Introduction
Hepatitis B virus (HBV) infection is a worldwide
epidemic. According to the World Health Organization (WHO), ~2
billion people worldwide have been infected with HBV, of whom 240
million have chronic HBV infection (1). In total, ~650,000 people succumb to
mortality as a result of liver failure (LF), liver cirrhosis (LC)
or hepatocellular carcinoma (HCC) due to HBV infection each year
(2). China has a high prevalence of
HBV infection, with ~93 million patients with hepatitis B, and 60%
of LC cases and 80% of HCC cases caused by HBV infection (3). HBV-related acute-on-chronic LF
(HBV-ACLF), HBV-related LC (HBV-LC), and HBV-related HCC (HBV-HCC)
represent severe end-stage liver diseases with rapid progression
and extremely high mortality (4).
Early identification and precise evaluation of the severity and
stages of these diseases may facilitate timely and proper clinical
treatment, thereby reducing mortality.
Multiple scoring systems are available for assessing
liver function and the severity of liver injury, including the the
Child-Turcotte-Pugh (CTP) score; the Model for End-Stage Liver
Disease (MELD) score; and the MELD-sodium (MELD-Na) and integrated
MELD (iMELD) scores, which were established based on the original
MELD score (5–7). The CTP and MELD scoring systems are
most commonly used; however, these scoring systems require multiple
variants and have certain limitations. The CTP scoring system, with
a total of 15 points, has a relatively narrow grading range, and
its assessment indicators of ascites and hepatic encephalopathy are
strongly subjective, lacking objective quantification (8). The indicators of the MELD scoring
system are all objective, and the inclusion of creatinine (Cr)
reflects the effect of complicating hepatorenal syndrome on the
prognosis of LC patients to a certain extent (9). However, the MELD score is typically
significantly affected by large variations in the international
normalized ratio (INR) due to measurement by different laboratories
with different methods (10).
Moreover, the MELD scoring system does not take the effects of
basic disease history, age, bleeding, ascites, bacterial infection,
or hepatopulmonary syndrome, among other factors, into account when
assessing disease outcomes, and the effects of different treatment
regimens on outcomes is not considered either (11).
The albumin (Alb)-bilirubin (ALBI) score is a newly
developed, simple, and objective scoring system for assessing the
severity of liver function damage via only two indicators:
bilirubin and Alb. The ALBI score may be used to evaluate the liver
function damage and prognosis of patients with liver cancer
(12,13). This score has been reported to have
predictive value for in-hospital mortality in patients with primary
biliary cirrhosis or LC combined with upper gastrointestinal
bleeding (14,15). Few studies on the value of the ALBI
score in assessing the conditions of liver function damage in
various HBV-related liver diseases have been performed (16,17). The
present study retrospectively analyzed data from patients with
HBV-ACLF, HBV-LC or HBV-HCC treated in the Department of Infectious
Diseases of Taihe Hospital (Shiyan, China) and evaluated the value
of the ALBI score in assessing liver function damage occurring in
these HBV-related end-stage liver diseases.
Materials and methods
Patients
Patients with HBV-related end-stage liver diseases
admitted to the Department of Infectious Diseases of Taihe Hospital
between November 2013 and October 2016 were enrolled in the present
retrospective study. In total, there were 138 HBV-ACLF patients
(age, 45.80±11.01 years; male:female, 111:27), 130 HBV-LC patients
(age, 49.00±11.13 years; male:female, 86:44), and 127 HBV-HCC
patients (age, 53.61±10.45 years; male:female, 118:9). The present
study conformed to ethical requirements and was approved by the
Ethics Committee of Taihe Hospital.
