Introduction
Lung cancer is a malignant tumor with high morbidity
and mortality rates that causes serious harm to patients and their
families. With the increasing deterioration of the environment, the
morbidity and mortality rates of lung cancer have shown a gradual
upward trend (1,2). Some scholars suggested that the immune
function of patients becomes abnormal in the occurrence and
development of lung cancer (3).
The dendritic cell (DC) is the most powerful
antigen-presenting cell (APC) in the body, and plays a key role in
immunological stress and immunoregulation of the body (4,5). DC is a
useful cell in tumor biological immunotherapy. Most scholars in
China and worldwide hope to enhance the effect of immunotherapy by
basis for the clinical judgment of prognosis improving the immune
function and number of autologous DCs in patients, but the in
vivo effect of DC immunotherapy is currently still
significantly inferior to the in vitro effect. It has been
confirmed that the number of DCs in the tumor-bearing host is
reduced and it is defective with regard to its function compared
with normal DCs (6,7). This result is universally recognized as
one of the important mechanisms of tumors evading immune
surveillance in the body. Studies have shown that the changes in
DC1, DC2 and DC1/DC2 ratio in peripheral blood can well reflect the
current immune function status of the body (8–10).
Therefore, the distribution and number of DC subsets
in peripheral blood in patients with non-small cell lung cancer
(NSCLC) after DC immunotherapy were detected in the present study
to investigate the clinical significance of DC subset
detection.
Subjects and methods
Subjects
A total of 55 patients, aged 19–77 years, who were
diagnosed as NSCLC from January, 2016 to January, 2017 were
selected. Exclusion criteria for the study were: Patients with
dysfunction in heart, liver, kidney or hematopoietic function;
child patients, pregnant women or mentally-ill patients; and
patients without clear pathological diagnosis, or whose estimated
survival was less than six months. Eighteen healthy subjects, aged
22–75 years, underwent physical examination and were selected as
normal controls.
All the subjects signed informed consent, and this
study was approved by the Dezhou Hospital Ethics Committee (Dezhou,
China).
Reagents and instruments
The reagents and instruments used in the study were:
BD dendritic cell flow cytometry reagent (Guangzhou Shuoheng
Biotechnology Co., Ltd., Guangzhou, China); mouse mAb-BDCA-1 and
mouse mAb-BDCA-3 (Shanghai Runwelltac Industrial Co., Ltd.,
Shanghai, China) and the corresponding secondary antibody and
TO-PRO-3 (Shanghai Jiwei Biotechnology Co., Ltd., Shanghai, China);
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA);
laser scanning confocal microscope (LSCM) (Bio-Rad, Hercules, CA,
USA) and small desktop high-speed centrifuge (BD Biosciences).
Detection using flow cytometer
In strict accordance with the instructions of BDDC
flow cytometry reagent, DC flow cytometry reagent was added into
the five heparin anticoagulant tubes, each containing 100 µl
peripheral blood, and the mixture was shaken gently and evenly,
followed by reaction at room temperature in the dark for 15 min.
Then, 2–3 ml hemolysin was added and the mixture was shaken gently
and evenly for splitting for 10 min, followed by centrifugation at
1,080 × g for 5 min. The supernatant was discarded, and the mixture
was washed with phosphate-buffered saline (PBS) three times, added
with 300 µl PBS and gently agitated in the dark at 4°C. The DC
subsets were detected using the FACSCalibur flow cytometer. Cells
(5×104) were collected into tubes. The threshold value
was set in FSC to eliminate the interference of debris on the
results. CellQuest software was used to obtain and analyze the
HLA-DR positive, Lin-1 weakly positive and negative cell groups in
the HLA-DR/Lin-1 point diagram. The results were recorded using the
percentage of positive cells in CD11c (DC1) and CD123 (DC2)
fluorescent antibody staining, while the DC1/DC2 ratio was
calculated.
