Introduction
Ovarian cancer is the second most prevalent
gynecological cancer type and the fifth most common cause of
cancer-associated mortality in women, of which epithelial ovarian
carcinoma is the most common pathological type, accounting for
85–90% of ovarian cancer cases (1–3).
Consistent with the poor prognosis due to the extensive metastasis
of ovarian cancer cells into the peritoneal cavity, the majority of
ovarian cancer patients experience a relapse within 2 years
(2,4). Despite the development of several
approaches for targeted therapies for ovarian cancer, the prognosis
of patients with advanced disease has not improved much in the last
2 decades (1,5,6). This
may be due to chemoresistance and difficulties in early detection,
as most patients are not diagnosed with ovarian cancer until
reaching the advanced stages (stage III or IV) (7,8).
Therefore, it is urgently required to identify novel therapeutic
and diagnostic targets to help improve the prognosis of ovarian
cancer patients.
MicroRNAs (miRNAs) are small RNA molecules of ~22
nucleotides in length that mediate the post-transcriptional
regulation of gene expression by binding mainly to the 3′-end of
mRNA transcripts to induce translational repression and/or mRNA
degradation (9,10). miRNAs have numerous critical roles in
the regulation of the proliferation, differentiation, apoptosis,
invasion and metastasis of tumor cells (11–13).
Emerging evidence suggests a role of miRNAs in cancer, with
potential use as novel disease-associated biomarkers (14–16).
Changes in tissue and circulating levels of miRNA have been
described in esophageal cancer (17)
and lung cancer (13). Accordingly,
the development of miRNAs as diagnostic biomarkers and therapeutic
targets for cancer is feasible, and has prospective clinical
applications.
miR-423-5p was identified as a circulating biomarker
for heart failure (18) and inducer
of apoptosis in cardiomyocytes by targeting β-linked
N-acetylglucosamine (O-GlcNAc) transferase (19). In tumors, miR-423-5p has been
demonstrated to contribute to the development of malignant
phenotypes and temozolomide resistance in glioblastoma (20), and increase autophagy in
hepatocellular carcinoma cells (21). Furthermore, plasma miR-423-5p levels
have promising potential to serve as a novel biomarker for
colorectal cancer detection, particularly at its early stage
(22). miR-423-5p was observed to
respond to Sorafenib therapy for hepatocellular carcinoma, since
75% of patients with increased plasma miR423-5p levels achieved
partial remission or stable disease after 6 months from the
beginning of therapy (21). These
results demonstrated the potential role of miR-423-5p in the
diagnosis and therapy of cancer. However, the expression and role
of miR-423-5p in ovarian cancer has remained to be determined,
which was therefore the aim of the present study.
Materials and methods
Clinical samples
The subjects of the present study were 40 ovarian
cancer patients treated at Sichuan Provincial People's Hospital
(Chengdu, China) and the Second People's Hospital of Neijiang City
(Neijiang, China) from January 2016 to May 2017. All patients were
diagnosed with epithelial ovarian cancer and complete clinical data
for these patients were available (age range, 52 to 75 years old;
mean age, 61.2; 15 patients were diagnosed with metastasis;
Table I). The control group
consisted of 20 patients that had been diagnosed with ovarian
endometriosis (age range 51 to 64 years old; mean age, 58.6). Human
ovarian cancer tissues and normal ovarian endometriosis tissues
were obtained with signed written informed consent under a general
waiver from the Academic Medical Center institutional review board
for the proper secondary use of human material (Sichuan, China).
Fasting peripheral blood (5 ml) was drawn from each patient and
placed in anti-coagulative tubes at room temperature for 30 min,
followed by centrifugation at 4,000 × g for 5 min at 4°C. The
plasma supernatant was collected and stored at −80°C until use. All
of the experiments described were approved by the ethics committee
of Sichuan Provincial People's Hospital (Chengdu, China).
 | Table I.Clinicopathological characteristics of
patients with ovarian cancer. |
Table I.
Clinicopathological characteristics of
patients with ovarian cancer.
| Characteristic | N (%) |
|---|
| Age (years) |
|
| ≤60 | 23 (57.5) |
|
>60 | 17 (42.5) |
| TNM stage (%) |
|
| I | 8 (20) |
|
II–III | 19 (47.5) |
| IV | 13 (32.5) |
| Metastasis |
|
|
Yes | 15 (37.5) |
| No | 25 (62.5) |
Cell culture and transfection
The human ovarian cancer cell lines A2780s, A2780cp,
SKOV3, CAOV3 and PA-1 were obtained from the American Type Culture
Collection (Manassas, VA, USA), and the normal ovarian epithelial
cell line HOEC and was obtained from Jennio Biotech (Guangzhou,
China). HOEC cells were and passaged for <10 passages in the
laboratory. The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere containing 5%
CO2. The molecular agomir miR-423-5p expression system
(cat. no. miR40004748-1-2) and negative control (miR-NC; cat. no.
miR04201-1-10) were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). miR-423-5p and miR-NC were transfected into
cells using miRNA transfection agent (riboFECT CP; cat. no. C10511;
RiboBio Co., Ltd., Guangzhou, China) following the manufacturer's
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from ovarian (cancer)
tissues and cell using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
miRNeasy Serum/Plasma kit (cat. no. 217184; Qiagen, Hilden,
Germany) was used to extract miRNA from plasma. qPCR for miR-423-5p
was performed using miRNA primers obtained from Guangzhou RiboBio
Co., Ltd. The sequences were designed with the
Bulge-Loop™ primer set, but not specified due to the
rules of the company. RT was performed on the isolated total RNA
using a Reverse Transcription kit (cat. no. RR047A; Takara Bio,
Inc., Otsu, Japan) and qPCR was performed using a Real Time PCR kit
(cat. no. RR430A; Takara Bio, Inc.). RT was performed using 1 µg
total RNA in 2 µl water and the reaction conditions were 65°C for 5
min, 30°C for 10 min, 42°C for 10–30 min and 2°C for 3 min. The
qPCR conditions were as follows: Denaturation at 94°C for 2 min,
amplification for 30 cycles at 94°C for 0.5 min, annealing at 58°C
for 0.5 min and extension at 72°C for 1 min, followed by a terminal
elongation step at 72°C for 10 min. The qPCR analysis was performed
on a Bio-Rad CFX96 thermal cycler (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). mRNA expression was quantified using the
2−ΔΔCq method (23). RT
and qPCR Experiments were performed three times. U6 was used as the
internal control.
