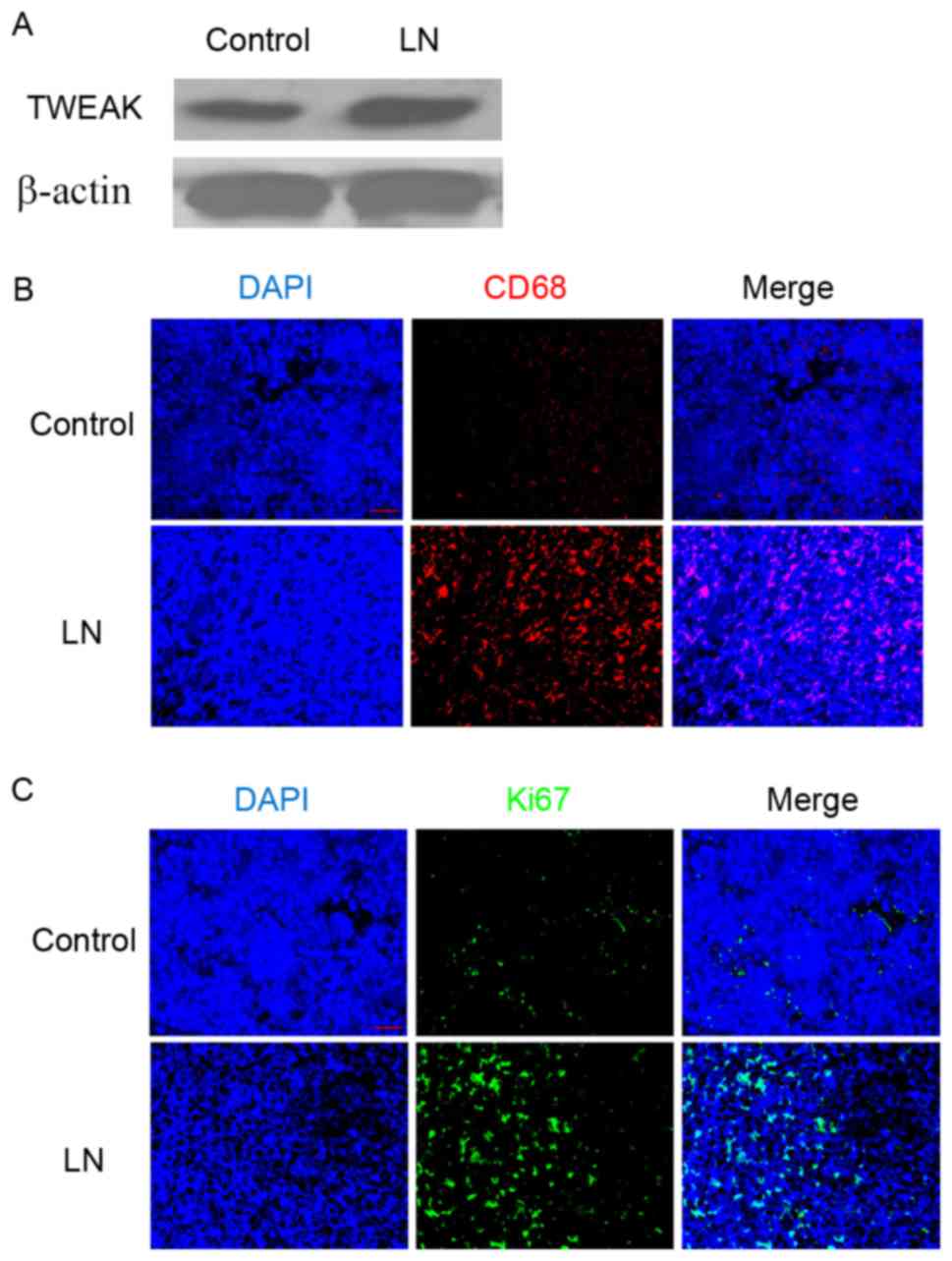
Western blotting demonstrated that, compared with
the adjacent normal renal tissue of patients with renal tumors, the
renal cortex of patients with LN exhibited markedly increased
protein expression of TWEAK (Fig.
