Introduction
Nephrotic syndrome is the most clinically common
kidney-related disease at present, posing a serious threat to
physical health (1). In its
pathogenesis, immune dysfunction is the main pathogenic and
initiative factors (2). At the same
time, patients are often accompanied with malnutrition due to the
loss of a large number of plasma proteins (3).
In the treatment of this disease, symptomatic and
supportive treatment is dominant and nutritional support is
strengthened for patients, as well as infection prevention,
reduction of body aseptic inflammation and anti-immunosuppression
(4). Recombinant human growth
hormone is mainly secreted by the pituitary gland, which belongs to
the common water-soluble protein in the body with the effects of
promoting growth, development and metabolism of the body (5). In addition, it can strengthen the
body's immune regulation function, and its therapeutic value in
chronic nephrotic syndrome has been confirmed by the basic research
(6).
In this study, the effects of recombinant human
growth hormone on nutritional status and inflammatory factors, in
patients with chronic nephrotic syndrome were investigated. The
results showed that, chronic nephrotic syndrome can improve the
clinical symptoms more rapidly, regulate the protein metabolism and
reduce the inflammatory response in the body, which also has fewer
adverse reactions and higher safety.
Materials and methods
General materials
Eighty patients with chronic nephrotic syndrome were
admitted to our hospital, and were included in the study, from
January 2015 to December 2016. All the patients were informed
before enrollment and signed the informed consent. The study was
approved by the Ethics Committee of the Zhumadian Central Hospital
(Henan, China). Patients were aged 18–60 years and received
education in junior high school and above. Those who were treated
with glucocorticoid and/or immunosuppressor within 30 days before
enrollment, complicated with mental illness, inflammation or
cardiopulmonary insufficiency, hepatic dysfunction, systemic
infection, immune system diseases due to other causes, malignant
tumors, unclear pathogenesis or drug allergy or who refused to be
enrolled were excluded. According to the random number method, the
patients were divided into two groups (40 cases in each group). The
observation group comprised 21 males and 19 females, aged 18–60
years, with an average of 35.3±2.1 years, and onset time of
nephrotic syndrome was 1 week to 5 months with an average of
1.5±0.2 months. The control group comprised 23 males and 17
females, aged 18–60 years, with an average of 35.4±2.1 years, and
the onset time of nephrotic syndrome was 1 week to 5 months with an
average of 1.5±0.3 months. There were no statistically significant
differences in the sex, age and onset time of nephrotic syndrome
between the two groups (P>0.05).
Methods
The patients enrolled received symptomatic and
supportive treatment, such as the application of diuretics,
immunosuppressor, anti-inflammatory drugs and cytotoxic drugs. At
the same time, patients complicated with infection were treated
with antibacterial drugs for the prevention and treatment of
infection, and anti-coagulation, regulation of plasma proteins,
prevention and delay of renal failure and other treatments were
also provided. On this basis, the observation group was injected
subcutaneously with recombinant human growth hormone (Swiss
Selangor Pharmaceutical Co., batch no. S2015010015) 3 times a week
(4 units each time). Continuous treatment for 4 weeks was 1 course
of treatment and the 3 courses of treatment constituted 1 treatment
cycle. The control group was treated with Shenyankangfu tablets
(Tianjin Tongrentang Group Co., Ltd., Tianjin, China; batch no.
201501132) at a dose of 5 tablets (0.48 g/tablets) each time, once
in the morning, afternoon and evening. Continuous treatment for 1
month was 1 course of treatment and, the 3 courses of treatment
constituted 1 treatment cycle.
Observational indexes
The recovery time of clinical symptoms, change in
serum protein, the body's caloric intake and protein metabolism
after intervention were compared between the two groups. The
changes in serum cystatin C, insulin-like growth factor-1
(IGF-1) and interleukin-2 (IL-2) before intervention,
and at 1 week, 1 month and 3 months after intervention were
detected. Adverse reactions in the two groups were observed during
the treatment.