Inclusion and exclusion criteria
The inclusion criteria were as follows: The
diagnostic criteria for hepatitis B were based on ‘The Guideline of
Prevention and Treatment for Chronic Hepatitis B: A 2015 Update’
(18). The ACLF diagnostic criteria
were based on the ‘Diagnostic and Treatment Guidelines for Liver
Failure (2012 version)’ (19). Due
to the chronic liver disease, acute or subacute clinical liver
function, decompensation syndrome occurred in the short term and
manifested as: i) Extreme weakness with obvious gastrointestinal
symptoms; ii) rapid jaundice, serum total bilirubin (TBil) levels
>10 times the upper limit of normal [defined as 0–17.1 µmol/l
(18)], or a daily increase ≥17.1
µmol/l; iii) bleeding tendency, prothrombin activity (PTA) ≤40% (or
INR ≥1.5) with exclusion of other causes; iv) decompensated
ascites; and v) with or without hepatic encephalopathy. Patients
with HBV-ACLF were divided into early, middle, and late stages upon
admission based on the previously mentioned guidelines. Early stage
was diagnosed as follows: i) Extreme fatigue with serious
gastrointestinal symptoms such as obvious anorexia, vomiting and
abdominal distension; ii) progressively increasing jaundice (serum
TBil ≥171 µmol/l or a daily increase ≥17.1 µmol/l); iii) bleeding
tendency, 30% <PTA ≤40% (or 1.5 <INR ≤1.9); and iv) no
hepatic encephalopathy or other complications. Middle stage was
diagnosed as liver failure aggravated beyond that of the early
stage with one of the following two conditions: i) Hepatic
encephalopathy below degree II and/or obvious ascites and
infection; and ii) bleeding tendency (bleeding or blood stasis),
20% <PTA ≤30% (or 1.9 <INR ≤2.6). Late stage was diagnosed
when the disease was further aggravated beyond that of the middle
stage with a severe bleeding tendency, PTA ≤20% (or INR >2.6),
and at least one of the following four symptoms was observed:
Hepatorenal syndrome, upper gastrointestinal bleeding, severe
infection or degree II hepatic encephalopathy.
The exclusion criteria were as follows: Patients
with viral hepatitis other than hepatitis B, alcoholic liver
disease, autoimmune liver disease, or drug-induced liver injury;
patients with other concomitant severe primary diseases of the
heart, lung, or other organs; patients with metastatic HCC; and
patients with primary carcinomas in other organs.
Additionally, patients with concurrent liver tumors
or tumors in other organs were excluded from the HBV-ACLF group,
patients with concomitant liver tumors or liver failure were
excluded from the HBV-LC group and patients with liver failure were
excluded from the HBV-HCC group.
Data collection
The parameters of TBil, Alb, Cr, prothrombin time
(PT) and INR required for ALBI, CTP, and MELD scoring were all
measured in the Department of Laboratory Tests at Taihe Hospital
within 24 h following admission. The ascites conditions were also
evaluated by imaging examinations within 24 h following admission.
Data were retrieved from the electronic medical record system of
Taihe Hospital and then validated via the clinical test data system
and imaging system of the hospital.
ALBI, CTP, and MELD scoring
criteria
The ALBI score was calculated as: ALBI = [Log10TBil
(µmol/l) × 0.66] + [Alb (g/l) x-0.085], wherein the ALBI grades
included grades 1 (ALBI score ≤-2.6), 2 (−2.59 <ALBI score
<-1.39), and 3 (ALBI score ≥-1.39) (20).
The CTP score was the sum of the scores of five
items (ascites, hepatic encephalopathy, TBil, Alb, and PT extension
time). Each item consisted of 1–3 points, for a total maximum score
of 15 points (21,22).
The MELD score was calculated as follows: MELD=3.8 ×
loge [TBil (mg/dl)] + 11.2 × loge (INR) + 9.6 × loge [Cr(mg/dl)] +
6.4 × etiology. Cholestasis and alcoholic liver disease had a value
of 0 and other etiologies had a value of 1 (23).
Statistical analysis
The statistical analysis was performed with GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Data
are presented as the mean ± standard deviation. Multiple sample
means of the measurement data were compared using one-way analysis
of variance, and Scheffe's post hoc tests when appropriate.
Pairwise comparisons were conducted using a Student's t-test and
count data were compared using the chi-squared test. Linear
regression analysis was used to compare the correlation between
ALBI score and CTP or MELD scores. P<0.05 was considered to
indicate a statistically significant difference.
Results
HBV-ACLF patients exhibit higher ALBI,
CTP, and MELD scores than patients with HBV-LC or HBV-HCC
The mean ALBI scores of the HBV-ACLF, HBV-LC, and
HBV-HCC patients were −1.17±0.55, −1.76±0.66 and −2.59±0.62,
respectively. Additionally, the respective mean CTP scores were
10.70±1.81, 8.19±1.25 and 5.81±1.22, and the respective mean MELD
scores were 19.93±7.44, 11.10±4.39 and 7.01±3.22. The HBV-ACLF
patients had the highest scores for all three scoring systems and
predominantly exhibited an ALBI grade of 3, whereas the lowest
scores for all three scoring systems were demonstrated by HBV-HCC
patients, who mainly exhibited an ALBI grade of 1. Intergroup
comparisons of the ALBI, CTP, and MELD scores indicated
statistically significant differences between all groups
(P<0.0001; Fig. 1; Table I).
