Introduction
Fructose is widely used as a sweetener in many
processed foods and beverages. The consumption of processed foods
and beverages has increased dramatically over the past few decades.
There is increasing evidence that high dietary fructose consumption
causes dyslipidemia, insulin resistance, obesity, and endothelial
dysfunction (1,2). All of these factors are associated with
increased risk of cardiovascular diseases (CVD), one of the main
cause of morbidity and mortality worldwide (3,4).
Endothelial dysfunction is considered one of the
initial stages in the development of atherosclerosis and CVD, and a
crucial target for the prevention of CVD (5). Vascular endothelium plays an important
role in modulating vascular tone through the synthesis and release
of several vasoactive factors including nitric oxide (NO). NO is
generated from the conversion of L-arginine to L-citrulline by
endothelial NO synthase (eNOS) and has a potent vasodilator,
anti-inflammatory, and antithrombotic properties (6). Reduction of NO bioavailability, caused
by reduced eNOS activity and/or accelerated NO degradation, results
in impaired endothelium-dependent vasorelaxation, increased
thrombus formation, and progressive atherogenesis (7). There is growing evidence that fructose
fed animals exhibited impaired endothelium-dependent relaxation
(8–11) and decreased NO bioavailability
(12–14). High fructose consumption is also
reported to induce oxidative and nitrative stress in vascular
tissues and that oxidative/nitrative stress seems to play a major
role in endothelial dysfunction. Indeed, elevated levels of
reactive oxygen species (ROS) cause a decrease in bioavailability
of NO and an increase in production of powerful oxidant
peroxynitrite that induces eNOS inactivation (15,16). A
number of studies reported that decreased eNOS expression and
increased nitrotyrosine expression were found in aortae from
fructose fed rats (8,17,18).
Therefore, the increased oxidative stress and the decreased NO
synthesis have been proposed to be involved in high
fructose-induced endothelial dysfunction.
Naringin (4′,5,7-trihydroxyflavone
7-rhamnoglucoside) is one of the major constituents of the
flavonoids in citrus fruit, especially grapefruit (19). Naringin and its colonic metabolite,
naringenin, have been reported to possess antihyperglycemic,
anti-inflammatory, anti-oxidative, and antihyperlipidemic effects
(19–24). Moreover, naringin has been shown to
exhibit cardiovascular protective effects in animal models by
preserving endothelial function, enhancing NO bioavailability, and
reducing oxidative stress (21,25).
Based on these findings, we hypothesized that naringin may improve
endothelial dysfunction through its modulation of NO bioavailabity
and oxidative stress. However, to our knowledge, the effect of
naringin on high fructose-induced endothelial dysfunction has never
been reported. Therefore, the aim of this study was to determine
whether naringin could attenuate endothelial dysfunction in
fructose-fed rats and to elucidate the mechanism underlying the
alleviation of endothelial dysfunction.
Materials and methods
Chemicals
Naringin, acethycholine (ACh),
NG-nitro-L-arginine, indomethacin, phenylephrine
(PE), and sodium nitroprusside were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany), and a nitrate/nitrite (NOx) assay
kit was purchased from Cayman Chemical Company, (Ann Arbor, MI, U).
Antibodies against eNOS, phosphorylated eNOS at Ser1177 and β-actin
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA), and the antibody against nitrotyrosine and HRP-conjugated
anti-rabbit IgG were from Merck KGaA. Fructose was obtained from
Charoentavorn Supply Co. (Samutprakarn, Thailand).
Animals and experimental design
Male Sprague-Dawley (SD) rats weighing 180–200 g
were obtained from the National Laboratory Animal Center, Mahidol
University, Bangkok, Thailand. All animals used in this study were
housed at a constant temperature of 20–22°C, with a 12 h light-dark
cycle and allowed free access to tap water and the standard rat
feed for one week. After acclimatization, the rats were randomly
divided into three experimental groups: Group I, the control group
(C) which received normal drinking water; Group II, the fructose
group (F) which received fructose solution and the vehicle 0.1%
carboxymethylcellulose (CMC); and Group III, the fructose plus
naringin group (FN), which received fructose solution and naringin
(100 mg/kg/day) suspended in 0.1% CMC. The fructose solution was
prepared as 10% fructose (w/v) in the drinking water and
administered every day to the rats for 12 weeks ad libitum.
Naringin (100 mg/kg/day) or the vehicle 0.1% CMC was administered
daily by oral gavage for the last 4 weeks of fructose feeding. The
concentration of naringin used in the present study was selected
according to previous studies using animal models which indicated
that this dosage exerted improving vascular dysfunction (21).
All animal procedures were performed within the
institutional guidelines for the care and use of laboratory animals
and were approved by the Animal Ethics Committee of Naresuan
University, Thailand (approval number: 57040023).
Preparation of serum and aortic
tissues
At the end of 12 weeks of fructose feeding,
overnight fasting rats were anesthetized with an intraperitoneal
injection of pentobarbital (50 mg/kg) and blood samples were
collected by cardiac puncture. Blood was then centrifuged at 1,500
× g for 15 min at 4°C to obtain serum, which was stored at −20°C
until further analysis. The descending thoracic rat aorta was
isolated and divided into two parts: One part was placed in Krebs
bicarbonate solution [composition (mM): NaCl 118.0,
NaH2CO3 25.0, glucose 11.0, CaCl2
1.6, KCl 4.7, KH2PO4 1.2 and MgSO4
1.18] for determination of vasorelaxant response, and the other
part was frozen by liquid nitrogen and stored at −80°C until used
for western blot analysis.