Evaluation methods
In clinical symptoms, the improvement of
proteinuria, hypoproteinemia, edema and hyperlipidemia in nephrotic
syndrome was observed. The normal level of proteinuria was <3.5
g/day and that of plasma albumin was >30 g/l. In serum lipid
parameters, the level of total cholesterol, triglyceride and
low-density lipoprotein was 2.8–5.17, 0.56–1.7 and 0–3.1 mmol/l,
respectively, which was normal. Transferrin, pre-albumin and
albumin were detected using the enzyme-linked immunosorbent assay
(ELISA), and the normal reference values were 2.20–4.0 g/l (220–400
mg/dl), 280–360 mg/l and 35–50 g/l, respectively. The caloric
intake was calculated according to the daily diet, combined with
Calorie Counter; and the normal concentration of serum cystatin C
in serum and plasma was 0.51–1.09 mg/l. IGF-1 was detected
using a spectrophotometer spectrophotometer (Hitachi, Tokyo, Japan)
and its normal reference value was 80–485 µg/l. IL-2 was
detected via ELISA and its normal reference value was 0.5–1.5
µg/l.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
21.0 (IBM, Armonk, NY, USA) software was used for statistical
analysis. Measurement data were presented as mean ± standard
deviation. A t-test was used for the comparison of means between
the two groups, while the Chi-square test was used for comparison
of the ratio between the two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
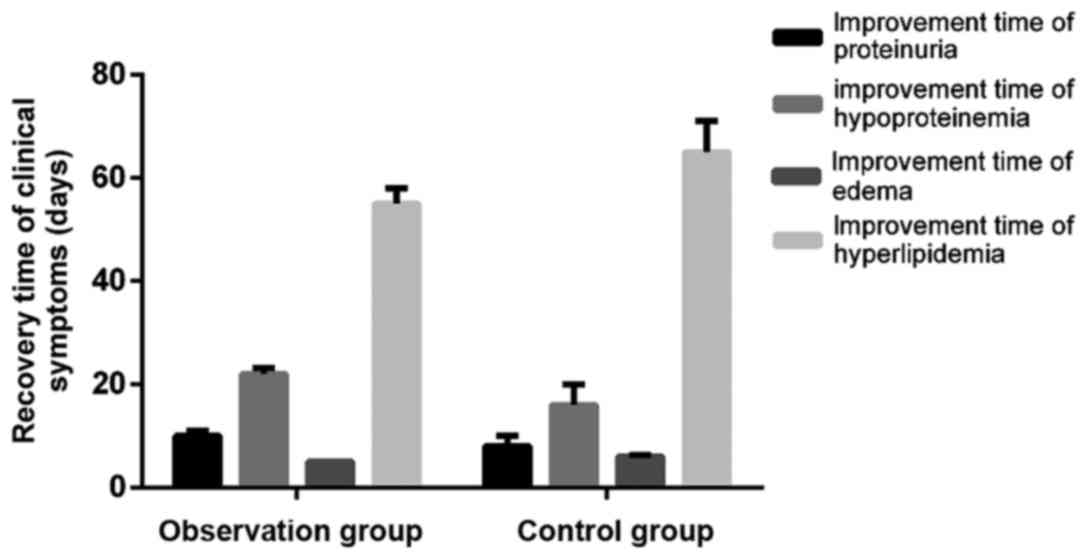
Comparison of recovery time of
clinical symptoms after intervention between the two groups
After intervention, the improvement time of
proteinuria, hypoproteinemia, edema and hyperlipidemia in the
observation group was significantly shorter than that in the
control group (t=9.803, 5.979, 21.503 and 17.481, P<0.05)
(Fig. 1).
Comparison of serum protein changes
between the two groups before and after intervention
There were no statistically significant differences
in transferrin, pre-albumin, albumin and total protein between the
two groups before intervention (P>0.05). After intervention, the
expression of transferrin, pre-albumin, albumin and total protein
in the observation group was significantly increased compared to
that in the observation group before intervention and, the control
group after intervention (P<0.05) (Table I).
 | Table I.Comparison of serum protein changes
between the two groups before and after intervention (g/l, mean ±
SD). |
Table I.
