Introduction
Disseminated intravascular coagulation (DIC) is a
severe clinical condition that is caused by an underlying disease
(1). The primary symptom is marked,
continuous, widespread coagulation activation in circulating blood
and the formation of microvascular thrombi (1). It has been reported that the underlying
diseases that most commonly cause DIC include sepsis, aortic
aneurysm, solid cancer, clotting disorders, acute promyelocytic
leukemia and fibrinolysis (2).
Sepsis-induced DIC models have been investigated by many studies
(3–5).
Sepsis is characterized by physiologic, pathologic
and biochemical abnormalities and has been identified as a
life-threatening organ dysfunction (6). Sepsis arises when the body's response
to infection injures its own tissues and organs and severe sepsis
may cause serious complications (7),
including acute kidney injury (8).
Renal inflammation is one of the main pathological changes observed
in acute kidney injury (9).
Lipopolysaccharide (LPS), an important inflammatory factor, is the
product of Gram-negative bacteria and has been used to establish
renal inflammation models for the investigation of
inflammation-associated renal diseases (10–12).
At present, 13 mammalian aquaporins (AQPs) have been
identified, comprising two subgroups: Water-selective channels
called orthodox AQPs and aquaglyceroporins that may be permeated by
water, glycerol and other small molecules (13). Of the known AQPs, eight (AQP-1, −2,
−3, −4, −6, −7, −8 and −11) are expressed in mammalian kidneys
(13). However, the expression and
functions of AQPs in LPS-induced HK-2 cells remain to be
elucidated. In the present study, it was demonstrated that, of the
eight AQPs expressed, AQP-1 had the lowest expression in
LPS-induced HK-2 cells. It has been reported that AQP-1
overexpression in hepatocytes improves LPS-induced cholestasis
(14). Furthermore, a previous study
demonstrated that AQP-1 overexpression inhibits the aristolochic
acid I-induced epithelial-mesenchymal transition of HK-2 cells,
suggesting that AQP-1 may be a target for aristolochic acid
nephropathy clinical therapy (15).
AQP-1 overexpression significantly reversed LPS-induced damage in
HK-2 cells and reduced the levels of tumor necrosis factor (TNF)-α,
interleukin (IL)-8, IL-1β and monocyte chemoattractant protein
(MCP)-1. The results of the present study also revealed that
LPS-induced p38 and extracellular signal-regulated kinase (ERK)1/2
pathways were blocked in AQP-1-overexpressing cells and were
aggravated in AQP-1-knockdown cells. However, AQP-1 did not affect
the c-Jun N-terminal kinase (JNK) pathway. These results suggest
that AQP-1 is able to reverse the LPS-induced decrease in cell
viability, increase in apoptosis and inflammation in HK-2 cells,
possibly via the p38 and ERK1/2 pathways.
Materials and methods
Cell culture and transfection
Renal tubular epithelial HK-2 cells (a proximal
tubular cell line derived from normal kidney; CRL-2190), were
purchased from the ATCC (Manassas, VA, USA) and incubated at 37°C
for 2 days in a humidified atmosphere containing 5% CO2
with DMEM-F12 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin.
Cell transfection
HK-2 cells were transfected with 100 nM pcDNA3.1,
pcDNA-AQP-1, small interfering-negative control RNA (si-NC) or
si-AQP-1 for 48 h (Guangzhou RiboBio Co., Ltd., Guangzhou, China)
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols.
Following transfection, cells were stimulated with 8 µg/ml LPS
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and harvested.
Total RNA and protein prepared from HK-2 cells were used to check
the levels of mRNA or proteins.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HK-2 cells using TRIzol
reagent (Axygen; Corning Incorporated, Corning, NY, USA) according
to the manufacturer's protocols. Purifed RNA (0.5 µg/µl) was mixed
with nuclease-free water for the cDNA synthesis using a Script cDNA
Synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at
37°C for 15 min and 85°C for 5 sec. RT-qPCR was performed using a
Bio-Rad CFX96 real-time PCR System (Bio-Rad Laboratories, Inc.).
The qPCR reaction volume was 50 µl and included 1 µl dNTPs (10 mM),
5 µl cDNA solution, 1 µl of each primer (50 pM), 1 µl Taq DNA
polymerase (Invitrogen; Thermo Fisher Scientific, Inc.), 33 µl
water, 3 µl of MgCl2 (15 mM), and 5 µl of PCR buffer
(10X). The amplification was performed according to the following
condition: Denaturation was performed at 94°C for 1 min, annealing
at 59°C for 1 min and elongation at 72°C for 1 min for 32 cycles,
followed by 72°C for 10 min. The expression of AQP-1, AQP-2, AQP-3,
AQP-4, AQP-6, AQP-7, AQP-8, AQP-11, TNF-α, IL-8, IL-1β and MCP-1
was detected by using the SYBR-Green PCR kit (Takara Bio, Inc.,
Otsu, Japan). Primers are listed in Table I and GAPDH was used as an internal
reference. Results were normalized using the 2−ΔΔCq
method (16) and each experiment was
performed in triplicate.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Direction | Primer sequence
(5′-3′) |
|---|
| AQP-1 | Forward |
CTGGGCATCGAGATCATCGG |
|
| Reverse |
ATCCCACAGCCAGTGTAGTCA |
| AQP-2 | Forward |
GCTCCGCTCCATAGCCTTC |
|
| Reverse |
GGGTGCCAATACCCAAGCC |
| AQP-3 | Forward |
GGGGAGATGCTCCACATCC |
|
| Reverse |
AAAGGCCAGGTTGATGGTGAG |
| AQP-4 | Forward |
AGCAGTCACAGCGGAATTTCT |
|
| Reverse |
TCTGTTCCACCCCAGTTGATG |
| AQP-6 | Forward |
GTCTTCGCTTCCACCGACAG |
|
| Reverse |
GCGGGCTGGATTCATGGAG |
| AQP-7 | Forward |
ACCCGTGGCTCCAAAATGG |
|
| Reverse |
GGAACCAAGGCCGAATACCA |
| AQP-8 | Forward |
GCGAGTGTCCTGGTACGAAC |
|
| Reverse |
CAGGCACCCGATGAAGATGAA |
| AQP-11 | Forward |
TGACCCAGTATCACGTCAGC |
|
| Reverse |
TGACCGCTTTGAGCAAGTCG |
| Tumor necrosis
factor-α | Forward |
CACCACTTCGAAACCTGGGA |
|
| Reverse |
TGTAGGCCCCAGTGAGTTCT |
| IL-8 | Forward |
ACCACCGGAAGGAACCATCT |
|
| Reverse |
AGCACTCCTTGGCAAAACTG |
| IL-1β | Forward |
AACCTCTTCGAGGCACAAGG |
|
| Reverse |
GGCGAGCTCAGGTACTTCTG |
| Monocyte
chemoattractant protein-1 | Forward |
GATCTCAGTGCAGAGGCTCG |
|
| Reverse |
TTTGCTTGTCCAGGTGGTCC |
| GAPDH | Forward |
CACCCACTCCTCCACCTTTG |
|
| Reverse |
CCACCACCCTGTTGCTGTAG |
Cell viability
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay was performed to assess
cell viability. Normal or transfected HK-2 cells were seeded in
96-well plates at a density of 2×104 cells/well for 24
h. Following LPS treatment, CCK-8 reagents were separately added to
well. The cell viability rates were assessed by measuring the
optical density at 450 nm using a microplate reader (Thermo Fisher
Scientific, Inc.).