1A). Additionally, immunofluorescence staining identified an
elevated population of CD68-positive cells (Fig. 1B) and increased Ki-67 protein
expression (Fig. 1C) in the LN renal
tissues. As CD68 is typically used as a marker of the macrophage
lineage (24), and Ki-67 is
established as a marker for cell proliferation (25), these results suggested that
macrophage infiltration and cell proliferation were enhanced in the
renal cortex of patients with LN.
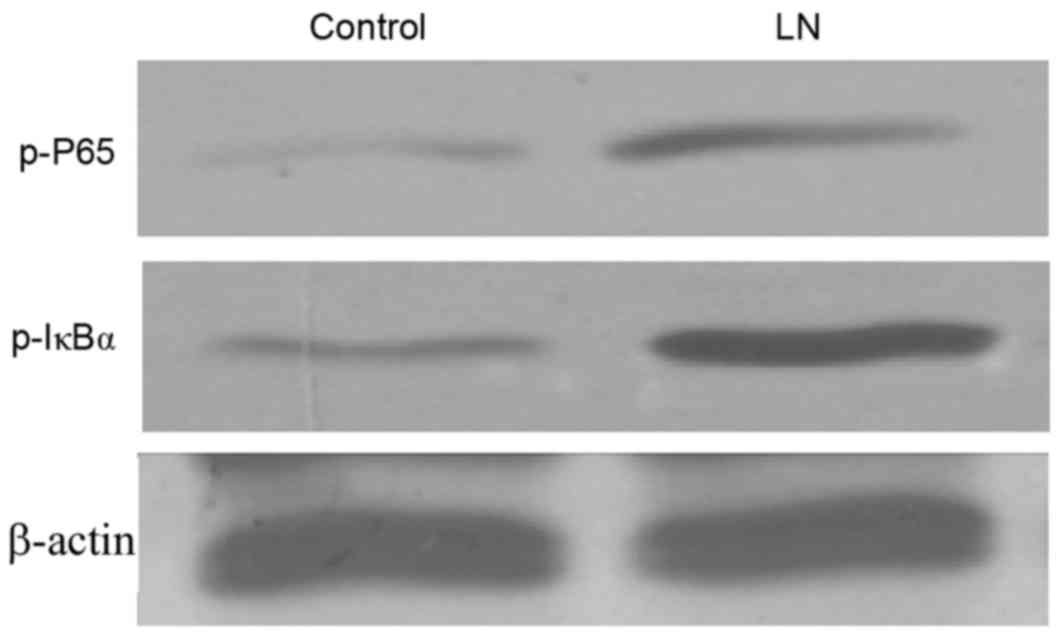
NF-κB activity in LN renal tissue
As the NF-κB signaling pathway serves an important
role in cell growth and immune regulation, and the regulation of
NF-κB by the TWEAK-Fn14 system has previously been indicated, the
present study investigated the transcriptional activity of NF-κB in
the renal tissue of patients with LN. In accordance with the
results on TWEAK expression, western blotting indicated that the
activation of NF-κB protein was notably elevated in the renal
cortex of patients with LN, as indicated by the elevated levels of
nuclear p-p65 and p-IκBα (Fig. 2),
which are indicators of NF-κB activation (17,26).