Comparison of serum protein changes
between the two groups before and after intervention (g/l, mean ±
SD).
| Groups | Transferrin | Pre-albumin | Albumin | Total protein |
|---|
| Observation
group |
| Before
intervention |
1.52±0.03 |
0.20±0.01 |
32.15±0.09 |
56.89±0.15 |
| After
intervention |
1.98±0.06a,b |
0.26±0.01a,b |
38.98±0.12a,b |
69.53±0.09a,b |
| Control group |
| Before
intervention |
1.53±0.03 |
0.21±0.01 |
32.16±0.09 |
56.90±0.15 |
| After
intervention |
1.73±0.04a |
0.03±0.01a |
34.56±0.07a |
66.33±0.07a |
Comparisons of caloric intake and
protein metabolism in the body before and after intervention
There were no statistically significant differences
in the caloric intake, protein intake and urea nitrogen survival
rate between the two groups before intervention (P>0.05). After
intervention, the caloric intake, protein intake and urea nitrogen
survival rate in the observation group were significantly superior
to those in the observation group before intervention and the
control group after intervention (P<0.05) (Table II).
 | Table II.Comparisons of caloric intake and
protein metabolism in the body before and after intervention (mean
± SD). |
Table II.
Comparisons of caloric intake and
protein metabolism in the body before and after intervention (mean
± SD).
| Groups | Caloric intake
[kJ/(kg·day)] | Protein intake
[g/(kg·day)] | Urea nitrogen
