Introduction
It is well established that dyslipidemia is one of
the most important risk factors for coronary heart disease (CHD)
(1,2). For patients with established CHD, the
use of lipid-lowering drugs is important in order to reduce the
risk of recurrent cardiovascular events (3). Based on a series of clinical trials
over the last two decades, statins block the rate-limiting step in
the biosynthesis of cholesterol and are the first-line treatments
used to decrease low-density lipoprotein-cholesterol (LDL-C) levels
(4,5). The United States guidelines recommend
using high-intensity statin therapy in coronary patients in order
to achieve a lowering of LDL-C by at least 50% (6). European guidelines recommend an LDL-C
goal of <1.8 mmol/l (70 mg/dl) or at least a 50% reduction of
LDL-C in patients with established CHD (2,7).
Certain patients are resistant or poorly tolerant to
statin treatment. Other lipid-lowering agents may be combined with
statins to reduce lipid levels. Ezetimibe and colesevelam
hydrocholoride are two cholesterol-lowering agents often used as
add-on therapy to statins to further lower LDL-C levels when
therapeutic goals are not achieved with statins alone. Colesevelam
is the second-generation medication of bile acid sequestrant, which
has a high binding affinity for specific bile acids (8,9). Studies
have demonstrated that colesevelam in combination with other
lipid-lowering drugs effectively lowers plasma lipid levels
(10,11). By contrast, ezetimibe lowers LDL-C
levels by inhibiting intestinal cholesterol absorption when used
alone or with statin therapy (12,13). The
results of the IMProved Reduction of Outcomes: Vytorin Efficacy
International Trial indicated that significantly more CHD patients
treated with a combination of a statin and ezetimibe met LDL-C
goals than patients treated with statin alone (14,15).
Ezetimibe-statin combination therapy reduces cardiovascular
outcomes in patients following vascular surgery or acute coronary
syndrome (16). The effectiveness
and safety of combined therapy with statins and other
lipid-lowering agents is worthy of further investigation.
Given the importance of LDL-C reduction in
influencing long-term risks of cardiovascular events, it's
important to investigate the clinical effectiveness of
cholesterol-targeted agents (17).
Only few studies have been performed to investigate the use
combinational therapy in CHD patients. The present study
investigated the effectiveness and safety of statin/colesevelam
combination therapy, statin/ezetimibe combination therapy and
high-intensity statin monotherapy in patients with CHD. The present
study may provide guidance for the management of dyslipidemia in
CHD patients.
Patients and methods
Enrollment of participants
The enrolled CHD patients with hypercholesterolemia
were hospitalized and received percutaneous coronary intervention
(PCI) at the Department of Cardiovascular Medicine of Linyi Central
Hospital (Linyi, China) between January 2016 and June 2016. All of
the participants were Chinese. The study was approved by the Ethics
Committee of Linyi Central Hospital (Linyi, China). Patients
provided written informed consent prior to the study commencing.
None of the patients took any lipid-lowering agents within one
month prior to admission and those with plasma LDL-C levels of
>100 mg/dl were eligible for inclusion. Patients were required
to have liver alanine aminotransferase (ALT) or aspartate
aminotransferase (AST) and creatine phosphokinase (CK) of <50%
above the upper limit of normal (ULN). Pregnant or lactating
patients, patients with kidney or liver diseases, and patients with
malignant tumors, autoimmune diseases or hypothyroidism were
excluded from the study. In total, 180 patients were enrolled in
the study.
Patient grouping
After receiving standard treatments for CHD,
patients were randomly divided into three groups that received
different lipid-lowering therapies: Statin/colesevelam combined
therapy (statin/col; 20 mg atorvastatin and 10 mg colesevelam
daily), statin/ezetimibe combined therapy (statin/eze; 20 mg
atorvastatin and 10 mg ezetimibe daily) and high-intensity statin
monotherapy (statin mono; 30 mg atorvastatin daily). The drugs were
taken once a day. Plasma levels of lipids and enzymes were measured
on admission and eight weeks after lipid-lowering therapy. Patients
were followed up for one year after treatment for monitoring of
adverse events.