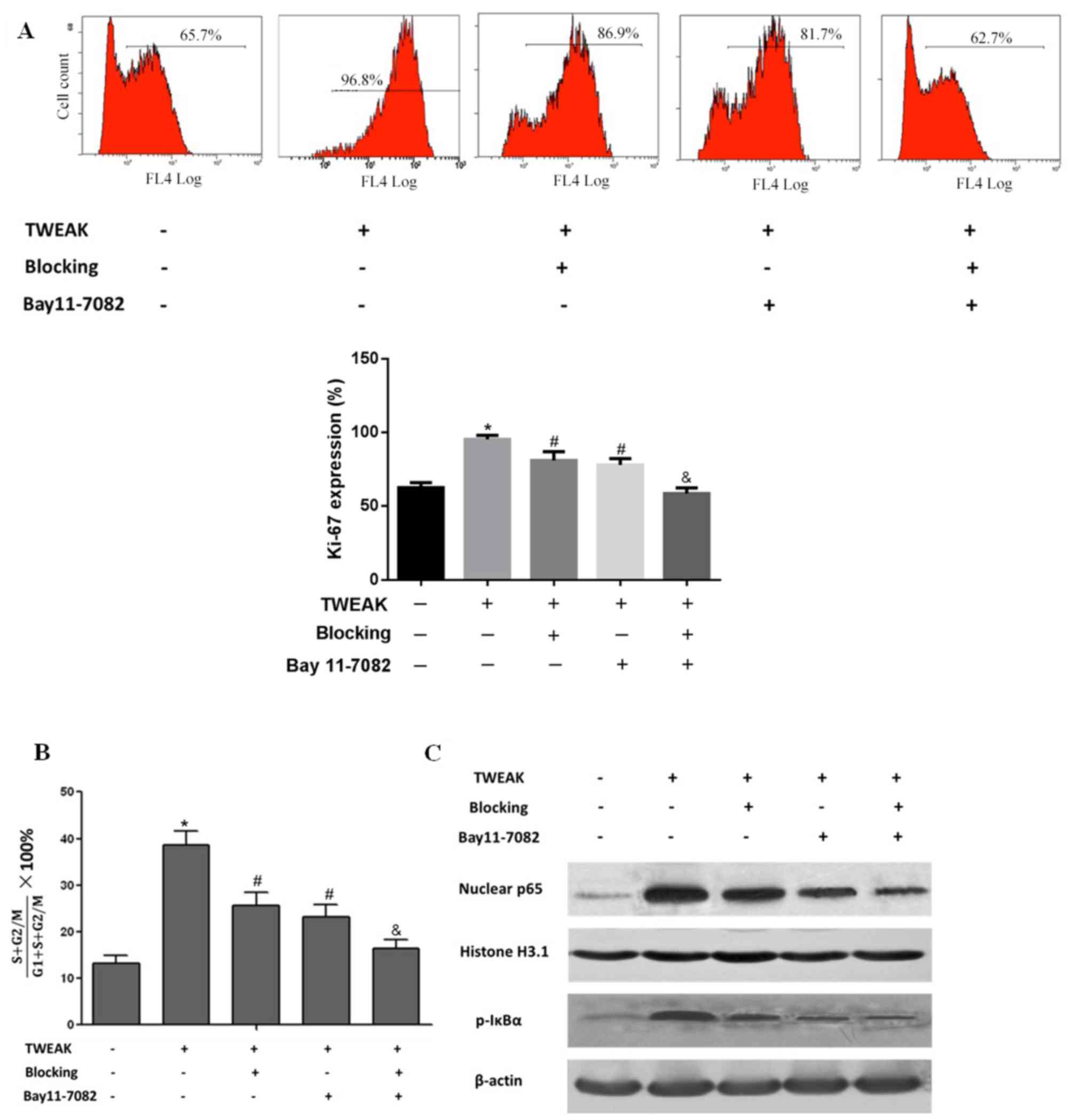
Effect of TWEAK on NF-κB
transcriptional activity, HMC proliferation and the cell cycle
Mesangial cell proliferation is among the primary
lesion characteristics of LN (27),
and the immunofluorescence results suggested that cell
proliferation was increased in the biopsied tissue of patients with
LN (Fig. 1C). Thus, the impact of
TWEAK on HMC proliferation was investigated in vivo. On flow
cytometry analysis, it was observed that treatment with recombinant
TWEAK significantly enhanced HMC proliferation, indicted by
elevated Ki-67 expression in the HMCs when compared with the normal
(untreated) control group (P<0.05; Fig. 3A). Cell cycle progression was also
indicated to be promoted in the TWEAK-treated HMCs, as the ratio of
cells in S+G2/M was significantly increased (P<0.05) compared
with the normal control group (Fig.
3B). Additionally, compared with the normal control group, IκBα
phosphorylation and NF-κB p65 nuclear translocation were notably
increased following TWEAK treatment, which directly suggested that
TWEAK enhanced NF-κB transcriptional activity (Fig. 3C). Following treatment with TWEAK
blocking antibody, Ki-67 expression was significantly decreased
(P<0.05), cell cycle progression was significantly attenuated
(P<0.05) and NF-κB transcriptional activity was markedly reduced
compared with the TWEAK positive group. Similar effects were
observed following treatment with the NF-κB specific inhibitor BAY
11–7082. Additionally, combined inhibition with BAY 11–7082 and
blocking antibody significantly enhanced their inhibitory effects
on cell proliferation (P<0.01), cell cycle progression
(P<0.01) and to some extent, NF-κB transcriptional activity
(Fig. 3A-C). These results suggested
that TWEAK may elevate NF-κB transcriptional activity and promote
HMC proliferation.