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) analysis
Flow cytometry was performed to quantify apoptotic
cells using an Annexin V-FITC/PI Apoptosis Detection kit
(BioVision, Inc., Milpitas, CA, USA). HK-2 cells were harvested and
washed in ice-cold PBS twice and double-stained with Annexin V-FITC
and PI at room temperature for 30 min in the dark. All samples were
quantitatively analyzed using a FACSCalibur flow cytometer at 488
nm emission and 570 nm excitation (BD Biosciences, San Jose, CA,
USA) and analyzed by CellQuest software (version 3.0; BD
Biosciences).
Apoptosis detection
According to a previous study (17), apoptosis was determined using a Cell
Death Detection ELISAPLUS kit (cat. no. 11774425001;
Roche Diagnostics, Basel, Switzerland) that measures cytoplasmic
DNA-histone complexes generated during apoptotic DNA fragmentation.
Cell apoptosis detection was performed according to the
manufacturer's protocol and monitored at 405 nm.
Caspase-3 activity assay
A caspase-3 fluorescent assay kit (Nanjing KeyGen
Biotech. Co., Ltd., Nanjing, China) was used to detect caspase
activity according to previous study (14). Briefly, cells were lysed using the
lysis buffer provided by the kit and centrifuged at 10,000 × g for
1 min at 4°C. Supernatants were collected, equal amounts (30 µg) of
protein were reacted with the synthetic fluorescent substrates,
which were provided by the kit, at 37°C for 90 min and fluorescence
was measured at 405 nm using a microplate reader.
Western blot analysis
Following treatment, HK-2 cells were washed twice in
cold PBS and lysed in radioimmunoprecipitation assay lysis buffer
(EMD Millipore, Billerica, MA, USA) with protease inhibitor
cocktail (Roche Diagnostics) to extract proteins. The protein
concentration was quantified using a BCA Protein Assay kit
(Beyotime Institute of Biotechnology, Haimen, China) and samples
(50 µg/lane) were separated by 10% SDS-PAGE. Blots were transferred
to polyvinylidene fluoride membranes (EMD Millipore) bloc which
were subsequently blocked in 5% skimmed milk diluted with TBST at
room temperature for 2 h and incubated overnight at 4°C with the
following primary antibodies: Anti-AQP-1 antibody (ab15080;
1:1,000; Abcam, Cambridge, MA, USA), phosphorylated (p)-p38
(#4511), total (t)-p38 (#8690), p-ERK1/2 (#8544), t-ERK1/2 (#4695),
p-JNK (#9251), t-JNK (#9252), anti-B-cell lymphoma (Bcl)-2 antibody
(#3498) and anti-v-2-associated protein X (Bax) antibody (#2772;
all 1:1,000; all Cell Signaling Technology, Inc., Danvers, MA,
USA). Membranes were subsequently incubated with a goat anti-mouse
IgG conjugated to horseradish peroxidase (#7076; 1:1,000; Cell
Signaling Technology, Inc.) for 2 h at room temperature. Proteins
were visualized using enhanced chemiluminescence-plus reagents (GE
Healthcare Life Sciences, Little Chalfont, UK). The density of the
bands was measured using the Image J software (version 1.45s;
National Institutes of Health, Bethesda, MA, USA) and values were
normalized to the densitometric values of α-tubulin (T5168;
1:1,000; Sigma-Aldrich; Merck KGaA), GAPDH (#5174) or β-actin
(#3700; both 1:1,000; both Cell Signaling Technology, Inc.).
Measurement of TNF-α, IL-8, IL-1β and
MCP-1 levels
The supernatants of HK-2 cells were collected after
treatment and the concentrations of TNF-α, IL-8, IL-1β and MCP-1
were measured using a sandwich ELISA kit (R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's instruction
and as previously described (9).
Briefly, the primary antibody (provided in kit) was coated onto
ELISA plates and incubated for 2 h at room temperature. Samples and
standards were added to the wells and incubated for 1 h, following
which wells were washed and a biotinylated antibody (provided in
kit) was added for 1 h at room temperature. The plates were washed
again and streptavidin conjugated to horseradish peroxidase was
added for 10 min at room temperature. Plates were washed and
tetramethylbenzidine was added for color development about 30 min
at room temperature and the reaction was terminated with 1 mol/l
H2SO4. Absorbance was measured at 490 nm by
using an automated ELISA reader (Thermo Fisher Scientific, Inc.).
Concentrations in the samples were calculated using a standard
curve and values were expressed as pg/ml.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Data are presented as the mean ± standard error of the mean. Data
were statistically analyzed using a two-tailed Student's t-test or
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
LPS-induced injury in HK-2 cells
HK-2 cells were stimulated with 0, 1, 2, 4, 8 and 16
µg/ml LPS for 8 h and the results indicated that cell viability was
significantly decreased with 2 µg/ml LPS (Fig. 1A). HK-2 cells were stimulated with 8
µg/ml LPS for 0, 1.5, 3, 6, 12 and 24 h and cell viability was
significantly decreased following 6 h treatment (Fig. 1B). These results suggest that LPS is
able to damage HK-2 cells in a concentration- and time-dependent
manner and 8 µg/ml LPS treatment for 12 h was selected for
following experiments. Flow cytometry and ELISA assays revealed
that LPS dramatically induced apoptosis inHK-2 cells (Fig. 1C and D). Furthermore, Bax expression
was increased and Bcl-2 expression was decreased following LPS
treatment (Fig. 1E). A caspase-3
assay was performed and the results revealed that LPS treatment
significantly increase caspase-3 activity (Fig. 1F).
