Introduction
Postoperative cognitive dysfunction often leads to
mental disorders, personality changes, memory loss, anxiety and
other mental symptoms, which is caused by central nervous system
damage due to surgery or anesthesia, seriously affecting the life
quality of patients (1,2). It has been reported that the
commonly-used anesthetics, such as isoflurane and ketamine, can
cause postoperative cognitive dysfunction. Studies have shown that
the application of isoflurane during surgery can lead to a
significant decline in cognitive and memory abilities of patients
(3,4). Li et al (5) found that the hippocampus cell apoptosis
is the reason for the decline in cognitive and memory abilities,
and the overexpression of inflammatory factors [p38 and
interleukin-1β (IL-1β)] is the leading cause of apoptosis.
Gastrodin is the extract of Gastrodia elata Bl., which has
liver-calming, wind-extinguishing and spasm-stopping effects,
mainly used for the treatment of headache, dizziness, epilepsy and
convulsion. Recent studies show that it also has the
intelligence-enhancing, brain-strengthening and senescence-delaying
effects (6–9). In this study, the protective effect of
gastrodin on apoptosis of hippocampus cells induced by desflurane
was investigated and its possible mechanism was studied to provide
a theoretical basis for the clinically reasonable and standardized
use of anesthetics.
Materials and methods
Instruments and materials
Desflurane (Shenzhen RWD Life Science Co., Ltd.,
Shenzhen China); dimethylsulfoxide (DMSO) (Sigma, St. Louis, MO,
USA); TRIzol kit; reverse transcription kit (both from Invitrogen,
Carlsbad, CA, USA); rabbit anti-rat p38, IL-1 and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) polyclonalantibodies; anti-rabbit
horseradish peroxidase-labeled rabbit secondary polyclonal antibody
(cat. nos. 8690, 12703, 2118 and 7074; both from Cell Signaling
Technology, Beverly, MA, USA); electrochemiluminescence (ECL)
liquid; developing powder (both from Invitrogen); skim milk powder
(Guangzhou Sijia Biotechnology Co., Ltd., Guangzhou, China);
polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA);
pipettor (Eppendorf, Hamburg, Germany); polymerase chain reaction
(PCR) instrument (ABI, Foster City, CA, USA); ultraviolet imaging
system (Biometra, Goettingen, Germany); electronic balance (BP121S;
Sartorious, Göttingen, Germany); −80°C refrigerator (Thermo Fisher
Scientific, Darmstadt Germany); instruments of novel object
recognition test and Morris water maze (Guangzhou Jiebeisi Animal
Instrument Co., Ltd., Guangzhou, China); low-temperature centrifuge
(Thermo Fisher Scientific); other related equipment and reagents
are described in relevant parts.
Experimental animals and grouping
Male Sprague-Dawley (SD) rats weighing 220–250 g
were purchased from Guangdong Animal Experimental Center
[experimental animal certificate no. SCXK (Guangdong) 2013–0015].
Animals adapted to the feeding environment for 1 week before the
experiment, following the circadian rhythms, in a quiet environment
at 25°C, and animals were fed with food and water freely. The above
36 SD rats were randomly divided into three groups: blank control
group (C group, n=12), desflurane anesthesia group (DF group, n=12)
and gastrodin treatment group (GT group, n=12). Rats in DF group
were treated with anesthesia using desflurane (1.2%) for 4 h for a
total of 7 days. Rats in GT group were treated with gavage using
gastrodin (0.5 g/kg) at 1 h before desflurane anesthesia, as well
as the same treatment as DF group. The study was approved by the
Ethics Committee of Hospital of Stomatology, Jilin University
(Jilin, China).
Novel object recognition test
Rats in each group were placed in the open box
before the test to adapt to the environment for 300 sec. After
that, each rat was disinfected using alcohol to remove the odor.
Then rats were placed in an open box with two identical black
objects for object recognition training. When the distance of
smelling object was <2 cm or they touched the object with the
limbs, it was defined as the recognition time. The time was
recorded for a total of 300 sec. The novel object recognition test
was performed at 24 h after the training: One of the black objects
was replaced with one white object, and the novel object
recognition time and the recognition index were recorded;
recognition index = novel object recognition time/total object
recognition time.
Morris water maze test
One platform was placed in a swimming pool with a
diameter of 120 cm and a depth of 50 cm, and the water in the pool
was kept 1 cm above the platform. Ink was added into the pool to
make the platform invisible. The pool was divided into four
quadrants, and one fixed point in each quadrant was determined as
the entry point, from which the animals were put into the pool to
let them swim freely and find the platform. When they stayed on the
platform for 20 sec, it was deemed as finding the platform. The
escape latency (T1) was the time from coming into the pool to
finding the platform, and the target quadrant exploration time (T2)
was the residence time of rats in the quadrant where the platform
was located. All rats were trained for 5 days, and all data were
recorded by the supporting image tracking and recording software of
the instrument. T1 and T2 were recorded accurately and the rats
were wiped dry after that. After the training, the platform was
removed and exploratory experiment was performed. T2 of each group
was recorded and analyzed statistically.
