Introduction
Sepsis is one of the most common complications that
occur in pediatric patients following extensive burns (1). In addition, infection is one of the
most common and severe complications of a major burn injury
associated with pediatric burn sepsis (2). Clinical data have indicated that sepsis
occurs in >30% of the pediatric burn patients (3). A previous study reviewed the
characteristics of patients with pediatric burn sepsis, including
patient categorization, diagnosis and treatment and it was
identified that the mortality rate of pediatric patients with burn
sepsis is significantly higher mortality than that of adult
patients (4). Although an increasing
number of strategies have been explored for the treatment of
pediatric burn sepsis, the mortality rate remains high (5,6).
Additionally, the physiological characteristics, drug treatment and
inflammatory responses to burn injury in pediatric patients differ
significantly compared with those in adult patients (7–9).
Therefore, it is important to develop more effective therapeutic
strategies and drugs for the treat pediatric patients with burn
sepsis in order to improve their survival rate.
Simvastatin is a statin that is a
3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, which is
currently used to manage blood cholesterol levels and prevent
cardiovascular disease (10,11). It has been reported that simvastatin
increases the survival of patients with sepsis or infection by
inhibiting sepsis-induced mortality and preventing acute kidney
injury (12). It has also been
demonstrated that induces anti-inflammatory effects in abdominal
sepsis (13). Furthermore,
simvastatin treatment improves the survival rate in a murine model
of burn sepsis by elevating interleukin (IL)-6 levels (14). However, simvastatin treatment alone
is not sufficient to attenuate inflammation and the pathological
processes of pediatric burn sepsis patients in clinical practice
(15).
Kallistatin is an anthropogenic kallikrein-binding
plasma protein that has been demonstrated to exert multiple
functions, including inhibition of inflammation, oxidative stress
and apoptosis in endothelial cells (16). A previous study revealed that
kallistatin presented beneficial anti-inflammation and
anti-fibrosis effects by decreasing the antioxidative stress and
inhibiting the reactive oxygen species-induced expression of
proinflammatory cytokines and transforming growth factor-β1
(17). In addition, kallistatin
treatment inhibited vascular inflammation by antagonizing tumor
necrosis factor α (TNF-α)-induced nuclear factor κB (NF-κB)
activation (18). Furthermore, human
kallistatin administration reduced organ injury, as well as
inflammatory responses and improves the survival rate in a mouse
model of polymicrobial sepsis (19).
However, further investigations are required to examine the
efficacy of kallistatin in the treatment of pediatric burn sepsis
patients.
In the present study, combined treatment with
simvastatin and kallistatin was investigated in pediatric burn
sepsis therapy. In addition, the study analyzed the
anti-inflammatory and anti-apoptotic effects of the combined
treatment in human endothelial cells (HECs) collected from the
pediatric burn sepsis patients.
Materials and methods
Study design, subjects and
sampling
A total of 72 pediatric patients with burn sepsis
from the Children's Hospital Affiliated to the Medical College of
Zhejiang University were recruited in the present retrospective
study between January 2014 and May 2015. The patients included 30
girls and 42 boys aged 2.8–10.4 years old. The inclusion criteria
for the individuals with severe pediatric burn sepsis were
diagnosed by inflammatory marks and hemodynamic indexes (20). The inclusion criteria for the current
study were patients <12 years old with severe pediatric burn
sepsis diagnosed using systemic inflammatory response syndrome
criteria (21) were included in the
present study. Pediatric patients with congenital heart disease,
renal failure or cancer were excluded from the study. The study
protocol was approved by the Central Ethics Committee of the
Children's Hospital Affiliated to the Medical College of Zhejiang
University (Hangzhou, China). Written informed consent was obtained
from the parents of all included patients. The body temperature of
patients was recorded every day and used to calculate the frequency
of fever in the current study.
Drug administration
The 72 included patients were randomized into three
treatment groups as follows: A simvastatin (n=30), kallistatin
(n=26) and combination treatment (n=16) group. Patients in the
simvastatin group received 40 mg/day simvastatin orally (Jiangsu
Yellow River Pharmaceutical Co., Ltd., Suqian, China), while
patients in the kallistatin group received 20 mg/day kallistatin
(Wuhan Huamei Biological Engineering Co., Ltd., Wuhan, China).
Patients in the combination group received 40 mg/day simvastatin
and 20 mg/day kallistatin orally. The treatment lasted 28 days and
all the patients completed the study following a 28 day follow-up
period.
ELISA
Plasma samples were prepared by centrifugation of
peripheral venous blood at 2,000 × g for 10 min at 4°C. Blood was
centrifuged immediately following collection from pediatric
patients with burn sepsis treated with simvastatin and/or
kallistatin on days 0, 7, 14, 21 and 28. The serum levels of blood
urea nitrogen (cat. no., EIABUN, Thermo Fisher Scientific, Inc.),
creatinine, (cat. no., EIACUN, Thermo Fisher Scientific, Inc.),
TNF-α (cat. no., DTA00C, Bio-Rad Laboratories, Inc., Hercules, CA,
USA), IL-1β (cat. no., DY201, Bio-Rad Laboratories, Inc.), IL-6
(cat. no., D6050, Bio-Rad Laboratories, Inc.), human leukocyte
antigen-D related (HLA-DR; cat. no., CSB-EL010497HU, Cusabio,
Wuhan, China) and procalcitonin (PCT; cat. no., EHPCT; Thermo
Fisher Scientific, Inc.) were then analyzed using the corresponding
ELISA kits according to the manufacturer's protocol. The serum
concentration levels of IL-1β, TNF-α, IL-6, HLA-DR and PCT were
measured using a microplate reader at 570 nm.
Inflammation severity score
On days 0, 7, 14, 21 and 28, treatment efficacy was
evaluated according to the inflammation severity score as the
efficacy criteria. The mean inflammation severity score of patients
was evaluated according to previous studies (22,23).