Cell viability assay
At 48 h post miR-423-5p and miR-NC transfection, the
transfected cells were collected and counted. Cells were seeded in
96-well plates at 1,000 cells/well in 0.1 ml DMEM with 10% FBS.
Following 0, 24, 48 and 72 h of incubation, a Cell Counting Kit-8
(CCK-8; Dojindo, Shanghai, China) was used to determine cell
viability. Subsequent to 3 h of incubation with CCK-8 reagent, the
absorbance of each well was measured at 450 nm using a micro-plate
reader (Thermo Fisher Scientific, Inc.). Four independent
experiments were performed.
Colony formation assay
At 48 h post miR-423-5p and miR-NC transfection, the
transfected cells were collected and counted. Cells were seeded on
6-well plates at 1,000 cells/well in 2.0 ml DMEM with 10% FBS. The
cells were cultured for ~14 days and then fixed with 4%
paraformaldehyde for 15 min, followed by staining with crystal
violet (Beyotime Institute of Biotechnology, Haimen, China) at room
temperature for 15 min. The number of colonies (>50 cells) in
each well was counted and analyzed. Three independent experiments
were performed.
Matrigel®-based invasion
assay
The Matrigel®-based invasion assay was
performed as described in a previous study (24). miR-423-5p and miR-NC transfected
cells (2,000 cells) in serum-free medium (0.1 ml) were seeded in
the upper chamber of a 24-well Transwell insert (pore size, 8 µm;
BD Biosciences, Bedford, MA, USA) with the filter pre-coated with
50 µl Matrigel® (BD Biosciences) diluted at 1:5 in DMEM
medium. The lower chambers were filled with 500 µl DMEM containing
10% FBS as a chemoattractant. After 48 h of incubation, the cells
that had not invaded through the pores were carefully wiped off
with a wet cotton swab and the inserts were fixed with 4%
paraformaldehyde for 15 min at room temperature, followed by
staining with crystal violet (Beyotime Institute of Biotechnology)
for 15 min at room temperature. The number of invaded cells in each
millicell was counted and analyzed. Three independent experiments
were performed.
Statistical analysis
Values are expressed as the mean ± standard
deviation. For statistical comparison of quantitative data between
two groups, Students' t-test was performed. If multiple groups were
present, one-way analysis of variance followed by Dunnett's
multiple comparisons test was used. All statistical analyses were
performed using SPSS 20.0 statistical software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-423-5p is downregulated in ovarian
cancer
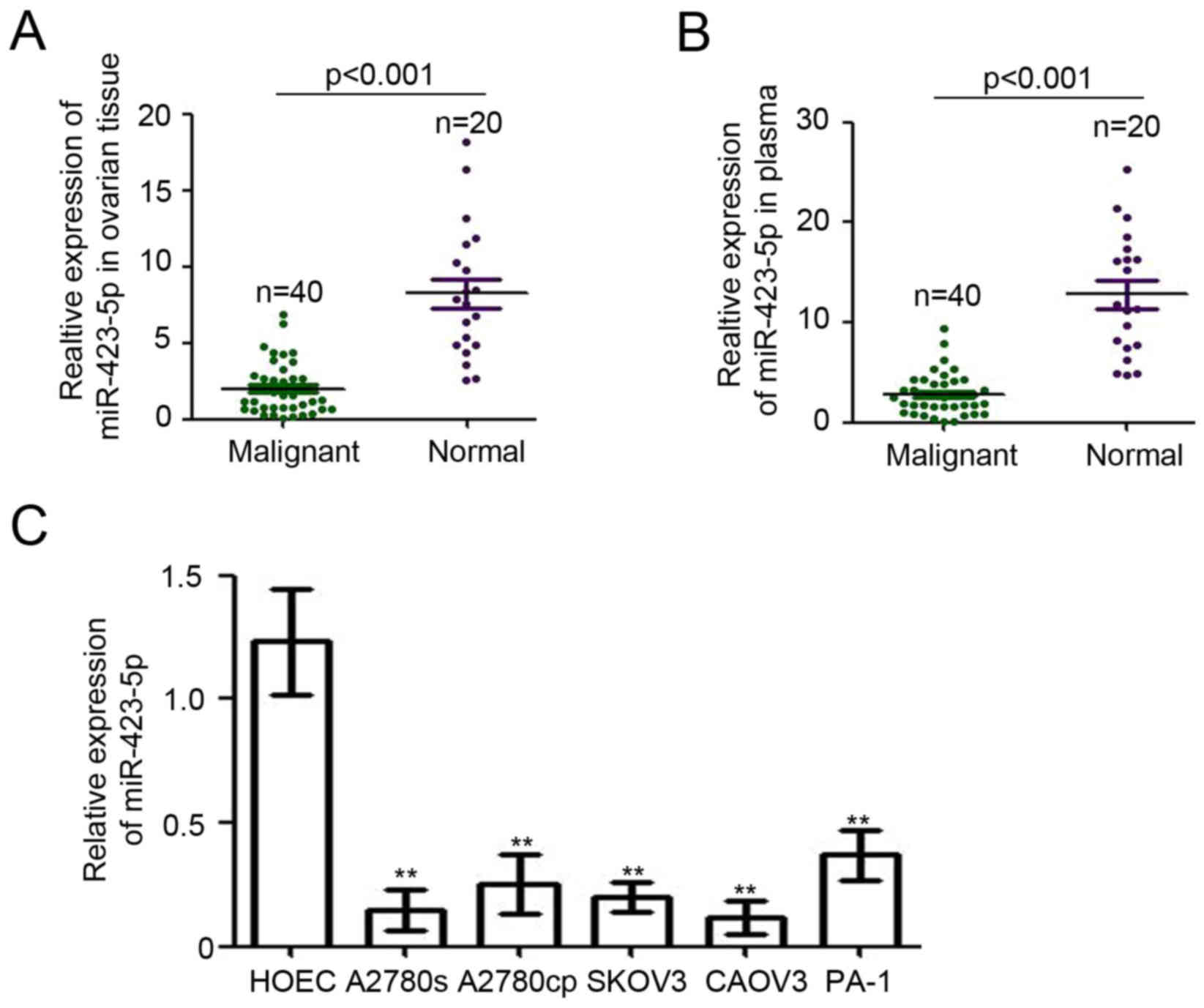
To determine the expression of miR-423-5p in
patients with ovarian cancer, RT-qPCR was performed. Epithelial
ovarian tissues of 40 ovarian cancer patients with different tumor
grades (Table I) and 20 adjacent
noncancerous (endometriosis) ovarian tissues were collected for
this analysis. Compared with that in ovarian endometriosis tissues,
miR-423-5p expression relative to U6 was significantly reduced in
ovarian cancer tissues (8.24±0.97 vs. 2.0±0.27; Fig. 1A). Furthermore, miR-423-5p levels in
plasma were also determined. As presented in Fig. 1B, the levels of miR-423-5p in the
plasma from ovarian cancer patients were determined to be lower
than those in patients with ovarian endometriosis (2.80±0.32 vs.