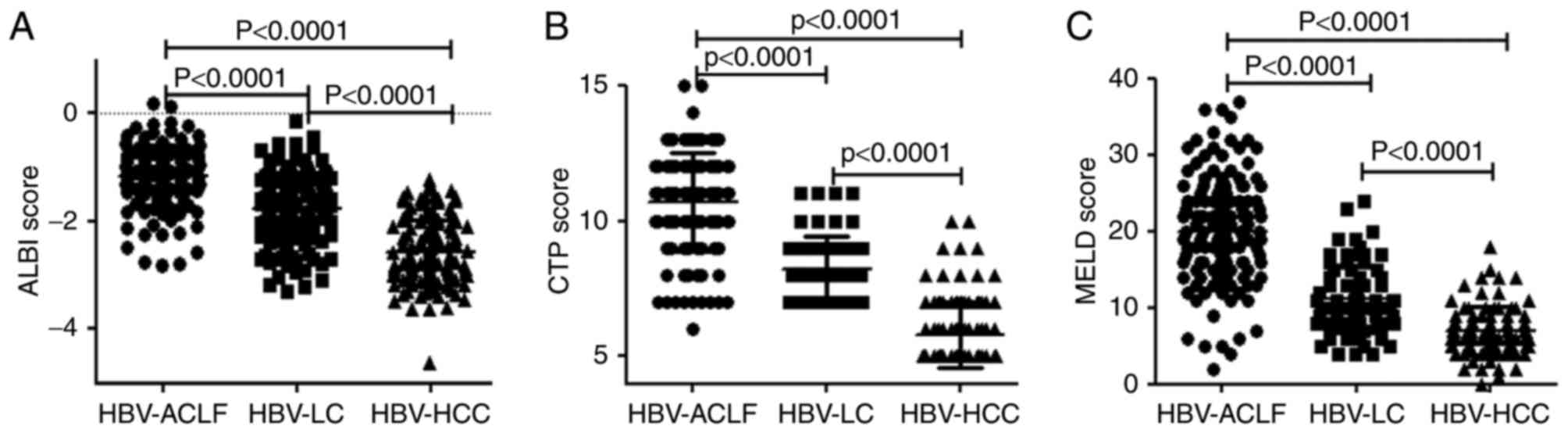 | Figure 1.Comparison of the scoring systems
among HBV-ACLF, HBV-LC, and HBV-HCC patients. Comparison of the (A)
ALBI score, (B) CTP score and (C) MELD score. Data are presented as
the mean ± standard deviation. HBV, hepatitis B virus; HBV-ACLF,
HBV-related acute-on-chronic liver failure; HBV-LC, HBV-related
liver cirrhosis; HBV-HCC, HBV-related hepatocellular carcinoma;
ALBI, albumin-bilirubin; CTP, Child-Turcotte-Pugh; MELD, model for
end-stage liver disease. |
 | Table I.The ALBI grades of patients with
HBV-ACLF, HBV-LC, and HBV-HCC. |
Table I.
The ALBI grades of patients with
HBV-ACLF, HBV-LC, and HBV-HCC.