Revised trauma score (RTS)
The RTS scoring system examines the injury severity
of burn sepsis patients in three groups and includes evaluation of
the Glasgow Coma scale (24),
systolic blood pressure and respiratory rate, evaluated on days 0
and 14. The score ranges between 0 and 12. In the ‘simple triage
and rapid treatment’ triage, a patient with an RTS score of 12 is
considered to require delayed care, a score of 11 indicates that
urgent care is required and a score of 3–10 indicates that
immediate care is required. Patients with an RTS of <3 should
receive emergency care, which includes the administration of
anti-inflammatory medication and antibiotics and admittance into
the intensive care unit, as survival is highly unlikely without a
significant amount of medication.
HEC culture
HECs were harvested from the pediatric burn sepsis
patients (n=3 in each group) as previously described (25). Briefly, on day 30, endothelial
specimens were minced with dissecting scissors into ≤2-mm sections
followed by digestion with collagenase type IV (2 mg/ml;
Worthington Biochemical Corp., Lakewood, NJ, USA), Dispase II (1.2
U/ml; Worthington Biochemical Corp.) and 2 mM CaCl2 in
phosphate-buffered saline (PBS) at 37°C for 60 min. Enzymes and
thus, the reaction was terminated following the addition of
endothelial basal medium (EBM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.). Next, cells were filtered through a
sterile 50-µm nylon mesh cell strainer (Thermo Fisher Scientific,
Inc.), centrifuged at 300 × g for 5 min at 4°C and cultured in
hemolytic buffer (containing 155 mM NH4Cl, 10 mM
KHCO3 and 0.1 mM EDTA in H2O) for 15 min at
37°C. Subsequently, the cells were cultured in complete EBM with
20% FBS, growth factor supplement kit (Lonza Group Ltd., Basel
Switzerland) and penicillin 100 U/ml (Gibco; Thermo Fisher
Scientific, Inc.) for 12 h at 37°C.
MTT assay
HECs (5×103 cells/well) isolated from
pediatric patients with burn sepsis subsequent to simvastatin
and/or kallistatin treatment were grown in 12-well plates to ~95%
monolayer cells for 24 h at 37°C. MTT at a concentration of 5 mg/ml
(50 µl; Amresco, LLC, Solon, OH, USA), was added to the cells and
incubated for 4 h. DMSO (100 µl) was then added and incubated for
30 min to dissolve the precipitate following removal of the
supernatant. The results were determined using a spectrophotometer
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 570 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was obtained from HECs using the RNAeasy
Mini kit (Qiagen, Hilden, Germany). RNA was reversed transcribed
using a PrimeScript RT Master Mix kit (Takara Bio, Inc., Otsu,
Japan). All the forward and reverse primers were synthesized by
Thermo Fisher Scientific, Inc., and were as follows: TLR4,
5′-AAACTCAGCAAAGTCCCTGATGAC-3′ (forward) and
5′-CGTAGAAACTGTAAGTCGTTGACAG-3′ (reverse); SOCS3,
5′-ACCTTCAGCTCCAAAAGCGAGTAC-3′ (forward) and
5′-CGCTCCAGTAGAATCCGCTCTC-3′ (reverse); high mobility group box-1
(HMGB1), 5′-GGCGAGCATCCTGGCTTATC-3′ (forward) and
5′-AGGCAGCAATATCCTTCTCATAC-3′ (reverse); β-actin,
5′-AGGCCGGTGCTGAGTATGTC-3′ (forward) and 5′-TGCCTGCTTCACCACCTTCT-3′
(reverse). qPCR was performed using a qPCR system (Invitrogen;
Thermo Fisher Scientific, Inc.) with SYBR Green Master Mix
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 45 amplification cycles were
performed, including 94°C for 30 sec, denaturation at 95°C for 5
sec, primer annealing at 63°C for 5 sec with touchdown to 54°C for
15 sec and applicant extension at 72°C for 10 sec. The relative
mRNA expression changes were calculated by the 2−ΔΔCq
method (26) and the results are
expressed as the fold of β-actin, which served as the internal
control.
Western blotting
HECs were homogenized in lysate buffer containing
protease-inhibitor (Sigma-Aldrich; Merck KGaA) and then centrifuged
at 6,000 × g at 4°C for 10 min. The supernatant was collected and
used for the analysis of the target protein expression. Protein
concentration was measured using a BCA protein assay kit (Thermo
Fisher Scientific Inc.). Protein samples (10 µg) were separated on
12.5% SDS-PAGE and transferred onto polyvinylidene difluoride
membranes (Millipore, Massachusetts, USA). Membrane were blocked
with 5% skimmed milk for 1 h at 37°C and then incubated with the
following rabbit anti-human primary antibodies: B-cell lymphoma 2
(Bcl-2; 1:500; cat. no., ab692), P53 (1:500; cat. no., ab26),
Bcl-2-associated X protein (1:500; cat. no., ab22048), caspase-3
(1:500, cat. no., ab13847) and β-actin (1:500; cat. no., ab8227;
all purchased from Abcam, Shanghai, China) for 12 h at 4°C. Samples
were then incubated with horseradish peroxidase (HRP)-conjugated
anti-rabbit IgG antibody (1:5,000, PV-6001, ZSGB-BIO, Beijing,
China) for 24 h at 4°C. The membranes were then visualized using
WesternBright ECL Chemiluminescent HRP Substrate (Advansta, Menlo
Park, CA, USA). The density of the bands was analyzed using
Quantity one software version 4.62 (Bio-Rad Laboratories,
Inc.).