Biochemical analysis
At the end of experimental period, blood glucose
levels were measured using an Accu-Check glucometer (Roche
Diagnostics GmbH, Mannheim, Germany). Serum concentrations of total
cholesterol (TC), triglyceride (TG), and high density lipoprotein
cholesterol (HDL-C) were measured colorimetrically with commercial
kits (Human Company, Wiesbaden, Germany). The serum low density
lipoprotein cholesterol (LDL-C) level was calculated using the
formula of Friedewald: LDL=TC-HDL-(TG/5) (26). The serum levels of NOx, the final
products of NO metabolism, were determined by using NOx
fluorometric assay kit (Cayman Chemical Company).
Measurement of vascular reactivity in
the thoracic aorta
As previously described (27), after the removal of superficial
connective tissue and fat surrounding the aorta, the isolated
thoracic aorta was cut into rings (3–4 mm in length) and mounted on
stainless steel hooks in an organ bath chamber containing
oxygenated Krebs bicarbonate solution (95% O2 and 5%
CO2) maintained at 37°C. The isometric tension of aortic
ring was recorded using a force transducer (model no. FT03, Grass
Medical Instruments) connected to a Powerlab Data Acquisition
System (AD Instruments, Sydney, Australia). Before starting the
experiment, the resting tension of each ring was adjusted to 1 g
and equilibrated for 60 min. Each aortic ring was maximally
contracted with an isotonic, high potassium salt solution (KPSS,
123 mM). To investigate the effect of the treatment on the relaxant
responses of the aortic rings, the endothelium-dependent relaxation
to ACh (10−9-10−5 M) and the
endothelium-independent relaxation to sodium nitroprusside (SNP,
10−9-10−5 M) were tested on the aortic rings
precontracted submaximally with PE (10−9-10−6
M). All changes in the tension were expressed as a percentage of
the precontraction. To assess the contribution of the endothelial
vasodilator factors in response to ACh, concentration-response
curves to ACh were performed in the absence or presence of the NO
synthase (NOS) inhibitor NG-nitro-L-arginine
(L-NNA; 100 mmol/l), the cyclo-oxygenase inhibitor indomethacin (10
mmol/l), or L-NNA plus indomethacin.
Western blot analysis of eNOS, p-eNOS
and nitrotyrosine
Aortic tissue samples were homogenized in ice-cold
RIPA buffer containing protease and phosphatase inhibitor and
centrifuged at 15,000 × g for 20 min at 4°C. The supernatants were
collected and the protein concentrations were measured by the
bicinchoninic acid protein assay kit (Merck KGaA). Each sample of
aortic homogenates (40 µg protein) was separated by 7.5% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and then
transferred to polyvinylidenedifluoride membranes which were
blocked with 5% nonfat dry milk for 1 h and then incubated with
anti-eNOS (1:500), anti-phospho-eNOSSer1177 (1:500),
anti-nitrotyrosine (1:1,000) and anti-β actin (1:5,000) at 4°C
overnight. The membranes were then washed with tris-buffered saline
with Tween-20 (TBST) and incubated with anti-rabbit horseradish
peroxidase conjugated secondary antibody (1:5,000) at room
temperature for 1 h. Protein expression was visualized using
Luminata forte HRP detection reagent (Merck KGaA). Protein bands
were quantified by densitometry using a Bio-Rad image analysis
system (Quantity One; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and normalized to β-actin protein expression.
Statistical analysis
The results are expressed as the mean ± SEM.
Relaxation responses to ACh or SNP were expressed as a percentage
of PE induced precontraction. Concentration-response to agonists
were fitted to a sigmoidal curve using GraphPad Prism, version 5
(GraphPad Software Inc., San Diego, CA, USA) to calculate the
sensitivity of each agonist (pEC50). Maximum relaxation
(Rmax) to ACh or SNP was calculated as a percentage of
precontraction to PE. The pEC50 and Rmax
values were compared among groups using one-way analysis of
variance (ANOVA) with post hoc multiple comparisons using
Newman-Keuls or Dunnett's test (GraphPad Software Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect on body weight and metabolic
parameters
As shown in Table I,
there was no difference in body weight gain among the three
experimental groups by the end of the experiment. The rats that had
consumed fructose in drinking water for 12 weeks showed
significantly increased levels of blood glucose, TC, TG and LDL-C,
compared to control rats. Naringin (100 mg/kg/day) treatment for 4
weeks significantly attenuated fructose-induced metabolic changes
in rats (Table I). These results
indicate that naringin improved metabolic abnormalities in fructose
fed rats including hyperglycemia and hyperlipidemia.
 | Table I.Effect of oral administration (4
weeks) of naringin (100 mg/kg/day) on body weight and blood
parameters in fructose fed rats. |
Table I.