survival rate (g/day) |
|---|
| Observation
group |
| Before
intervention |
123.5±2.5 |
0.78±0.11 |
12.3±0.05 |
| After
intervention |
149.6±3.3a,b |
0.96±0.12a,b |
15.1±0.12a,b |
| Control group |
| Before
intervention |
124.0±2.5 |
0.79±0.11 |
12.6±0.05 |
| After
intervention |
133.5±2.6a |
0.86±0.13a |
13.3.35±0.08a |
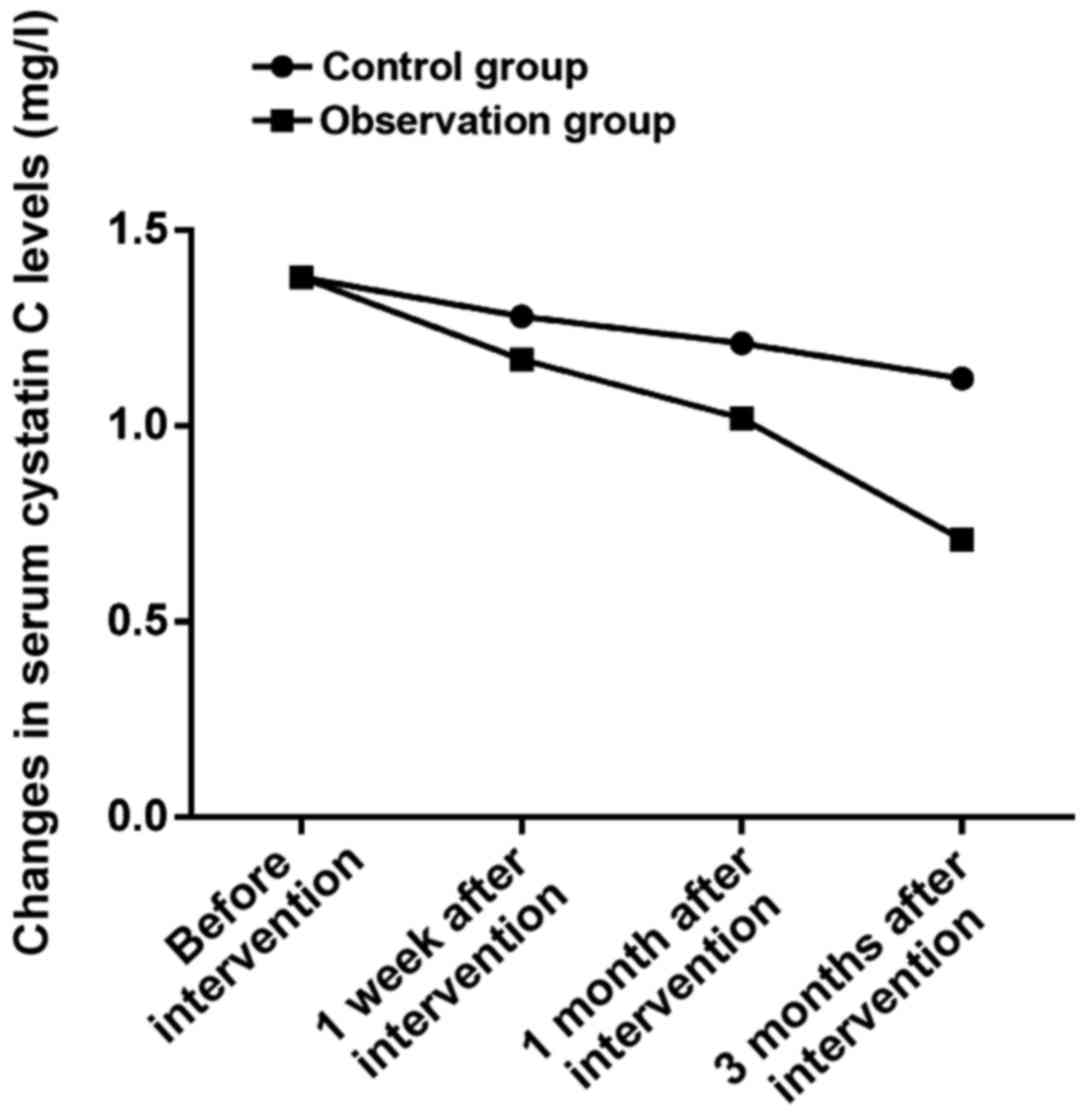
Changes in serum cystatin C levels in
the two groups during the intervention
There was no statistically significant difference in
the serum cystatin C level between the two groups before
intervention (P>0.05). At 1 week, 1 month and 3 months after
intervention, the levels of serum cystatin C in the observation
group were 1.15±0.02, 1.02±0.01 and 0.69±0.01 mg/l, which were
significantly lower than those in the control group (1.26±0.03,
1.21±0.02 and 1.10±0.02 mg/l) during the same period (t=19.295,
53.740 and 166.877, P<0.05) (Fig.
2).
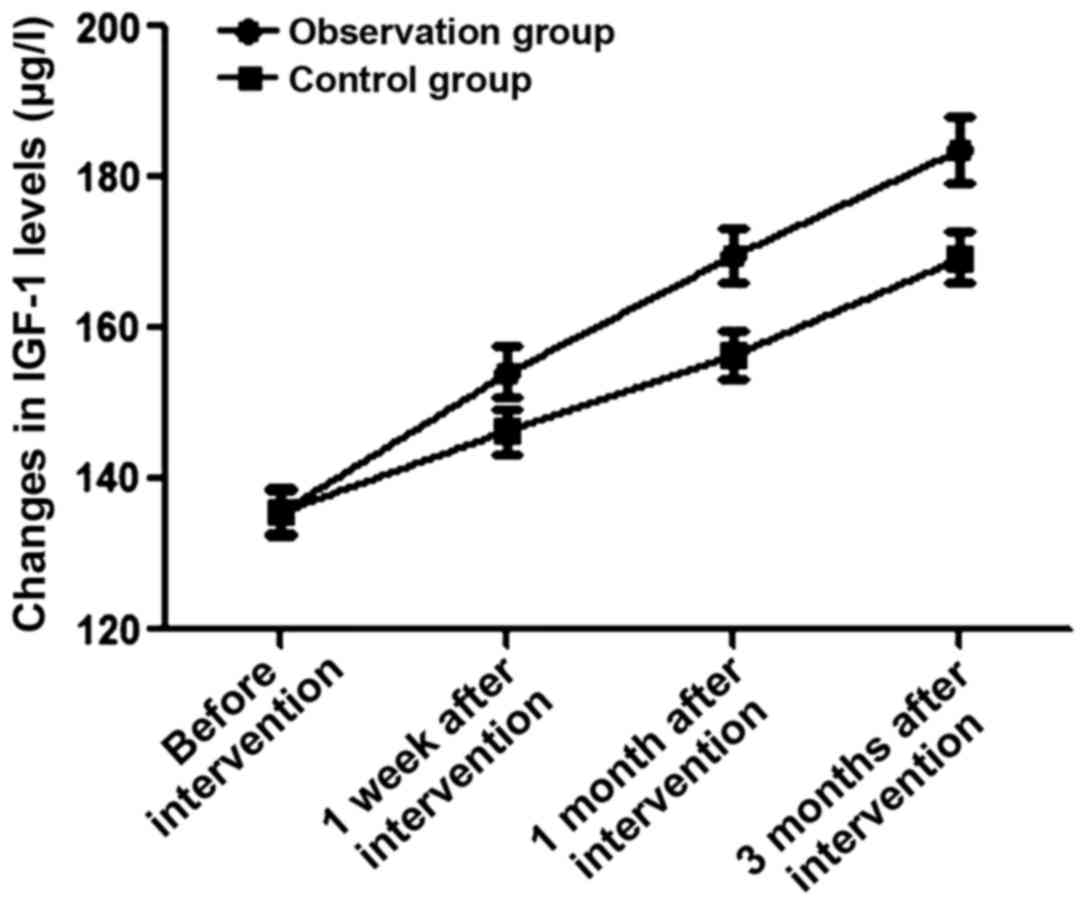
Changes in the IGF-1 level in the two
groups during intervention
There was no statistically significant difference in
the IGF-1 level between the two groups before intervention
(P>0.05). At 1 week, 1 month and 3 months after intervention,
the levels of IGF-1 in the observation group were 154.2±3.3,
169.6±3.6 and 183.6±4.3 µg/l, which were significantly lower than
those in the control group (146.3±3.0, 156.5±3.2 and 169.4±3.4