Analysis of TLR4-positive cells
Blood samples were collected on days 0, 7, 14, 21
and 28 following simvastatin and/or kallistatin treatments. Blood
samples were placed into a test tube containing EDTA and peripheral
blood mononuclear cells were isolated by density-gradient
centrifugation using Ficoll-Paque Premium (GE Healthcare Life
Sciences) according to the manufacturer's protocol. Samples were
blocked by 5% bovine serum albumin (Thermo Fisher Scientific, Inc.)
for 2 h at 4°C were then incubated with anti-TLR4 (cat. no. 551964,
1:1,000) and anti-CD14 antibodies (cat. no. 555397; 1:1,000, BD
Biosciences, Franklin Lakes, NJ, USA) for 1 h at 4°C and then with
anti-immunoglobulin G H&L secondary antibody (Alexa
Fluor® 488; cat. no., ab150117, Abcam) for 2 h at 4°C
following washing with PBS three times at room temperature.
TLR4/CD14 double-positive peripheral blood mononuclear cells were
then identified using a flow cytometer. The number of TLR4-positive
peripheral blood mononuclear cells was calculated in terms of the
mean fluorescent intensity.
Apoptosis assay
HECs were isolated from pediatric patients with burn
sepsis on days 0, 7, 14, 21 and 28 after simvastatin and/or
kallistatin treatment. HECs were adjusted to 1×106
cells/ml with PBS and labeled with Annexin V-FITC and PI using a
kit (BD Biosciences) according to the manufacturer's protocol.
Subsequently, cells were analyzed with a FACScan flow cytometer (BD
Biosciences). The treatments were performed in triplicate and the
percentage of labeled cells undergoing apoptosis in each group was
determined and calculated. The ratios of apoptotic cells were
analyzed using Expo32-ADC v. 1.2B software (Beckman Coulter, Inc.,
Brea, CA, USA).
NF-κB activation
HECs were isolated from pediatric patients with burn
sepsis on days 0 and 28 following simvastatin and/or kallistatin
treatment. Subsequently, HECs were seeded at a density of
2×105 cells/well in 24-well plates and cultured in EBM.
The 3′-untranslated region (3′-UTR) sequence of NF-κB, predicted to
the effects of simvastatin and kallistatin on NF-κB activity, was
cloned into the pGL3 control vector (Promega Corporation, Madison,
WI, USA). These constructs were designated as wild-type (wt)
NF-κB-3′-UTR or mutant NF-κB-3′-UTR, respectively. For the reporter
assay, HECs (1×104) were seeded in 24-well plates and
transfected with the aforementioned constructs or negative control
(mutant NF-κB-3′-UTR) using lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). After 48 h, the cells were collected and