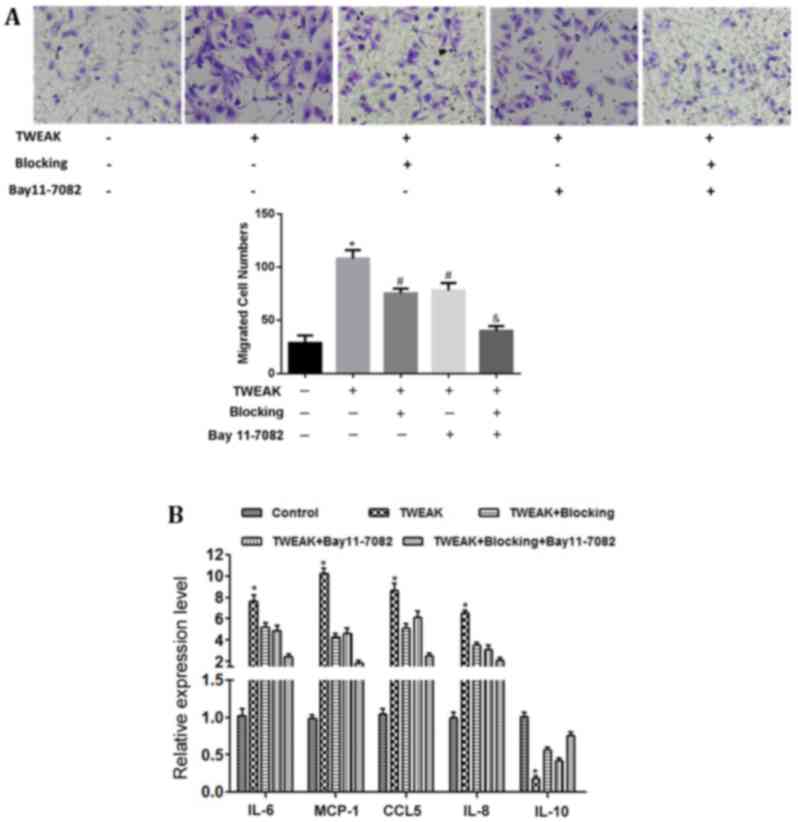
Effect of TWEAK on HMC cytokine
secretion and macrophage migration
Macrophage infiltration in the renal tissue of
patients with LN was indicated (Fig.
1B), which suggested that renal tissue may contain factors that
promote macrophage migration. Therefore, the impact of HMCs on
macrophage migration was investigated in vitro by Transwell
assay. The results demonstrated that a limited number of
macrophages migrated through the chamber membrane when co-cultured
with the normal control group. Conversely, numerous macrophages
migrated to the lower chamber when the chamber was occupied by
TWEAK-treated HMCs; however, TWEAK blocking antibody or BAY 11–7082
treatment significantly reduced this macrophage migratory activity
(P<0.05). Macrophage migration was further lowered on co-culture
with the combined inhibition group (P<0.01; Fig. 4A).
To investigate the role of chemotactic factors in
macrophage migration, RT-qPCR was performed to assess the mRNA
expression of chemotactic factors in HMCs. The results demonstrated
that the mRNA levels of IL-6, IL-8, MCP-1, and CCL5 were
significantly increased (P<0.05), while IL-10 mRNA was
significantly decreased (P<0.05) in the HMCs following TWEAK
treatment (Fig. 4B). Blocking TWEAK
function and/or inhibiting NF-κB activity significantly reversed
these effects of TWEAK treatment (P<0.05), most notably in the
combined inhibition group (P<0.01; Fig. 4B).
Discussion
In recent years, various studies have focused on the
combination of SLE biomarkers and simple clinical indicators for
improving the sensitivity and specificity of predictions regarding
LN activity and disease progression (28,29).