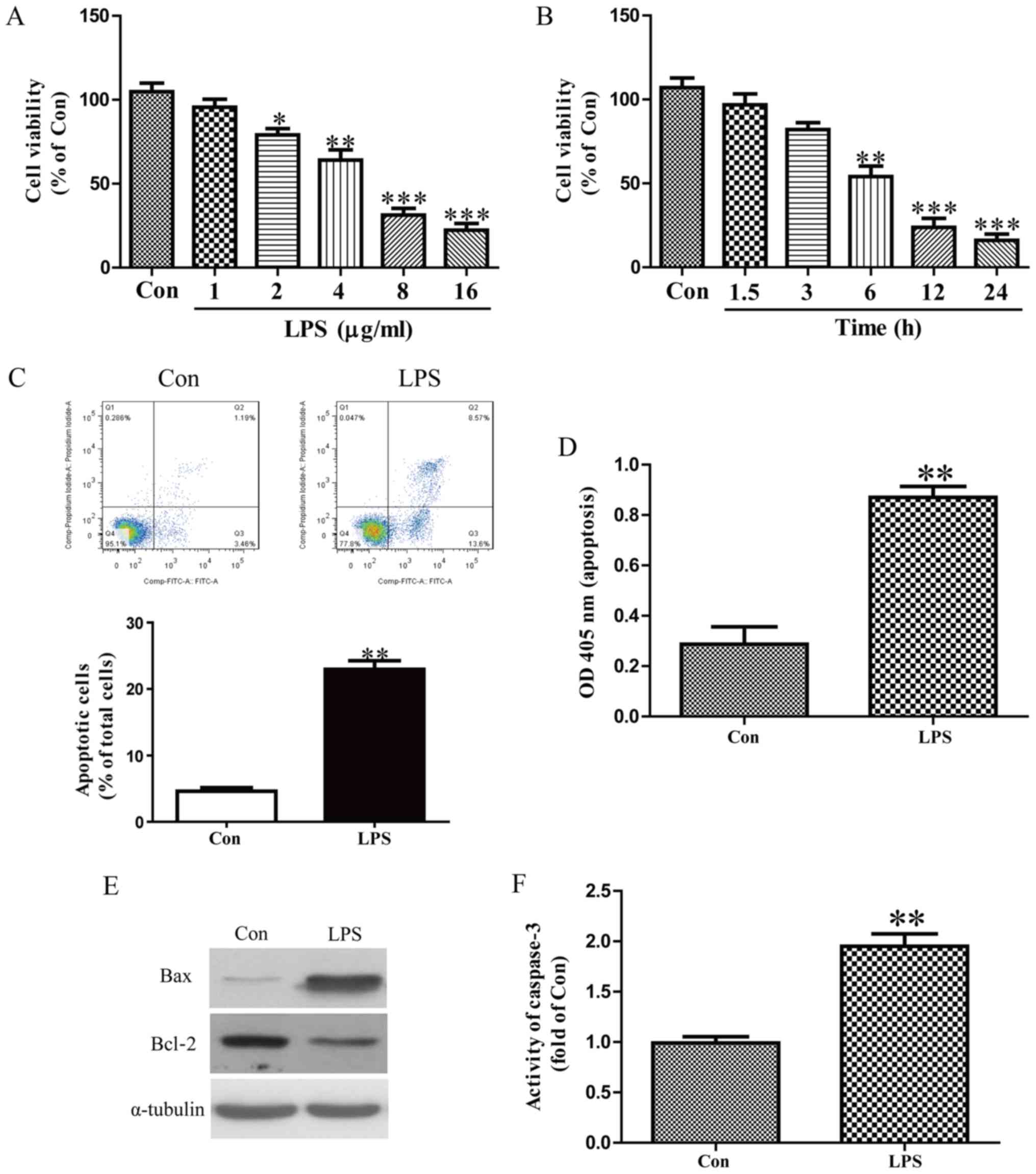 | Figure 1.LPS-induced damage in HK-2 cells. HK-2
cells were treated with (A) 0, 1, 2, 4, 8 and 16 µg/ml of LPS for 8
h or (B) 8 µg/ml LPS for 0, 1.5, 3, 6, 12 and 24 h. HK-2 cells were
treated with 8 µg/ml LPS for 12 h and cell apoptosis was measured
by (C) flow cytometric analysis and (D) nucleosomal degradation,
respectively. (E) The expression of Bcl-2 and Bax were determined
by western blotting. (F) The activity of caspase-3 was determined
using a caspase-3 activity detection assay. *P<0.05, **P<0.01
and ***P<0.001 vs. Con. LPS, lipopolysaccharide; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated protein X; Con, control; FITC,
fluorescein isothiocyanate; OD, optical density. |
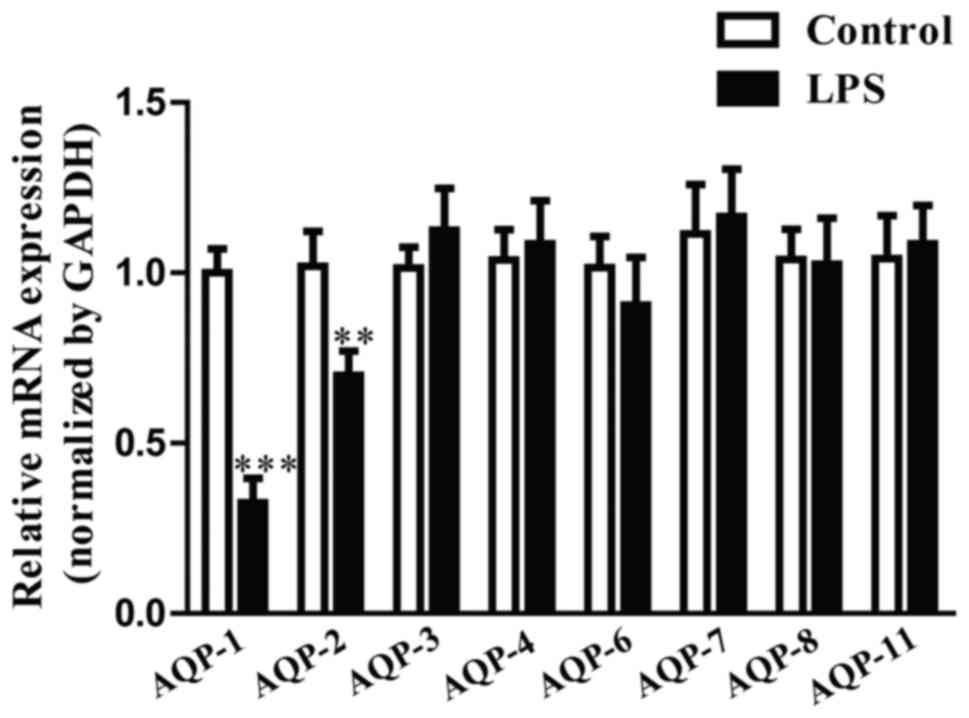
AQP-1 expression in LPS-induced HK-2
cells
AQP-1, −2, −3, −4, −6, −7, −8 and −11 are expressed
in the mammalian kidney (13).