No differences in the demographic data were noted
among the three groups of patients. The baseline characteristics of
the patients are summarized in Table
I. The majority of the patients were male. The age (mean ±
standard deviation) was 61±9.1 years in the statin/col group,
60±8.7 years in the statin/eze group and 60±8.5 years in the statin
monotherapy group.
 | Table I.Baseline characteristics of
patients. |
Table I.
Baseline characteristics of
patients.
| Characteristics | Statin/col
(n=60) | Statin/eze
(n=60) | Statin mono
(n=60) |
|---|
| Demographic data |
|
|
|
| Age
(years) | 61±9.1 | 60±8.7 | 60±8.5 |
|
Males | 38 (63.3) | 40 (66.7) | 37 (61.7) |
|
Hypertension | 34 (56.7) | 33 (55) | 35 (58.3) |
| Smoking
history | 27 (45) | 29 (48.3) | 28 (46.7) |
| Clinical
presentation |
|
|
|
|
STEMI | 19 (31.7) | 20 (33.3) | 17 (28.3) |
|
NSTE-ACS | 41 (68.3) | 40 (66.7) | 43 (71.7) |
Effectiveness and safety
measurements
Treatments were considered as effective when the
LDL-C-lowering goals were achieved. The goal was defined as LDL-C
<70 mg/dl (1.8 mmol/l) or a reduction of LDL-C by at least 50%.
The effectiveness rates in the three groups of patients were
compared. The percentage changes of LDL-C from baseline after eight
weeks of treatment were recorded and compared. Plasma lipid levels
were measured. Safety was assessed by recording the occurrence of
adverse cardiovascular events, including all-cause death,
recurrence of myocardial infarction, coronary revascularization and
stroke. Adverse cardiovascular events were measured at one year
after treatment of CHD.
Statistical analyses
Categorical variables were presented as absolute
values or percentages. The Chi-square test was used for comparisons
among three groups of patients. Continuous variables were described
as the mean ± standard deviation and differences between groups
were assessed by one-way analysis of variance followed by a Least
Significant Difference test. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed with SPSS 20 (IBM Corp., Armonk, NY, USA) or GraphPad
prism 5 software (GraphPad, Inc., La Jolla, CA, USA).
Results
Baseline information of patients
The mean baseline LDL-C was 3.1±0.6, 2.9±0.4 and
3.0±0.6 mmol/l in the statin/col, statin/eze and statin mono group,
respectively. Baseline values of plasma lipids and safety
parameters were similar among the three groups (Table II).
 | Table II.Baseline levels of plasma lipids and
enzymes of patients in the three groups. |
Table II.
Baseline levels of plasma lipids and
enzymes of patients in the three groups.