Detection of the number of DC subsets
in lung cancer, para-carcinoma and normal lung tissues
The lung cancer, para-carcinoma (~2 cm around the
cancer) and normal lung tissues were snap frozen and stored in
liquid nitrogen (−196°C). The above three kinds of tissues stored
in the liquid nitrogen were taken, washed with the pre-cooled PBS
and placed in the blocking solution containing 10% goat serum for
45 min. Then, the tissues were placed in the diluent with donkey
anti-goat BDCA-1 and BDCA-3 polyclonal antibodies (dilution, 1:20;
cat. nos. AF5910 and AF3894;) for incubation at 4°C overnight.
Tissues were washed with pre-cooled PBS and goat anti-mouse
secondary polyclonal antibody (dilution, 1:400; cat. no. AF109) was
used for incubation at room temperature for 2 h, followed by
re-staining via TO-PRO-3 and sealing.
LSCM detection
LSCM was equipped with three kinds of ion lasers,
namely argon-krypton (480 nm), chlorine-neon (543 nm) and
helium-neon (633 nm). Using the objective and water lens (×20),
confocal images were taken on the z-axis of the slide at an
interval of 500 nm, and the visual field with the most DCs was the
field observed. The number of DC1- and DC2-positive cells were
observed and counted by two individuals, and the average count was
taken; the count difference between the two individuals was not
>20%.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used. Measurement data are presented as mean ± SD. The ANOVA
was used for the intergroup comparison and LSD test was used as
post hoc test. The Chi-square test was used for the comparisons of
enumeration data or rate; α=0.05.
Results
Comparisons of DC subsets in
peripheral blood between NSCLC patients and normal controls
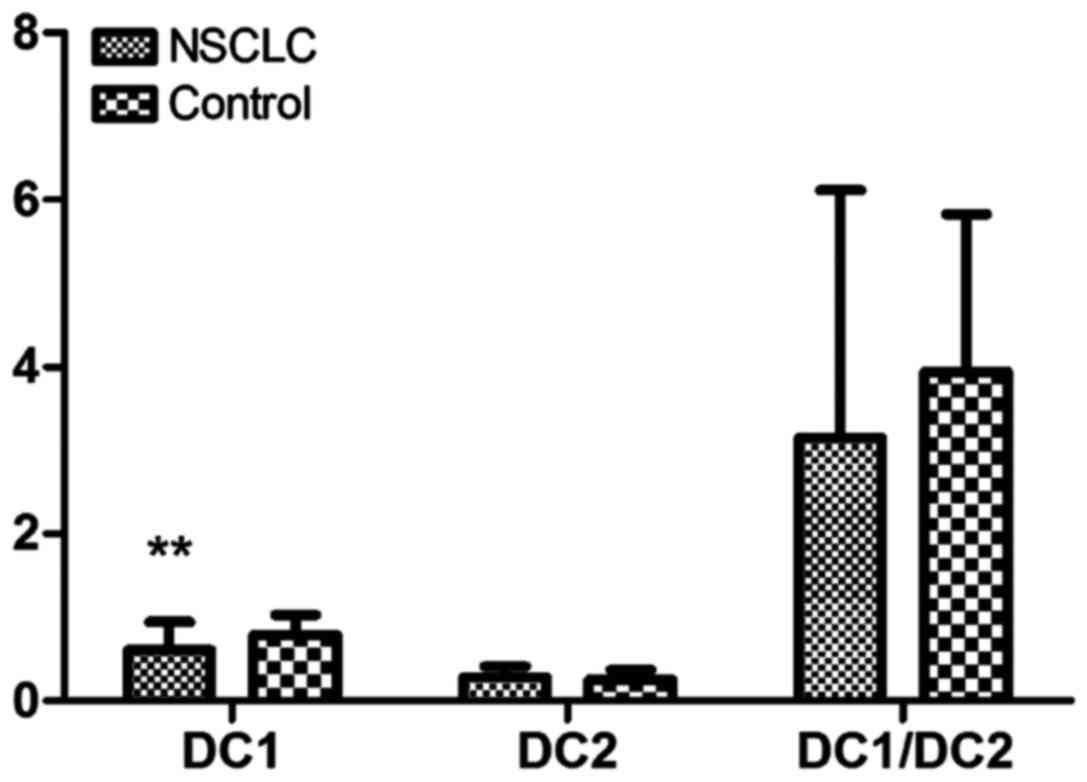
The expression of DC1 in peripheral blood in NSCLC
patients was significantly decreased compared with that in the
normal control group, and the difference was statistically
significant (P<0.01). The expression of DC2 did not change
significantly in the NSCLC and normal control groups. Compared with
that in the normal control group, the DC1/DC2 ratio in the NSCLC
group was lower, but there was no statistically significant
difference between the groups (P>0.05) (Table I and Fig.