Effect of oral administration (4
weeks) of naringin (100 mg/kg/day) on body weight and blood
parameters in fructose fed rats.
| Parameters | C | F | FN |
|---|
| Initial body weight
(g) | 256±3 | 250±3 | 251±2 |
| Final body weight
(g) | 487±7 | 535±6 | 483±6 |
| Blood glucose
(mg/dl) | 99±9 | 149±13 | 114±4 |
| TC (mg/dl) | 60±7a | 78±5 | 63±3a |
| TG (mg/dl) | 61±7a | 82±5 | 52±4a |
| HDL-C (mg/dl) | 30±4 | 26±2 | 27±1 |
| LDL-C (mg/dl) | 24±5a | 37±2 | 26±3a |
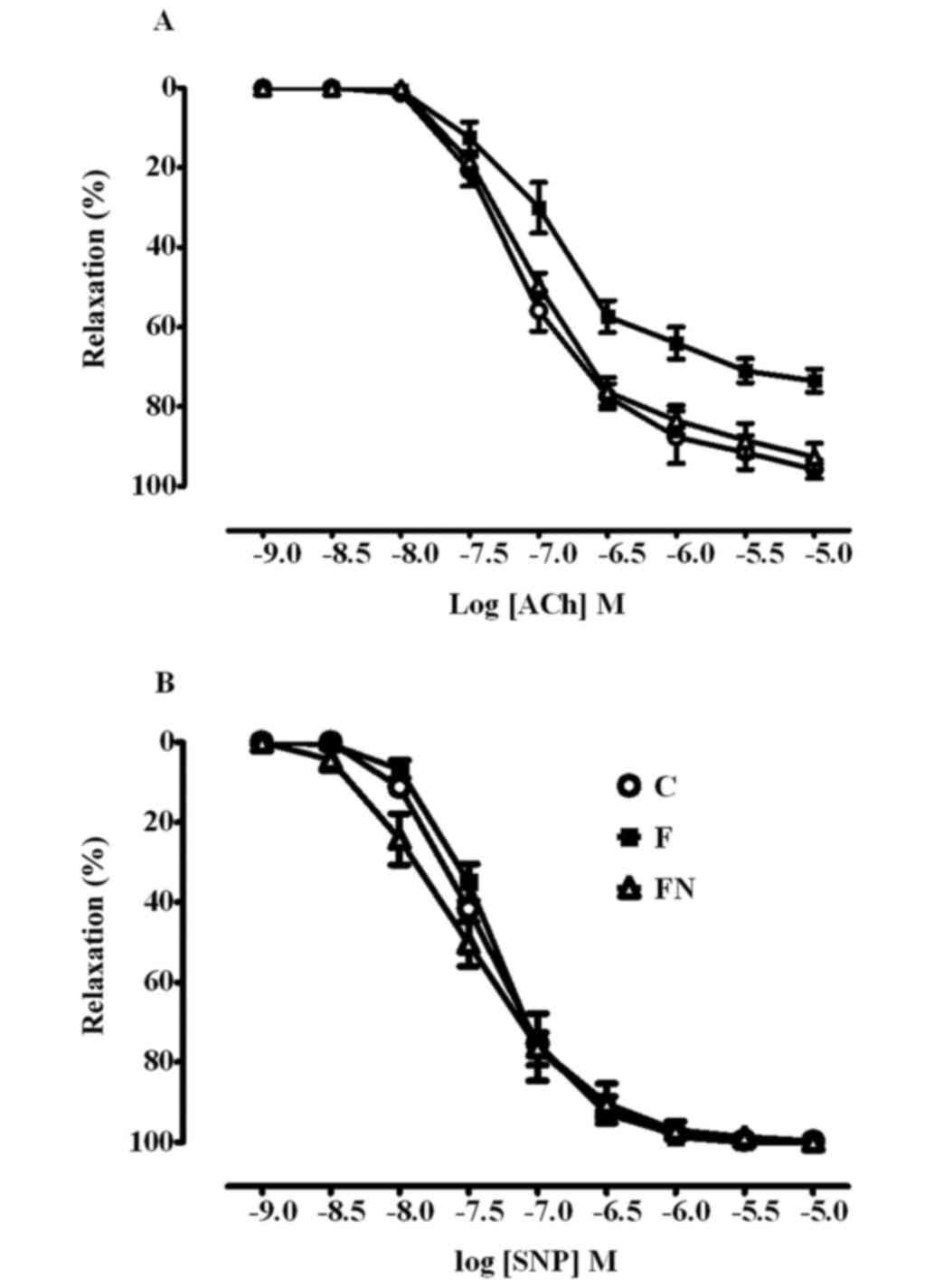
Effect on endothelial function
Endothelium-dependent and independent relaxation to
ACh and SNP respectively, are shown in Fig. 1 and Table
II. Fructose feeding significantly decreased the maximum
relaxation but not the sensitivity to ACh in aortae compared to the
control rats, indicating that fructose impaires endothelial
function. Relaxation responses to SNP were not significantly
different between the control and fructose fed rats, indicating
that vascular smooth muscle function was unaffected by fructose
treatment. The 4-week treatment of the high fructose fed rats with
naringin significantly restored ACh induced vasorelaxaton to the
levels observed in the control rats but had no effect on
SNP-induced relaxation.
 | Table II.Comparison of the sensitivity
(pEC50) and maximum response (Rmax) to ACh
and SNP in aortic rings from control, fructose-fed rats, and
fructose fed rats with naringin treatment (100 mg/kg/day). |
Table II.