TWEAK is a novel member of the tumor necrosis factor ligand super
family that is distributed in a range of organs, including the
pancreas, heart, intestine, kidney, brain, ovaries, liver, spleen,
lymph nodes and skeletal muscle (30). TWEAK is also expressed in multiple
cell types, including lymphocytes, macrophages, natural killer
cells, renal tubular epithelial cells and glomerular mesangial
cells (31). TWEAK combined with its
receptor Fn14 activates the NF-κB signaling pathway to participate
in inflammation, angiogenesis, cell proliferation and apoptosis
(18). Multiple studies have
suggested the value of TWEAK in assessing renal damage (10–12). Liu
et al (32) reported that
TWEAK expression in the peripheral blood mononuclear cells of
patients with LN was significantly higher than that in patients
with rheumatoid arthritis and healthy controls. In addition, its
elevation was positively correlated with SLEDAI score,
anti-double-stranded DNA content and MCP-1 expression, which
suggested that TWEAK was associated with LN activity, and that
abnormal chemokine levels may also participate in active LN
occurrence (32). The present study
identified that TWEAK content in the urine of patients with LN was
significantly increased compared with that in non-LN SLE patients
and healthy controls. Furthermore, TWEAK levels in patients with
active LN were significantly increased compared with that in
patients with inactive LN, which indicated that TWEAK may not only
be associated with LN occurrence, but also with LN activity.
However, the present study failed to observe a significant
difference in the serum levels of TWEAK among the groups, which may
be attributed to localized expression of TWEAK in LN kidney
lesions, potentially resulting in its elevation in the urine via
the glomerular filtration membrane (33). Western blotting results also
indicated that the protein levels of TWEAK in the renal tissue of
patients with LN were increased compared with the controls.
LN is a type of autoimmune disease mediated by the
immune complex (34). Immune
function disorder, inflammatory factor secretion and inflammatory
cell infiltration are considered to be the key pathogenic factors
directly involved in the pathogenesis of LN (34). In addition, dysfunction in the
cytokine regulation network may cause abnormal cell proliferation
and apoptosis, which has also been indicated as a notable factor in
LN (35). For instance, abnormal
cell cycle progression and hyperplasia have been confirmed to
participate in LN occurrence in glomerular mesangial cells, and
cell proliferation was suggested as one of the predominant
pathological features of LN (36).
Immunofluorescence analysis in the present study identified CD68
and Ki-67 to be overexpressed in LN renal tissue compared with
normal control tissue, which suggested that macrophage infiltration
and cell proliferation were increased in the renal tissue of
patients with LN.
NF-κB is a transcription factor that predominantly
resides in the cytoplasm in the form of a p65-p50 heterodimer
(17). NF-κB participates in the
immune inflammatory response, cell differentiation, cell growth and
apoptosis regulation (17). TWEAK
may activate the NF-κB signaling pathway through binding with Fn14,
the effects of which serve to regulate cell proliferation (37), apoptosis (38), and migration (39). By comparing p65 and IκBα protein
phosphorylation levels, the present findings indicated that NF-κB
transcriptional activity was elevated in the renal tissue of
patients with LN when compared with the controls, which suggested
that TWEAK may be involved in the pathogenesis of LN by affecting
NF-κB transcriptional activity.
To investigate whether TWEAK affected cell
proliferation and macrophage infiltration through NF-κB, a series
of in vitro experiments were performed for verification.
HMCs were treated with recombinant TWEAK cytokine (50 ng/ml), and
the results suggested that NF-κB activity was enhanced, which was
consistent with the observations in pathological tissues. In
addition, HMC proliferation and cell cycle progression were
promoted, and macrophage chemotaxis was enhanced. In turn, blocking
TWEAK cytokine and/or inhibiting NF-κB transcriptional activity
reversed these effects of TWEAK treatment, most notably with
combined inhibition These results demonstrated that TWEAK may alter
the biological functions of HMCs in the pathogenesis of LN by
influencing NF-κB activity.