1).
 | Table I.Comparisons of DC subsets in
peripheral blood between NSCLC patients and normal controls (mean ±
SD). |
Table I.
Comparisons of DC subsets in
peripheral blood between NSCLC patients and normal controls (mean ±
SD).
| Groups | No. | DC1 | DC2 | DC1/DC2 |
|---|
| NSCLC | 55 |
0.61±0.33a | 0.28±0.13 | 3.15±2.97 |
| Control | 18 | 0.79±0.24 | 0.26±0.11 | 3.94±1.89 |
| t-value |
| 2.884 | 0.537 | −1.526 |
| P-value |
| 0.008 | 0.745 | 0.183 |
Relationship between DC subsets and
clinical features in NSCLC group
There were no statistically significant differences
in the sex, age, pathological type, CEA and other DC subsets for
the 55 NSCLC patients (P>0.05). There were statistically
significant differences in DC1 and DC1/DC2 ratio in NSCLC patients
with different tumor staging (P=0.029 and P=0.001), while there
were statistically significant differences in patients with a
different Karnofsky performance status (KPS) scores (P=0.025)
(Table II).
 | Table II.Relationship between DC subsets and
clinical features in NSCLC group. |
Table II.
Relationship between DC subsets and
clinical features in NSCLC group.
| Characteristics | No. | DC1 | P-value | DC2 | P-value | DC1/DC2 | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
| Male | 34 | 0.58±0.33 | 0.336 | 0.24±0.18 | 0.295 | 2.96±2.05 | 0.903 |
|
Female | 21 | 0.51±0.24 |
| 0.21±0.13 |
| 2.79±1.88 |
|
| Age (years) |
|
|
|
|
|
|
|
|
<70 | 40 | 0.62±0.34 | 0.103 | 0.25±0.13 | 0.135 | 2.67±1.75 | 0.194 |
| ≥70 | 15 | 0.48±0.29 |
| 0.21±0.15 |
| 3.86±3.27 |
|
| Staging |
|
|
|
|
|
|
|
|
Early | 18 | 0.77±0.28 | 0.029a | 0.26±0.15 | 0.894 | 4.47±3.01 | 0.001b |
| Late | 37 | 0.52±0.23 |
| 0.25±0.13 |
| 2.56±1.73 |
|
| Pathology |
|
|
|
|
|
|
|
|
Adenocarcinoma | 36 | 0.59±0.32 | 0.406 | 0.27±0.12 | 0.184 | 2.89±2.15 | 0.243 |
| Squamous
carcinoma | 15 | 0.63±0.36 |
| 0.31±0.19 |
| 2.46±1.63 |
|
|
Others | 4 | 0.74±0.44 |
| 0.24±0.15 |
| 4.41±2.74 |
|
| KPS |
|
|
|
|
|
|
|
|
<60 | 20 | 0.48±0.31 | 0.025a | 0.26±0.12 | 0.184 | 2.62±1.84 | 0.402 |
| ≥60 | 35 | 0.72±0.38 |
| 0.29±0.18 |
| 3.14±3.08 |
|
| CEA |
|
|
|
|
|
|
|
|
Normal | 26 | 0.68±0.41 | 0.091 | 0.29±0.18 | 0.724 | 3.62±2.96 | 0.056 |
|
Abnormal | 29 | 0.57±0.38 |
| 0.26±0.13 |
| 2.38±1.44 |
|
Comparisons of DC subsets in
peripheral blood before and after three DC treatments
In NSCLC, DC1 expression was low, and the immune
function of body was decreased significantly. The DC1 content and
DC1/DC2 ratio after three DC treatments were significantly
increased compared with those before treatment (Table III).