Comparison of the sensitivity
(pEC50) and maximum response (Rmax) to ACh
and SNP in aortic rings from control, fructose-fed rats, and
fructose fed rats with naringin treatment (100 mg/kg/day).
|
| ACh | SNP |
|---|
|
|
|
|
|---|
| Group |
pEC50 |
Rmax |
pEC50 |
Rmax |
|---|
| C | 7.17±0.09 | 94±3 | 7.42±0.13 | 100±0.1 |
| F | 6.91±0.08 | 73±4a | 7.23±0.07 | 100±0.1 |
| FN | 7.05±0.51 | 95±3b | 7.76±0.03 | 100±0.2 |
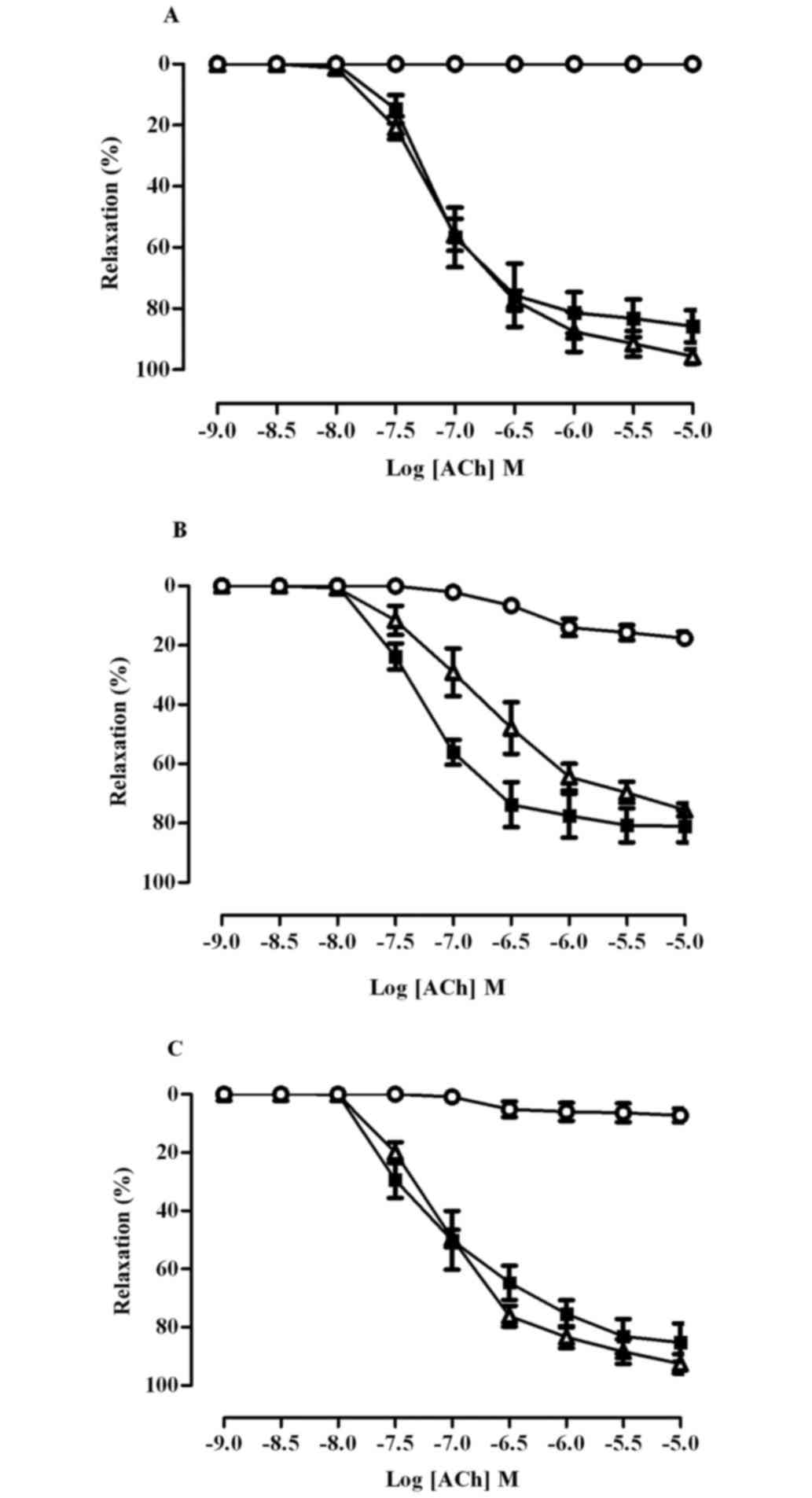
As shown in Fig. 2,
pre-incubation of aortic rings with indomethacin did not affect the
vasodilator response to ACh in any group (Rmax control
86±5; fructose 81±5; fructose+naringin 85±6%), indicating that
cyclo-oxygenase products, including prostacyclin, did not
contribute to endothelium dependent relaxation. In the presence of
L-NNA, ACh induced relaxation was completely abolished in aortic
rings from the control rats, but was only partially inhibited in
aortae from the fructose fed rats (Rmax control 0;
fructose 18±2%, P<0.01). In the FN groups, the relaxant response
to ACh was almost completely inhibited by L-NNA
(Rmaxfructose+naringin 7±2; fructose 18±2%, P<0.01),
indicating that endothelium-derived NO plays an important role in
the vascular effect of naringin. The combination of L-NNA plus
indomethacin totally abolished the response to ACh in aortic rings
of all rats.
Effect on NO levels
Serum NOx levels were measured to estimate the
nitric oxide bioactivity. After 8 weeks, the serum NOx levels of
the fructose fed rats were significantly lower than that of the
control rats (control 0.26±0.03; fructose 0.13±0.02 µM/ml,
P<0.01). However, naringin treatment of the fructose fed rats
significantly increased NOx levels compared to the fructose fed
group (Group 2, the F group), (fructose+naringin 0.21±0.01;
fructose 0.13±.0.02 µM/ml, P<0.01).