µg/l) during the same period (t=11.162, 16.856 and 16.254,
P<0.05) (Fig. 3).
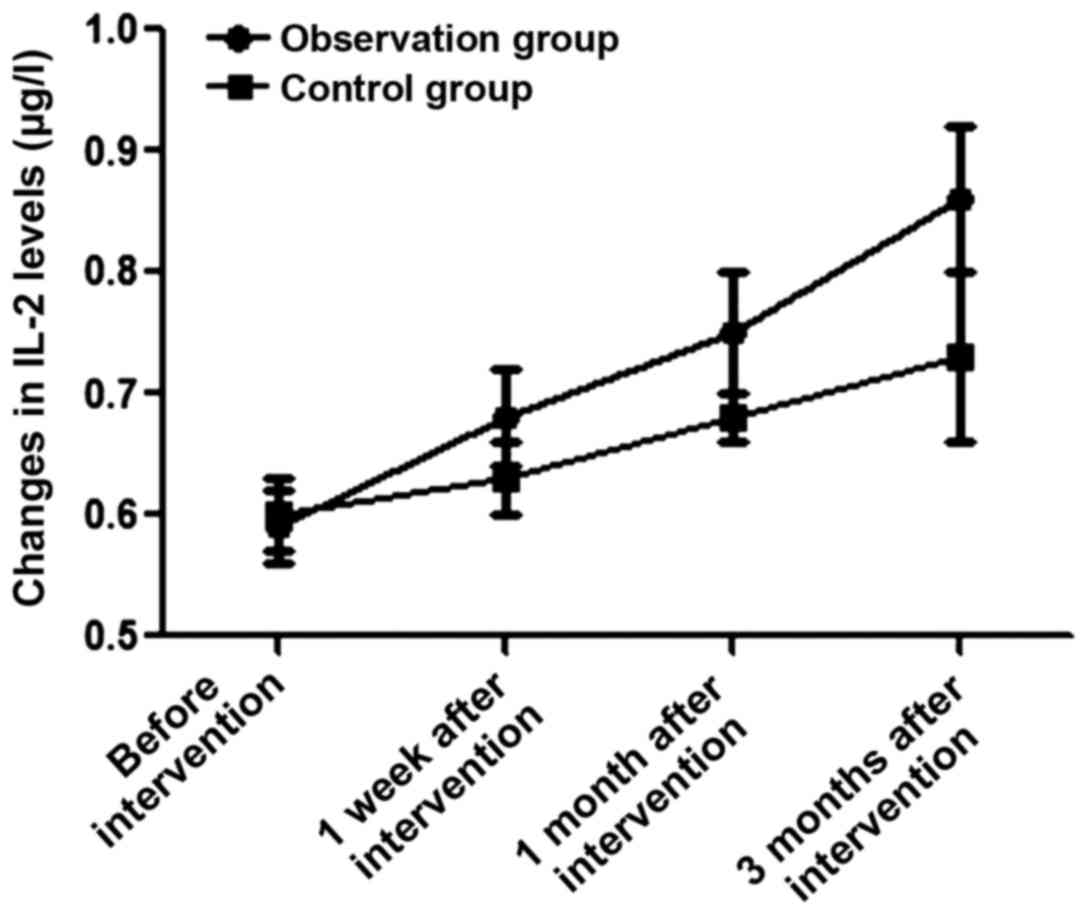
Changes in the IL-2 level in the two
groups during the intervention
There was no statistically significant differences
in the IL-2 level between the two groups before intervention
(P>0.05). At 1 week, 1 month and 3 months after intervention,
the levels of IL-2 in the observation group were 0.68±0.04,
0.75±0.05 and 0.86±0.06 µg/l, which were significantly lower than
those in the control group (0.63±0.03, 0.68±0.06 and 0.73±0.07
µg/l) during the same period (t=6.325, 5.668 and 8.918, P<0.05)
(Fig. 4).
Comparison of adverse reactions
between the two groups during treatment
The total proportion of allergy, systemic pruritus,
nausea and vomiting, abdominal distension and abdominal pain in the
observation group was obviously lower than that in the control
group (P<0.05) (Table III).
 | Table III.Comparison of adverse reactions
between the two groups during treatment [n (%)]. |
Table III.
Comparison of adverse reactions
between the two groups during treatment [n (%)].
| Variable | Allergy | Systemic
pruritus | Nausea and
vomiting | Abdominal distension
and abdominal pain | Total incidence
rate |
|---|
| Observation
group | 1 | 1 | 1 | 1 | 4
(10.0%) |
| Control group | 3 | 4 | 3 | 3 | 13 (32.5%) |
| χ2
test |
|
|
|
| 4.781 |
| P-value |
|
|
|
| 0.029 |
Discussion
The body of patients with nephrotic syndrome occurs
mostly at an accelerated catabolism status, combined with increased
protein consumption and loss and malnutrition (7). With the progression of disease, they
are accompanied with inflammatory hypoproteinemia and
hyperlipidemia (8). Additionally,
the body's nutritional metabolism is in a state of disorder, and
long-term malnutrition seriously affects the prognosis of patients
(9). Previous findings have shown
that hypoproteinemia is treated by the infusion of exogenous human
albumin for symptomatic support treatment; however, albumin is
expensive (10). Additionally, there
are certain infusion complications, leading to loss of albumin
while the total time of maintaining plasma protein after infusion
is short (11). Therefore, it cannot
fundamentally correct the hypoproteinemia and malnutrition of the
body. Traditional Chinese medicine has also been applied in recent
studies (12). However its treatment
period was long, taking effect slowly over a short-term period and
patient treatment compliance was poor (13). Long-term medication has a negative
impact on renal function (14).
Therefore, a clinically effective method with rapid effect is
needed for nephrotic syndrome at present (15), especially for hypoproteinemia and
increased inflammation in the body (16).