Renilla luciferase activity in cells was measured using the
Dual-Luciferase Reporter assay system (Promega Corporation)
following the manufacturer's protocol, as described previously
(27). Luciferase activity was
normalized to that of Renilla luciferase. Results were
obtained from three independent experiments performed in
duplicate.
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation and were analyzed by Student's t-test. All data
were analyzed using SPSS 19.0 statistical software (IBM Corp.,
Armonk, NY, USA) along with the use of Microsoft Excel (Microsoft
Corp., Redmond, WA, USA). Unpaired data were determined by
Student's t-test, while comparisons of data between multiple groups
were conducted by analysis of variance. A P-value of ≤0.05 was
considered to indicate a difference that was statistically
significant.
Results
Effect of combined treatment with
simvastatin and kallistatin on the levels of
inflammation-associated cytokines in pediatric burn sepsis
patients
Pediatric burn sepsis patients received simvastatin,
kallistatin or combined treatment with simvastatin and kallistatin
and the levels of inflammatory cytokines TNF-α, IL-1β, IL-10 and
HLA-DR in the serum were determined on days 0, 7, 14, 21 and 28. As
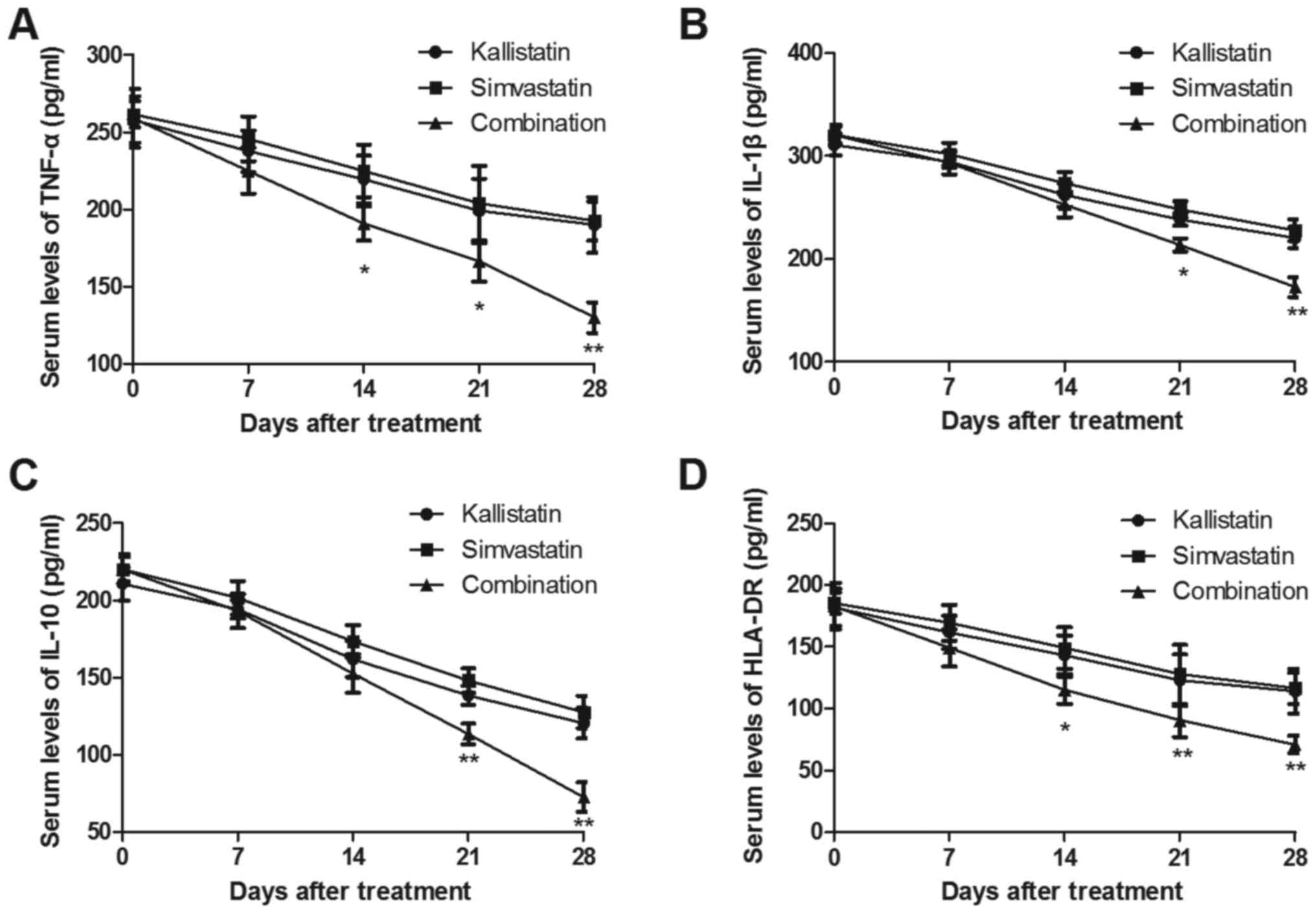
presented in Fig. 1A and B, the
serum levels of TNF-α and IL-1β were significantly decreased in the
combined treatment group, compared with those that received
simvastatin or kallistatin alone. However, the anti-inflammatory
cytokines IL-10 and HLA-DR were markedly downregulated in the serum
of pediatric burn sepsis patients subsequent to combined treatment
(Fig. 1C and D). Furthermore, the
results indicated that the levels of TNF-α and IL-1β were not
significantly altered between the simvastatin alone and kallistatin
alone group.
Effect of combined treatment with
simvastatin and kallistatin on blood urea nitrogen and serum
creatinine levels, PCT and RTS in pediatric burn sepsis
patients
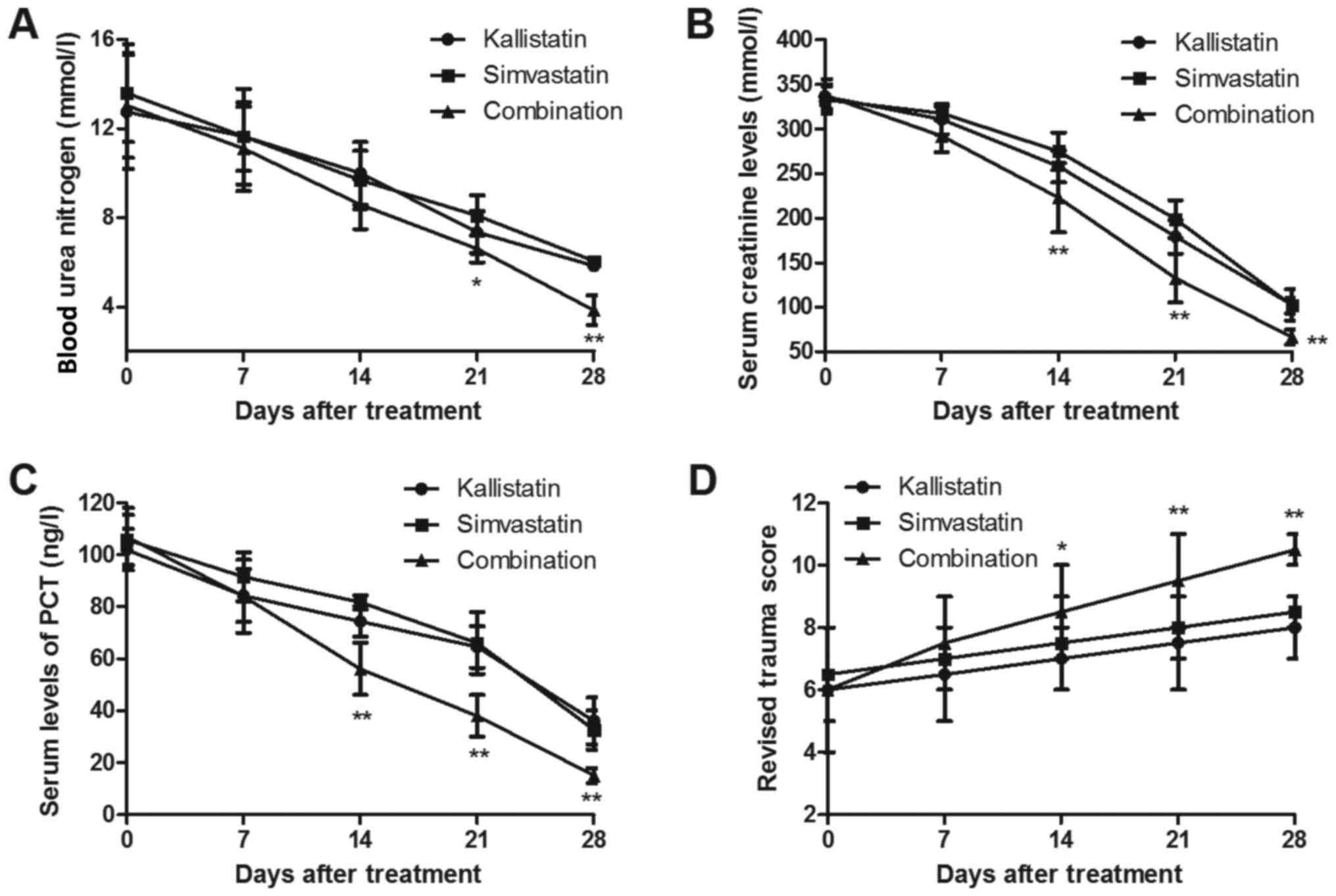
Several important biochemical indicators were
investigated in pediatric patients with burn sepsis subsequent to
receiving simvastatin, kallistatin or combined treatment. The
results revealed that combined treatment significantly decreased
blood urea nitrogen in pediatric burn sepsis patients on days 21
and 28 and exhibited decreased serum creatinine levels on days 14,
21 and 28 (Fig. 2A and B).