Previous research has indicated that a number of
cytokines may be involved in the immune inflammatory reaction in
LN, and that imbalances between pro- and anti-inflammatory factors
determine the severity and scope of the inflammatory response
(35). Chemokines are a type of low
molecular weight protein that promote leukocyte migration and serve
an important role in the inflammatory response (40). Cytokines with critical effects on
macrophage chemotaxis include MCP-1, CCL5, IL-6 and IL-8 (41). In chronic arthritis, a type of
chronic inflammatory disease, it has been identified that TWEAK may
induce chronic arthritis synovial cells to produce a variety of
chemokines, including MMP-1, IL-6, IL-8, CCL5 and IL-10, which led
to inflammatory cell migration and invasion (42). Additionally, a previous study
reported that TWEAK increased MCP-1, IL-6, IL-8 and matrix
metallopeptidase-9 expression and secretion in macrophages
(43). Thus, the present study
investigated whether TWEAK could promote macrophage migration and
invasion through HMC-expressing chemokines; the mRNA levels of
chemokines in HMCs were determined to verify the influence of
chemokine expression on macrophages. The results indicated that
TWEAK significantly elevated the mRNA expression of IL-6, IL-8,
MCP-1 and CCL5 in HMC cells, which suggested these factors may be
involved in promoting TWEAK-induced macrophage migration and
invasion. IL-10 is a type of anti-inflammatory factor expressed and
secreted by multiple immune cells, including macrophages, T
lymphocytes and B lymphocytes (44).
It exerts an anti-inflammatory effect by inhibiting the expression
and secretion of a variety of inflammatory factors, including
interferon-γ, IL-2 and tumor necrosis factor-α (45). Previous results have indicated that
abnormal IL-10 expression was associated with renal disease
(46). Furthermore, IL-10 may
prevent glomerular cell proliferation, decrease IL-1β and
intercellular adhesion molecule-1 expression, and alleviate
inflammatory cell infiltration (47). The present results demonstrated that
TWEAK reduced the mRNA level of IL-10 in HMCs, suggesting that
TWEAK may promote macrophage migration by downregulating IL-10
expression.
In conclusion, TWEAK levels were increased in the
renal tissue of patients with LN, and more notably, in the urine of
patients with active LN. Therefore, the urine level of TWEAK may be
a novel marker of LN activity status. Additionally, the present
results suggested that blocking TWEAK and the downstream NF-κB
signaling pathway may reduce HMC proliferation and macrophage
chemotaxis, thus implicating these as novel methods and targets in
the clinical treatment of LN.
Acknowledgements
The present study was supported by the Yantai
Science and Technology Development Project (grant no.
2013WS237).
References
|
1
|
Manson JJ and Isenberg DA: The
pathogenesis of systemic lupus erythematosus. Neth J Med.
61:343–346. 2003.PubMed/NCBI
|
|
2
|
Mok CC and Lau CS: Pathogenesis of
systemic lupus erythematosus. J Clin Pathol. 56:481–490. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Birmingham DJ and Hebert LA: The
complement system in lupus nephritis. Semin Nephrol. 35:444–454.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cross J and Jayne D: Diagnosis and
treatment of kidney disease. Best Pract Res Clin Rheumatol.
19:785–798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuste C, Gutierrez E, Sevillano AM,
Rubio-Navarro A, Amaro-Villalobos JM, Ortiz A, Egido J, Praga M and
Moreno JA: Pathogenesis of glomerular haematuria. World J Nephrol.
4:185–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ortega LM, Schultz DR, Lenz O, Pardo V and
Contreras GN: Review: Lupus nephritis: Pathologic features,
epidemiology and a guide to therapeutic decisions. Lupus.
19:557–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karsh J, Klippel JH, Balow JE and Decker
JL: Mortality in lupus nephritis. Arthritis Rheum. 22:764–769.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reyes-Thomas J, Blanco I and Putterman C:
Urinary biomarkers in lupus nephritis. Clin Rev Allergy Immunol.
40:138–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kopetschke K, Klocke J, Griessbach AS,
Humrich JY, Biesen R, Dragun D, Burmester GR, Enghard P and
Riemekasten G: The cellular signature of urinary immune cells in
lupus nephritis: New insights into potential biomarkers. Arthritis
Res Ther. 17:942015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crispin JC: A TWEAK in lupus nephritis.