Detection of apoptosis in rat
hippocampus tissues via terminal
deoxynucleotidyltransferase-mediated dUTP nick end labelling
(TUNEL)
Hippocampus tissue sections (20 µm) of rats were
made according to immunohistochemical methods. After sealing,
proteinase K solution was dropped onto the section, and the section
was digested at 37°C for 10 min in the wet box for permeable
treatment. After that, the section was washed with
phosphate-buffered saline (PBS) 3 times, and soaked in 4%
paraformaldehyde for fixation for 5 min. Then 40 µl TdT and
DIG-d-UTP mixed solution was dropped onto the section, and the
section was placed in the wet box for labeling at 4°C for 2 h.
After that, the liquid was dried and the section was soaked in PBS
for 10 min. Then 40 µl blocking solution was dropped onto the
section, and the section was sealed at room temperature for 30 min.
The biotinylated antibody (1:100) was added and the section was
placed in the wet box, followed by incubation at 37°C for 40 min
and washing with PBS 3 times. Then the secondary antibody (1:100)
was applied and the section was placed in the wet box for
incubation at 37°C for 40 min, followed by washing with PBS 3
times. The anti-fluorescence quenching sealing fluid was used,
followed by observation and photography under a fluorescence
microscope (Olympus, Tokyo, Japan). Those with yellow-green
fluorescence were the positive cells, namely the apoptotic
cells.
Detection of mRNA expression levels
via semi-quantitative PCR
The total RNA in rat hippocampus sample in each
group was extracted, and the reverse transcription was performed
according to the instructions of PrimeScript RT reagent kit to
obtain the cDNA, and the expression levels of p38 and IL-1β were
detected via semi-quantitative PCR using GAPDH as the internal
reference. Primer sequence is shown in Table I, and the primers were synthesized by
Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China). Two
microliters cDNA sample and RT-PCR reaction solutions were added to
prepare the 25 µl reaction system. The mixture was shaken and mixed
evenly, centrifuged at 100 × g for 30 sec and placed in the RT-PCR
instrument for amplification. The reaction conditions were as
follows: pre-degeneration at 95°C for 5 min, 95°C for 30 sec, 64°C
for 25 sec, 72°C for 30 sec, a total of 35 cycles, and then
extension at 72°C for 7 min. After the reaction, 2% agarose gel
electrophoresis was performed to separate the band under 120 V
until the electrophoresis exceeded more than half of the gel. The
ultraviolet imaging system was used to photograph after
electrophoresis, and the results were analyzed and compared with
p38/GAPDH and IL-1β/GAPDH as the indexes.
 | Table I.PCR primers. |
Table I.
PCR primers.
| Gene | Primer sequences |
|---|
| p38 | F:
5-GTACCACGATCCTGATGATG-3 |
|
| R:
5-CAGTAGTGGGATCAACAG-3 |
| IL-1β | F:
5-GACCTGAGCACCTTCTTTCC-3 |
|
| R:
5-CTGGAGGTGGAGAGCTTTCA-3 |
| GAPDH | F:
5-CTCCTCCACCTTTGACGCTG-3 |
Detection of the expression levels of
related proteins via western blotting
The hippocampus tissues of rats in each group were
taken and the total protein was extracted from the hippocampus
tissues using the protein extraction kit (Beyotime Biological
Medicine Technology Co., Ltd., Shanghai, China). Then the protein
was quantified using the BCA protein quantitative kit (Invitrogen).
Loading sample in the equal concentration was prepared according to
the protein content. Twelve percent separation gel and 5% spacer
gel were prepared and 20 µl sample was added into each well for
sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresed
under 80 V. After the sample was separated till the bottom of the
gel, the gel was electrophoresis. Besides, after the gel and PVDF
membrane were prepared into membrane transfer ‘sandwich’, the
protein was transferred onto the PVDF membrane for membrane
transfer under 100 V. Then 5% skim milk was prepared for sealing
for 2 h. After sealing, the target band was cut, and p38 and IL-1β
primary antibodies (1:1,000) were used for incubation overnight at
4°C, followed by comparative analysis with GAPDH as the internal
reference. After the membrane was washed three times with TBST (5
min/time), the secondary antibody (1:5,000) was incubated for 2 h
at room temperature; then after the membrane was washed three times
with TBST, appropriate amount of ECL fluid (solution A and B were
mixed at 1:1) was added onto each band in the dark. The plastic
wrap covered bubbles were removed and the black box was firmly
closed. According to the protein band fluorescence intensity, the
time of tableting was determined, followed by color development and
fixation in the dark, and then the band was scanned and the gray
value was analyzed using ImageJ software.
Statistical analysis
Data in this study are presented as mean ± standard
deviation. SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was
used for data processing, and the t-test for intergroup comparison,
and analysis of variance was used for comparison among groups.
Homogeneity test of variance was performed. If the variance is
homogeneous, Bonferroni method was used for pairwise comparison;
otherwise, Welch's method was used. Dunnett's T3 method was used
for multiple comparisons. P<0.05 indicated that the difference
was statistically significant.