12.79±1.36). Next, the expression of miR-423-5p was examined in
ovarian cancer cell lines. The normal ovarian epithelial cell line
HOEC was used as a normal control. The results indicated that
miR-423-5p expression was downregulated in all ovarian cancer cell
lines compared with that in the normal ovarian epithelial cell line
(Fig. 1C). Collectively, these
results demonstrated the downregulation of miR-423-5p in the tumor
tissues and plasma of ovarian cancer patients as well as in ovarian
cancer cell lines.
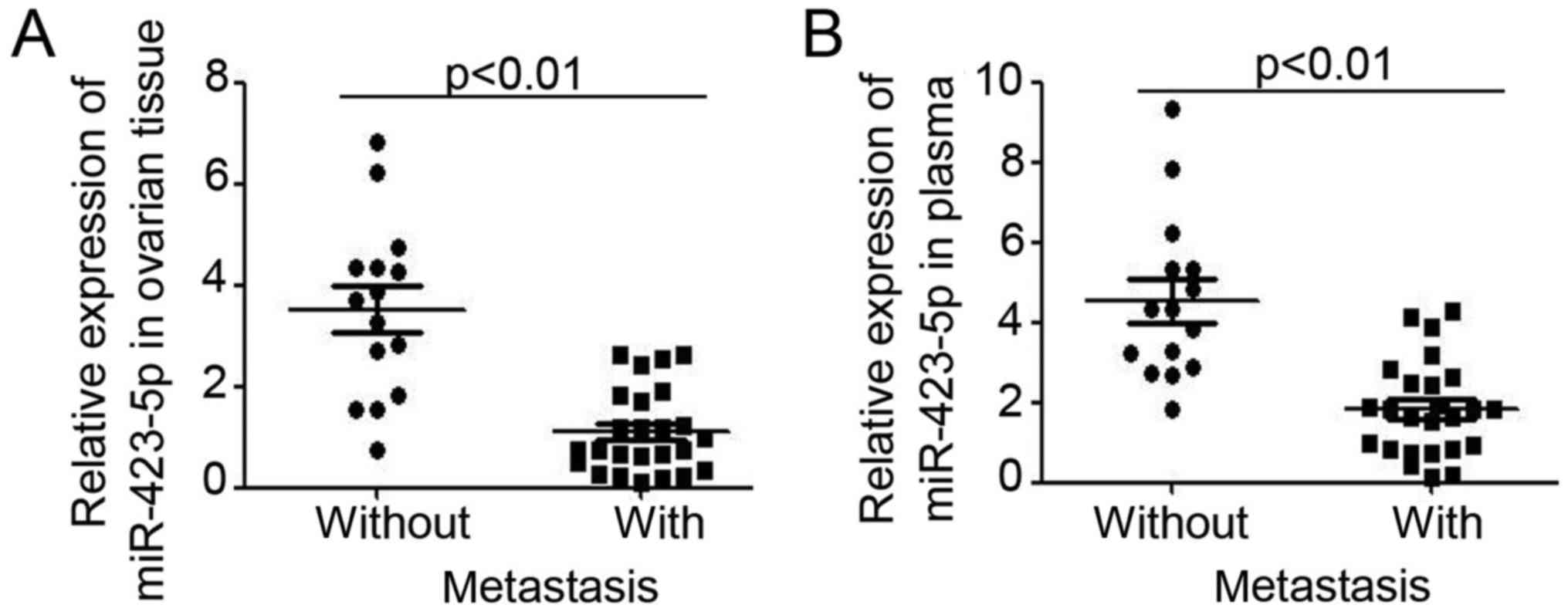
miR-423-5p is inversely associated
with ovarian cancer progression
Next, the relative expression of miR-423-5p was
analyzed in ovarian cancer patients stratified into two groups
depending on the absence or presence of metastasis. As presented in
Fig. 2A, lower miR-423-5p expression
was demonstrated in ovarian cancer tissues from patients with
metastasis. Furthermore, analysis of miR-423-5p levels in plasma
also indicated lower levels in ovarian cancer patients with
metastasis (Fig. 2B). miR-423-5p
expression in ovarian tissues and plasma was then compared for
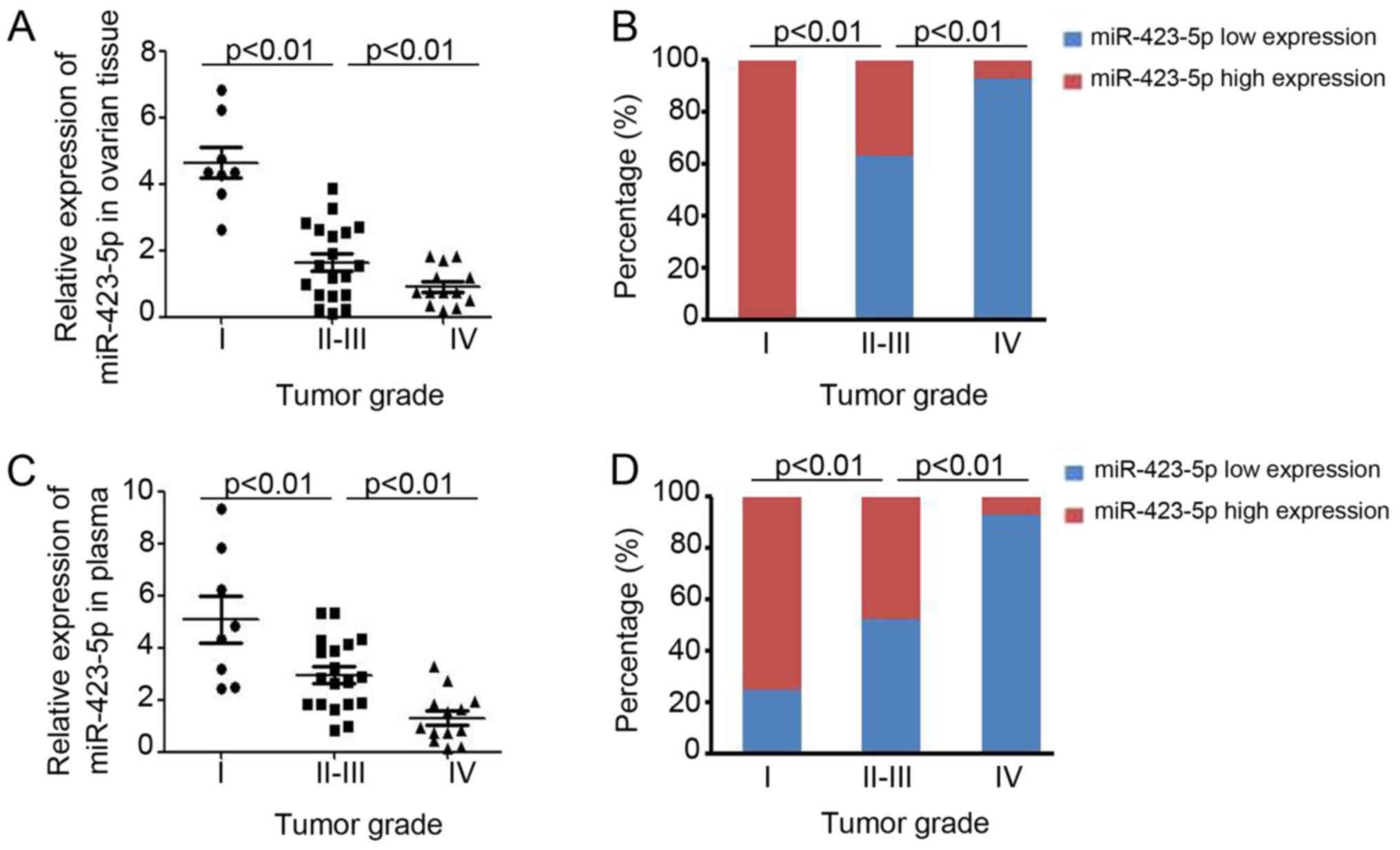
ovarian cancer patients at different stages, which were stratified
using International Federation of Gynecology and Obstetrics staging