 | Table III.Comparisons of DC subsets in
peripheral blood before and after three DC treatments. |
Table III.
Comparisons of DC subsets in
peripheral blood before and after three DC treatments.
| Groups | No. | DC1 | DC2 | DC1/DC2 |
|---|
| Control | 18 | 0.79±0.24 | 0.26±0.11 | 3.94±1.89 |
| Before
treatment | 55 | 0.61±0.33 | 0.28±0.13 | 3.15±2.97 |
| After
treatment | 55 |
0.72±0.26a | 0.27±0.12 |
3.54±2.38b |
Relationship between peripheral blood
subsets and prognosis of NSCLC patients
After three DC treatments, 49 NSCLC patients were
followed up for >1 year and 32 patients were lost to follow-up
or died, and the 1-year survival rate was 34.7%. There were no
statistically significant differences in the comparisons of DC1 and
DC2, limited by 1-year survival time (P=0.122 and P=0.916), but
there was statistically significant difference in the DC1/DC2 ratio
between patients with the survival time of greater than and less
than one year (P=0.019) (Table
IV).
 | Table IV.Relationship between peripheral blood
subsets and prognosis of NSCLC patients. |
Table IV.
Relationship between peripheral blood
subsets and prognosis of NSCLC patients.
|
| Survival time |
|
|
|---|
|
|
|
|
|
|---|
| Groups | ≥1 year | <1 year | t-value | P-value |
|---|
| DC subset |
|
|
|
|
|
DC1 | 0.71±0.33 | 0.65±0.36 | −1.488 | 0.122 |
|
DC2 | 0.29±0.17 | 0.27±0.15 | −0.174 | 0.916 |
|
DC1/DC2 | 2.94±1.24 | 1.73±1.27 | −2.546 | 0.019 |
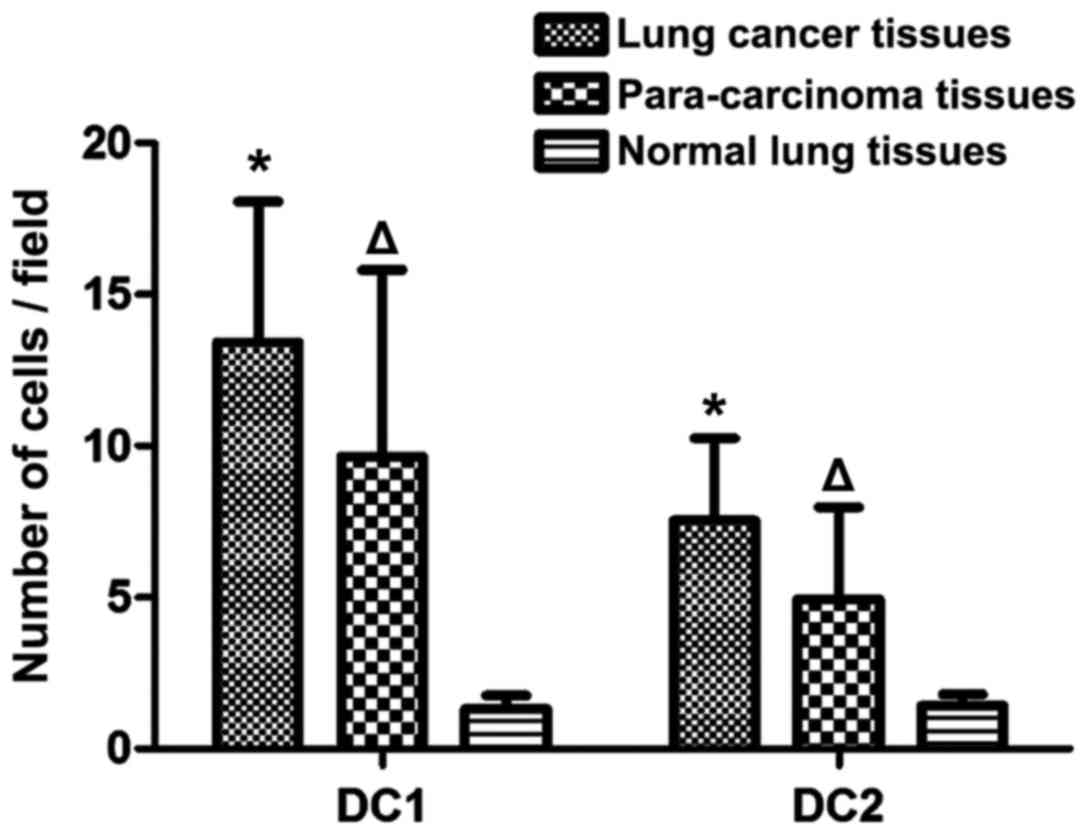
The number of DC subsets in lung
cancer, para-carcinoma and normal lung tissues
The number of DC1 in para-carcinoma and normal lung
tissues was significantly lower than that in lung cancer tissues,
and the difference was statistically significant (P<0.05). The
number of DC2 in para-carcinoma and normal lung tissues was also
evidently lower than that in lung cancer tissues, and the
difference was statistically significant (P<0.05). Compared with
those in normal lung tissues, the number of DC1 and DC2 in
para-carcinoma tissues was increased significantly, and the
differences were statistically significant (P<0.05) (Fig. 2).
Discussion
With the aggravation of environmental pollution, the
morbidity and mortality rates of lung cancer have been on the