However, the expression and function of these genes in
LPS-stimulated HK-2 cells remain to be elucidated. The results of
the present study revealed that AQP-1 mRNA expression levels were
the lowest of the AQP genes in LPS-induced HK-2 cells (Fig. 2). AQP-1 was therefore selected for
the following experiments.
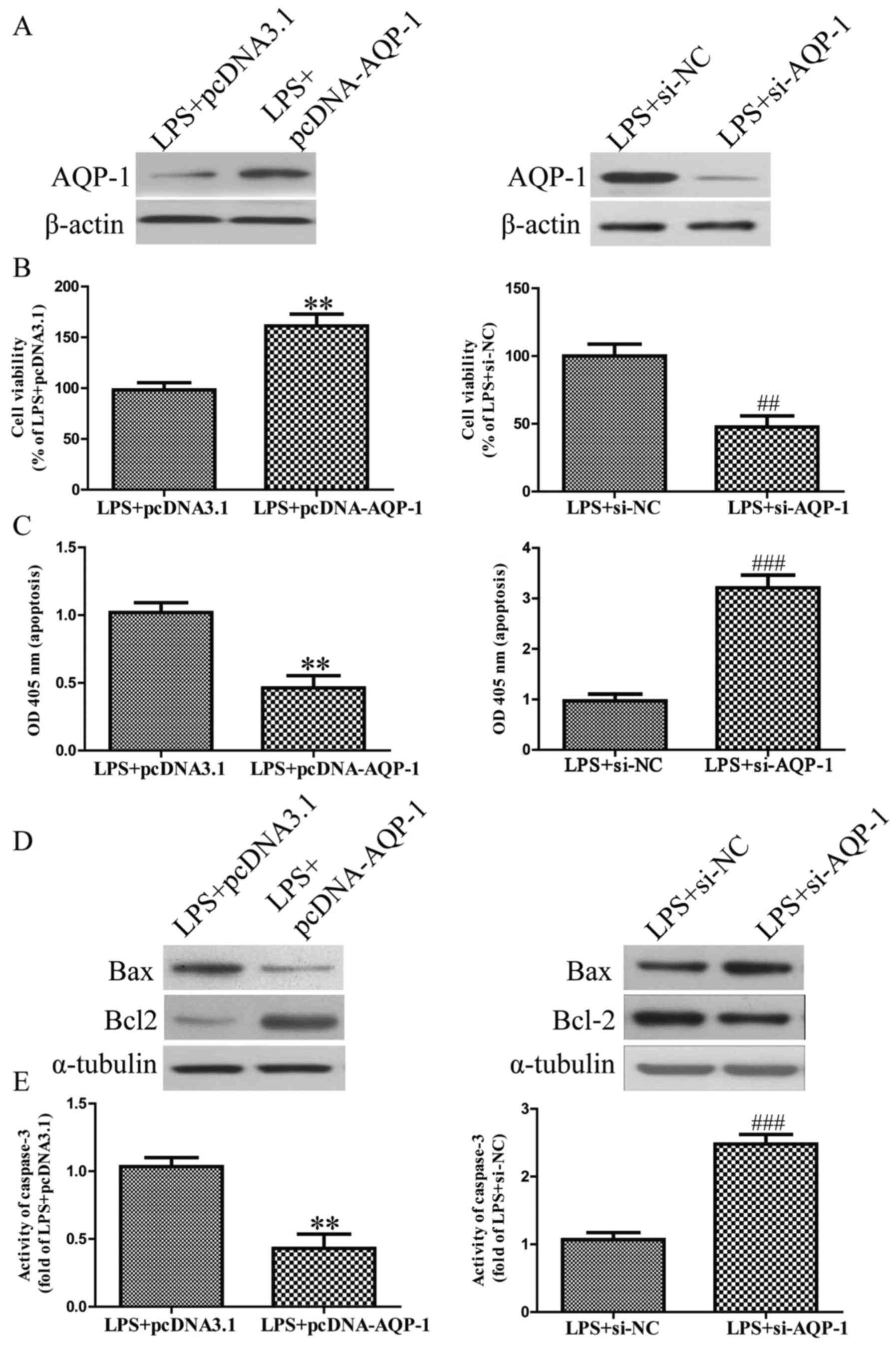
The effects of AQP-1 in LPS-stimulated
apoptosis in HK-2 cells
Following transfection with pcDNA-AQP-1 or si-AQP-1,
the expression of AQP-1 was significantly increased or decreased in
HK-2 cells, respectively (Fig. 3A).
The results revealed that AQP-1 overexpression significantly
increased the viability of LPS-induced HK-2 cells (Fig. 3B). Furthermore, AQP-1 overexpression
significantly reversed the LPS-induced increase in apoptosis
(Fig. 3C). Furthermore, AQP-1
overexpression significantly downregulated Bax expression and
upregulated Bcl2 expression (Fig.
3D), as well as inhibiting caspase-3 activity in LPS-induced
HK-2 cells (Fig. 3E). However, AQP-1
knockdown aggravated the pro-apoptotic effect of LPS on HK-2 cells
(Fig. 3A-E). These results indicate
that AQP-1 overexpression is able to effectively protect HK-2 cells
from LPS-induced apoptosis.
Effect of AQP-1 on LPS-induced
inflammatory cytokine and chemokine expression in HK-2 cells
The expression levels of TNF-α, IL-8, IL-1β and
MCP-1 mRNA and protein were significantly upregulated in
LPS-induced HK-2 cells (Figs. 4 and
5). LPS-induced increases in TNF-α,
IL-8, IL-1β and MCP-1 mRNA and protein were significantly
aggravated in si-AQP-1-transfected HK-2 cells (Fig. 4). However, the effects of LPS on
TNF-α, IL-8, IL-1β and MCP-1 were significantly reduced in
pcDNA-AQP-1-transfected HK-2 cells (Fig.