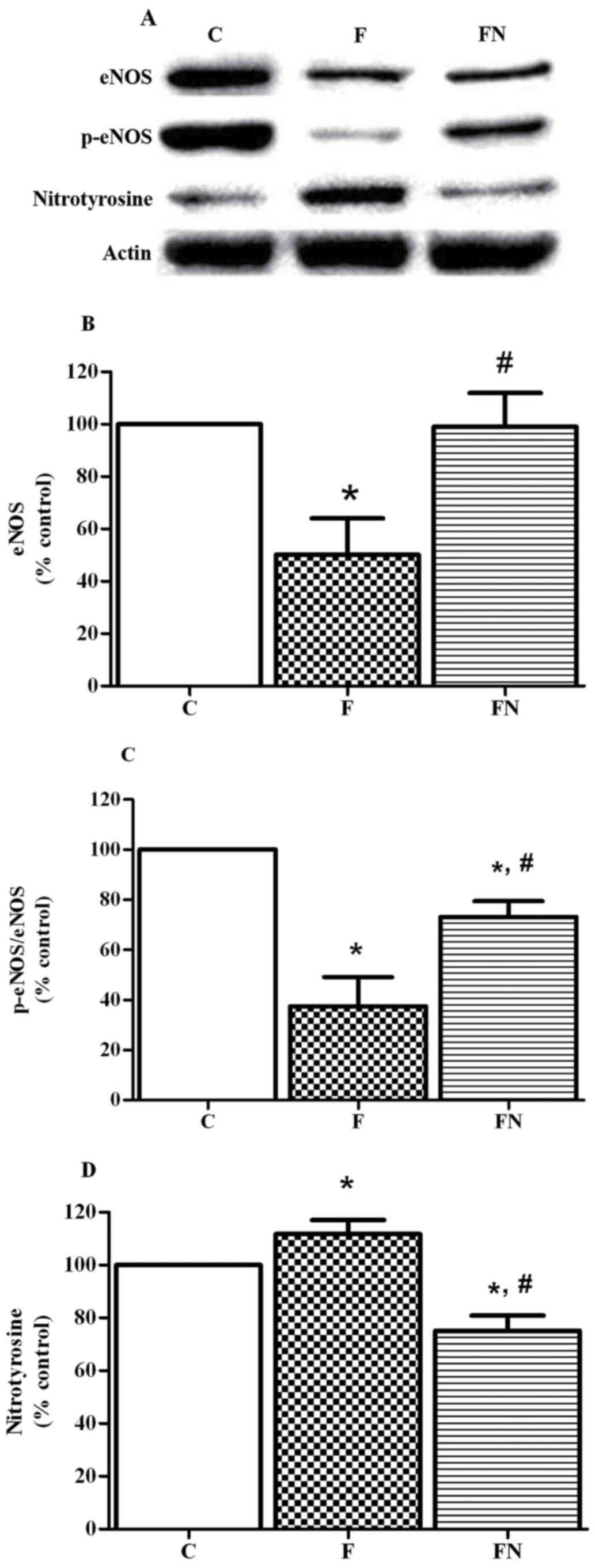
Effect of eNOS, p-eNOS, and
nitrotyrosine expression in aortic rings
The fructose fed rats (the F group) exhibited a
significant decrease in protein expressions of eNOS and
phosphorylation of eNOS at Ser1177 in the aortic tissues compared
to the control rats. Treatment of the fructose fed rats with
naringin (the FN group) significantly increased aortic eNOS and
phosphorylation of eNOS expression. In addition, fructose feeding
significantly increased the expression of nitrotyrosine in aortic
tissues, but this was reversed by treatment with naringin (Fig. 3).
Discussion
The present study demonstrated that treatment of
fructose fed rats with naringin (100 mg/kg/d) for 4 weeks improved
the impaired endothelial dependent relaxation in aortic rings and
increased serum NO level. These effects of naringin may be
associated with enhanced aortic eNOS and p-eNOS expression, and
reduced expression of nitrotyrosine in aortic tissues of fructose
fed rats.
The model of fructose-drinking rats has been widely
reported to develop metabolic abnormalities, including
hypertriglyceridemia, hyperglycemia, hyperinsulinemia and obesity,
as well as endothelial dysfunction (1,11,12,18).
These abnormalities are closely associated with the development of
CVD (3–5,9).
However, the concentration of fructose used in our experiment and
in most animal studies was higher than that consumed by human,
thereby increasing significant metabolic changes (1,28–30).
This study demonstrated that rats given 10% fructose in drinking
water for 12 weeks, exhibited increased blood glucose and developed
dyslipidemia, as indicated by the elevated levels of serum TC,
LDL-C and TG. Oral administration of naringin to rats for 4 weeks
ameliorated high fructose-induced hyperglycemia and
hyperlipidemia.
There is evidence to support the beneficial effect
of naringin on these metabolic alterations in animals. Previous
studies have shown that naringin reduced blood glucose and lipid
levels in several animal models of diabetes and diet-induced
metabolic syndromes (19,20,22,31). In
type 2 diabetic db/db mice, naringin lowered
hyperlipidemia and hyperglycemia through regulating the lipid
metabolism and affecting the gene expression of glucose-regulating
enzymes (32). In mice fed high fat
diet, naringin could activate AMPK-mediated MAPKs signaling
pathway, resulting in the reduction of insulin resistance,
hyperglycemia, and hyperlipidemia (22).