increase, seriously threatening human health (11). At present, the main types of therapy
are radiotherapy, chemotherapy and biological immunotherapy. DC is
the most powerful APC known currently, playing an important role in
triggering the primary immune response in the body for the first
time, which can effectively control tumor cell growth and
metastasis (12). There are two
sources of DC in the body. One is the myeloid-derived DC from bone
marrow CD34+ cells, which can stimulate the T-helper
cell 1; consequently, it is also known as DC1. Such a cell is the
most important source of DC cells at present, which can induce the
secretion of a variety of cytokines from T cells in the body, thus
responding to Th1 and finally triggering immune rejection in the
body. The other one is lymphocytic DC derived from the thymus,
which can stimulate the T-helper cell 2. Consequently, it is also
known as DC2. After contacting with the tumor cells in the body, it
immediately secretes a variety of cytokines, including
interferon-α, triggering Th2 immune response, which is closely
related to the immune tolerance response in the body (13,14).
In the present study, the number of DCs and its
subsets were detected via indirect immunofluorescence and LSCM. The
results of the statistical analysis revealed that DC1 and DC2 in
lung cancer tissues were significantly increased compared with
those in para-carcinoma and normal lung tissues (P<0.05), and
the number of DC1 and DC2 in para-carcinoma tissues was increased
compared with that in the normal tissues (P<0.05). The
expression of DC subsets in peripheral blood in NSCLC patients
before and after DC immunotherapy were detected using a flow
cytometer. The results revealed that the ratio of DC1 in peripheral
blood in the normal control group was significantly higher than
that in NSCLC patients (P<0.01). There were significant
differences in DC1 and DC1/DC2 ratio in NSCLC patients with
different tumor staging, and they also had obvious differences in
patients with different KPS scores. Compared with those before
treatment, DC1 and DC1/DC2 were significantly increased after three
treatments, and there was a significant difference in DC1/DC2
between NSCLC patients with the survival time greater than and less
than one year.