(25) (Fig. 3). In ovarian cancer patients with a
higher tumor stage, lower expression of miR-423-5p was demonstrated
in ovarian cancer tissues (stage I vs. stage II–III vs. stage IV:
4.64±0.47 vs. 1.64±0.26 vs. 0.92±0.16; Fig. 3A) and in plasma (stage I vs. stage
II–III vs. stage IV: 5.08±0.89 vs. 2.13±0.75 vs. 1.29±0.27;
Fig. 3C). Further analysis also
demonstrated that miR-423-5p expression is inversely correlated
with the tumor grade (Fig. 3B and
D). Collectively, these results proved the capacity of
miR-423-5p levels in ovarian cancer tissues and plasma to indicate
ovarian cancer progression.
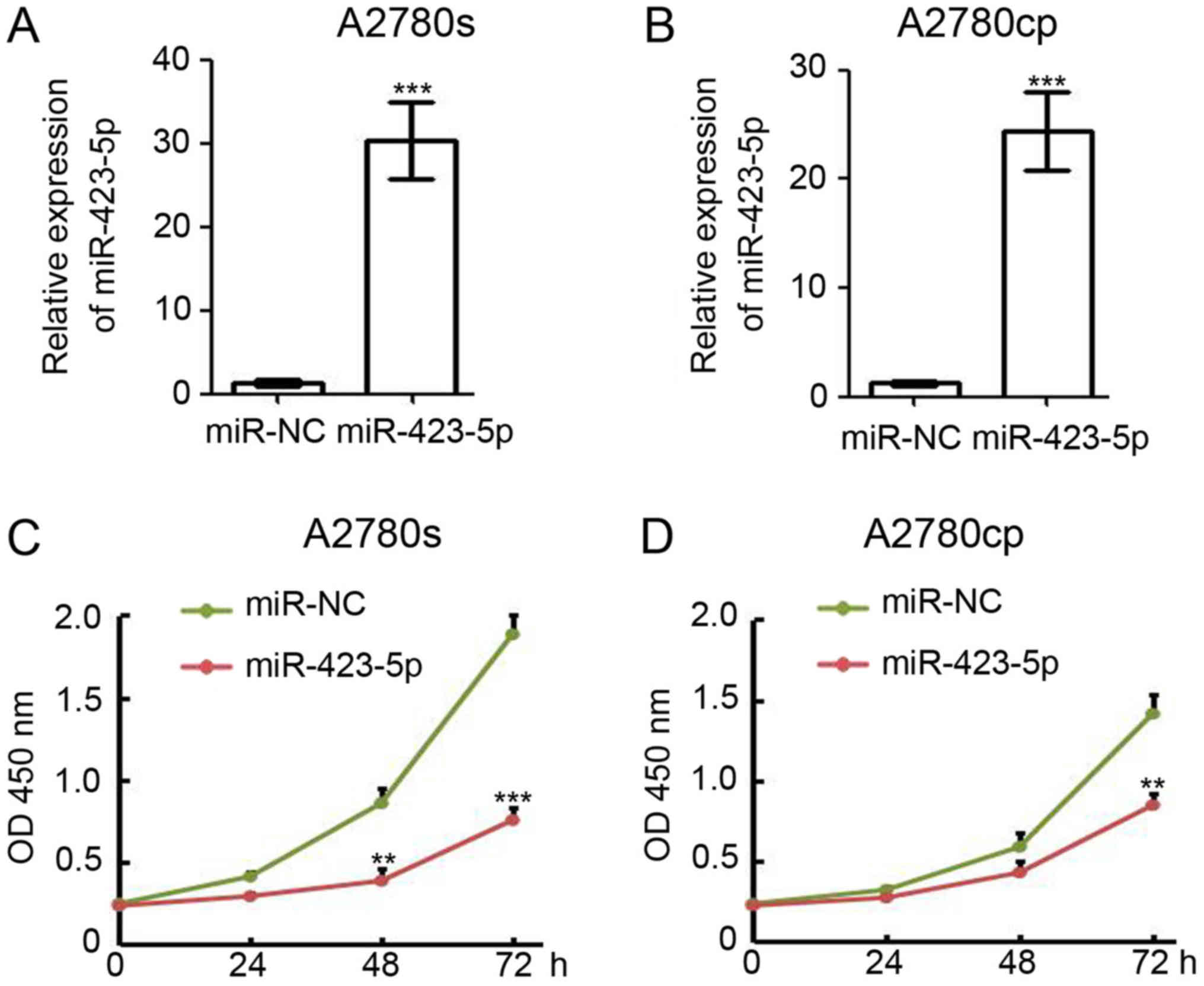
Overexpression of miR-423-5p inhibits
ovarian cancer cell proliferation
Based on the results obtained with the clinical
samples in the present study, miR-423-5p is a potential tumor
suppressor in ovarian cancer. Thus, a gain-of-function approach was
employed to investigate the function of miR-423-5p in A2780s and
A2780cp cells. The cells were transfected with miR-423-5p and
miR-NC (negative control) and collected for total RNA extraction at
48 h post-transfection. RT-qPCR analysis indicated that miR-423-5p
was efficiently expressed in miR-423-5p-transfected A2780s cells
(miR-423-5p/miR-NC expression ratio, 30.3±2.7; Fig. 4A) and A2780cp cells