Results
Effects of gastrodin and desflurane on
novel object recognition of rats
The effects of gastrodin and desflurane on the
cognitive ability of rats were studied via the novel object
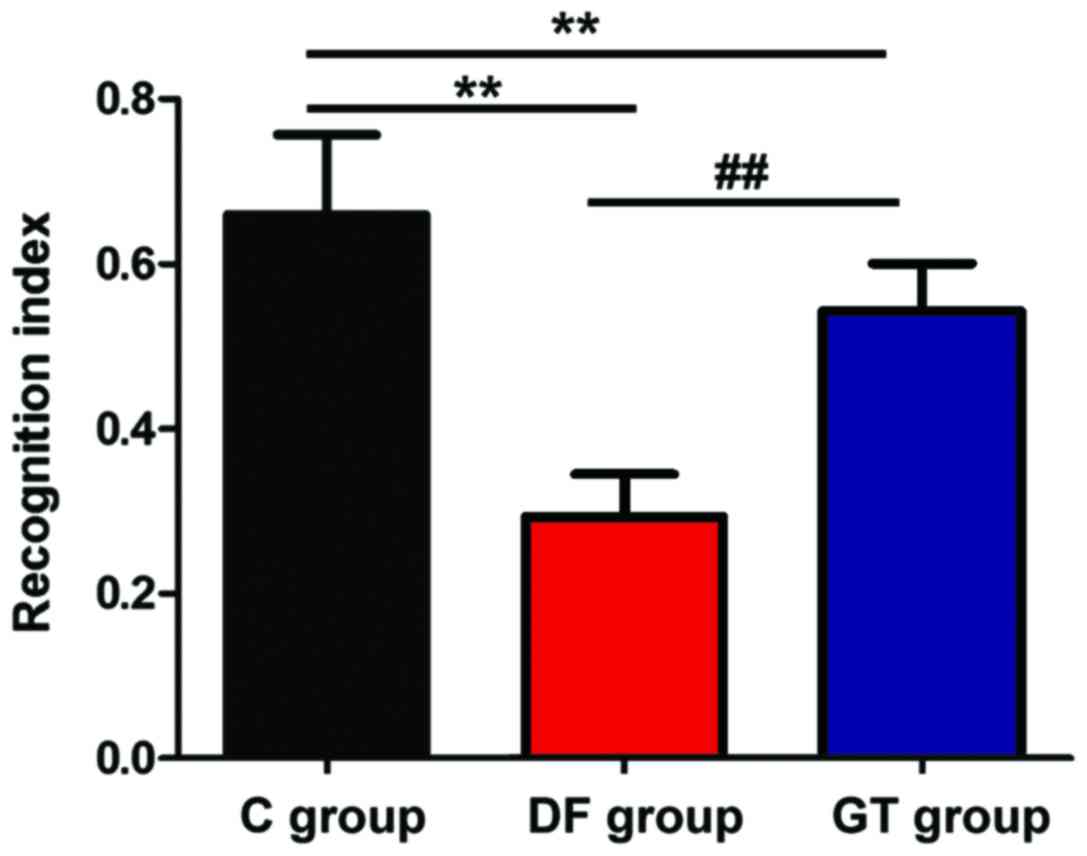
recognition test. The results are shown in Fig. 1. It can be seen from the recognition
index that compared with that in C group, the exploration
capacities of novel objects in DF group and GT group were
significantly decreased, and the differences were statistically
significant (P<0.01); the exploration capacity of novel objects
in GT group was higher than that in DF group (P<0.01).
Effects of gastrodin and desflurane on
spatial learning-memory ability of rats
The spatial learning-memory ability of rats was
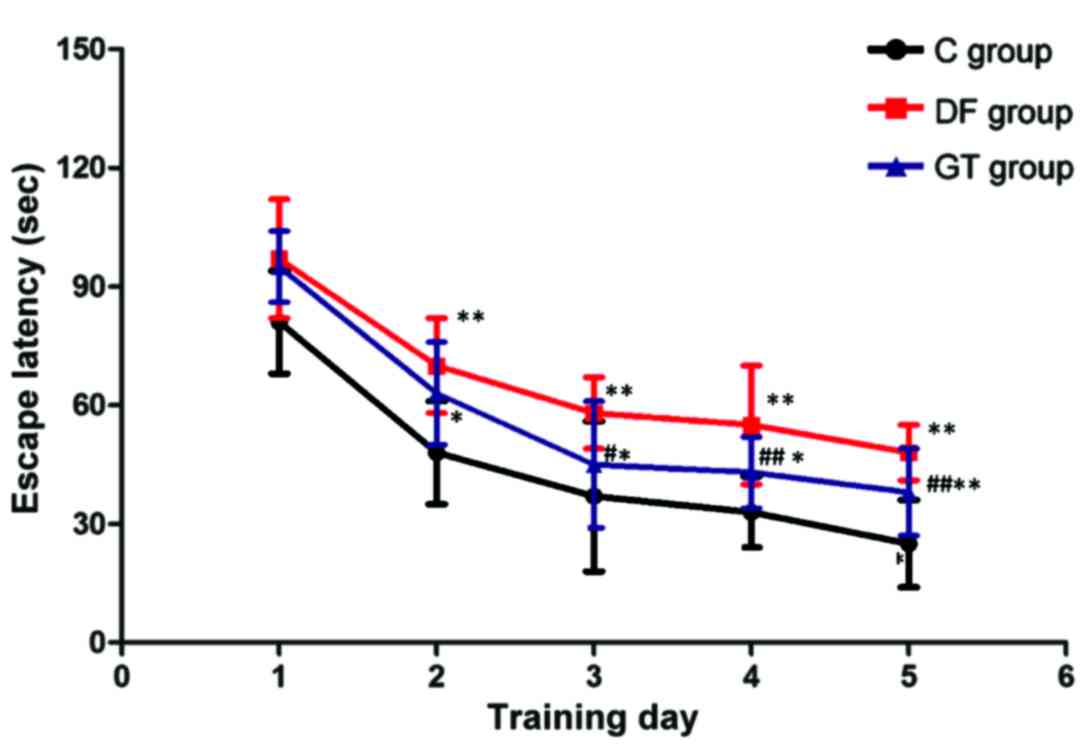