5).
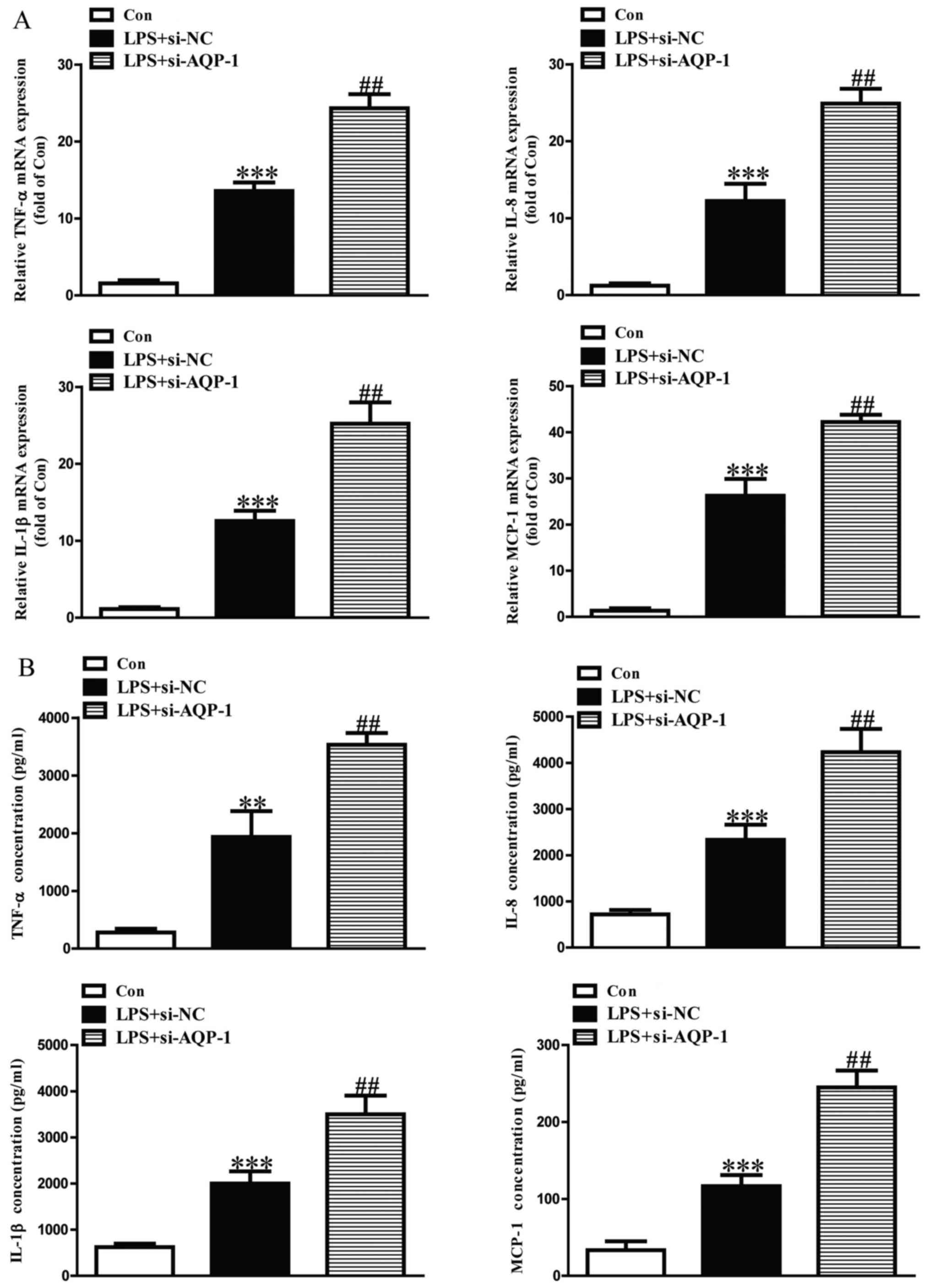 | Figure 4.Effects of AQP-1 knockdown on
LPS-induced inflammatory cytokine and chemokine expression in HK-2
cells. Following transfection with si-AQP-1, HK-2 cells were
treated with 8 µg/ml LPS for 12 h. The expression levels of TNF-α,
IL-8, IL-1β and MCP-1 protein and mRNA were determined by (A)
reverse transcription-quantitative polymerase chain reaction and
(B) ELISA, respectively. The Con group was treated with vehicle
alone. **P<0.01 and ***P<0.001 vs. Con.