Both dyslipidemia and hyperglycemia have been widely
reported to be a leading cause of vascular endothelial dysfunction,
which is an early marker of atherosclerosis and cardiovascular
disease (33,34). Endothelial dysfunction is generally
characterized by a decrease in endothelial dependent relaxation.
The vascular endothelium plays a vital role in the regulation of
basal vascular tone through the synthesis and release of several
vasodilators including nitric oxide (NO), prostaglandin
I2 (PGI2) and endothelium-derived
hyperpolarizing factor (EDHF) (7).
It has been reported that endothelium-derived NO is a predominant
mediator of endothelial-dependent relaxation in aortae, and its
bioavailability is impaired in several pathophysiological states
such as hyperlipidemia, metabolic syndrome, diabetes and
hypertension (35,36). There is increasing evidence that high
fructose induced endothelial dysfunction is associated with the
decreased NO bioavailability in the vasculature (8,12).
This study demonstrated that the vasorelaxation
response to ACh but not SNP was decreased in aortae from fructose
fed rats, indicating that fructose feeding caused an impairment of
endothelium dependent relaxation in rat aortae. These findings are
consistent with previous reports (10,17),
which demonstrated that an impaired endothelium function was found
in aortic rings of fructose fed animals. The presence of NOS
inhibitor L-NNA partially inhibited ACh-induced relaxation in
aortic rings of fructose fed rats, indicating that fructose feeding
impaired the contribution of NO to endothelium-dependent relaxation
in aortic tissues. An impaired endothelium derived NO was also
confirmed by reduced serum nitrite/nitrate concentration in
fructose fed rats.
Previous studies have reported that naringin
treatment improved endothelial dysfunction in stroke-prone
spontaneously hypertensive rats (25) and high carbohydrate, high fat
diet-fed rats (21). In this study,
the treatment of fructose fed rats with naringin for 4 weeks
restored ACh-induced relaxation in aortae to levels similar to
those observed in control rats (Fig.
1 and Table II). The
possibility that naringin improved endothelium dependent relaxation
by increasing NO bioavailability was investigated. This study
demonstrated that the relaxation to ACh was totally abolished in
the presence of L-NNA but unaffected by indomethacin (Fig. 2). We also found that naringin
increased serum nitrite/nitrate levels in fructose fed rats. These
results indicate that the beneficial effects of naringin on
endothelial dysfunction in fructose fed rats are due to its ability
to restore endothelial derived NO.
Possible mechanisms for maintaining NO
bioavailability and improving endothelium dependent relaxation in
fructose fed rats by naringin treatment may be related to an
increase in NO production and/or decrease in NO degradation by ROS.
The production of NO is regulated by eNOS activity, which is
activated by phosphorylation at an activation site such as Ser1177,
Ser633 and Ser614 (37,38). There is evidence that phosphorylation
of eNOS at serine 1117 is a crucial target for intervention to
improve endothelial dysfunction (15). In addition, there is growing evidence
that an overproduction of ROS, which is generally generated by a
cellular disturbance in glucose or/and lipid metabolism leads to
the degradation of NO (39–41). Superoxide rapidly reacts with NO to
form the powerful oxidant peroxynitrite which causes the nitration
of proteins leading to the impairing of the function of cellular
proteins including eNOS protein (15). Therefore, in our study, nitrotyrosine
was detected to indirectly indicate ROS mediated NO inactivation
and peroxynitrite formation. It has been reported that fructose-fed
rats exhibited a decreased expression of eNOS and p-eNOS in several
tissues including aorta (8,42,43). In
addition fructose has been demonstrated to generate peroxynitrite
through increased superoxide production and enhanced methyglyoxal
formation (16).
Consistent with the impairment of endothelium
dependent relaxation and the decrease in NO levels, it was found
that the reduction of expression of eNOS and p-eNOS, and the
elevation of nitrotyrosine expression in fructose fed rats.
Previous studies demonstrated that naringin elevated expression of
p-eNOS (Ser1177) and reduced expression of nitrotyrosine in
myocardial ischemia reperfusion injury (44). In this study, it was found that oral
administration of naringin to fructose fed rats for 4 weeks
increased the expression of eNOS and p-eNOS (Ser1177), and
decreased the expression of nitrotyrosine in aortic tissues
(Fig. 3). These results suggest that
naringin treatment increases NO bioavailability through enhanced
eNOS activity and attenuated NO inactivation to nitrotyrosine,
resulting in the improved endothelial dysfunction in fructose fed
rats. However, the mechanisms of increased eNOS and p-eNOS
expression in naringin and fructose-fed rats were still unclear and
further study is needed to clarify the mechanisms underlying the
upregulated eNOS and p-eNOS protein expression.
In conclusion, the present study suggests that
treatment with naringin improves endothelial function in the aortic
rings of fructose fed rats. The vascular effect of naringin may be,
in part, due to improving NO bioavailability, increasing eNOS
activity, and preventing the generation of peroxynitrite.
Acknowledgements
This study was supported by grants from the National
Research Council of Thailand. The authors would like to thank Mr.
Roy Morien of the naresuan university language centre for his
editing assistance and advice on english expression in this
document. The authors declare no conflict of interest.