|
|
| Grade 1 | Grade 2 | Grade 3 |
|---|
|
|
|
|
|
|
|---|
| Group | Patients (n) | Patients (n) | Constituent frequency
(%) | Patients (n) | Constituent frequency
(%) | Patients (n) | Constituent frequency
(%) |
|---|
| HBV-ACLF | 138 | 3 | 2.17 | 35 | 25.36 | 100 | 72.46 |
| HBV-LC | 130 | 20 | 15.38 | 71 | 54.62 | 39 | 30.00 |
| HBV-HCC | 127 | 72 | 56.69 | 54 | 42.52 |
1 | 0.79 |
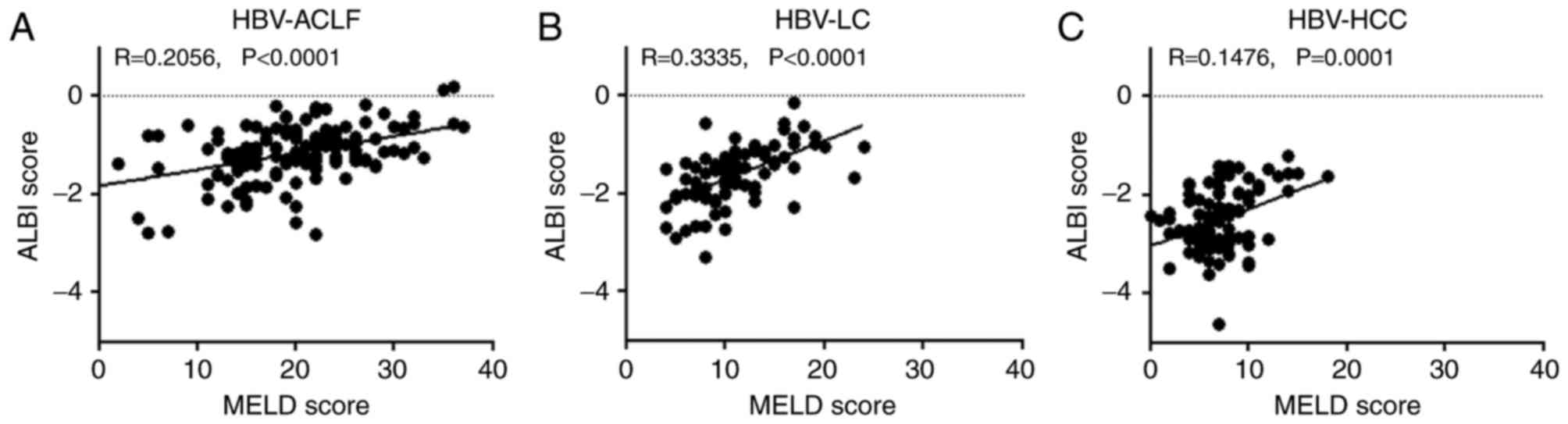
ALBI scores are positively correlated
with MELD scores
A positive correlation was observed between the ALBI
score and the MELD score for HBV-ACLF, HBV-LC, and HBV-HCC
patients, with correlation coefficients of 0.2056, 0.3335 and
0.1476, respectively (P<0.01; Fig.
2).
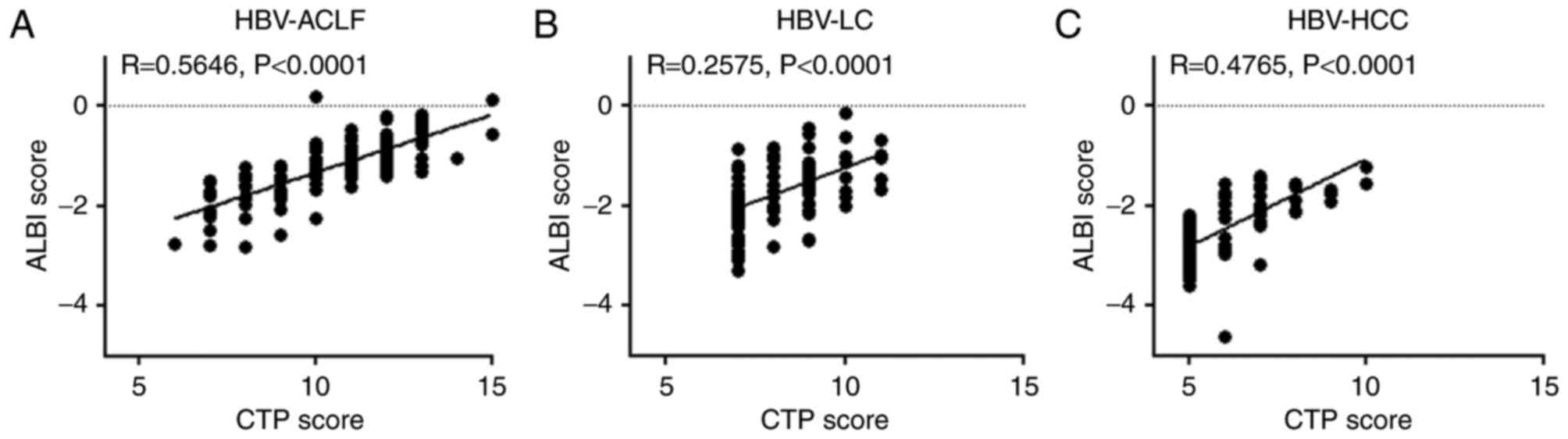
ALBI scores are positively correlated
with CTP scores
A positive correlation was also observed between the
ALBI and CTP scores of HBV-ACLF, HBV-LC, and HBV-HCC patients, with
correlation coefficients of 0.5646, 0.2575 and 0.4765, respectively
(P<0.0001; Fig. 3). Furthermore,
the ALBI grading of HBV-ACLF patients was more complex than CTP
grading. The only patient with a CTP grade of A exhibited an ALBI
grade of 1, and the majority of patients with a CTP grade of C had
an ALBI grade of 3, whereas patients with a CTP grade of B had
varying ALBI grades (Table II).
 | Table II.The association between the CTP and
ALBI grades in patients with HBV-ACLF. |
Table II.