In this study, based on conventional treatment,
patients with nephrotic syndrome in the observation group were
treated with recombinant human growth hormone, while those in the
control group were treated with traditional Chinese medicine
Shenyankangfu tablets. The comparison of recovery time of clinical
symptoms between the two groups after intervention showed that the
improvement time of proteinuria, hypoproteinemia, edema and
hyperlipidemia in the observation group after intervention was
significantly shorter than that in the control group, indicating
that the application of recombinant human growth hormone can
improve the clinical symptoms of patients more quickly. In
addition, the study on protein metabolism and caloric intake in the
body before and after intervention revealed that the transferrin,
pre-albumin, albumin and total protein, and caloric intake, protein
intake and urea nitrogen survival rate in the observation group
after intervention were significantly superior to those in the
observation group before intervention and the control group after
intervention, suggesting that the application of recombinant human
growth hormone in patients with chronic nephrotic syndrome can
effectively improve the patient's protein metabolism and maintain
the positive nitrogen balance compared with the traditional Chinese
medicine Shenyankangfu tablets in the control group. At the same
time, the study on changes in serum cystatin C, IGF-1 and
IL-2 levels in the two groups during intervention showed
that at 1 week, 1 month and 3 months after intervention, the levels
of serum cystatin C, IGF-1 and IL-2 in the
observation group were obviously lower than those in the control
group during the same period, indicating that application of the
recombinant human growth hormone in patients with chronic nephrotic
syndrome has a positive significance effect in improving glomerular
filtration and reabsorption and reducing the body's inflammatory
response. Finally, it was found in the comparisons of adverse
reactions in the two groups during the treatment that the total
proportion of allergy, systemic pruritus, nausea and vomiting,
abdominal distension and abdominal pain in the observation group
was obviously lower than that in the control group, suggesting that
the intramuscular injection of recombinant human growth hormone has
fewer adverse reactions and higher safety.
Recombinant human growth hormone is a kind of
protein synthesis-promoting hormone in the body, which can reduce
protein catabolism, reduce urea nitrogen excretion and promote the
fat metabolism in the body (17),
thereby improving the efficiency of protein synthesis. In addition,
the recombinant human growth hormone has a certain effect of
improving the immune function and reducing the incidence of
infection (18). Through the
indirect growth-promoting effect and direct anti-insulin function,
it is used to improve the body's growth and development, increase
the protein synthesis rate, enhance the albumin messenger RNA
synthesis in liver (19), improve
the amino acid utilization of muscle tissues, and promote the
body's anabolism (20).
In conclusion, compared with the traditional Chinese
medicine Shenyankangfu tablets applied in the control group, the
recombinant human growth hormone used for patients with chronic
nephrotic syndrome can improve the clinical symptoms more quickly,
regulate the protein metabolism and reduce the inflammatory
response in the body, which also has less adverse reactions and
higher safety.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GD was involved in drafting the manuscript and data
analysis. DW helped with acquisition of data. HD performed and
analyzed ELISA. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zhumadian Central Hospital (Zhumadian, China). Written informed
consents were signed by the patients and/or guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Akchurin OM, Kogon AJ, Kumar J, Sethna CB,
Hammad HT, Christos PJ, Mahan JD, Greenbaum LA and Woroniecki R:
Approach to growth hormone therapy in children with chronic kidney
disease varies across North America: The Midwest Pediatric
Nephrology Consortium report. BMC Nephrol. 18:1812017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cho SM, Lee SH, Lee D, Lee JH, Chang GT,
Kim H and Lee JY: The Korean herbal formulation Yukmijihwangtang
stimulates longitudinal bone growth in animal models. BMC
Complement Altern Med. 17:2392017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cernaro V, Lucisano S, Canale V, Bruzzese
A, Caccamo D, Costantino G, Buemi M and Santoro D: Acetate-free
biofiltration to remove fibroblast growth factor 23 in hemodialysis
patients: A pilot study. J Nephrol. 11:1007–1010. 2017.
|
|
4
|
Johar DR and Bernstein LH: Biomarkers of
stress-mediated metabolic deregulation in diabetes mellitus.