Furthermore, the results indicated that the PCT level was evidently
downregulated by combined treatment with simvastatin and
kallistatin in the serum of burn sepsis patients from day 14
(Fig. 2C). The RTS values were also
significantly improved on days 14, 21 and 28 in patients receiving
combined treatment (Fig. 2D). These
outcomes suggest that combined treatment with simvastatin and
kallistatin improves the prognosis of pediatric burn sepsis
patients.
Efficacy of combined treatment with
simvastatin and kallistatin on the improvement of
inflammation-associated gene levels in pediatric burn sepsis
patients
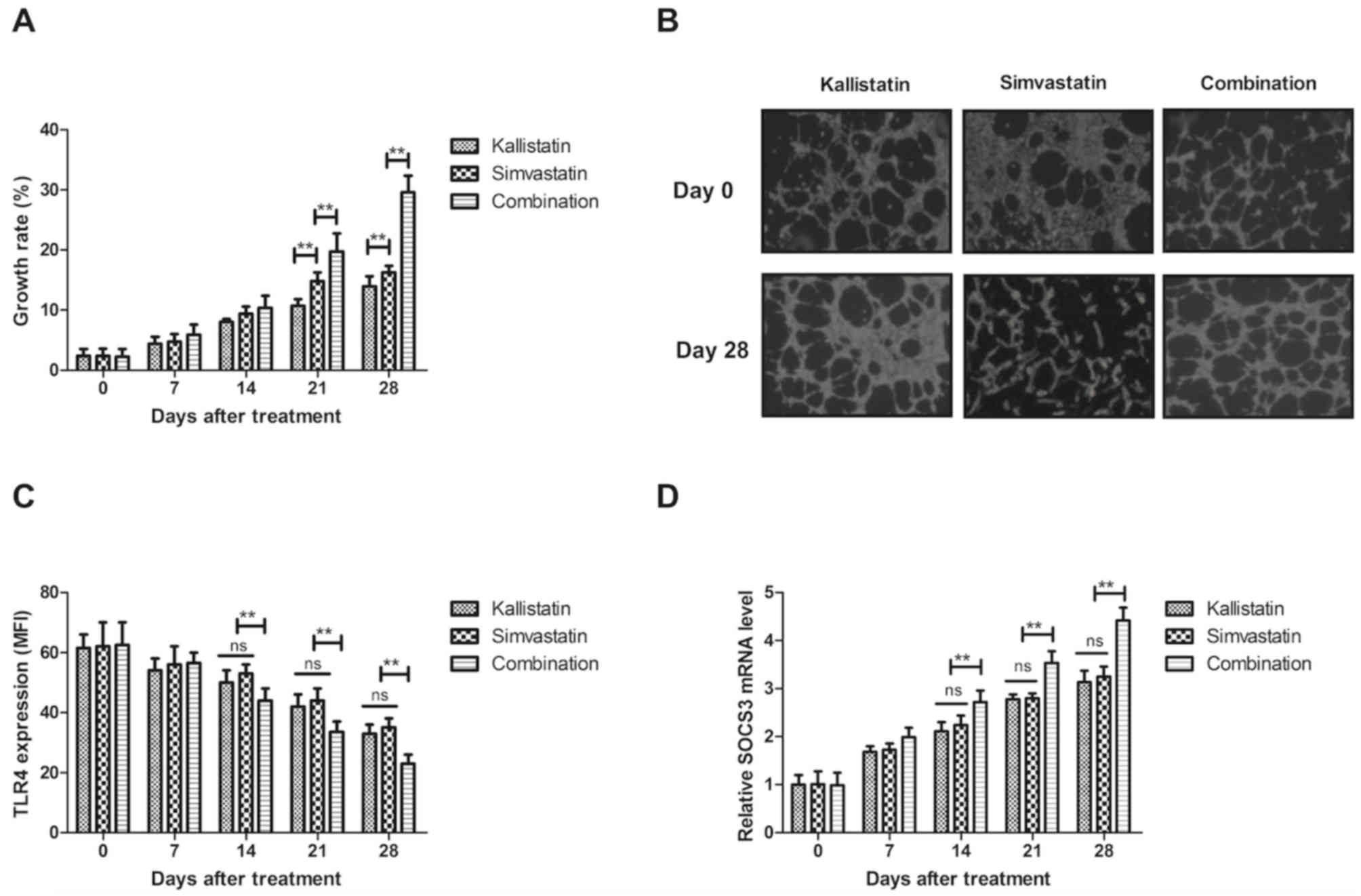
The present study further analyzed alterations in
the expression levels of inflammation-associated genes in HECs. The
results revealed that combined treatment with simvastatin and
kallistatin significantly promoted the viability of HECs compared
with that of the single treatment groups (Fig. 3A). Treatment with simvastatin and
kallistatin increased the viability of HECs compared with the
groups treated with simvastatin or kallistatin alone. In addition,
it was demonstrated that combined treatment with simvastatin and
kallistatin improved the morphology of HECs (Fig. 3B). The results further indicated that
TLR4 expression on the surface of monocytes was markedly decreased
and SOCS3 expression was markedly increased in the combined
treatment group compared with the levels in the kallistatin or
simvastatin only groups on days 14, 21 and 28 (Fig. 3C and D). Notably, it was demonstrated
that HMGB1 levels and HMGB1-induced NF-κB p65 activation were
inhibited in HECs in three groups (Fig.
3E and F), with a significant difference observed in the
combination treatment as compared with the kallistatin or
simvastatin groups after 14-day treatment. Furthermore, it was
observed that the inflammation severity score was significantly
decreased following combined treatment as compared with that in the
single treatment groups (Fig. 3G).