(miR-423-5p/miR-NC expression ratio, 24.4±2.1; Fig. 4B). To determine whether miR-423-5p
has any effect on cell proliferation in vitro, 1,000
miR-423-5p and miR-NC transfected cells were seeded into the wells
of a 96-well plate for the CCK-8 assay. The results indicated that
ectopic expression of miR-423-5p significantly reduced the
proliferation of A2780s cells (Fig.
4C). miR-423-5p expression also markedly reduced A2780cp cell
proliferation (Fig. 4D).
Collectively, these results suggest that miR-423-5p suppresses
ovarian cancer cell proliferation.
Overexpression of miR-423-5p impairs
colony formation of ovarian cancer cells
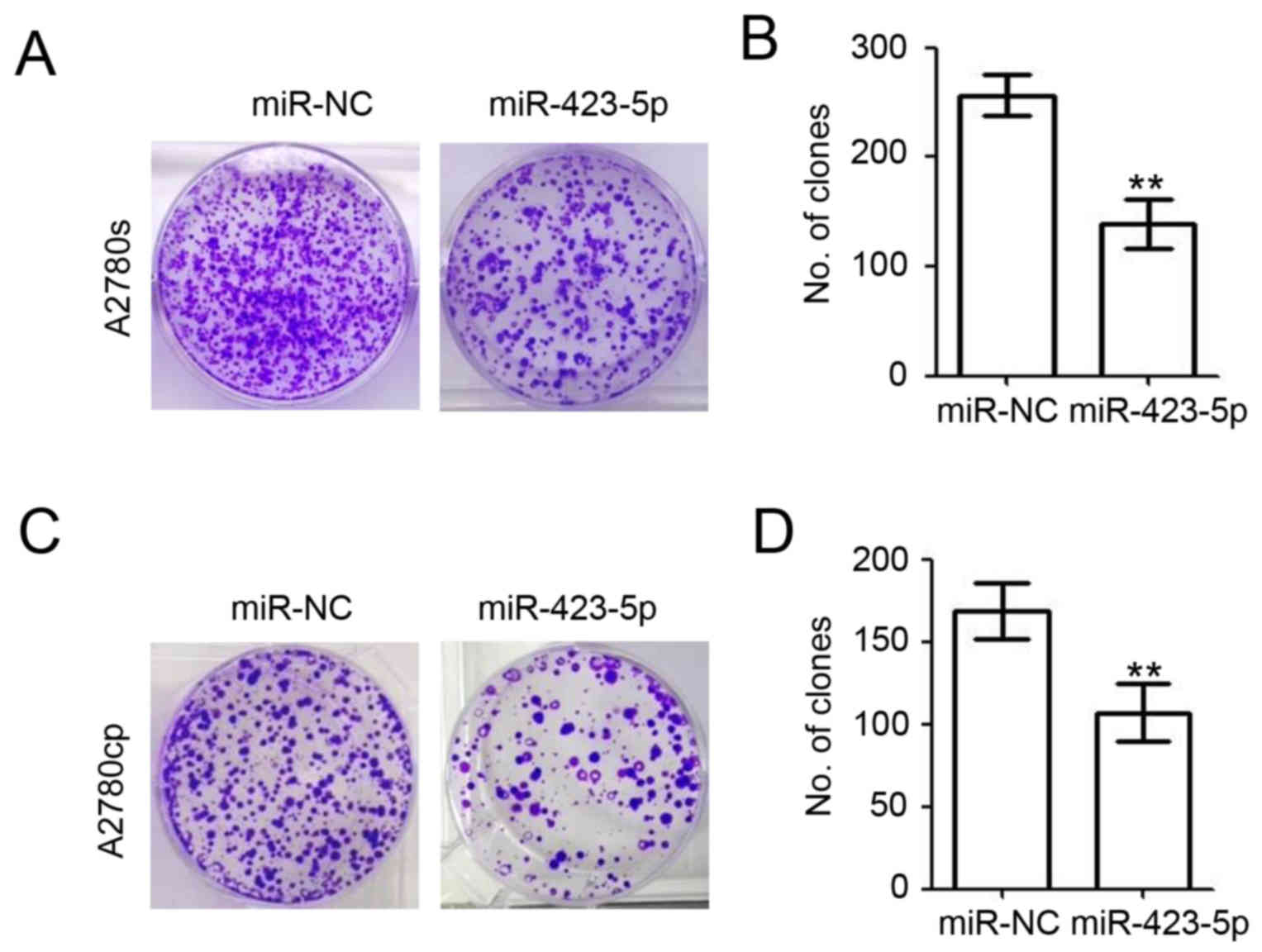
A colony formation assay was performed to further
investigate the roles of miR-423-5p in ovarian cancer (Fig. 5). At 48 h post miR-NC and miR-423-5p
transfection, 1,000 A2780s and A2780cp cells were seeded into the
wells of a 6-well plate containing 2 ml DMEM with 10% FBS. Ten days
later, crystal violet was used to stain the colonies of A2780s
(Fig. 5A) and A2780cp cells
(Fig. 5C). The results also
demonstrated the inhibitory role of miR-423-5p regarding the colony
formation ability of A2780s cells (miR-423-5p vs. miR-NC: 132.0±5.7
vs. 187.3±10.3; decreased by 29.5%; Fig.