Glossary
Abbreviations
Abbreviations:
|
TC
|
total cholesterol
|
|
TG
|
triglyceride
|
|
HDL-C
|
high density lipoprotein
cholesterol
|
|
LDL-C
|
low density lipoprotein
cholesterol
|
|
NO
|
nitric oxide
|
|
eNOS
|
endothelial nitric oxide synthase
|
|
ACh
|
acethycholine
|
|
SNP
|
sodium nitroprusside
|
|
PE
|
phenylephrine
|
|
NOx
|
nitrate/nitrite
|
References
|
1
|
Tappy L, Lê KA, Tran C and Paquot N:
Fructose and metabolic diseases: New findings, new questions.
Nutrition. 26:1044–1049. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson RJ, Segal MS, Sautin Y, Nakagawa
T, Feig DI, Kang DH, Gersch MS, Benner S and Sánchez-Lozada LG:
Potential role of sugar (fructose) in the epidemic of hypertension,
obesity and the metabolic syndrome, diabetes, kidney disease, and
cardiovascular disease. Am J Clin Nutr. 86:899–906. 2007.PubMed/NCBI
|
|
3
|
Nelson RH: Hyperlipidemia as a risk factor
for cardiovascular disease. Prim Care. 40:195–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lorber D: Importance of cardiovascular
disease risk management in patients with type 2 diabetes mellitus.
Diabetes Metab Syndr Obes. 7:169–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Favero G, Paganelli C, Buffoli B, Rodella
LF and Rezzani R: Endothelium and its alterations in cardiovascular
diseases: Life style intervention. Biomed Res Int. 2014:8018962014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fadel PJ: Nitric oxide and cardiovascular
regulation: Beyond the endothelium. Hypertension. 69:778–779. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vanhoutte PM, Shimokawa H, Feletou M and
Tang EH: Endothelial dysfunction and vascular disease-a 30th
anniversary update. Acta Physiol (Oxf). 219:22–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pektaş MB, Sadi G and Akar F: Long-term
dietary fructose causes gender-different metabolic and vascular
dysfunction in rats: Modulatory effects of resveratrol. Cell
Physiol Biochem. 37:1407–1420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia G, Aroor AR, Whaley-Connell AT and
Sowers JR: Fructose and uric acid: Is there a role in endothelial
function? Curr Hypertens Rep. 16:4342014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kho MC, Lee YJ, Cha JD, Choi KM, Kang DG
and Lee HS: Gastrodia elata ameliorates high-fructose diet-induced
lipid metabolism and endothelial dysfunction. Evid Based Complement
Alternat Med. 2014:1016242014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tran LT, Yuen VG and McNeill JH: The
fructose-fed rat: A review on the mechanisms of fructose-induced
insulin resistance and hypertension. Mol Cell Biochem. 332:145–159.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El-Bassossy H, Badawy D, Neamatallah T and
Fahmy A: Ferulic acid, a natural polyphenol, alleviates insulin
resistance and hypertension in fructose fed rats: Effect on
endothelial-dependent relaxation. Chem Biol Interact. 254:191–197.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li T, Lu X, Sun Y and Yang X: Effects of
spinach nitrate on insulin resistance, endothelial dysfunction
markers and inflammation in mice with high-fat and high-fructose
consumption. Food Nutr Res. 60:320102016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nade VS, Kawale LA and Patel KM:
Protective effect of sitagliptin and rosuvastatin combination on
vascular endothelial dysfunction in type-2 diabetes. Indian J Pharm
Sci. 77:96–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Vanhoutte PM and Leung SW:
Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci. 129:83–94.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Meng QH, Chang T and Wu L:
Fructose-induced peroxynitrite production is mediated by
methylglyoxal in vascular smooth muscle cells. Life Sci.
79:2448–2454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Babacanoglu C, Yildirim N, Sadi G, Pektas
MB and Akar F: Resveratrol prevents high-fructose corn
syrup-induced vascular insulin resistance and dysfunction in rats.
Food Chem Toxicol. 60:160–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Litterio MC, Vazquez Prieto MA, Adamo AM,
Elesgaray R, Oteiza PI, Galleano M and Fraga CG: (−)-Epicatechin
reduces blood pressure increase in high-fructose-fed rats: Effects
on the determinants of nitric oxide bioavailability. J Nutr
Biochem. 26:745–751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chanet A, Milenkovic D, Deval C, Potier M,
Constans J, Mazur A, Bennetau-Pelissero C, Morand C and Bérard AM:
Naringin, the major grapefruit flavonoid, specifically affects
atherosclerosis development in diet-induced hypercholesterolemia in
mice. J Nutr Biochem. 23:469–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adebiyi OA, Adebiyi OO and Owira PM:
Naringin reduces hyperglycemia-induced cardiac fibrosis by
relieving oxidative stress. PLoS One. 11:e01498902016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alam MA, Kauter K and Brown L: Naringin
improves diet-induced cardiovascular dysfunction and obesity in
high carbohydrate, high fat diet-fed rats. Nutrients. 5:637–650.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pu P, Gao DM, Mohamed S, Chen J, Zhang J,
Zhou XY, Zhou NJ, Xie J and Jiang H: Naringin ameliorates metabolic
syndrome by activating AMP-activated protein kinase in mice fed a
high-fat diet. Arch Biochem Biophys. 518:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma AK, Bharti S, Ojha S, Bhatia J,
Kumar N, Ray R, Kumari S and Arya DS: Up-regulation of PPARγ, heat
shock protein-27 and −72 by naringin attenuates insulin resistance,
β-cell dysfunction, hepatic steatosis and kidney damage in a rat
model of type 2 diabetes. Br J Nutr. 106:1713–1723. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Wang C, Peng J, Liang J, Jin Y, Liu
Q, Meng Q, Liu K and Sun H: Naringin inhibits TNF-α induced
oxidative stress and inflammatory response in HUVECs via Nox4/NF-κ
B and PI3K/Akt pathways. Curr Pharm Biotechnol. 15:1173–1182. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ikemura M, Sasaki Y, Giddings JC and
Yamamoto J: Preventive effects of hesperidin, glucosyl hesperidin
and naringin on hypertension and cerebral thrombosis in
stroke-prone spontaneously hypertensive rats. Phytother Res.