| Items | Statin/col
(n=60) | Statin/eze
(n=60) | Statin mono
(n=60) |
|---|
| Baseline plasma lipid
values (mmol/l) |
|
|
|
|
LDL-C | 3.1±0.6 | 2.9±0.4 | 3.0±0.6 |
| Total
C | 4.7±0.7 | 4.6±0.5 | 4.7±0.7 |
|
HDL-C | 1.2±0.3 | 1.2±0.2 | 1.3±0.3 |
|
Triglycerides | 1.8±0.5 | 1.9±0.5 | 1.9±0.6 |
| Baseline plasma
enzyme levels (U/l) |
|
|
|
|
AST | 25.6±6.2 | 26.3±5.7 | 27.1±6.9 |
|
ALT | 26.1±7.8 | 27.0±8.1 | 25.3±8.4 |
| CK | 105.1±40.0 | 117.2±45.3 | 110.4±39.5 |
Effectiveness of the three therapeutic
strategies
After eight weeks of treatment, the levels of LDL-C
in the three groups of participants were all reduced (Table III). Combinational treatments
resulted in significantly greater reductions in mean LDL-C levels
as compared with those achieved by high-intensity statin
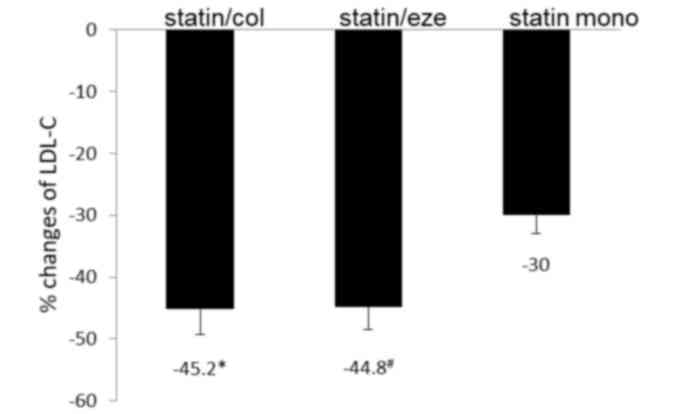
monotherapy (Fig. 1). The reductions
of the mean LDL-C values in the two combinational treatment groups
were not significantly different (Fig.
1). The proportions of patients achieving the LDL-C-lowering
goal were 68, 72 and 50% in the statin/col, statin/eze and statin
monotherapy group, respectively (data not shown). In the
combinational treatment groups, a higher percentage of patients
achieved the LDL-C goals.
 | Table III.Plasma lipid levels (mmol/l) after
eight weeks of treatment. |
Table III.
Plasma lipid levels (mmol/l) after
eight weeks of treatment.
| Plasma lipid | Statin/col
(n=60) | Statin/eze
(n=60) | Statin mono
(n=60) |
|---|
| LDL-C |
1.7±0.4a | 1.9±0.3a | 2.1±0.4 |
| Total C | 3.1±0.6 | 3.3±0.4 | 3.5±0.6 |
| HDL-C | 1.3±0.3 | 1.1±0.3 | 1.2±0.3 |
| Triglycerides | 1.4±0.4 | 1.4±0.4 | 1.5±0.5 |
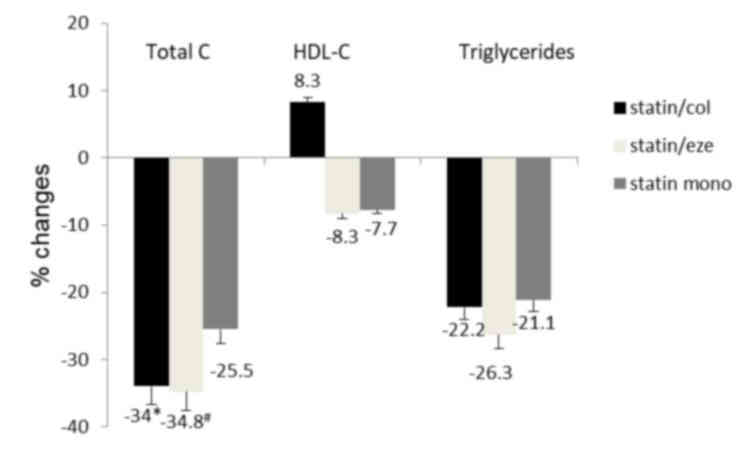
The percentage reductions of total cholesterols were
34.0, 34.8 and 25.5% in the statin/col, statin/eze and statin mono
group, respectively. Combinational treatments achieved higher
effectiveness in reducing total cholesterol (Fig. 2). Changes in triglycerides were
similar among the three treatment groups. Levels of HDL-C were
increased in the statin/col group and decreased in statin/eze and
statin mono groups compared with the baseline levels. The
difference was not significant among the three groups.