The association between the CTP and
ALBI grades in patients with HBV-ACLF.
|
|
| ALBI grade 1 | ALBI grade 2 | ALBI grade 3 |
|---|
| Group | Patients (n) | Patients (n) | Constituent frequency
(%) | Patients (n) | Constituent frequency
(%) | Patients (n) | Constituent frequency
(%) |
|---|
| CTP grade A |
1 | 1 | 100 | 0 | 0 | 0 | 0 |
| CTP grade B | 31 | 2 | 6.45 | 26 | 83.87 | 3 | 9.68 |
| CTP grade C | 106 | 0 | 0 | 9 | 8.49 | 97 | 91.51 |
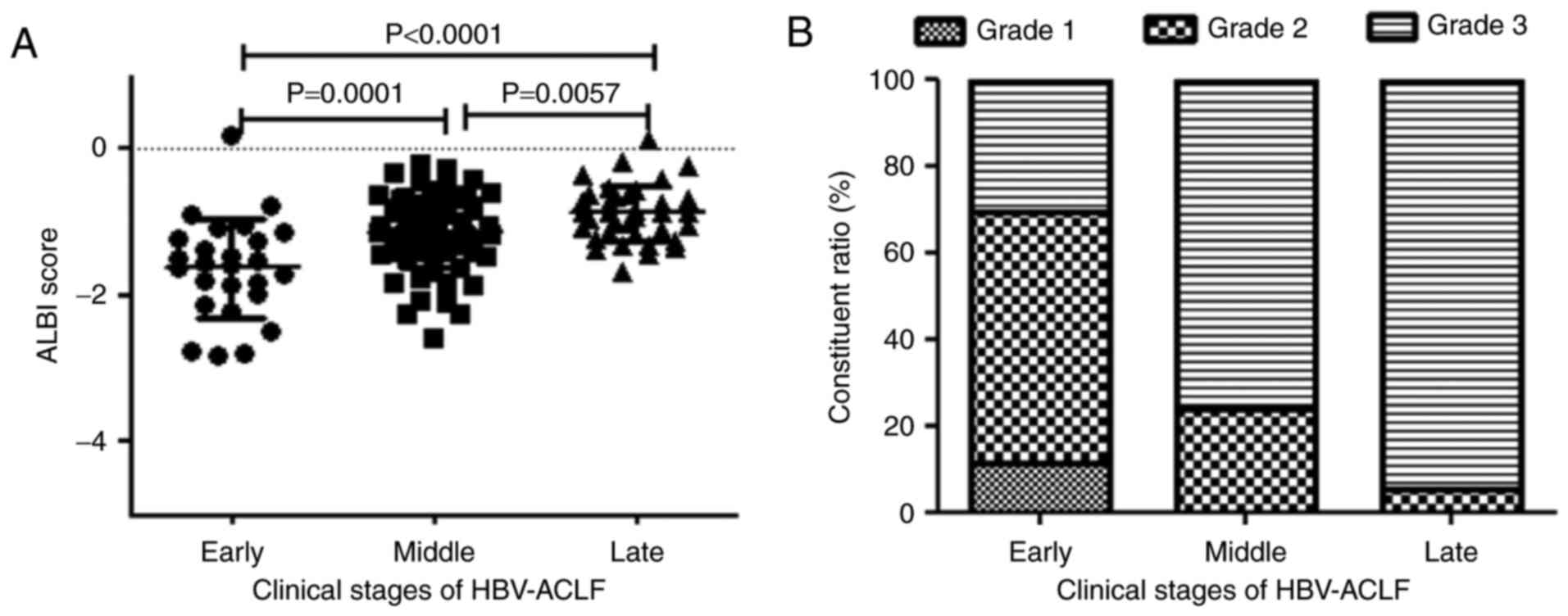
ALBI score is associated with the
clinical stages of HBV-ACLF patients
The mean ALBI scores of patients with early-,
middle- and late-stage HBV-ACLF were −1.63±0.68, −1.14±0.47 and
−0.88 ± 0.39, respectively. A later stage of HBV-ACLF resulted in a
higher ALBI score and a greater frequency of an ALBI grade of 3
(P<0.01; Table III; Fig. 4).
 | Table III.The ALBI score and grades of HBV-ACLF
patients in different clinical stages. |
Table III.