studied via water maze test. The results are shown in Fig. 2. During the 5-day training, T1 in DF
group and GT group from the 2nd day to the 5th day of training was
significantly longer than that in C group (P<0.05 and
P<0.01). T1 in GT group was significantly shorter than that in
DF group from the 3rd day to the 5th day of training, and the
difference was statistically significant (P<0.01). After the
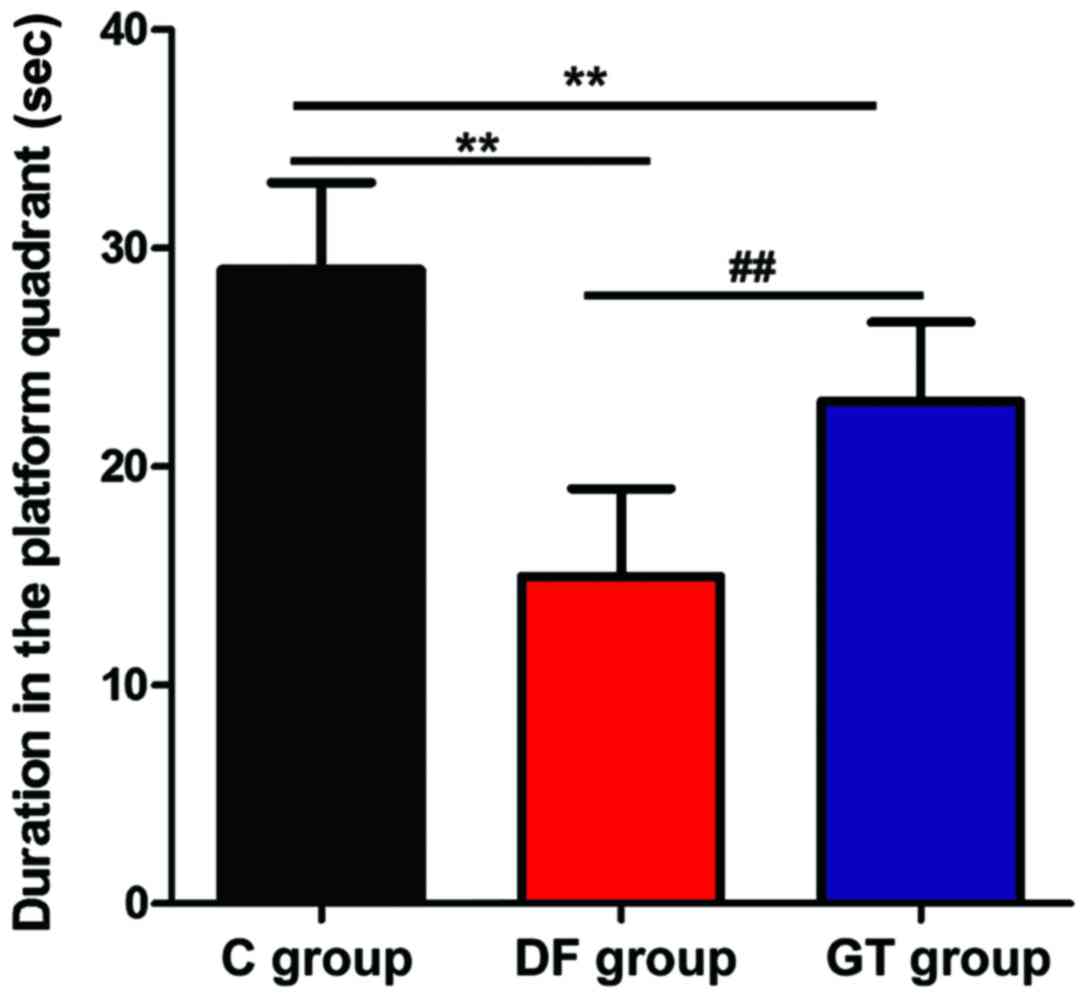
training, the exploratory experiment was performed and the results
are shown in Fig. 3. The residence
time in the target quadrant of rats in DF group and GT group was
significantly shorter than that in C group (P<0.01), and the
residence time in the target quadrant of rats in DF group was
shorter than that in GT group, and the differences were
statistically significant (P<0.01).