Safety assessment
The proportion of patients reporting serious adverse
events, including strokes, coronary artery diseases and
mortalities, was similar among the three treatment groups. During
the 12-month follow-up, one case of stroke was reported in the
statin mono group, one coronary artery bypass grafting in the
statin/col group and one transient ischemic attack in the
statin/eze group. These serious events were considered not to
associated with the drugs. The number and rate of other observed
drug-associated events, which were mainly muscle-associated adverse
events, were similar among the three groups (P=0.73). No patient
was reported to have increased ALT or AST ≥3X ULN. Two patients had
CK ≥5X ULN. These two patients also had symptoms of myalgia. A
summary of the safety assessment is presented in Table IV.
 | Table IV.Summary of safety data. |
Table IV.
Summary of safety data.
| Item | Statin/col
(n=60) | Statin/eze
(n=60) | Statin mono
(n=60) |
|---|
| Serious adverse
events | 1 (1.7) | 1 (1.7) | 1 (1.7) |
| Drug-associated
adverse events | 4 (6.7) | 3 (5) | 3 (5) |
| Serious
drug-associated adverse events | 0 | 0 | 0 |
| ALT/AST ≥3X
ULN | 0 | 0 | 0 |
| CK ≥5X ULN | 1 (1.7) | 0 | 1 (1.7) |
Discussion
The importance of reducing the levels of LDL-C in
CHD patients has been well recognized. When the first-line statin
medication is insufficient or poorly tolerated, a second-line
treatment option, including ezetimibe or colesevelam, may be
considered. These second-line treatments are known to reduce LDL-C
levels. However, studies investigating the effectiveness and side
effects of combinational treatment in CHD patients, particularly in
Asian populations, are currently lacking. The present study
compared the effectiveness of statin/ezetimibe, statin/colesevelam
and high-intensity statin monotherapy in the management of plasma
lipids in CHD patients, and the side effects were also observed.
The present study indicated that the combined therapies were more
effective in reducing LDL-C than high-dose statin monotherapy in
CHD patients. Furthermore, a larger percentage of patients achieved
lowering LDL-C goals in the combined therapy groups than in the
statin monotherapy group. The safety was similar among the three
therapeutic methods.
It has been reported that in patients with
hypercholesterolemia and CHD, achieving LDL-C targets often fails
(18). Moderate dosages of statin
therapy may not be sufficient for achieving the LDL-C treatment
goals (19). Physicians often
prescribe low or moderate doses of statins instead of
high-intensity statins in order to reduce adverse effects (20,21).
Indeed, certain patients are intolerant or resistant to high doses
of statin therapy. Statins combined with other lipid-lowering
agents may be a choice for those patients. Several studies have
investigated the effects of statin/eze as compared with
high-intensity statin monotherapy (22–25).
Although the dosages of agents used may differ between various
studies, the present results were consistent or similar to those of
previous studies. As atorvastatin is usually prescribed at a daily
dosage of 20 mg in China, patients in the high-intensity statin
group received 30 mg atorvastatin daily and patients in the
combined therapy groups received 20 mg atorvastatin plus 10 mg
ezetimibe or colesevelam. A review estimated that compared with
high-intensity statin monotherapy, mid-intensity statin combined
with ezetimibe may achieve a further decrease of LDL-C by 5–15%
(26). The results of the present
study indicated that statin combined with ezetimibe reduced LDL-C
by 44.8% as compared with 30% by statin alone. The proportion of
patients reaching the LDL-C-lowering goal was also higher in the
statin/eze group, indicating that combining ezetimibe with a
moderate dose of statin is more effective in reducing LDL-C than
high-intensity statin therapy alone. Statins inhibit the production
of cholesterol, which in turn may upregulate the absorption of
cholesterol, whereas ezetimibe reduces the absorption of
cholesterol by inhibiting the Niemann-Pick C1-like 1 protein. This
may reflect that the two agents have different mechanisms by which
they reduce LDL. Colesevelam is another type of lipid-lowering
agent, which acts as a bile acid sequestrant (27,28).