5B). Overexpression of miR-423-5p also impaired the colony
formation ability of A2780cp cells (miR-423-5p vs. miR-NC:
157.3±7.8 vs. 256.7±10.9; decreased by 38.7%; Fig. 5D). These results demonstrated the
inhibitory role of miR-423-5p regarding the colony formation of
ovarian cancer cells.
Overexpression of miR-423-5p reduces
the invasion ability of ovarian cancer
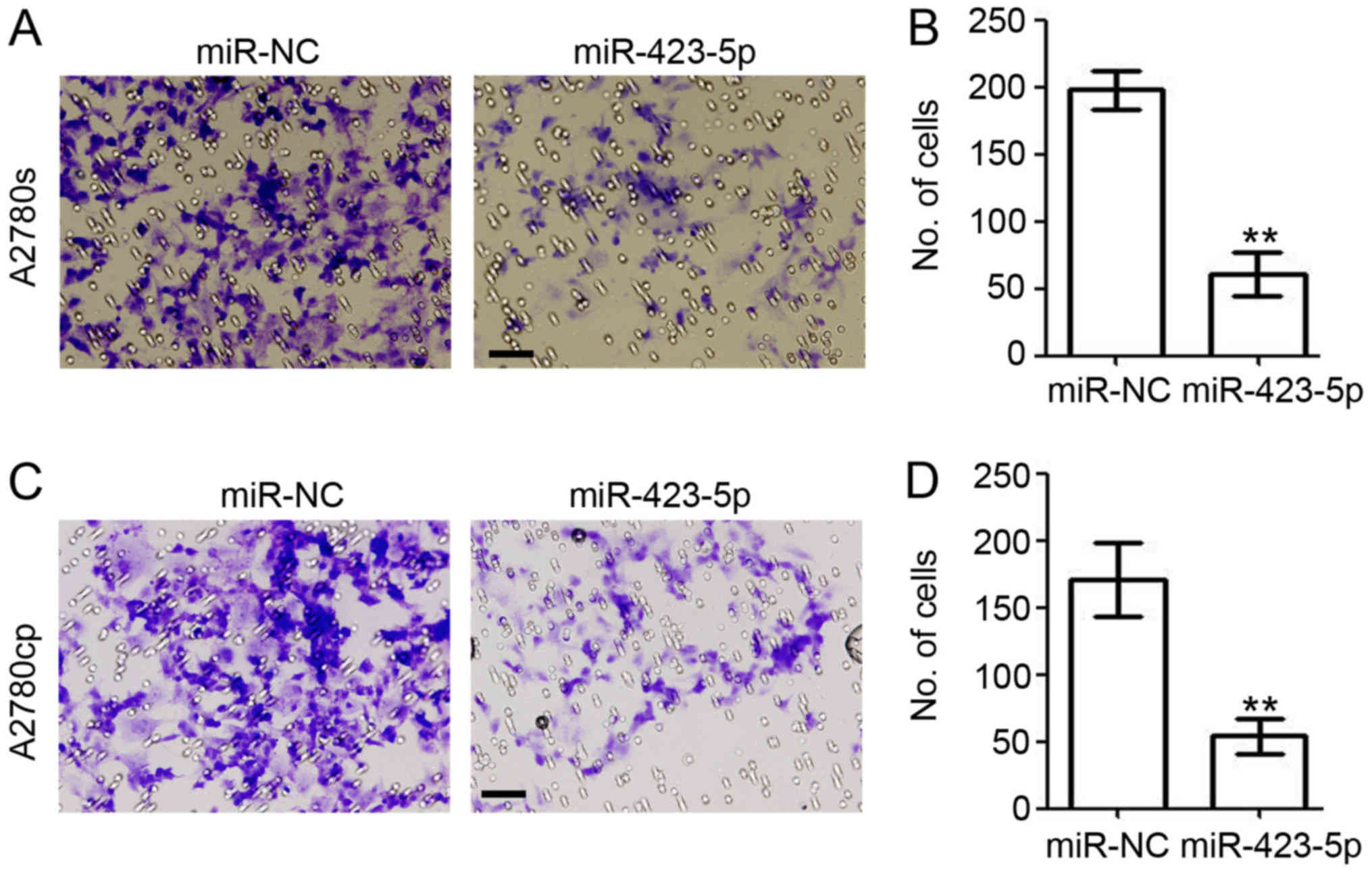
Next, the present study investigated the role of
miR-423-5p in cell invasion by performing a
Matrigel®-based invasion assay (Fig. 6). In the miR-423-5p-transfected
A2780s cells, the amount of invaded cells was reduced by 70.4%
(miR-423-5p vs. miR-NC: 60.7±9.2 vs. 198.0±8.2; Fig. 6A and B). In A2780cp cells, ectopic
expression of miR-423-5p also significantly reduced cell invasion
(miR-423-5p vs. miR-NC: 54.0±7.4 vs. 171.0±15.9; Fig. 6C and D). Collectively, the present
results further demonstrated the role of miR-423-5p in inhibiting
the invasion of ovarian cancer cells.