Effects of gastrodin and desflurane on
hippocampus cell apoptosis of rats
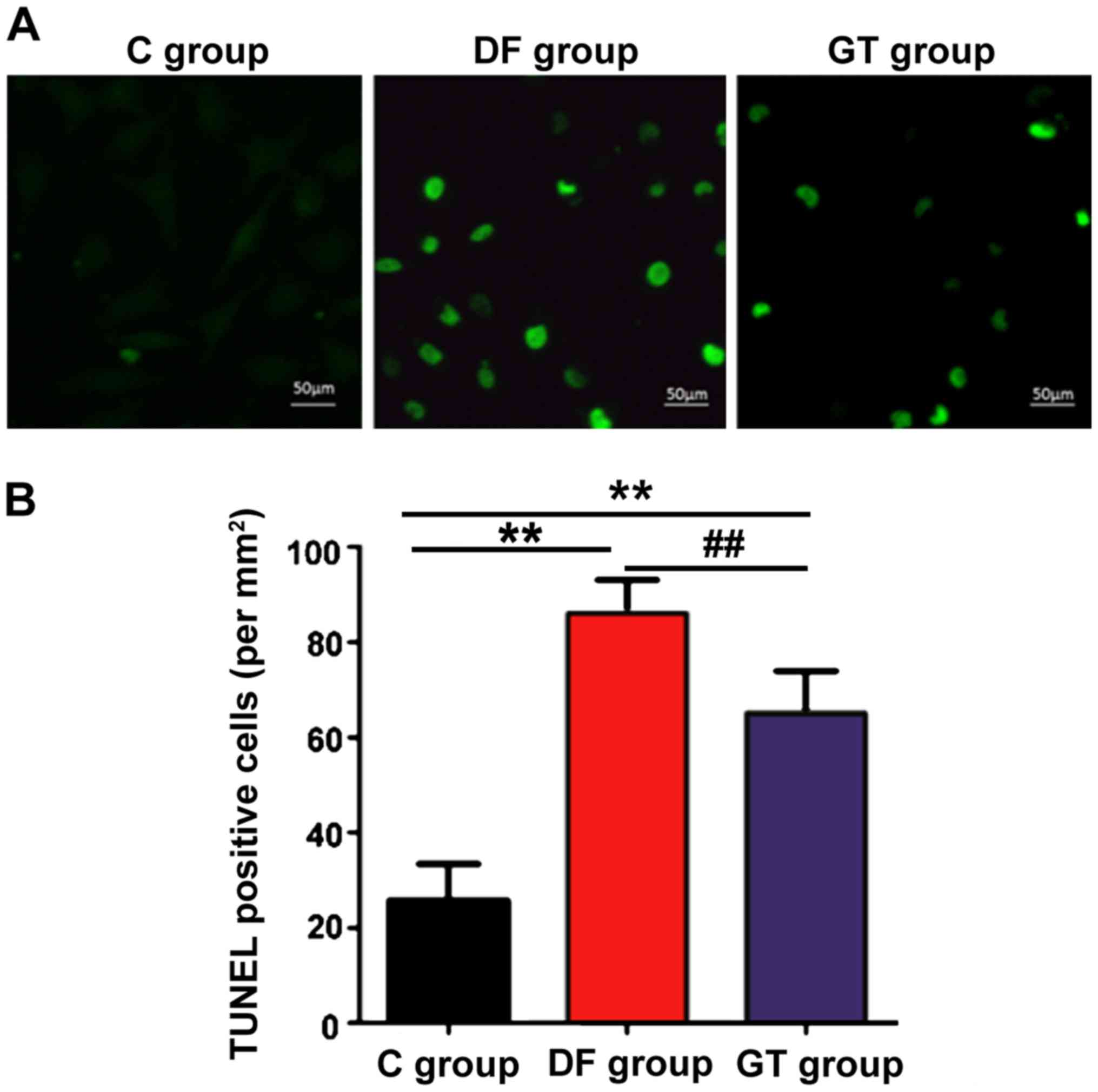
The cell apoptosis in rat hippocampus tissues in
each group was detected using the TUNEL kit, the number of positive
cells was recorded and the apoptotic index was calculated. The
results are shown in Fig. 4.
Compared with that in C group, the hippocampus cell apoptosis in DF
group and GT group was increased significantly, and the differences
were statistically significant (P<0.01); the number of apoptotic
cells in DF group was more than that in GT group (P<0.01).
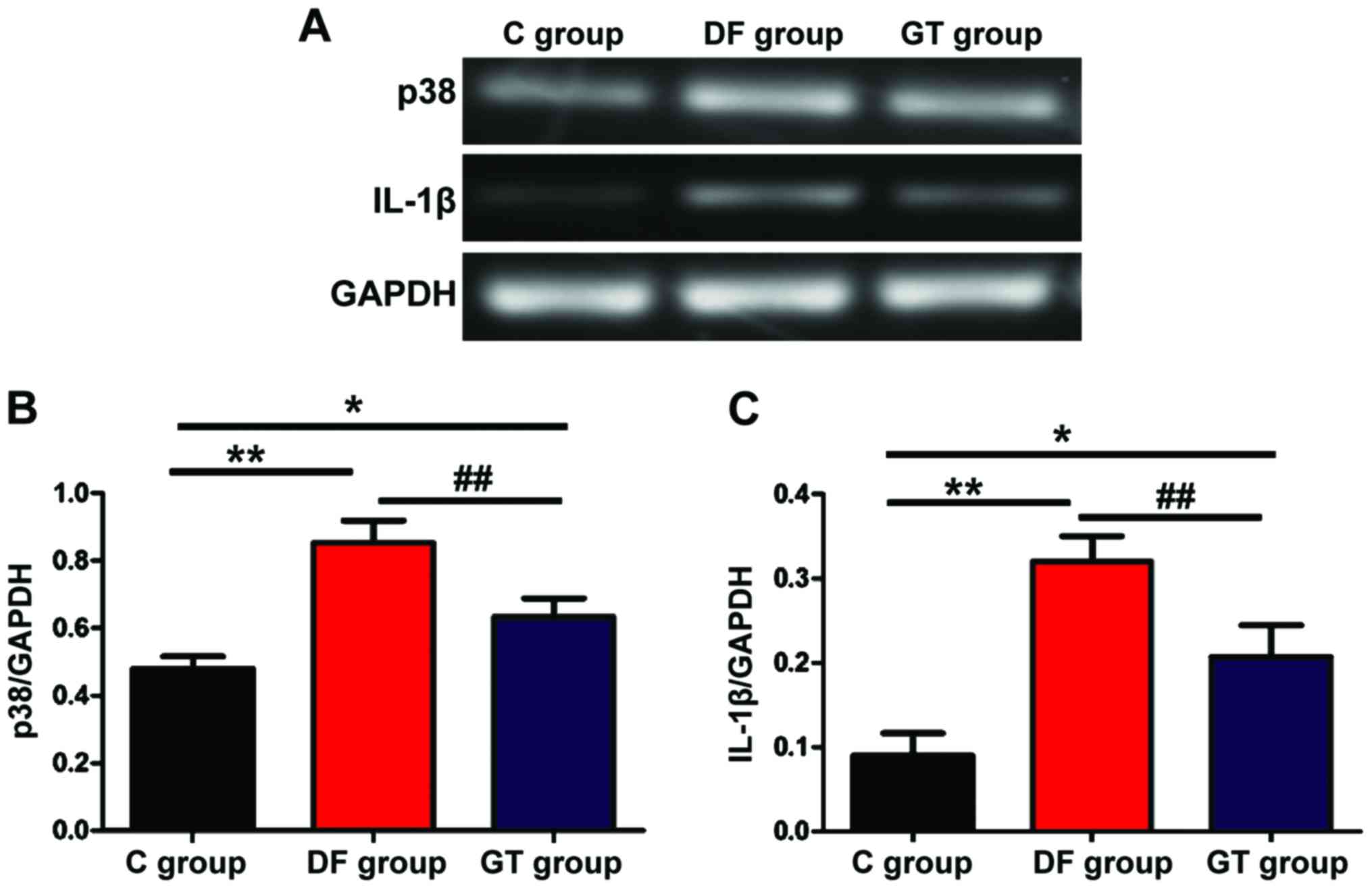
Detection of mRNA expression via
semi-quantitative PCR
The mRNA expression of p38 and IL-1β was detected
via semi-quantitative PCR. The results are showed in Fig. 5. The mRNA expression levels of p38