No significant differences were observed between the kallistatin
and simvastatin groups. These outcomes suggested that combined
treatment with simvastatin and kallistatin improved the levels
inflammation-associated genes and inflammation severity score in
HECs in pediatric burn sepsis patients.
Anti-apoptotic efficacy of combined
treatment with simvastatin and kallistatin in pediatric burn sepsis
patients
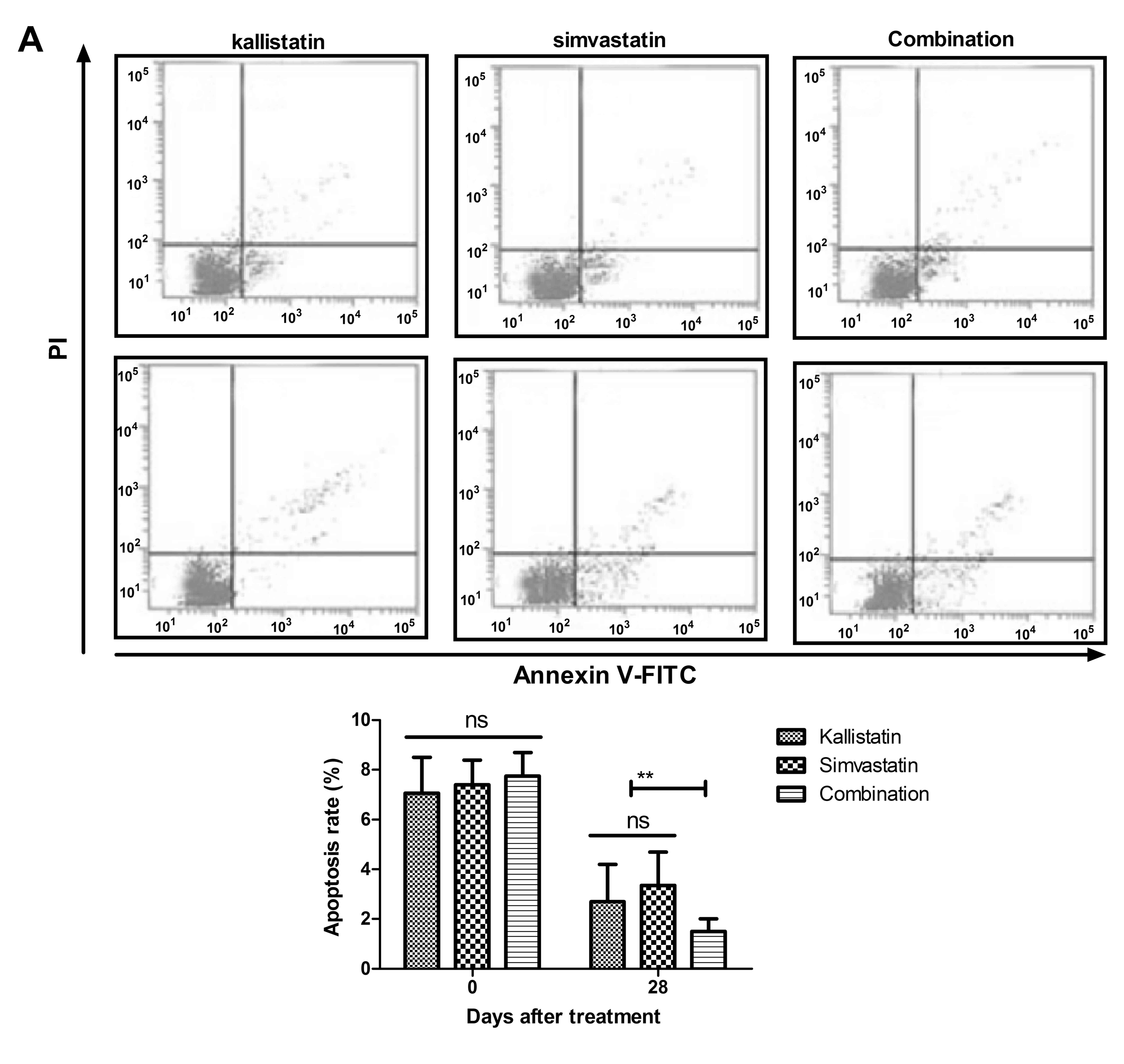
The anti-apoptotic efficacy of combined treatment
with simvastatin and kallistatin was investigated in HECs obtained
from pediatric burn sepsis patients. As presented in Fig. 4A, it was observed that combined
treatment with kallistatin and simvastatin significantly decreased
the apoptosis of HECs on day 28 compared with either treatment
alone. Additionally, combined treatment upregulated the Bcl-2 and
P53 expression in HECs on day 28 (Fig.
4B), while the expression of the pro-apoptotic Bax and
caspase-3 proteins were downregulated (Fig. 4C). Notably, the findings indicated
that combined treatment significantly improved the fever frequency
on day 28 compared with the single treatment groups (Fig. 4D). These results suggested that
combined treatment with simvastatin and kallistatin inhibited the
apoptosis of HECs and improved the fever frequency in pediatric
burn sepsis patients.
Discussion
Burn sepsis is a systemic inflammatory response
syndrome and pediatric patients with burn wound-induced sepsis have
a relative higher mortality rate compared with that of adult
patients with sepsis (2). Currently,
an increasing number of drugs are applied for the treatment of
pediatric burn sepsis (28,29). Previous studies have indicated that
simvastatin is able to attenuate the sepsis-induced blood-brain
barrier integrity loss, which also inhibits the apoptosis of
endothelial cells induced by sepsis by upregulating the expression
of Bcl-2 and downregulating Bax (15,30). In
addition, a previous study suggested that kallistatin is able to
protect HECs against inflammation and apoptosis, as well as improve
sepsis-associated acute lung injury (31). In the current study, in order to
improve the therapeutic efficacy of clinical treatment, combined
treatment with simvastatin and kallistatin, or treatment with
simvastatin or kallistatin alone were administered to patients with
burn sepsis. The results of the current study indicated that
combined treatment with simvastatin and kallistatin may be a
potential therapeutic regimen for the treatment of pediatric burn
sepsis patients in clinical practice.