The ALBI score and grades of HBV-ACLF
patients in different clinical stages.
|
|
|
| Grade 1 | Grade 2 | Grade 3 |
|---|
|
|
|
|
|
|
|
|---|
| Clinical stage | Patients (n) | ALBI score (mean ±
SD) | Patients (n) | Constituent frequency
(%) | Patients (n) | Constituent frequency
(%) | Patients (n) | Constituent frequency
(%) |
|---|
| Early stage | 26 |
−1.63±0.68 | 3 | 11.54 | 15 | 57.69 | 8 | 30.77 |
| Middle stage | 76 |
−1.14±0.47 | 0 | 0 | 18 | 23.68 | 58 | 76.32 |
| Late stage | 36 |
−0.88±0.39 | 0 | 0 | 2 |
5.56 | 34 | 94.44 |
Discussion
HBV-ACLF, HBV-LC, and HBV-HCC are the main clinical
types of end-stage liver diseases. Given the severity of the
diseases and their poor prognoses, a precise assessment of the
disease conditions is pivotal for proper treatment planning and
accurate prognosis prediction. Scoring systems, such as the CTP and
MELD scores, have been used to assess the severity and prognosis of
end-stage liver diseases, although their multiple requirements
restrain their usage. In contrast, the ALBI score requires only two
factors, which are also objective and easily available. The ALBI
score has recently been validated to assess the severity of liver
function damage in patients with HCC (12). The ALBI score has also been shown to
be more accurate than the CTP score in predicting in-hospital
mortality following hepatic carcinectomy in HCC patients (20). For the assessment of liver function
in patients with liver cancer and treated with sorafenib, the
resolution of the ALBI score is similar to that of the CTP score
for patients with a CTP grade of A, whereas the ALBI grading is
superior to the CTP grading for all HCC patients (21). The ALBI score has a predictive
efficacy similar to that of the CTP and MELD scores in terms of
predicting in-hospital mortality in LC patients with acute upper
gastrointestinal bleeding and in ACLF patients with concurrent LC
(22,24). In the present study, the ALBI score
was compared with the CTP and MELD scores to assess their value in
evaluating the liver function of patients with HBV-ACLF, HBV-LC, or
HBV-HCC.
ACLF is an acute severe liver injury that occurs due
to chronic liver disease. ACLF results in massive liver cell
necrosis and dysfunctions in liver synthesis, detoxification,
excretion, and biotransformation, with a mortality rate >50%
(25). The present study
demonstrated that, among the patients with end-stage liver
diseases, HBV-ACLF patients had the highest ALBI scores, which was
consistent with the CTP and MELD scores. The differences in the
ALBI, CTP, and MELD scores among the three groups suggested that
the degrees of liver function damage vary among HBV-ACLF, HBV-LC,
and HBV-HCC patients. The highest ALBI, CTP, and MELD scores,
observed in HBV-ACLF patients, represent the most severe liver
injury, and the lowest scores, observed in HBV-HCC patients,
represent the least severe liver injury. Intergroup differences
measured using the ALBI scoring system were consistent with the
differences measured using the CTP and MELD scoring systems, which
suggested that the ALBI score may also reflect liver function
damage that occurs in HBV-HCC, HBV-LC and HBV-ACLF patients.
Similar to the CTP scoring system, the ALBI scoring system divided
the patients into three grades according to disease severity, with
higher ALBI grades indicating a more serious disease status. In the
present study, analysis of the ALBI grades of HBV-ACLF, HBV-LC, and
HBV-HCC patients revealed that the HBV-ACLF group predominantly
exhibited grades of 3 and that the HBV-HCC group mainly exhibited
grades of 1. Therefore, the ALBI classification accurately reflects
liver injury severity in HBV-ACLF, HBV-LC, and HBV-HCC
patients.