##P<0.01 vs. LPS+si-NC. AQP, aquaporin; LPS,
lipopolysaccharide; si, small interfering RNA; TNF, tumor necrosis
factor; IL, interleukin; MCP, monocyte chemoattractant protein;
Con, control; NC, negative control. |
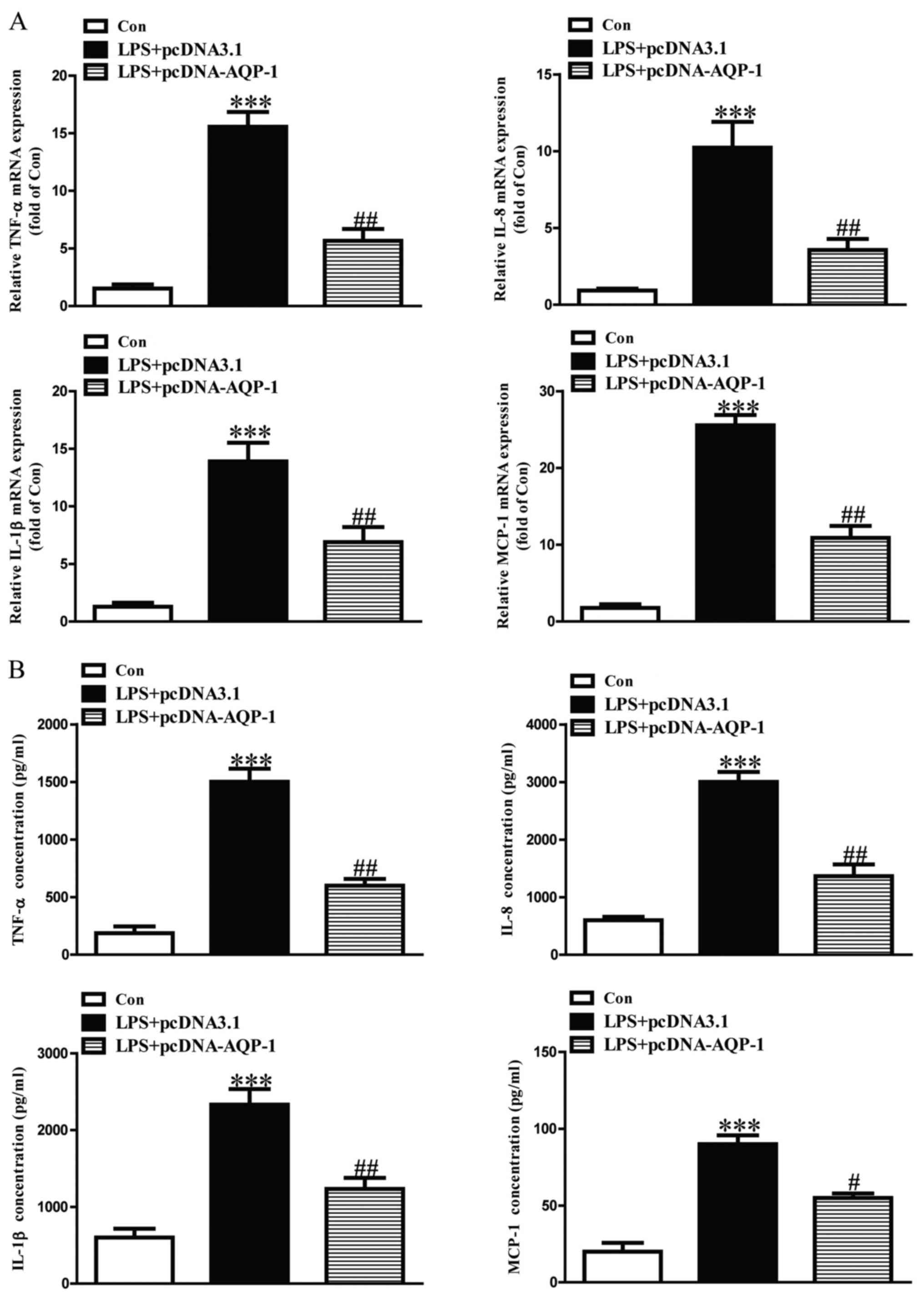 | Figure 5.Effects of AQP-1 overexpression on
LPS-induced inflammatory cytokines and chemokines in HK-2 cells.
Following transfection with pcDNA-AQP-1, HK-2 cells were treated
with 8 µg/ml LPS for 12 h. The expression levels of TNF-α, IL-8,
IL-1β and MCP-1 protein and mRNA were determined by (A) reverse
transcription-quantitative polymerase chain reaction and (B) ELISA,
respectively. The Con group was treated with vehicle alone.
***P<0.001 vs. Con. #P<0.05 and
##P<0.01 vs. LPS+pcDNA3.1. AQP, aquaporin; LPS,
lipopolysaccharide; TNF, tumor necrosis factor; IL, interleukin;
MCP, monocyte chemoattractant protein; Con, control; NC, negative
control; si, small interfering RNA. |
Effects of AQP-1 on LPS-induced renal
MAPK p38, ERK1/2 and JNK signaling pathways
The effects of AQP-1 on LPS-stimulated MAPK p38,
ERK1/2 and JNK phosphorylation in HK-2 cells were assessed. The
results revealed that AQP-1 knockdown increased the LPS-induced
upregulation of p-p38 and p-ERK1/2 (Fig.
6A). By contrast, the expression of p-p38 and p-ERK1/2 was
significantly reduced in HK-2 cells followed by transfection with
pcDNA-AQP-1 (Fig. 6B). The
expression of t-p38 and t-ERK1/2 protein was not affected. No
significant differences in p-JNK or t-JNK were observed between
groups (Fig. 6A and B). These
results suggest that AQP-1 may protect HK-2 cells against
LPS-induced inflammation and apoptosis via inhibiting the p38 and
ERK1/2 signaling pathways.
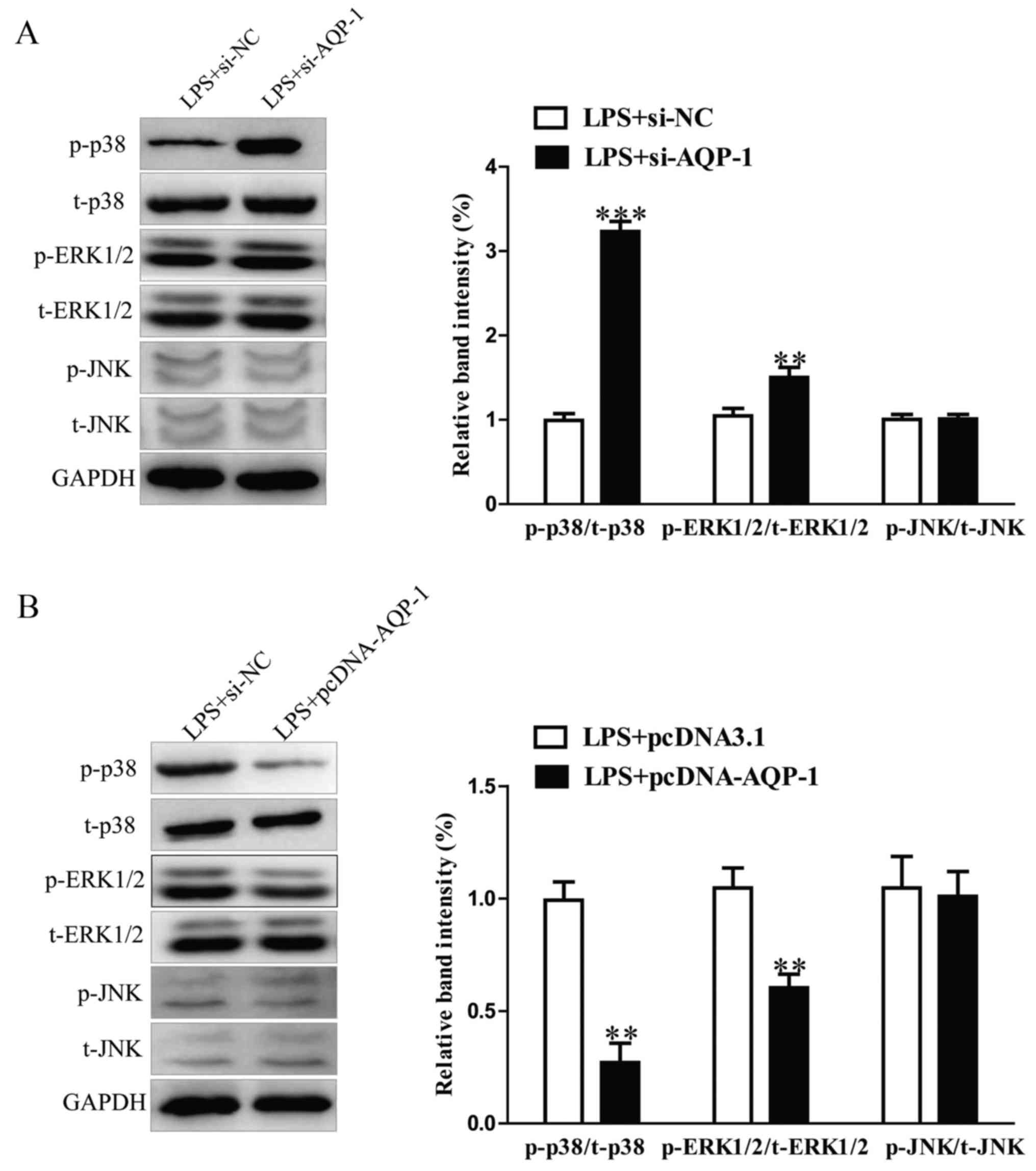 | Figure 6.Effects of AQP-1 on LPS-activated
renal MAPK p38, ERK1/2 and JNK signaling. Following transfection
with pcDNA-AQP-1 or si-AQP-1, HK-2 cells were treated with 8 µg/ml
LPS for 12 h. (A and B) The p-p38, t-p38, p-ERK1/2, t-ERK1/2, p-JNK
and t-JNK protein levels were measured using western blotting.