and IL-1β in hippocampus tissues in DF group and GT group were
significantly increased compared with those in C group, and the
differences were statistically significant (P<0.05 and
P<0.01). Compared with those in DF group, the mRNA expression
levels of p38 and IL-1β in hippocampus tissues in GT group were
decreased (P<0.01).
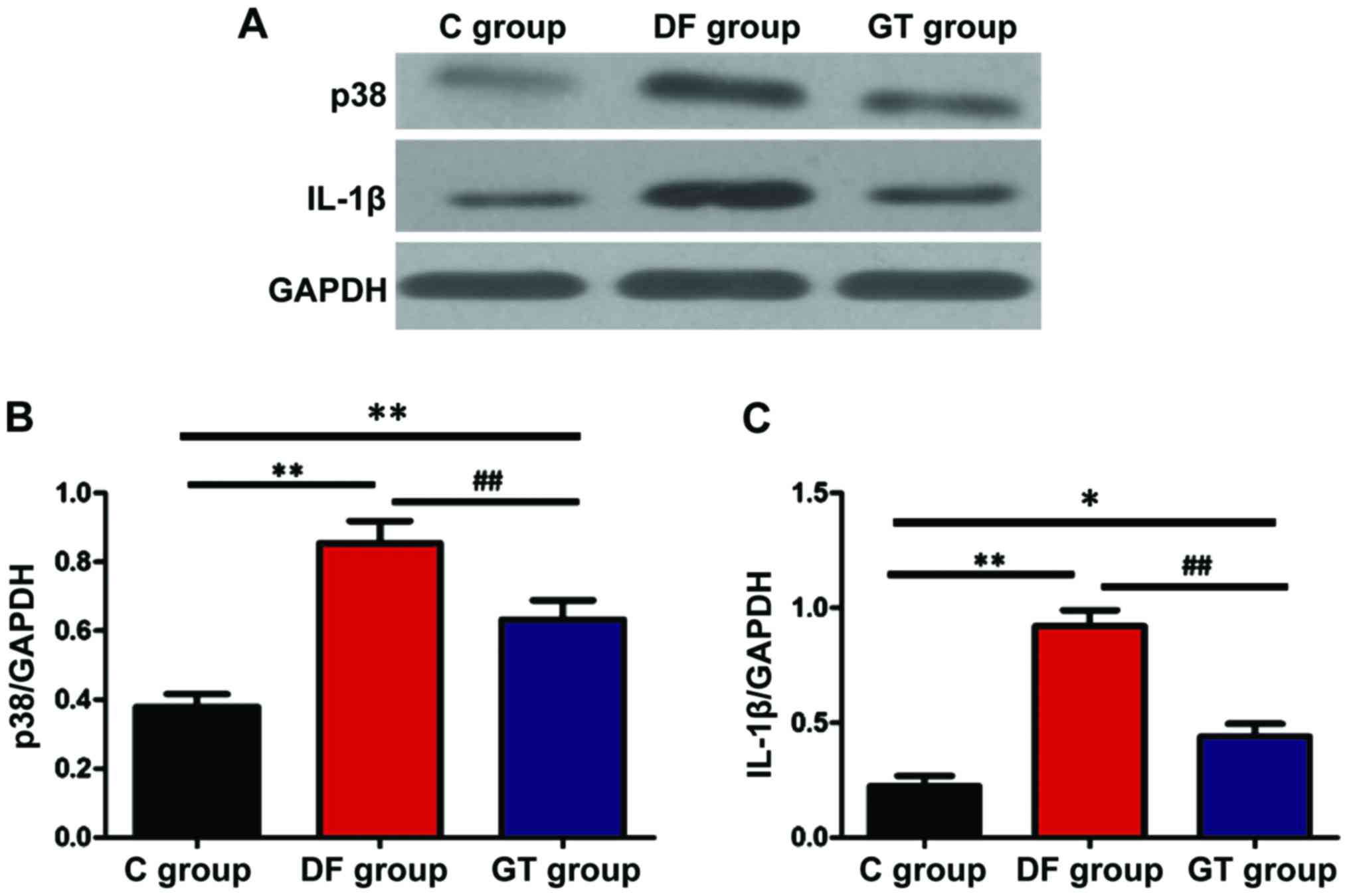
Detection of protein expression via
western blotting
The protein expression of p38 and IL-1β was detected
via western blotting and the results are shown in Fig. 6. The protein expression levels of p38
and IL-1β in hippocampus tissues in DF group and GT group were
significantly increased compared with that in C group (P<0.05
and P<0.01). Compared with DF group, the protein expression
levels of p38 and IL-1β in hippocampus tissues in GT group were
decreased (P<0.01).