Additionally, the present study demonstrated that
the ALBI scores of HBV-ACLF, HBV-LC, or HBV-HCC patients were
positively correlated with the CTP and MELD scores, further
validating that the ALBI score and the CTP and MELD scores had
similar efficacy in assessing liver injury in patients with
HBV-related end-stage liver diseases. Comparison of the CTP and
ALBI grading in patients with HBV-ACLF demonstrated that all CTP
grade A patients had an ALBI grade of 1, whereas the patients with
CTP grades of B and C had varying ALBI grades, suggesting that the
ALBI score may be more accurate than the CTP score.
For HBV-ACLF patients, later stages indicate more
severe disease conditions. To further investigate the association
between the ALBI score and disease severity, the ALBI score and the
ALBI grade of HBV-ACLF patients in different disease stages were
compared. The results showed significant differences in the ALBI
scores and the constitutive grades among the early, middle, and
late stages of liver failure, with the later stages associated with
higher ALBI scores. ALBI grade 1 was only observed among
early-stage HBV-ACLF patients, whereas all middle-stage patients
exhibited ALBI grade 2 or grade 3 and the late-stage patients
predominantly exhibited grade 3. This finding suggests that ALBI
scoring and grading reflect the severity of liver function damage
in HBV-ACLF patients and are positively correlated with the
severity of liver disease, with more severe liver injury associated
with higher ALBI scores and grades.
In conclusion, similar to the CTP and MELD scoring
systems, the ALBI score is a good indicator of the severity of
liver function damage in patients with HBV-ACLF, HBV-LC, or
HBV-HCC. Different diseases and even different stages of the same
disease exhibit different ALBI scores, and ALBI grading may
accurately classify disease severity, with higher ALBI grades
indicating more severe disease conditions. Given that the ALBI
score requires only two simple factors and displays versatility in
evaluating the severity of HBV-related end-stage liver diseases, it
is expected to be extensively clinically applied as a simple
scoring system. However, the present study was limited, as the
values of the ALBI score for the evaluation of HBV-related
end-stage liver diseases were only investigated at a single center
at a single time point (admission). Multi-center studies with large
sample sizes are required to further validate the efficacy and
prognostic role of the ALBI score in other types of liver
diseases.
Acknowledgements
The present study was partly supported by the
National Natural Science Foundation of China (grant no. 81541140),
the Natural Science Foundation of Hubei Province of China (grant
no. 2014CFB645), the Research and Development Project of Science
and Technology Plan of Hubei Provinc e (grant no. 2011BCB030), the
Foundation for Innovative Research Team of Hubei University of
Medicine (grant no. 2014CXG05) and the Key Program for Precision
Medicine of Taihe Hospital (grant no. 2016JZ05).
References
|
1
|
Ott JJ, Stevens GA, Groeger J and Wiersma
ST: Global epidemiology of hepatitis B virus infection: new
estimates of age-specific HBsAg seroprevalence and endemicity.
Vaccine. 30:2212–2219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang FS, Fan JG, Zhang Z, Gao B and Wang
HY: The global burden of liver disease: The major impact of China.
Hepatology. 60:2099–2108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jalan R, Gines P, Olson JC, Mookerjee RP,
Moreau R, Garcia-Tsao G, Arroyo V and Kamath PS: Acute-on chronic
liver failure. J Hepatol. 57:1336–1348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng XP, Zhao J, Chen Y, Meng FK, Xu B,
Yu HW, Meng QH, Liu YM, Zhang SB, Meng S, et al: Comparison of the
ability of the PDD-ICG clearance test, CTP, MELD, and MELD-Na to
predict short-term and medium-term mortality in patients with
decompensated hepatitis B cirrhosis. Eur J Gastroenterol Hepatol.
28:444–448. 2016.PubMed/NCBI
|
|
6
|
Ling Q, Dai H, Zhuang R, Shen T, Wang W,
Xu X and Zheng S: Predicting short-term survival after liver
transplantation on eight score systems: A national report from
China liver transplant registry. Sci Rep. 7:422532017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Ghannam MT, Hassanien MH, El-Talkawy
MD, Saleem A, Sabry AI and Abu TH: Performance of disease-specific
scoring models in intensive care patients with severe liver
diseases. J Clin Diagn Res. 11:OC12–OC16. 2017.PubMed/NCBI
|
|
8
|
Durand F and Valla D: Assessment of
prognosis of cirrhosis. Semin Liver Dis. 28:110–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong G, Lee KW, Suh S, Yoo T, Kim H, Park
MS, Choi Y, Yi NJ and Suh KS: The model for end-stage liver disease
score-based system predicts short term mortality better than the
current Child-Turcotte-Pugh score-based allocation system during
waiting for deceased liver transplantation. J Korean Med Sci.