It is currently known that DC1 in peripheral blood
is reduced in a variety of malignant tumors, such as pancreatic,
colorectal and liver cancer (15–17).
However, there are also exceptions, such as myeloma (18). Previous findings have shown that the
number of DC1 in peripheral blood does not change significantly in
myeloma patients. Additionally, the increase of expression levels
of various cytokines in serum is closely related to the decrease in
DC in peripheral blood (18). The
experiment further confirmed that the supernatant of tumor cells
cultured in vitro can inhibit the differentiation of DC
in vitro, and it is speculated that the tumor cells secrete
some cytokines, thus inhibiting the differentiation of DC precursor
in vivo (19–21). This may be one of the reasons why the
anti-tumor effect of DC therapy in vivo is less than that
in vitro.
In conclusion, the immune function of NSCLC patients
is improved after DC immunotherapy. The survival time of NSCLC
patients is closely related to the DC1/DC2 ratio in peripheral
blood. The detection of DC subsets in peripheral blood can help
clinicians understand the immune function of NSCLC patients and
provide a basis for the clinical judgment of prognosis of NSCLC
patients.
Acknowledgements
The abstract of the present study was published in J
Clin Oncol 35 (Suppl 15): e17574, 2017.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY contributed to analysis using flow cytometry and
wrote the manuscript. FD performed LSCM detection. LM performed and
analysed detection of the number of DC subsets. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Dezhou People's
Hospital Ethics Committee (Dezhou, China). All the subjects signed
informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Teramoto K, Ozaki Y, Hanaoka J, Sawai S,
Tezuka N, Fujino S, Daigo Y and Kontani K: Predictive biomarkers
and effectiveness of MUC1-targeted dendritic-cell-based vaccine in
patients with refractory non-small cell lung cancer. Ther Adv Med
Oncol. 9:147–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang L, Yang X, Sun Z, Li J, Zhu H, Li J
and Pang Y: Dendritic cell vaccine and cytokine-induced killer cell
therapy for the treatment of advanced non-small cell lung cancer.
Oncol Lett. 11:2605–2610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li R, Fang F, Jiang M, Wang C, Ma J, Kang
W, Zhang Q, Miao Y, Wang D, Guo Y, et al: STAT3 and NF-κB are
simultaneously suppressed in dendritic cells in lung cancer. Sci
Rep. 7:453952017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendoza L: Dendritic cell vaccines against
non-small cell lung cancer - an emerging therapeutic alternative.
Klin Onkol. 27:294–298. 2014.PubMed/NCBI
|
|
5
|
Liu X, Li J and Liu Y, Ding J, Tong Z and
Liu Y, Zhou Y and Liu Y: Calreticulin acts as an adjuvant to
promote dendritic cell maturation and enhances antigen-specific
cytotoxic T lymphocyte responses against non-small cell lung cancer
cells. Cell Immunol. 300:46–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou C, Liu D, Li J, Sun H, Zheng X, Wang
S, Hong G, Mallampati S, Sun H, Zhou X, et al: Chemotherapy plus
dendritic cells co-cultured with cytokine-induced killer cells
versus chemotherapy alone to treat advanced non-small-cell lung
cancer: A meta-analysis. Oncotarget. 7:86500–86510. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rafei H, El-Bahesh E, Finianos A,
Nassereddine S and Tabbara I: Immune-based therapies for non-small
cell lung cancer. Anticancer Res. 37:377–387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi SB, Tang XY, Tian J, Chang CX, Li P
and Qi JL: Efficacy of erlotinib plus dendritic cells and
cytokine-induced killer cells in maintenance therapy of advanced
non-small cell lung cancer. J Immunother. 37:250–255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao M, Li H, Li L and Zhang Y: Effects of
a gemcitabine plus platinum regimen combined with a dendritic
cell-cytokine induced killer immunotherapy on recurrence and
survival rate of non-small cell lung cancer patients. Exp Ther Med.