Inflammatory response is a major cause of mortality
in pediatric patients with burn sepsis (32). The results of the present study
revealed that simvastatin treatment decreased TNF-α and IL-1β
levels, while it increased the serum levels of the
anti-inflammatory cytokines IL-10 and HLA-DR in pediatric burn
sepsis patients. A previous study reviewed the inflammatory
responses and anti-inflammatory strategies in experimental models
of sepsis and hemorrhage, and indicated that the regulation of
inflammatory pathways contributed to the treatment of pediatric
burn sepsis through different pathophysiological processes
(33). Simvastatin treatment has a
beneficial effect in patients with sepsis and severe sepsis by
decreasing the inflammation (34).
In HECs isolated from pediatric burn sepsis patients, simvastatin
treatment resulted in markedly decreased TLR4, HMGB1 and NF-κB
levels, as well as in increased SOCS3 expression.
It has been reported that kallistatin may be a
potential therapeutic agent to treat pediatric burn sepsis
(31). In the present study, the
therapeutic effects of kallistatin we also investigated in
pediatric burn sepsis patients. The outcomes indicated that
kallistatin treatment exerted anti-inflammatory effects and
inhibited the apoptosis of HECs isolated from pediatric burn sepsis
patients, with no marked differences detected between the
simvastatin and kallistatin-treated groups. Zhou et al
(35) have suggested that
kallistatin treatment significantly improved the oxidative stress
and inflammation, indicating that kallistatin was able to regulate
the inflammation. The current study revealed that kallistatin
treatment also decreased the levels of TLR4, HMGB1 and NF-κB, as
well as increased SOCS3 expression, in HECs isolated from pediatric
burn sepsis patients.
In the present study, it was reported that combined
treatment with kallistatin and simvastatin significantly decreased
the apoptosis of HECs on day 28. Lee et al (36) have demonstrated that simvastatin
presented an anti-apoptotic effect by increasing P53 expression.
Another study indicated that simvastatin regulated neuronal
apoptosis through the modulation of Bcl-2 expression in a rat
quinolinic acid model of Huntington's disease (37). Furthermore, Chao et al
(38) indicated that kallistatin
protected against myocardial ischemia-reperfusion injury by
preventing apoptosis and inflammation. The present study results
revealed that combined treatment with kallistatin and simvastatin
significantly increased Bcl-2 and P53 expression levels, whereas it
decreased Bax and caspase-3 levels in HECs. It was also observed
that combined treatment significantly improved the incidence of
fever as compared with that in the single treatment group.
In conclusion, the present study reported the
efficacy of combined treatment with simvastatin and kallistatin in
pediatric burn sepsis patients. The outcomes demonstrated that
combined treatment exhibited increased beneficial effects for these
patients as compared with simvastatin or kallistatin treatment
alone in terms of anti-inflammation and anti-apoptosis. These
results indicated that combined therapy with simvastatin and
kallistatin resulted in successful outcomes for numerous pediatric
patients with burn sepsis.
References
|
1
|
Housinger TA, Brinkerhoff C and Warden GD:
The relationship between platelet count, sepsis, and survival in
pediatric burn patients. Arch Surg. 128:65–67. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu T, Stockmann C, Healy DP, Olson J, Wead
S, Neely AN, Kagan RJ, Spigarelli MG and Sherwin CM: Determination
of optimal amikacin dosing regimens for pediatric patients with
burn wound sepsis. J Burn Care Res. 36:e244–e252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sheridan RL: Sepsis in pediatric burn
patients. Pediatr Crit Care Med. 6 3 Suppl:S112–S119. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng YZ: Clinical characteristics and
diagnosis of sepsis in pediatric burn patients. Zhonghua Shao Shang
Za Zhi. 29:1–3. 2013.(In Chinese). PubMed/NCBI
|
|
5
|
Scott HF, Paul R and Balamuth F: The
spectrum of pediatric sepsis: ‘Septicemia’ misses severe cases. Ann
Emerg Med. 66:685–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruth A, McCracken CE, Fortenberry JD and
Hebbar KB: Extracorporeal therapies in pediatric severe sepsis:
Findings from the pediatric health-care information system. Crit
Care. 19:3972015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Odetola FO, Gebremariam A and Freed GL:
Patient and hospital correlates of clinical outcomes and resource
utilization in severe pediatric sepsis. Pediatrics. 119:487–494.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alexandre-Treilles M, Chenaud M, Kacet N,
Ego A and Truffert P; OMBREL de Soins Périnatals de Lille
Métropole, : Pediatric management of early-onset neonatal sepsis:
Guidelines adherence in Lille's perinatal care network. Arch
Pediatr. 13:341–345. 2006.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moloney-Harmon PA: Pediatric sepsis: The
infection unto death. Crit Care Nurs Clin North Am. 17:417–429, xi.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramadan WH and Kabbara WK:
Sitagliptin/Simvastatin: A first combination tablet to treat type 2
diabetes and hypercholesterolemia-a review of its characteristics.
Vasc Health Risk Manag. 11:125–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taveira-DaSilva AM, Jones AM,
Julien-Williams PA, Stylianou M and Moss J: Retrospective review of
combined sirolimus and simvastatin therapy in
lymphangioleiomyomatosis. Chest. 147:180–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yasuda H, Yuen PS, Hu X, Zhou H and Star
RA: Simvastatin improves sepsis-induced mortality and acute kidney
injury via renal vascular effects. Kidney Int. 69:1535–1542. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Souza Neto JL, Araújo Filho I, Rego AC,
Dominici VA, Azevedo IM, Egito ES, Brandão-Neto J and Medeiros AC:
Effects of simvastatin in abdominal sepsis in rats. Acta Cir Bras.