28:1207–1212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Porte RJ, Lisman T, Tripodi A, Caldwell SH
and Trotter JF; Coagulation in Liver Disease Study Group, : The
international normalized ratio (INR) in the MELD score: Problems
and solutions. Am J Transplant. 10:1349–1353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vitale A, Bertacco A, Gambato M, D'Amico
F, Morales RR, Frigo AC, Zanus G, Burra P, Angeli P and Cillo U:
Model for end-stage liver disease-sodium and survival benefit in
liver transplantation. Transpl Int. 26:138–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toyoda H, Lai PB, O'Beirne J, Chong CC,
Berhane S, Reeves H, Manas D, Fox RP, Yeo W, Mo F, et al: Long-term
impact of liver function on curative therapy for hepatocellular
carcinoma: Application of the ALBI grade. Br J Cancer. 114:744–750.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan AW, Chan RC, Wong GL, Wong VW, Choi
PC, Chan HL and To KF: New simple prognostic score for primary
biliary cirrhosis: Albumin-bilirubin score. J Gastroenterol
Hepatol. 30:1391–1396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou D, Qi X, Zhu C, Ning Z, Hou F, Zhao J,
Peng Y, Li J, Deng H and Guo X: Albumin-bilirubin score for
predicting the in-hospital mortality of acute upper
gastrointestinal bleeding in liver cirrhosis: A retrospective
study. Turk J Gastroenterol. 27:180–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen RC, Cai YJ, Wu JM, Wang XD, Song M,
Wang YQ, Zheng MH, Chen YP, Lin Z and Shi KQ: Usefulness of
albumin-bilirubin grade for evaluation of long-term prognosis for
hepatitis B-related cirrhosis. J Viral Hepat. 24:238–245. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen B and Lin S: Albumin-bilirubin (ALBI)
score at admission predicts possible outcomes in patients with
acute-on-chronic liver failure. Medicine (Baltimore). 96:e71422017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou JL and Lai W: The guideline of
prevention and treatment for chronic hepatitis B: A 2015 update.
Zhonghua Gan Zang Bing Za Zhi. 23:888–905. 2015.PubMed/NCBI
|
|
19
|
Liver Failure And Artificial Liver Group
CSOI and Severe Liver Diseases And Artificial Liver Group CSOH, .
Diagnostic and treatment guidelines for liver failure (2012
version). Zhonghua Gan Zang Bing Za Zhi. 21:177–183.
2013.PubMed/NCBI
|
|
20
|
Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD,
Xiang BD, Ma L, Qi LN, Ou BN and Li LQ: Albumin-bilirubin versus
Child-Pugh score as a predictor of outcome after liver resection
for hepatocellular carcinoma. Br J Surg. 103:725–734. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edeline J, Blanc JF, Johnson P,
Campillo-Gimenez B, Ross P, Ma YT, King J, Hubner RA, Sumpter K,
Darby S, et al: A multicentre comparison between Child Pugh and
Albumin-Bilirubin scores in patients treated with sorafenib for
Hepatocellular Carcinoma. Liver Int. 36:1821–1828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou D, Qi X, Zhu C, Ning Z, Hou F, Zhao J,
Peng Y, Li J, Deng H and Guo X: Albumin-bilirubin score for
predicting the in-hospital mortality of acute upper
gastrointestinal bleeding in liver cirrhosis: A retrospective
study. Turk J Gastroenterol. 27:180–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamath PS, Wiesner RH, Malinchoc M,
Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER and Kim
WR: A model to predict survival in patients with end-stage liver
disease. Hepatology. 33:464–470. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng Y, Qi X, Tang S, Deng H, Li J, Ning
Z, Dai J, Hou F, Zhao J, Wang R, et al: Child-Pugh, MELD, and ALBI
scores for predicting the in-hospital mortality in cirrhotic
patients with acute-on-chronic liver failure. Expert Rev
Gastroenterol Hepatol. 10:971–980. 2016.PubMed/NCBI
|
|
25
|
Garg H, Kumar A, Garg V, Sharma P, Sharma
BC and Sarin SK: Clinical profile and predictors of mortality in
patients of acute-on-chronic liver failure. Dig Liver Dis.
44:166–171. 2012. View Article : Google Scholar : PubMed/NCBI
|