Discussion
Early detection has long been key to the successful
treatment of multiple life-threatening diseases, including ovarian
cancer. It has been reported that miR-141 and miR-200a/b/c are the
most significantly overexpressed miRs, whereas miR-199, miR140,
miR-145 and miR-125b are significantly downregulated in ovarian
cancer (26). Decreased miR-145
expression was detected in serum of patients with malignant and
benign ovarian tumors compared with that in healthy controls, and
miR-145 may potentially serve as a biomarker for the detection of
ovarian cancer (27). The present
study was the first to report that miRNA-423-5p is downregulated in
ovarian tissues and plasma from ovarian cancer patients. miR-423-5p
expression in ovarian tissue and plasma was identified to be
significantly associated with metastasis and tumor progression of
ovarian cancer. Furthermore, miR-423-5p was indicated to function
as a tumor suppressor in ovarian cancer by inhibiting cell
proliferation, colony formation and invasion. Taken together, the
present results suggest that miRNA-423-5p functions as a tumor
suppressor in ovarian cancer and may potentially be utilized as a
negative diagnostic indicator.
Aberrant expression of miRNA-423-5p has been
reported in several cancer types. The plasma levels of miR-423-5p
were decreased in patients with colon cancer, but increased in
patients with inflammatory bowel disease (22). The sensitivity of miR-423-5p in
detecting colon cancer at the early stage was determined as 88.89%
and the plasma concentration of miR-423-5p was increased in
patients with clinical improvement after the surgery (22). Secretory miR-423-5p was upregulated
in vitro and in vivo by sorafenib treatment and its
increase was correlated with the response to therapy in
hepatocarcinoma (21). Furthermore,
in pancreatic cancer, miR-423-5p was either downregulated or
upregulated with a significant inter-individual variation (28). However, miR-423-5p was demonstrated
to be overexpressed in glioma tissues and corresponding glioma stem
cells (29). In the present study,
the ovarian tissue and plasma miRNA-423-5p levels in ovarian cancer
patients were detected by RT-qPCR, and patients with ovarian
endometriosis served as control subjects. The results indicated
that the average miRNA-423-5p expression was markedly lower in the
ovarian tumor tissues and plasma of ovarian cancer patients
compared with that in the samples of ovarian endometriosis
patients. Subgroup analysis revealed that the expression levels of
miRNA-423-5p were lower in the ovarian cancer tissue and plasma of
patients with a higher tumor stage or those with tumor metastasis.
The heterogeneity of miR-423-5p expression may be caused by the
heterogeneity of the tumor tissues. Collectively, these results
demonstrated that miR-423-5p is a potential diagnostic biomarker
and an indicator of tumor progression in ovarian cancer. In the
future, in order to analyze the receiver operator characteristic
curves for ovarian cancer diagnosis by plasma miRNA-423-5p, more
clinical samples should be collected for the determination of
miR-423-5p levels. Furthermore, the survival data of ovarian cancer
patients should be collected for exploring the correlation between
miR-423-5p expression and the clinical outcome for ovarian cancer
patients.
Previous studies have demonstrated that in different
tumor types, miR-423-5p may have the opposite role to that in
ovarian cancer. In gastric cancer, miRNA-423-5p was reported to
participate in proliferation/invasion-associated processes via
negatively regulating the expression of trefoil factor 1, which is
a tumor suppressor gene, in the stomach (30). Furthermore, miR-423-5p expression
enhanced glioma cell proliferation, angiogenesis and invasion via
targeting inhibitor of growth family member 4 and activating
important signaling molecules, including AKT and extracellular
signal-regulated kinase 1/2 (31).
In another study, miR-423-5p knockdown notably enhanced the
inhibitory effect of apigenin on the proliferation of glioma stem
cells and the promotion of their apoptosis through the
mitochondrial pathway (29).
However, hepatocellular cancer cells transfected with miR-423-5p
exhibited an increase in the S-phase population of the cell cycle,
paralleled by a similar-size increase in autophagic cells (21). miR-423-5p was indicated to be an
inducer of apoptosis in cardiomyocytes by targeting O-GlcNAc
transferase (19). The present
results suggested that miR-423-5p functions as a tumor suppressor
in ovarian cancer according to its inhibitory role on cell
proliferation, colony formation and cell invasion. However, the
direct targets of miR-423-5p in ovarian cancer remain to be
elucidated and further in-depth research is required.
In conclusion, miR-423-5p in the tumor tissues and
plasma of ovarian cancer patients was indicated to be inversely
associated with metastasis and tumor progression. Therefore,
miRNA-423-5p may serve as an important molecular marker for the
diagnosis of ovarian cancer and an indicator of its progression. In
the future, the correlation between miR-423-5p expression and the
clinical outcome of ovarian cancer patients should be further
explored.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data and materials supporting the findings of this
study are available within the article.
Authors' contributions
XT, XZ and YH were involved in acquisition of the
data. SC and FL were involved in the analysis and interpretation of
the data. XZ, GY and NY were involved in the collection of human
tissues. NY was involved in the conception and design of the
present study.
Ethics approval and consent to
participate
Human ovarian cancer tissues and ovarian tissues
from patients with ovarian endometriosis were obtained with written
and signed informed consent under a general waiver for the proper
secondary use of human material by the institutional review board
of the Academic Medical Center (Chengdu, China) and were obtained
from Sichuan Provincial People's Hospital and The Second People's
Hospital of Neijiang City (Neijiang, China).
Consent for publication
Not applicable.
Competing interests
All authors declare that there are no competing
interests.
References
|
1
|
Chang SJ, Bristow RE, Chi DS and Cliby WA:
Role of aggressive surgical cytoreduction in advanced ovarian
cancer. J Gynecol Oncol. 26:336–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kujawa KA and Lisowska KM: Ovarian
cancer-from biology to clinic. Postepy Hig Med Dosw (Online).
69:1275–1290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dinkelspiel HE, Champer M, Hou J, Tergas
A, Burke WM, Huang Y, Neugut AI, Ananth CV, Hershman DL and Wright
JD: Long-term mortality among women with epithelial ovarian cancer.