21 Suppl 4:S8–S12. 2006. View Article : Google Scholar
|
|
14
|
Beffa DC, Fischman AJ, Fagan SP, Hamrahi
VF, Paul KW, Kaneki M, Yu YM, Tompkins RG and Carter EA:
Simvastatin treatment improves survival in a murine model of burn
sepsis: Role of interleukin 6. Burns. 37:222–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu H, Wang QS, Luo Q, Tan S, Su H, Tang
SL, Zhao ZL and Huang LP: Simvastatin inhibits apoptosis of
endothelial cells induced by sepsis through upregulating the
expression of Bcl-2 and downregulating Bax. World J Emerg Med.
5:291–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang CR, Chen SY, Wu CL, Liu MF, Jin YT,
Chao L and Chao J: Prophylactic adenovirus-mediated human
kallistatin gene therapy suppresses rat arthritis by inhibiting
angiogenesis and inflammation. Arthritis Rheum. 52:1319–1324. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen B, Hagiwara M, Yao YY, Chao L and
Chao J: Salutary effect of kallistatin in salt-induced renal
injury, inflammation, and fibrosis via antioxidative stress.
Hypertension. 51:1358–1365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin H, Gao L, Shen B, Chao L and Chao J:
Kallistatin inhibits vascular inflammation by antagonizing tumor
necrosis factor-alpha-induced nuclear factor kappaB activation.
Hypertension. 56:260–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li P, Guo Y, Bledsoe G, Yang ZR, Fan H,
Chao L and Chao J: Kallistatin treatment attenuates lethality and
organ injury in mouse models of established sepsis. Crit Care.
19:2002015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chai JK: Diagnosis and comprehensive
management of sepsis after burn. Zhonghua Shao Shang Za Zhi.
29:105–108. 2013.PubMed/NCBI
|
|
21
|
Maguire PJ, Power KA, Downey AF, O'Higgins
AC, Sheehan SR and Turner MJ: Evaluation of the systemic
inflammatory response syndrome criteria for the diagnosis of sepsis
due to maternal bacteremia. Int J Gynaecol Obstet. 133:116–119.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hautamäki A, Luoma A and Immonen I:
Anterior chamber flare during bevacizumab treatment in eyes with
exudative age-related macular degeneration. Retina. 36:2183–2190.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nierhaus A, Linssen J, Wichmann D, Braune
S and Kluge S: Use of a weighted, automated analysis of the
differential blood count to differentiate sepsis from
non-infectious systemic inflammation: The intensive care infection
score (ICIS). Inflamm Allergy Drug Targets. 11:109–115. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kondo Y, Abe T, Kohshi K, Tokuda Y, Cook
EF and Kukita I: Revised trauma scoring system to predict
in-hospital mortality in the emergency department: Glasgow coma
scale, age, and systolic blood pressure score. Crit Care.
15:R1912011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hewett PW: Vascular endothelial cells from
human micro- and macrovessels: Isolation, characterisation and
culture. Methods Mol Biol. 467:95–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Collins PE, O'Carroll C and Carmody RJ:
Measurement of NF-κB transcriptional activity and identification of
NF-κB cis-regulatory elements using luciferase assays. Methods Mol
Biol. 1280:25–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riedel S and Carroll KC: Early
identification and treatment of pathogens in sepsis: Molecular
diagnostics and antibiotic choice. Clin Chest Med. 37:191–207.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brown KA, Brown GA, Lewis SM, Beale R and
Treacher DF: Targeting cytokines as a treatment for patients with
sepsis: A lost cause or a strategy still worthy of pursuit? Int
Immunopharmacol. 36:291–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang CH, Kao MC, Shih PC, Li KY, Tsai PS
and Huang CJ: Simvastatin attenuates sepsis-induced blood-brain
barrier integrity loss. J Surg Res. 194:591–598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin WC, Chen CW, Huang YW, Chao L, Chao J,
Lin YS and Lin CF: Kallistatin protects against sepsis-related
acute lung injury via inhibiting inflammation and apoptosis. Sci
Rep. 5:124632015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bird L: Inflammation: Hope for sepsis
treatment. Nat Rev Drug Discov. 9:516–517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai B, Deitch EA and Ulloa L: Novel
insights for systemic inflammation in sepsis and hemorrhage.
Mediators Inflamm. 2010:6424622010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shao H, Wang C, Zhu W, Huang X, Guo Z,
Zhang H and Qin B: Influence of simvastatin treatment on Toll-like
receptor 4 in monocytes of peripheral blood in patients with sepsis
and severe sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue.
28:159–163. 2016.(In Chinese). PubMed/NCBI
|
|
35
|
Zhou S, Sun Y, Zhuang Y, Zhao W and Chen
Y, Jiang B, Guo C, Zhang Z, Peng H and Chen Y: Effects of
kallistatin on oxidative stress and inflammation on renal
ischemia-reperfusion injury in mice. Curr Vasc Pharmacol.
13:265–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee SK, Kim YC, Song SB and Kim YS:
Stabilization and translocation of p53 to mitochondria is linked to
Bax translocation to mitochondria in simvastatin-induced apoptosis.
Biochem Biophys Res Commun. 391:1592–1597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Patassini S, Giampà C, Martorana A,
Bernardi G and Fusco FR: Effects of simvastatin on neuroprotection
and modulation of Bcl-2 and BAX in the rat quinolinic acid model of
Huntington's disease. Neurosci Lett. 448:166–169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chao J, Yin H, Yao YY, Shen B, Smith RS Jr
and Chao L: Novel role of kallistatin in protection against
myocardial ischemia-reperfusion injury by preventing apoptosis and
inflammation. Hum Gene Ther. 17:1201–1213. 2006. View Article : Google Scholar : PubMed/NCBI
|