Combining colesevelam with statins is an alternative to
high-intensity statin therapy. Several trials compared the effect
of statin monotherapy to combination therapy with bile acid
sequestrant in patients with hyperlipidemia (10,29–32). In
high-risk hyperlipidemic patients, low-intensity statin combined
with bile acid sequestrant decreased LDL-C levels 0–14% more than
moderate-intensity statin monotherapy (26). However, there is currently a lack of
studies investigating the lipid-lowering effects of colesevelam
plus statin in CHD patients. The results of the present study
indicated that colesevelam combined with moderate-intensity statin
therapy was more effective in lowering LDL-C than high-intensity
statin monotherapy. Colesevelam has been reported to improve the
lipoprotein particles; in the present study, HDL was increased by
colesevelam, which was consistent with the results of previous
studies (33,34). The proportion of patients achieving
the LDL-C lowering goal was 68% in the statin/col group as compared
with 50% in the statin monotherapy group. The effects of the two
combinational treatment methods were similar, suggesting that
administration of colesevelam or ezetimibe combined with
moderate-dose statin may be an alternative method for the
management of hyperlipidemia.
The safeties and tolerability of the three treatment
methods were studied and compared. There was no report of
drug-associated serious side effects. A total of 10 patients (5.6%)
developed musculoskeletal side effects and the symptoms were mainly
myalgia. The rate is consistent with that reported in a previous
study (35). None of the patients
had any increased ALT or AST by ≥3X ULN; however, two patients had
CK ≥5X ULN. The incidence of side effects was similar among the
three groups of patients. The major side effects of colesevelam are
reported to be gastrointestinal discomfort, including nausea,
abdominal cramps and impaired absorption of other medications
(8,36). None of these side effects were
observed in the present study. The treatments were generally well
tolerated and safe in the whole population of participants.
In conclusion, atorvastatin combined with
colesevelam or ezetimibe was more effective than high-intensity
statin monotherapy in reducing plasma LDL-C levels in patients with
CHD. The combined therapies are safe and well-tolerated. The
combinational therapeutic strategy may be an alternative to
high-intensity statin monotherapy for patients that are resistant
or intolerant to statins. One limitation of the present study is
that the number of patients is moderate. Multi-centered,
large-scale and randomized clinical trials are warranted to confirm
the results of the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CHL gathered the patients' clinical information, and
analyzed and interpreted the patient data. QWL helped to design the
study and was a major contributor in writing the study. XHX
designed the study and carried out the statistical analysis. All
authors read and approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Linyi Central Hospital (Linyi, China). Patients provided written
informed consent prior to the study commencing.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Graham I, Cooney MT, Bradley D, Dudina A
and Reiner Z: Dyslipidemias in the prevention of cardiovascular
disease: Risks and causality. Curr Cardiol Rep. 14:709–720. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perk J, De Backer G, Gohlke H, Graham I,
Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R,
et al: European Guidelines on cardiovascular disease prevention in
clinical practice (version 2012): The Fifth Joint Task Force of the
European Society of Cardiology and Other Societies on
Cardiovascular Disease Prevention in Clinical Practice (constituted
by representatives of nine societies and by invited experts).
Atherosclerosis. 223:1–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cholesterol Treatment Trialists' (CTT)
Collaboration, . Baigent C, Blackwell L, Emberson J, Holland LE,
Reith C, Bhala N, Peto R, Barnes EH, Keech A, et al: Efficacy and
safety of more intensive lowering of LDL cholesterol: A
meta-analysis of data from 170,000 participants in 26 randomised
trials. Lancet. 376:1670–1681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grundy SM, Cleeman JI, Merz CN, Brewer HB
Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr and Stone
NJ: National Heart, Lung, and Blood Institute, et al:
Implications of recent clinical trials for the national cholesterol
education program adult treatment panel III guidelines.