7:1403–1407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi H, Okamoto M, Shimodaira S,
Tsujitani S, Nagaya M, Ishidao T, Kishimoto J and Yonemitsu Y:
DC-vaccine study group at the Japan Society of Innovative Cell
Therapy (J-SICT): Impact of dendritic cell vaccines pulsed with
Wilms' tumour-1 peptide antigen on the survival of patients with
advanced non-small cell lung cancers. Eur J Cancer. 49:852–859.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong R, Han B and Zhong H: A prospective
study of the efficacy of a combination of autologous dendritic
cells, cytokine-induced killer cells, and chemotherapy in advanced
non-small cell lung cancer patients. Tumour Biol. 35:987–994. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aguilar-Cazares D, Meneses-Flores M,
Prado-Garcia H, Islas-Vazquez L, Rojo-Leon V, Romero-Garcia S,
Rivera-Rosales RM and Lopez-Gonzalez JS: Relationship of dendritic
cell density, HMGB1 expression, and tumor-infiltrating lymphocytes
in non-small cell lung carcinomas. Appl Immunohistochem Mol
Morphol. 22:105–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hradilova N, Sadilkova L, Palata O,
Mysikova D, Mrazkova H, Lischke R, Spisek R and Adkins I:
Generation of dendritic cell-based vaccine using high hydrostatic
pressure for non-small cell lung cancer immunotherapy. PLoS One.
12:e01715392017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu XP, Xu YH, Zhou J and Pan XF: A
clinical study evaluating dendritic and cytokine-induced killer
cells combined with concurrent radiochemotherapy for stage IIIB
non-small cell lung cancer. Genet Mol Res. 14:10228–10235. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Besse B, Charrier M, Lapierre V, Dansin E,
Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F,
Laplanche A, et al: Dendritic cell-derived exosomes as maintenance
immunotherapy after first line chemotherapy in NSCLC.
Oncoimmunology. 5:e10710082015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han RX, Liu X, Pan P, Jia YJ and Yu JC:
Effectiveness and safety of chemotherapy combined with dendritic
cells co-cultured with cytokine-induced killer cells in the
treatment of advanced non-small-cell lung cancer: A systematic
review and meta-analysis. PLoS One. 9:e1089582014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuanying Y, Lizhi N, Feng M, Xiaohua W,
Jianying Z, Fei Y, Feng J, Lihua H, Jibing C, Jialiang L, et al:
Therapeutic outcomes of combining cryotherapy, chemotherapy and
DC-CIK immunotherapy in the treatment of metastatic non-small cell
lung cancer. Cryobiology. 67:235–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kovarova L, Buchler T, Pour L, Zahradova
L, Ocadlikova D, Svobodnik A, Penka M, Vorlicek J and Hajek R:
Dendritic cell counts and their subsets during treatment of
multiple myeloma. Neoplasma. 54:297–303. 2007.PubMed/NCBI
|
|
19
|
Ma J, Liu H and Wang X: Effect of ginseng
polysaccharides and dendritic cells on the balance of Th1/Th2 T
helper cells in patients with non-small cell lung cancer. J Tradit
Chin Med. 34:641–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Xu Y, Shen J, He F, Zhang D, Chen
Z, Duan Y and Sun J: Feasibility study of DCs/CIKs combined with
thoracic radiotherapy for patients with locally advanced or
metastatic non-small-cell lung cancer. Radiat Oncol. 11:602016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao P, Bu X, Wei X, Sun W, Xie X, Li C,
Guo Q, Zhu D, Wei X and Gao D: Dendritic cell immunotherapy
combined with cytokine-induced killer cells promotes skewing toward
Th2 cytokine profile in patients with metastatic non-small cell
lung cancer. Int Immunopharmacol. 25:450–456. 2015. View Article : Google Scholar : PubMed/NCBI
|