26:1272–1277. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Friedewald WT, Levy RI and Fredrickson DS:
Estimation of the concentration of low-density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 18:499–502. 1972.PubMed/NCBI
|
|
27
|
Ali SF, Nguyen JC, Jenkins TA and Woodman
OL: Tocotrienol-rich tocomin attenuates oxidative stress and
improves endothelium-dependent relaxation in aortae from rats fed a
high-fat western diet. Front Cardiovasc Med. 3:392016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ventura EE, Davis JN and Goran MI: Sugar
content of popular sweetened beverages based on objective
laboratory analysis: Focus on fructose content. Obesity (Silver
Spring). 19:868–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toop CR and Gentili S: Fructose beverage
consumption induces a metabolic syndrome phenotype in the rat: A
systematic review and meta-analysis. Nutrients. 8:pii: E5772016.
View Article : Google Scholar
|
|
30
|
Zhang DM, Jiao RQ and Kong LD: High
dietary fructose: Direct or indirect dangerous factors disturbing
tissue and organ functions. Nutrients. 9:pii: E3352017. View Article : Google Scholar
|
|
31
|
Mahmoud AM, Ashour MB, Abdel-Moneim A and
Ahmed OM: Hesperidin and naringin attenuate hyperglycemia-mediated
oxidative stress and proinflammatory cytokine production in high
fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes
Complications. 26:483–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jung UJ, Lee MK, Park YB, Kang MA and Choi
MS: Effect of citrus flavonoids on lipid metabolism and
glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int
J Biochem Cell Biol. 38:1134–1145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tabit CE, Chung WB, Hamburg NM and Vita
JA: Endothelial dysfunction in diabetes mellitus: Molecular
mechanisms and clinical implications. Rev Endocr Metab Disord.
11:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Q, Qin L, Dai S, Zhang H, Pasula S,
Zhou H, Chen H and Min W: AIP1 suppresses atherosclerosis by
limiting hyperlipidemia-induced inflammation and vascular
endothelial dysfunction. Arterioscler Thromb Vasc Biol. 33:795–804.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Widmer RJ and Lerman A: Endothelial
dysfunction and cardiovascular disease. Glob Cardiol Sci Pract.
2014:291–308. 2014.PubMed/NCBI
|
|
36
|
Wong WT, Wong SL, Tian XY and Huang Y:
Endothelial dysfunction: The common consequence in diabetes and
hypertension. J Cardiovasc Pharmacol. 55:300–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu L, Liu Y, Qiu Z, Liu S, Gao X and Zhu
D: Cellular mechanisms and intracellular signaling pathways for the
modulation of eNOS in pulmonary arteries by 15-HETE. J Recept
Signal Transduct Res. 32:87–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li S, Li Q, Lv X, Liao L, Yang W, Li S, Lu
P and Zhu D: Aurantio-obtusin relaxes systemic arteries through
endothelial PI3K/AKT/eNOS-dependent signaling pathway in rats. J
Pharmacol Sci. 128:108–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Steven S, Daiber A, Dopheide JF, Münzel T
and Espinola-Klein C: Peripheral artery disease, redox signaling,
oxidative stress-Basic and clinical aspects. Redox Biol.
12:787–797. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lau YS, Tian XY, Huang Y, Murugan D,
Achike FI and Mustafa MR: Boldine protects endothelial function in
hyperglycemia-induced oxidative stress through an antioxidant
mechanism. Biochem Pharmacol. 85:367–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chtourou Y, Slima AB, Makni M, Gdoura R
and Fetoui H: Naringenin protects cardiac
hypercholesterolemia-induced oxidative stress and subsequent
necroptosis in rats. Pharmacol Rep. 67:1090–1097. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stanišić J, Korićanac G, Ćulafić T, Romić
S, Stojiljković M, Kostić M, Pantelić M and Tepavčević S: Low
intensity exercise prevents disturbances in rat cardiac insulin
signaling and endothelial nitric oxide synthase induced by high
fructose diet. Mol Cell Endocrinol. 420:97–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu X, Tu L, Wang L, Fang X and Wang DW:
CYP2J3 gene delivery reduces insulin resistance via upregulation of
eNOS in fructose-treated rats. Cardiovasc Diabetol. 10:1142011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rani N, Bharti S, Manchanda M, Nag TC, Ray
R, Chauhan SS, Kumari S and Arya DS: Regulation of heat shock
proteins 27 and 70, p-Akt/p-eNOS and MAPKs by Naringin Dampens
myocardial injury and dysfunction in vivo after
ischemia/reperfusion. PLoS One. 8:e825772013. View Article : Google Scholar : PubMed/NCBI
|