Circulation. 110:227–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davidson MH and Toth PP: Combination
therapy in the management of complex dyslipidemias. Curr Opin
Lipidol. 15:423–431. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stone NJ, Robinson JG, Lichtenstein AH,
Merz Bairey CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D,
Lloyd-Jones DM, et al: 2013 ACC/AHA guideline on the treatment of
blood cholesterol to reduce atherosclerotic cardiovascular risk in
adults: A report of the American College of Cardiology/American
Heart Association Task Force on Practice Guidelines. Circulation.
129 25 Suppl 2:S1–S45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
European Association for Cardiovascular
Prevention & Rehabilitation, . Reiner Z, Catapano AL, De Backer
G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman
MJ, et al: ESC/EAS Guidelines for the management of dyslipidaemias:
The Task Force for the management of dyslipidaemias of the European
Society of Cardiology (ESC) and the European Atherosclerosis
Society (EAS). Eur Heart J. 32:1769–1818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davidson MH, Dillon MA, Gordon B, Jones P,
Samuels J, Weiss S, Isaacsohn J, Toth P and Burke SK: Colesevelam
hydrochloride (cholestagel): A new, potent bile acid sequestrant
associated with a low incidence of gastrointestinal side effects.
Arch Intern Med. 159:1893–1900. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Insull W Jr, Toth P, Mullican W,
Hunninghake D, Burke S, Donovan JM and Davidson MH: Effectiveness
of colesevelam hydrochloride in decreasing LDL cholesterol in
patients with primary hypercholesterolemia: A 24-week randomized
controlled trial. Mayo Clin Proc. 76:971–982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones MR and Nwose OM: Role of colesevelam
in combination lipid-lowering therapy. Am J Cardiovasc Drugs.
13:315–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katsiki N, Athyros VG and Karagiannis A:
Exploring the management of statin intolerant patients: 2016 and
beyond. Curr Vasc Pharmacol. 14:523–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Engelking LJ, McFarlane MR, Li CK and
Liang G: Blockade of cholesterol absorption by ezetimibe reveals a
complex homeostatic network in enterocytes. J Lipid Res.
53:1359–1368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toth PP and Davidson MH: Cholesterol
absorption blockade with ezetimibe. Curr Drug Targets Cardiovasc
Haematol Disord. 5:455–462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reiner Z: Combined therapy in the
treatment of dyslipidemia. Fundam Clin Pharmacol. 24:19–28. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Špinar J, Špinarová L and Vitovec J:
IMProved reduction of outcomes: Vytorin efficacy international
trial (studie IMPROVE-IT). Vnitr Lek. 60:1095–1101. 2014.(In
Czech). PubMed/NCBI
|
|
16
|
Sando KR and Knight M: Nonstatin therapies
for management of dyslipidemia: A review. Clin Ther. 37:2153–2179.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Otokozawa S, Ai M, Asztalos BF, White CC,
Demissie-Banjaw S, Cupples LA, Nakajima K, Wilson PW and Schaefer
EJ: Direct assessment of plasma low density lipoprotein and high
density lipoprotein cholesterol levels and coronary heart disease:
Results from the Framingham Offspring Study. Atherosclerosis.
213:251–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Foley KA, Simpson RJ Jr, Crouse JR III,
Weiss TW, Markson LE and Alexander CM: Effectiveness of statin
titration on low-density lipoprotein cholesterol goal attainment in
patients at high risk of atherogenic events. Am J Cardiol.