**P<0.01, ***P<0.001 vs. LPS+pcDNA3.1 or LPS+si-NC. AQP,
aquaporin; LPS, lipopolysaccharide; MAPK, mitogen activated protein
kinase; ERK, extracellular signal-regulated kinase; JNK, c-JUN
N-terminal kinase; si, small interfering RNA; p, phosphorylated; t,
total; NC, negative control. |
Discussion
The aim of the present study was to determine the
functional roles of AQP-1 in LPS-stimulated HK-2 cells, an in
vitro septic acute kidney injury model of DIC, and to elucidate
the mechanism of AQP-1 in this model. The results revealed that LPS
treatment decreased HK-2 cell viability as well as increasing
apoptosis and the expression of proinflammatory cytokines and
chemokines. AQP-1 upregulation served a protective role by
reversing LPS-induced damage in HK-2 cells. Furthermore, AQP-1 was
able to modulate the p38 and ERK1/2 signaling pathways.
It has been reported that LPS treatment induces
apoptosis and caspase-3 cleavage in tubular epithelial cells
(18). In accordance with previous
reports, the results of the present study demonstrated that LPS
treatment dramatically reduced the viability of HK-2 cells and
induced cell apoptosis (8). Previous
studies have reported that the expression of AQP-1 and AQP-5 was
significantly reduced in rats with LPS-induced acute lung injury
(19–22). Furthermore, Jin et al
(23) demonstrated that the
expression of AQP5 was downregulated in a rat model of LPS-induced
lung DIC (23). The expression of
AQP-2 has been reported to be decreased in rats with LPS-induced
acute kidney injury (24). However,
the expression and role of AQPs in LPS-induced acute kidney injury
remain to be elucidated. In the present study, the expression
levels of AQPs in LPS-stimulated HK-2 cells were assessed in
vitro and the results revealed that AQP-1 levels were the
lowest of the eight AQPs expressed in HK-2 cells. The effects of
LPS on cell viability and apoptosis were partially reversed by
AQP-1 overexpression, suggesting that AQP-1 protects against
LPS-induced injury in HK-2 cells. It was also demonstrated AQP-1
knockdown aggravated the LPS-induced decrease in the Bax/Bcl-2
ratio and activated caspase-3, whereas overexpression of AQP-1
inhibited LPS-induced apoptosis by increasing the Bax/Bcl-2 ratio
and increasing caspase 3 activity.
It has been reported that LPS stimulation increases
the expression of proinflammatory cytokines and chemokines,
including TNF-α, IL-1β and IL-6 in human HK-2 cells, resulting in
endotoxemia and sepsis (25). The
results of the present study support these findings. AQP-1
overexpression partially the effects of LPS, whereas AQP-1
knockdown aggravated them. The results of the present study suggest
that AQP-1 may serve an anti-inflammatory role in LPS-stimulated
septic acute kidney injury.
NF-κB is a well-known transcription factor that
regulates the expression of multiple inflammation-associated
proteins in response to various infections (26). ERK, p38 and c-JNK are members of the
MAPK family and are able to activate NF-κB (27). ERK1/2 activation may further activate
and translocate NF-κB to the nucleus (28). Furthermore, LPS-induced NF-κB
activation may be associated with the phosphorylation of MAPKs. In
the present study it was demonstrated that AQP-1 overexpression was
able to significantly reduce the phosphorylation of p38 and ERK1/2,
whereas AQP-1 enhanced the phosphorylation of p38 and ERK1/2.
However, the phosphorylation of JNK was not affected by AQP-1.
These results suggest that AQP-1 exerts a cytoprotective role in
LPS-induced HK-2 cells via the p38 and ERK1/2 signaling
pathways.
In conclusion, the results of the present study
suggest that AQP-1 overexpression is able to partially reverse the
LPS-induced decrease in cell viability and increase in apoptosis,
as well as reducing inflammation in HK-2 cells. AQP-1 may have
anti-apoptotic and anti-inflammatory functional roles in HK-2 cells
and these effects may be achieved via the p38 and ERK1/2 signaling
pathways. These results may provide a basis for the used of AQP-1
as a treatment for septic acute kidney injury in DIC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Youth
Science Foundation of China (grant no. 81501825) and Youth Science
Foundation of Heilongjiang Province of China Grant (grant no.
QC2012C035).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the author for correspondence upon
reasonable request.