Clin Immunol. 145:139–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanz AB, Izquierdo MC, Sanchez-Niño MD,
Ucero AC, Egido J, Ruiz-Ortega M, Ramos AM, Putterman C and Ortiz
Ap: TWEAK and the progression of renal disease: Clinical
translation. Nephrol Dial Transplant. 29 Suppl 1:i54–i62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rayego-Mateos S, Morgado-Pascual JL, Sanz
AB, Ramos AM, Eguchi S, Batlle D, Pato J, Keri G, Egido J, Ortiz A
and Ruiz-Ortega M: TWEAK transactivation of the epidermal growth
factor receptor mediates renal inflammation. J Pathol. 231:480–494.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwartz N, Su L, Burkly LC, Mackay M,
Aranow C, Kollaros M, Michaelson JS, Rovin B and Putterman C:
Urinary TWEAK and the activity of lupus nephritis. J Autoimmun.
27:242–250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwartz N, Rubinstein T, Burkly LC,
Collins CE, Blanco I, Su L, Hojaili B, Mackay M, Aranow C, Stohl W,
et al: Urinary TWEAK as a biomarker of lupus nephritis: A
multicenter cohort study. Arthritis Res Ther. 11:R1432009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hotta K, Sho M, Yamato I, Shimada K,
Harada H, Akahori T, Nakamura S, Konishi N, Yagita H, Nonomura K
and Nakajima Y: Direct targeting of fibroblast growth
factor-inducible 14 protein protects against renal ischemia
reperfusion injury. Kidney Int. 79:179–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Z, Burkly LC, Campbell S, Schwartz N,
Molano A, Choudhury A, Eisenberg RA, Michaelson JS and Putterman C:
TWEAK/Fn14 interactions are instrumental in the pathogenesis of
nephritis in the chronic graft-versus-host model of systemic lupus
erythematosus. J Immunol. 179:7949–7958. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oeckinghaus A and Ghosh S: The NF-kappaB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1:a0000342009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Armstrong CL, Galisteo R, Brown SA and
Winkles JA: TWEAK activation of the non-canonical NF-κB signaling
pathway differentially regulates melanoma and prostate cancer cell
invasion. Oncotarget. 7:81474–81492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Michaelson JS, Wisniacki N, Burkly LC and
Putterman C: Role of TWEAK in lupus nephritis: A bench-to-bedside
review. J Autoimmun. 39:130–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith EL and Shmerling RH: The American
College of Rheumatology criteria for the classification of systemic
lupus erythematosus: Strengths, weaknesses and opportunities for
improvement. Lupus. 8:586–595. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mikdashi J and Nived O: Measuring disease
activity in adults with systemic lupus erythematosus: The
challenges of administrative burden and responsiveness to patient
concerns in clinical research. Arthritis Res Ther. 17:1832015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gladman DD, Ibañez D and Urowitz MB:
Systemic lupus erythematosus disease activity index 2002. J
Rheumatol. 29:288–291. 2000.
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stöger JL, Gijbels MJ, van der Velden S,
Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E and de
Winther MP: Distribution of macrophage polarization markers in
human atherosclerosis. Atherosclerosis. 225:461–468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sobecki M, Mrouj K, Camasses A, Parisis N,
Nicolas E, Llères D, Gerbe F, Prieto S, Krasinska L, David A, et
al: The cell proliferation antigen Ki-67 organises heterochromatin.
Elife. 5:e137222016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Christian F, Smith EL and Carmody RJ: The
regulation of NF-κB Subunits by phosphorylation. Cells. 5:E122016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wilhelmus S, Alpers CE, Cook HT, Ferrario
F, Fogo AB, Haas M, Joh K, Noël LH, Seshan SV, Bruijn JA and Bajema
IM: The revisited classification of GN in SLE at 10 Years: Time to
re-evaluate histopathologic lesions. J Am Soc Nephrol.
26:2938–2946. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Torres-Salido MT, Cortes-Hernandez J,
Vidal X, Pedrosa A, Vilardell-Tarres M and Ordi-Ros J: Neutrophil
gelatinase-associated lipocalin as a biomarker for lupus nephritis.
Nephrol Dial Transplant. 29:1740–1749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wolf BJ, Spainhour JC, Arthur JM, Janech
MG, Petri M and Oates JC: Development of biomarker models to
predict outcomes in lupus nephritis. Arthritis Rheumatol.