92:79–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kotseva K, Wood D, De Backer G, De Bacquer
D, Pyörälä K and Keil U: EUROASPIRE Study Group: EUROASPIRE III: A
survey on the lifestyle, risk factors and use of cardioprotective
drug therapies in coronary patients from 22 European countries. Eur
J Cardiovasc Prev Rehabil. 16:121–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reiner Ž and Tedeschi-Reiner E: Prevalence
and types of persistent dyslipidemia in patients treated with
statins. Croat Med J. 54:339–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Šimić I and Reiner Ž: Adverse effects of
statins-myths and reality. Curr Pharm Des. 21:1220–1226. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Averna M, Zaninelli A, Le Grazie C and
Gensini GF: Ezetimibe/simvastatin 10/20 mg versus simvastatin 40 mg
in coronary heart disease patients. J Clin Lipidol. 4:272–278.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conard SE, Bays HE, Leiter LA, Bird SR,
Rubino J, Lowe RS, Tomassini JE and Tershakovec AM: Efficacy and
safety of ezetimibe added on to atorvastatin (20 mg) versus
uptitration of atorvastatin (to 40 mg) in hypercholesterolemic
patients at moderately high risk for coronary heart disease. Am J
Cardiol. 102:1489–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barrios V, Amabile N, Paganelli F, Chen
JW, Allen C, Johnson-Levonas AO, Massaad R and Vandormael K:
Lipid-altering efficacy of switching from atorvastatin 10 mg/day to
ezetimibe/simvastatin 10/20 mg/day compared to doubling the dose of
atorvastatin in hypercholesterolaemic patients with atherosclerosis
or coronary heart disease. Int J Clin Pract. 59:1377–1386. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ballantyne CM, Blazing MA, King TR, Brady
WE and Palmisano J: Efficacy and safety of ezetimibe
co-administered with simvastatin compared with atorvastatin in
adults with hypercholesterolemia. Am J Cardiol. 93:1487–1494. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gudzune KA, Monroe AK, Sharma R,
Ranasinghe PD, Chelladurai Y and Robinson KA: Effectiveness of
combination therapy with statin and another lipid-modifying agent
compared with intensified statin monotherapy: A systematic review.
Ann Intern Med. 160:468–476. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tziomalos K, Karagiannis A, Mikhailidis DP
and Athyros VG: Colesevelam: A new and improved bile acid
sequestrant? Curr Pharm Des. 19:3115–3123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong NN: Colesevelam: A new bile acid
sequestrant. Heart Dis. 3:63–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Knapp HH, Schrott H, Ma P, Knopp R, Chin
B, Gaziano JM, Donovan JM, Burke SK and Davidson MH: Efficacy and
safety of combination simvastatin and colesevelam in patients with
primary hypercholesterolemia. Am J Med. 110:352–360. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schrott HG, Stein EA, Dujovne CA, Davidson
MH, Goris GB, Oliphant TH, Phillips JC and Shawaryn GG: Enhanced
low-density lipoprotein cholesterol reduction and
cost-effectiveness by low-dose colestipol plus lovastatin
combination therapy. Am J Cardiol. 75:34–39. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bays HE, Davidson M, Jones MR and Abby SL:
Effects of colesevelam hydrochloride on low-density lipoprotein
cholesterol and high-sensitivity C-reactive protein when added to
statins in patients with hypercholesterolemia. Am J Cardiol.
97:1198–1205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hunninghake D, Insull W Jr, Toth P,
Davidson D, Donovan JM and Burke SK: Coadministration of
colesevelam hydrochloride with atorvastatin lowers LDL cholesterol
additively. Atherosclerosis. 158:407–416. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goldberg RB, Rosenson RS, Hernandez-Triana
E, Misir S and Jones MR: Initial combination therapy with metformin
plus colesevelam improves lipoprotein particles in patients with
early type 2 diabetes mellitus. J Clin Lipidol. 6:318–324. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Davidson MH, Donovan JM, Misir S and Jones
MR: A 50-week extension study on the safety and efficacy of
colesevelam in adults with primary hypercholesterolemia. Am J
Cardiovasc Drugs. 10:305–314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ramkumar S, Raghunath A and Raghunath S:
Statin therapy: Review of safety and potential side effects. Acta
Cardiol Sin. 32:631–639. 2016.PubMed/NCBI
|
|
36
|
Jacobson TA, Armani A, McKenney JM and
Guyton JR: Safety considerations with gastrointestinally active
lipid-lowering drugs. Am J Cardiol. 99:47C–55C. 2007. View Article : Google Scholar : PubMed/NCBI
|