Authors' contributions
YW and WZ performed the experiments. GY and QL
analyzed the data. YJ made substantial contributions to conception
and design, acquisition of data, analysis and interpretation of
data, acquisition of funding. YW and YJ was involved in drafting
the manuscript and revising it critically for important
intellectual content. All authors gave their final approval of the
version to be published. YJ agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DIC
|
disseminated intravascular
coagulation
|
|
LPS
|
lipopolysaccharide
|
|
AQP-1
|
aquaporin-1
|
References
|
1
|
Minomo H, Inoue K, Sakaki S, Okazaki T,
Kobayashi K, Inoue K and Miyata A: Establishment of disseminated
intravascular coagulation (DIC) model by a single iv administration
of Escherichia coli-derived lipopolysaccharide (LPS) to
cynomolgus monkeys and evaluation of its pathophysiological status.
J Pharmacol Sci. 133:88–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asakura H: Classifying types of
disseminated intravascular coagulation: Clinical and animal models.
J Intensive Care. 2:202014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fink MP: Animal models of sepsis.
Virulence. 5:143–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wigton DH, Kociba GJ and Hoover EA:
Infectious canine hepatitis: Animal model for viral-induced
disseminated intravascular coagulation. Blood. 47:287–296.
1976.PubMed/NCBI
|
|
5
|
Song J, Hu D, He C, Wang T, Liu X, Ma L,
Lin Z and Chen Z: Novel biomarkers for early prediction of
sepsis-induced disseminated intravascular coagulation in a mouse
cecal ligation and puncture model. J Inflamm (Lond). 10:72013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Angus DC and van der Poll T: Severe sepsis
and septic shock. N Engl J Med. 369:840–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li C, Wu J, Li Y and Xing G:
Cytoprotective effect of heat shock protein 27 against
lipopolysaccharide-induced apoptosis of renal epithelial HK-2
cells. Cell Physiol Biochem. 41:2211–2220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong F, Chen H, Han L, Jin Y and Wang W:
Curcumin attenuates lipopolysaccharide-induced renal inflammation.
Biol Pharm Bull. 34:226–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bascands JL, Bachvarova M, Neau E,
Schanstra JP and Bachvarov D: Molecular determinants of LPS-induced
acute renal inflammation: Implication of the kinin B1 receptor.
Biochem Biophys Res Commun. 386:407–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang WJ, Wei H, Hagen T and Frei B:
Alpha-lipoic acid attenuates LPS-induced inflammatory responses by
activating the phosphoinositide 3-kinase/Akt signaling pathway.
Proc Natl Acad Sci USA. 104:4077–4082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee S, Kim W, Kang KP, Moon SO, Sung MJ,
Kim DH, Kim HJ and Park SK: Agonist of peroxisome
proliferator-activated receptor-gamma, rosiglitazone, reduces renal
injury and dysfunction in a murine sepsis model. Nephrol Dial
Transplant. 20:1057–1065. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nielsen S, Kwon TH, Frøkiaer J and Agre P:
Regulation and dysregulation of aquaporins in water balance
disorders. J Intern Med. 261:53–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marrone J, Danielli M, Gaspari CI and
Marinelli RA: Adenovirus-mediated human aquaporin-1 expression in
hepatocytes improves lipopolysaccharide-induced cholestasis. IUBMB
Life. 69:978–984. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Zhang M, Mao Y, Li Y, Zhang X, Peng
X and Yu F: The potential role of aquaporin 1 on aristolochic acid
I induced epithelial mesenchymal transition on HK-2 cells. J Cell
Physiol. 233:4919–4925. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Z, Li C, Xi H, Gao Y and Xu D:
Curcumin induces apoptosis in pancreatic cancer cells through
induction of forkhead box o1 (FOXO1) and inhibition of PI3K/Akt
pathway. Mol Med Rep. 12:5415–5422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Guo L, Qian P, Zhao Y, Liu A, Ji F,
Chen L, Wu X and Qian G: Lipopolysaccharide induces autophagic cell
death through the PERK-dependent branch of the unfolded protein
response in human alveolar epithelial A549 cells. Cell Physiol
Biochem. 36:2403–2417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiao G, Li E and Yu R: Decreased
expression of AQP1 and AQP5 in acute injured lungs in rats. Chin
Med J (Engl). 115:963–967. 2002.PubMed/NCBI
|
|
20
|
Su X, Song Y, Jiang J and Bai C: The role
of aquaporin-1 (AQP1) expression in a murine model of
lipopolysaccharide-induced acute lung injury. Respir Physiol
Neurobiol. 142:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang YX, Dai ZL, Zhang XP, Zhao W, Huang
Q and Gao LK: Dexmedetomidine alleviates pulmonary edema by
upregulating AQP1 and AQP5 expression in rats with acute lung
injury induced by lipopolysaccharide. J Huazhong Univ Sci Technolog
Med Sci. 35:684–688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong-Min F, Chun-Rong H, Rui Z, Li-Na S,
Ya-Jun W and Li L: CGRP 8–37 enhances lipopolysaccharide-induced
acute lung injury and regulating aquaporin 1 and 5 expressions in
rats. J Physiol Biochem. 73:381–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin Y, Yu G, Peng P, Zhang Y and Xin X:
Down-regulated expression of AQP5 on lung in rat DIC model induced
by LPS and its effect on the development of pulmonary edema. Pulm
Pharmacol Ther. 26:661–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui WY, Tian AY and Bai T: Protective
effects of propofol on endotoxemia-induced acute kidney injury in
rats. Clin Exp Pharmacol Physiol. 38:747–754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirschfeld M, Ma Y, Weis JH, Vogel SN and
Weis JJ: Cutting edge: Repurification of lipopolysaccharide
eliminates signaling through both human and murine toll-like
receptor 2. J Immunol. 165:618–622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lerolle N, Nochy D, Guérot E, Bruneval P,
Fagon JY, Diehl JL and Hill G: Histopathology of septic shock
induced acute kidney injury: Apoptosis and leukocytic infiltration.
Intensive Care Med. 36:471–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Chen L, Wang C, Chen J, Zhang X, Hu
Y, Niu X, Pei D, He Z and Bi Y: Extracellular matrix
metalloproteinase inducer enhances host resistance against
pseudomonas aeruginosa infection through MAPK signaling pathway. Am
J Transl Res. 8:5619–5627. 2016.PubMed/NCBI
|
|
28
|
Soubh AA, Abdallah DM and El-Abhar HS:
Geraniol ameliorates TNBS-induced colitis: Involvement of
Wnt/β-catenin, p38MAPK, NFκB, and PPARγ signaling pathways. Life
Sci. 136:142–150. 2015. View Article : Google Scholar : PubMed/NCBI
|