Discussion
Postoperative cognitive dysfunction is the result of
a variety of factors, and hippocampus cell injury is considered to
be the main factor of causing the decreased cognitive and memory
ability (10). The activation of a
large number of inflammatory factors in hippocampus activates the
relevant signal pathways to damage the hippocampus tissues, thereby
affecting the learning and memory ability (11). Ramírez-Jirano et al (12) found that the learning and memory
abilities of rats can be significantly damaged at 30 days after
anesthesia with low-dose isoflurane.
In this study, rats received gastrodin 5 days before
the desflurane anesthesia to explore the protective effect of
gastrodin on neurological damage caused by desflurane. The novel
object recognition test found that after the desflurane anesthesia,
the exploration time of novel objects of rats was significantly
decreased, and the water maze test showed that after the desflurane
anesthesia, T1 was significantly increased, while T2 was
significantly decreased compared with those in C group. The above
results showed that the learning and memory ability of rats was
damaged; in other words, desflurane can damage the learning and
memory ability of rats. Before desflurane anesthesia, the
intraperitoneal injection of gastrodin could increase the time of
exploration, decrease T1 and increase T2. The above results showed
that gastrodin could effectively resist the damage of rats caused
by desflurane and protect the hippocampal nerve. TUNEL is the most
commonly used method of studying apoptosis, which can visually
label the apoptotic cells and calculate the apoptotic index to
measure the apoptosis of tissue or cells. Wu et al (13) found that isoflurane has a direct
toxic effect on the rat neurons and PC12 cells, then inducing the
apoptosis, and it is thought that the toxicity of isoflurane may be
realized through the destruction of endoplasmic reticulum calcium
homeostasis. In this study, desflurane anesthesia significantly
increased apoptosis of hippocampus cells, and early administration
of gastrodin effectively protected the hippocampus tissues and
reduced apoptosis. Mani et al (14) found that the p38 activity was
increased in brain tissues of AD patients, and the same is true in
rats with overexpression of amyloid protein, indicating that p38
and p38 MAPK signaling pathways are closely related to the nerve
injury. IL-1β is one of the earliest pro-inflammatory cytokines,
and the overexpression of IL-1β can induce the aggregation of
neutrophils and injury; the activation of IL-1β will further
activate p38 activity, and then activate p38 MAPK signaling pathway
(15,16). Studies have shown that isoflurane
anesthesia can significantly increase the IL-1β expression level in
hippocampus tissues, thus causing neural inflammation, and a series
of central nervous system injury symptoms (17–20). In
this study, the expression of p38 and IL-1β were detected via
semi-quantitative PCR and western blotting. It was found that
compared with those in C group, the mRNA and protein expression
levels of p38 and IL-1β in DF group and GT group were significantly
increased, and the expression levels of p38 and IL-1β in GT group
were lower than those in DF group. The above results suggest that
the hippocampus tissue apoptosis caused by desflurane may be
realized by increasing the level of IL-1β, thus activating the p38
and p38 MAPK pathways.
In conclusion, desflurane anesthesia can cause
hippocampus tissue apoptosis in rats, and its mechanism may be the
activation of IL-1β and p38, thus activating the p38 MAPK signaling
pathway. Gastrodin can reduce the levels of IL-1β and p38, and
effectively protect the hippocampus tissues of rats from
desflurane, thus playing a protective role in the learning, memory
and cognitive abilities of rats.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW drafted this manuscript. HW and ZD was mainly
devoted on collecting and interpreting the data. JZ and WZ revised
it critically for important intellectual content. LW, JZ and WZ
contributed to the conception and design of the study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Hospital of Stomatology, Jilin University (Jilin, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng LQ, Hou LN, Song FX, Zhu HY, Zhao HY,
Chen G and Li JJ: Effect of pre-emptive analgesia by continuous
femoral nerve block on early postoperative cognitive function
following total knee arthroplasty in elderly patients. Exp Ther
Med. 13:1592–1597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geng YJ, Wu QH and Zhang RQ: Effect of
propofol, sevoflurane, and isoflurane on postoperative cognitive
dysfunction following laparoscopic cholecystectomy in elderly
patients: A randomized controlled trial. J Clin Anesth. 38:165–171.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia ZM, Hao HN, Huang ML, Ma DF, Jia XL
and Ma B: Influence of dexmedetomidine to cognitive function during
recovery period for children with general anesthesia. Eur Rev Med
Pharmacol Sci. 21:1106–1111. 2017.PubMed/NCBI
|
|
4
|
Devore EE, Fong TG, Marcantonio ER,
Schmitt EM, Travison TG, Jones RN and Inouye SK: Prediction of
long-term cognitive decline following postoperative delirium in
older adults. J Gerontol A Biol Sci Med Sci. Mar 15–2017.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Shen R, Wen G, Ding R, Du A, Zhou J,
Dong Z, Ren X, Yao H, Zhao R, et al: Effects of ketamine on levels
of inflammatory cytokines IL-6, IL-1β, and TNF-α in the hippocampus
of mice following acute or chronic administration. Front Pharmacol.