Diabetes Res Clin Pract. 126:222–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH
and Park MH: Interleukin 32, inflammation and cancer. Pharmacol
Ther. 174:127–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stephen J, Vilboux T, Mian L, Kuptanon C,
Sinclair CM, Yildirimli D, Maynard DM, Bryant J, Fischer R,
Vemulapalli M, et al: NISC Comparative Sequencing Program:
Mutations in KIAA0753 cause Joubert syndrome associated with growth
hormone deficiency. Hum Genet. 136:399–408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fragiadaki M, Lannoy M, Themanns M, Maurer
B, Leonhard WN, Peters DJ, Moriggl R and Ong AC: STAT5 drives
abnormal proliferation in autosomal dominant polycystic kidney
disease. Kidney Int. 91:575–586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Othman EM, Naseem M, Awad E, Dandekar T
and Stopper H: The plant hormone cytokinin confers protection
against oxidative stress in mammalian cells. PLoS One.
11:e01683862016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Curran IH, Liston V, Nunnikhoven A,
Caldwell D, Scuby MJ, Pantazopoulos P, Rawn DF, Coady L, Armstrong
C, Lefebvre DE, et al: Toxicologic effects of 28-day dietary
exposure to the flame retardant
1,2-dibromo-4-(1,2-dibromoethyl)-cyclohexane (TBECH) in F344 rats.
Toxicology. 377:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bu P, Le Y, Zhang Y and Cheng X: Hormonal
and chemical regulation of the Glut9 transporter in mice. J
Pharmacol Exp Ther. 360:206–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Dutra EH, Reichenberger EJ and Chen
IP: Dietary phosphate supplement does not rescue skeletal phenotype
in a mouse model for craniometaphyseal dysplasia. J Negat Results
Biomed. 15:182016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Batra J, Buttar RS, Kaur P, Kreimerman J
and Melamed ML: FGF-23 and cardiovascular disease: Review of
literature. Curr Opin Endocrinol Diabetes Obes. 23:423–429. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zvereva I, Semenistaya E, Krotov G and
Rodchenkov G: Comparison of various in vitro model systems of the
metabolism of synthetic doping peptides: Proteolytic enzymes, human
blood serum, liver and kidney microsomes and liver S9 fraction. J
Proteomics. 149:85–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao S, Vickers MH, Evans A, Stanley JL,
Baker PN and Perry JK: Comparison of pulsatile vs. continuous
administration of human placental growth hormone in female C57BL/6J
mice. Endocrine. 54:169–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobalava Z: SP 04-1 The role of
natriuretic peptides in the pathogenesis of cardiovascular
diseases. J Hypertens. 3 Suppl 1:e3772016. View Article : Google Scholar
|
|
16
|
Morgan SJ, Neumann S, Marcus-Samuels B and
Gershengorn MC: Thyrotropin and insulin-like growth factor 1
receptor crosstalk upregulates sodium-iodide symporter expression
in primary cultures of human thyrocytes. Thyroid. 26:1794–1803.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Angelis M, Giesert F, Finan B,
Clemmensen C, Müller TD, Vogt-Weisenhorn D, Tschöp MH and Schramm
KW: Determination of thyroid hormones in mouse tissues by
isotope-dilution microflow liquid chromatography-mass spectrometry
method. J Chromatogr B Analyt Technol Biomed Life Sci.
1033–1034:413–420. 2016. View Article : Google Scholar
|
|
18
|
Czarnecka AM, Matak D, Szymanski L,
Czarnecka KH, Lewicki S, Zdanowski R, Brzezianska-Lasota E and
Szczylik C: Triiodothyronine regulates cell growth and survival in
renal cell cancer. Int J Oncol. 49:1666–1678. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sexton DJ, O'Reilly MW, Geoghegan P,
Kinsella SM, Moran PJ and O'Regan AW: Serum fibroblastic growth
factor 23 in acute Sarcoidosis and normal kidney function.
Sarcoidosis Vasc Diffuse Lung Dis. 33:139–142. 2016.PubMed/NCBI
|
|
20
|
Nguyen-Yamamoto L, Karaplis AC, St-Arnaud
R and Goltzman D: Fibroblast growth factor 23 regulation by
systemic and local osteoblast-synthesized 1,25-dihydroxyvitamin D.
J Am Soc Nephrol. 28:586–597. 2017. View Article : Google Scholar : PubMed/NCBI
|