68:1955–1963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chicheportiche Y, Bourdon PR, Xu H, Hsu
YM, Scott H, Hession C, Garcia I and Browning JL: TWEAK, a new
secreted ligand in the tumor necrosis factor family that weakly
induces apoptosis. J Biol Chem. 272:32401–32410. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fragoso-Loyo H, Atisha-Fregoso Y,
Nuñez-Alvarez CA and Llorente L: Utility of TWEAK to assess
neuropsychiatric disease activity in systemic lupus erhytematosus.
Lupus. 25:364–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu ZC, Zhou QL, Li XZ, Yang JH, Ao X,
Veeraragoo P and Zuo XX: Elevation of human tumor necrosis
factor-like weak inducer of apoptosis in peripheral blood
mononuclear cells is correlated with disease activity and lupus
nephritis in patients with systemic lupus erythematosus. Cytokine.
53:295–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang C, Chen LL, Pan HF, Leng RX, Qin WZ
and Ye DQ: Expression of human tumor necrosis factor-like weak
inducer of apoptosis in patients with systemic lupus erythematosus.
Clin Rheumatol. 31:335–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lech M and Anders HJ: The pathogenesis of
lupus nephritis. J Am Soc Nephrol. 24:1357–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iwata Y, Furuichi K, Kaneko S and Wada T:
The role of cytokine in the lupus nephritis. J Biomed Biotechnol.
2011:5948092011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feng X, Hao J, Liu Q, Yang L, Lv X, Zhang
Y, Xing L, Xu N and Liu S: HMGB1 mediates IFN-γ-induced cell
proliferation in MMC cells through regulation of cyclin D1/CDK4/p16
pathway. J Cell Biochem. 113:2009–2019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen HN, Wang DJ, Ren MY, Wang QL and Sui
SJ: TWEAK/Fn14 promotes the proliferation and collagen synthesis of
rat cardiac fibroblasts via the NF-κB pathway. Mol Biol Rep.
39:8231–8241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwon OH, Kim JH, Kim SY and Kim YS:
TWEAK/Fn14 signaling mediates gastric cancer cell resistance to
5-fluorouracil via NF-κB activation. Int J Oncol. 44:583–590. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cherry EM, Lee DW, Jung JU and Sitcheran
R: Tumor necrosis factor-like weak inducer of apoptosis (TWEAK)
promotes glioma cell invasion through induction of
NF-kappaB-inducing kinase (NIK) and noncanonical NF-κB signaling.
Mol Cancer. 14:92015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cameron MJ and Kelvin DJ: Cytokines and
chemokines-their receptors and their genes: An overview. Adv Exp
Med Biol. 520:8–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Turner MD, Nedjai B, Hurst T and
Pennington DJ: Cytokines and chemokines: At the crossroads of cell
signalling and inflammatory disease. Biochim Biophys Acta.
1843:2563–2582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chicheportiche Y, Chicheportiche R, Sizing
I, Thompson J, Benjamin CB, Ambrose C and Dayer JM: Proinflammatory
activity of TWEAK on human dermal fibroblasts and synoviocytes:
Blocking and enhancing effects of anti-TWEAK monoclonal antibodies.
Arthritis Res. 4:126–133. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SH, Kang YJ, Kim WJ, Woo DK, Lee Y,
Kim DI, Park YB, Kwon BS, Park JE and Lee WH: TWEAK can induce
pro-inflammatory cytokines and matrix metalloproteinase-9 in
macrophages. Circ J. 68:396–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moore KW, de Waal Malefyt R, Coffman RL
and O'Garra A: Interleukin-10 and the interleukin-10 receptor. Annu
Rev Immunol. 19:683–765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Iyer SS and Cheng G: Role of interleukin
10 transcriptional regulation in inflammation and autoimmune
disease. Crit Rev Immunol. 32:23–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sinuani I, Beberashvili I, Averbukh Z and
Sandbank J: Role of IL-10 in the progression of kidney disease.
World J Transplant. 3:91–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kitching AR, Katerelos M, Mudge SJ,
Tipping PG, Power DA and Holdsworth SR: Interleukin-10 inhibits
experimental mesangial proliferative glomerulonephritis. Clin Exp
Immunol. 128:36–43. 2002. View Article : Google Scholar : PubMed/NCBI
|