8:1392017.PubMed/NCBI
|
|
6
|
Zhang Z, Zhou J, Song D, Sun Y, Liao C and
Jiang X: Gastrodin protects against LPS-induced acute lung injury
by activating Nrf2 signaling pathway. Oncotarget. 8:32147–32156.
2017.PubMed/NCBI
|
|
7
|
Jia J, Shi X, Jing X, Li J, Gao J, Liu M,
Lin CI, Guo X and Hua Q: BCL6 mediates the effects of gastrodin on
promoting M2-like macrophage polarization and protecting against
oxidative stress-induced apoptosis and cell death in macrophages.
Biochem Biophys Res Commun. 486:458–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L, Liu X, Wang H and Qu M: Gastrodin
attenuates pentylenetetrazole-induced seizures by modulating the
mitogen-activated protein kinase-associated inflammatory responses
in mice. Neurosci Bull. 33:264–272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun T, Wang J, Li X, Li YJ, Feng D, Shi
WL, Zhao MG, Wang JB and Wu YM: Gastrodin relieved complete
Freund's adjuvant-induced spontaneous pain by inhibiting
inflammatory response. Int Immunopharmacol. 41:66–73. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kline R, Wong E, Haile M, Didehvar S,
Farber S, Sacks A, Pirraglia E, de Leon MJ and Bekker A:
Peri-operative inflammatory cytokines in plasma of the elderly
correlate in prospective study with postoperative changes in
cognitive test scores. Int J Anesthesiol Res. 4:313–321.
2016.PubMed/NCBI
|
|
11
|
Rossetti AC, Paladini MS, Racagni G, Riva
MA, Cattaneo A and Molteni R: Genome-wide analysis of LPS-induced
inflammatory response in the rat ventral hippocampus: Modulatory
activity of the antidepressant agomelatine. World J Biol
Psychiatry. Mar 24–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramírez-Jirano LJ, Zenteno-Savín T,
Gaxiola-Robles R, Ramos-González EJ, Torres-Mendoza BM and
Bitzer-Quintero OK: The neuroprotective effect of erythropoietin in
rat hippocampus in an endotoxic shock model. Rev Invest Clin.
68:292–298. 2016.PubMed/NCBI
|
|
13
|
Wu J, Liu Z, Su J, Pan N and Song Q:
Anti-inflammatory activity of 3β-hydroxycholest-5-en-7-one isolated
from Hippocampus trimaculatus leach via inhibiting iNOS, TNF-α, and
IL-1β of LPS induced RAW 264.7 macrophage cells. Food Funct.
8:788–795. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mani R, Natesan V and Arumugam R:
Neuroprotective effect of chrysin on hyperammonemia mediated
neuroinflammatory responses and altered expression of astrocytic
protein in the hippocampus. Biomed Pharmacother. 88:762–769. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang R, Zhang H, Wang Y, Song F and Yuan
Y: Inhibitory effects of quercetin on the progression of liver
fibrosis through the regulation of NF-κB/IκBα, p38 MAPK, and
Bcl-2/Bax signaling. Int Immunopharmacol. 47:126–133. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alva-Murillo N, Ochoa-Zarzosa A and
López-Meza JE: Sodium octanoate modulates the innate immune
response of bovine mammary epithelial cells through the
TLR2/P38/JNK/ERK1/2 pathway: Implications during Staphylococcus
aureus internalization. Front Cell Infect Microbiol. 7:782017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Achanta P, Thompson KJ, Fuss M and
Martinez JL Jr: Gene expression changes in the rodent hippocampus
following whole brain irradiation. Neurosci Lett. 418:143–148.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong X, Liu L and Sheng X: Effects of
excessive zinc in fodder on brain development and abilities of
learning and memory and their mechanisms in young rats. Zhonghua Yu
Fang Yi Xue Za Zhi. 32:225–228. 1998.(In Chinese). PubMed/NCBI
|
|
19
|
Tang TY, Zhou XX, Huang H and Huang QD:
Relationship between IL-1β polymorphisms and obstructive sleep
apnea syndrome. Eur Rev Med Pharmacol Sci. 21:3120–3128.
2017.PubMed/NCBI
|
|
20
|
Sun L, Zhao ZY, Hu J and Zhou XL:
Potential association of lead exposure during early development of
mice with alteration of hippocampus nitric oxide levels and
learning memory. Biomed Environ Sci. 18:375–378. 2005.PubMed/NCBI
|