Gynecol Oncol. 138:421–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devi Uma K, Purushotham N and Jayashree N:
Management of ovarian cancer in younger women. Rev Recent Clin
Trials. 10:263–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gasparri ML, Attar R, Palaia I, Perniola
G, Marchetti C, Di Donato V, Farooqi AA, Papadia A and Panici PB:
Tumor infiltrating lymphocytes in ovarian cancer. Asian Pac J
Cancer Prev. 16:3635–3638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grisham RN, Hyman DM and Iyer G: Targeted
therapies for treatment of recurrent ovarian cancer. Clin Adv
Hematol Oncol. 12:158–162. 2014.PubMed/NCBI
|
|
7
|
Au KK, Josahkian JA, Francis JA, Squire JA
and Koti M: Current state of biomarkers in ovarian cancer
prognosis. Future Oncol. 11:3187–3195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong X, Men X, Zhang W and Lei P: Advances
in tumor markers of ovarian cancer for early diagnosis. Indian J
Cancer. 51 Suppl 3:e72–e76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Slezak-Prochazka I, Durmus S, Kroesen BJ
and van den Berg A: MicroRNAs, macrocontrol: Regulation of miRNA
processing. RNA. 16:1087–1095. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu J, Wang S, Zhang W, Qiu J, Shan Y,
Yang D and Shen B: Screening key microRNAs for castration-resistant
prostate cancer based on miRNA/mRNA functional synergistic network.
Oncotarget. 6:43819–43830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun X and Zhang J: Dysfunctional
miRNA-mediated regulation in chromophobe renal cell carcinoma. PLoS
One. 11:e01563242016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sathyapalan T, David R, Gooderham NJ and
Atkin SL: Increased expression of circulating miRNA-93 in women
with polycystic ovary syndrome may represent a novel, non-invasive
biomarker for diagnosis. Sci Rep. 5:168902015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Min L, Ren C, Xu X, Yang J, Sun X,
Wang T, Wang F, Sun C and Zhang X: miRNA-148a serves as a
prognostic factor and suppresses migration and invasion through
Wnt1 in non-small cell lung cancer. PLoS One. 12:e01717512017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zedan AH: Heterogeneity of miRNA
expression in localized prostate cancer with clinicopathological
correlations. PLoS One. 12:e01791132017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gautam A, Kumar R, Dimitrov G, Hoke A,
Hammamieh R and Jett M: Identification of extracellular miRNA in
archived serum samples by next-generation sequencing from RNA
extracted using multiple methods. Mol Biol Rep. 43:1165–1178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura K, Sawada K, Yoshimura A, Kinose
Y, Nakatsuka E and Kimura T: Clinical relevance of circulating
cell-free microRNAs in ovarian cancer. Mol cancer. 15:482016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li SP, Su HX, Zhao D and Guan QL: Plasma
miRNA-506 as a prognostic biomarker for esophageal squamous cell
carcinoma. Med Sci Monitor. 22:2195–2201. 2016. View Article : Google Scholar
|
|
18
|
Tijsen AJ, Creemers EE, Moerland PD, de
Windt LJ, van der Wal AC, Kok WE and Pinto YM: MiR423-5p as a
circulating biomarker for heart failure. Circ Res. 106:1035–1039.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo P, He T, Jiang R and Li G:
MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in
cardiomyocytes. Mol Med Rep. 12:1163–1168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Zeng A, Hu Q, Yan W, Liu Y and You
Y: miR-423-5p contributes to a malignant phenotype and temozolomide
chemoresistance in glioblastomas. Neuro Oncol. 19:55–65. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stiuso P, Potenza N, Lombardi A,
Ferrandino I, Monaco A, Zappavigna S, Vanacore D, Mosca N,
Castiello F, Porto S, et al: MicroRNA-423-5p promotes autophagy in
cancer cells and is increased in serum from hepatocarcinoma
patients treated with sorafenib. Mol Ther Nucleic Acids.
4:e2332015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang Z, Tang J, Bai Y, Lin H, You H, Jin
H, Lin L, You P, Li J, Dai Z, et al: Plasma levels of microRNA-24,
microRNA-320a and microRNA-423-5p are potential biomarkers for
colorectal carcinoma. J Exp Clin Cancer Res. 34:862015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai L, Cui X, Zhang X, Cheng L, Liu Y,
Yang Y, Fan P, Wang Q, Lin Y, Zhang J, et al: SARI inhibits
angiogenesis and tumour growth of human colon cancer through
directly targeting ceruloplasmin. Nat Commun. 7:119962016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Falcetta FS, Lawrie TA, Medeiros LR, da
Rosa MI, Edelweiss MI, Stein AT, Zelmanowicz A, Moraes AB, Zanini
RR and Rosa DD: Laparoscopy vs. laparotomy for FIGO stage I ovarian
cancer. Cochrane Database Syst Rev. 10:CD0053442016.PubMed/NCBI
|
|
26
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang H, Jiang Z, Xie G and Lu Y: Serum
microRNA-145 as a novel biomarker in human ovarian cancer. Tumour
Biol. 36:5305–5313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ali S, Saleh H, Sethi S, Sarkar FH and
Philip PA: MicroRNA profiling of diagnostic needle aspirates from
patients with pancreatic cancer. Br J Cancer. 107:1354–1360. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wan Y, Fei X, Wang Z, Jiang D, Chen H,
Wang M and Zhou S: miR-423-5p knockdown enhances the sensitivity of
glioma stem cells to apigenin through the mitochondrial pathway.
Tumour Biol. 39:10104283176955262017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Wang X, Yang X, Liu Y, Shi Y, Ren J
and Guleng B: miRNA423-5p regulates cell proliferation and invasion
by targeting trefoil factor 1 in gastric cancer cells. Cancer Lett.
347:98–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Zeng A, Hu Q, Yan W, Liu Y and You
Y: miR-423-5p contributes to a malignant phenotype and temozolomide
chemoresistance in glioblastomas. Neuro Oncol. 19:55–65. 2017.
View Article : Google Scholar : PubMed/NCBI
|