Introduction
An infantile hemangioma (IH), also known as
hemangioma, is a common benign endothelial cell-derived tumor and
often occurs in infancy with the incidence rate of approximately
5–10% (1). IHs can often be found on
the head and face, limbs and other regions of the body. An IH has a
unique clinical course, in which it rapidly proliferates in
children at the age of 3–9 months, and then enters the
self-extinction phase (2). Although
most IHs can naturally fade away, approximately 20% of the severe
IHs cannot fade away, which directly affects the vision and
breathing of infants and young children, and even directly
oppresses adjacent organs (3). At
the same time, IHs grow rapidly at the proliferative phase, which
may be accompanied by ulcers, bleeding and dysfunction of adjacent
parts. Under severe conditions, these symptoms will affect the
appearance, organ functions, growth and development of infants and
young children, thus bringing great psychological pressures to
patients' family members. Therefore, most children's parents expect
the intervention can be conducted as early as possible to achieve
early regression of IHs.
Although there are many clinical treatments of IHs,
which are generally divided into surgical resection, physical
therapy and drug therapy (4), each
has its own limitations with varying degrees of adverse reactions.
Besides, due to the lack of the standard treatment program for IHs
currently, to explore a highly efficient and safe treatment is very
urgent.
Glucocorticoid is a traditional first-line drug for
treating IHs (5), but due to its
large individual differences in the curative effect and relatively
more adverse reactions, it has been gradually used as an auxiliary
drug in the clinical treatment of IHs. In 2008, Izadpanah et
al (6) accidentally found that
propranolol has a relatively better curative effect in the
treatment of IHs, and since then, many scholars from various
countries paid attention to and studied propranolol. Now, it has
become the first-choice drug for clinical treatment of IHs. In
order to improve the curative effect of propranolol while reducing
the side effects of adverse reactions and complications, drug dose,
route of administration, maintenance treatment time and other
aspects are discussed in current studies (7), but there are relatively less studies on
the combination of drugs. From November 2015 to August 2016, the
authors adopted oral propranolol combined with oral prednisone for
the treatment of IHs on the head and face at the proliferative
phase, and conducted a preliminary study on the curative effect and
safety of it. The results are reported as follows:
Materials and methods
General data
Forty-four children with IHs on the head and face
treated in Jinan Center Hospital Affiliated to Shandong University
(Jinan, China) from January 2015 to August 2016 were selected,
including 10 males and 34 females aged 1–8 months with an average
age of 4.5 months. All children were eligible for the diagnostic
criteria of the World Health Organization (WHO) for IHs (8). Inclusion criteria: 1) Children aged
less than 9 months with IHs on the head and face; 2) children whose
IHs were growing rapidly and tumor bodies were increased by over 2
times within 1–2 weeks; 3) children receiving no other treatment
methods before; 4) children with no congenital cardiovascular
disease, pulmonary disease (bronchial asthma and bronchitis),
diabetes and visceral hemangioma; 5) children whose parents or
guardians signed the relevant informed consent before the
treatment. This study was approved by the medical Ethics Committee
of Jinan Center Hospital. Signed written informed consents were
obtained from the patients' guardians. The children studied were
divided into the treatment group (n=22) and the control group
(n=22) using a random number table. Comparisons showed that the
general data (including age, sex and complications) in this study
were not significantly different (P>0.05) (Table I), and the data were comparable and
met the needs of this study.
 | Table I.Comparisons of general data between
two groups of children. |
Table I.
Comparisons of general data between
two groups of children.
|
|
|
| Sex (n) | Complications
(n) |
|---|
|
|
|
|
|
|
|---|
| Groups | Case (n) | Age (m) | Male | Female | Deformed
appearance | Hemorrhage | Anabrosis |
|---|
| A | 22 | 4.5±3.0 | 6 | 16 | 3 | 2 | 3 |
| B | 22 | 3.5±3.5 | 4 | 18 | 4 | 3 | 2 |
Examinations before treatment
Before treatment, all children received
electrocardiogram, blood routine examination, liver and kidney
function examination, fasting blood glucose examination, Doppler
echocardiography, chest X-ray and examinations for other parts. In
addition, the location, size, color and surface texture of IHs at
admission were recorded for preparation as controls for those after
administration.
Treatment methods
Treatment methods in group A: Children took orally
prednisone in the first week at 2 mg/kg/day in two divided doses
and propranolol at 2 mg/kg/day in three divided doses. Within the
second week, the dose of prednisone was gradually reduced to zero,
but the dose of propranolol was unchanged. Then, children orally
took propranolol for 5 consecutive months, and finally the dose was
gradually reduced to zero within two weeks. Treatment methods in
group B: Children received monotherapy with the same dose of
propranolol as that in group A for 6 months. The clinical curative
effects and the incidence rates of adverse drug reactions of group
A and B were recorded. Finally, relevant conclusions were obtained
by statistical analysis.
Clinical observation and
follow-ups
During hospitalization, the pulse, blood pressure,
blood glucose, respiratory rate and other indexes were observed all
day. Adverse reactions were closely monitored and the corresponding
measures were taken. In particular, treatment plans were stopped
when the heart rate was <70% of the normal value, systolic blood
pressure was reduced by >25% of the normal value, bronchospasm
appeared or the symptomatic blood glucose was reduced.
The two groups were followed up for a total of 9
months (including 6 months during the treatment and 3 months after
the treatment). In the first month, children were followed up every
2 weeks, followed by once a calender month. The volume, texture and
color of IHs were recorded at each follow-up in detail, and the
dose of propranolol was adjusted according to the weight of the
children.
Evaluation of curative effects
The size of IHs was recorded by hemispheric
measurement (9), and the curative
effect was evaluated using the 4-Grade standard proposed by Achauer
et al (10). Grade I: Poor
curative effects with the tumor volume shrinking <25%; grade II:
moderate curative effects with the tumor volume shrinking 26–50%;
grade III: good curative effects with the tumor volume shrinking
51–75%; grade IV: excellent curative effects with the tumor volume
shrinking 76–100%. The total effective rate of treatment =
(excellent + good + moderate)/total number of cases ×100%.
Conditions of the color of IHs becoming light and
the surface becoming flat were recorded by a digital photography,
and the curative effect was analyzed using the fractional
evaluation method adopted by Hogeling et al (11), that is, 2 points for significant
changes; 1 point for moderate changes; 0 point for no change. The
total effective rate of treatment = (2 points + 1 points)/total
number of cases ×100%.
Evaluation of the effective rate of treatment: 1)
Recovery: the tumor completely disappeared, and the skin function
returned to normal; 2) effective treatment: the tumor volume was
significantly reduced, and most skin functions returned to normal;
3) ineffective treatment: there was no significant change in the
tumor or the tumor recurs after the treatment.
Statistical analysis
The data were analyzed by SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Measurement data were expressed as mean ±
SD. Intergroup comparisons were detected using the t-test.
Enumeration data were expressed as %, and intergroup comparisons
were detected using χ2 test. P<0.05 represent that
the difference was significant, and the results were statistically
significant.
Results
Changes in the tumor volume
The following results were obtained from different
treatments for two groups of IH patients with changes in the tumor
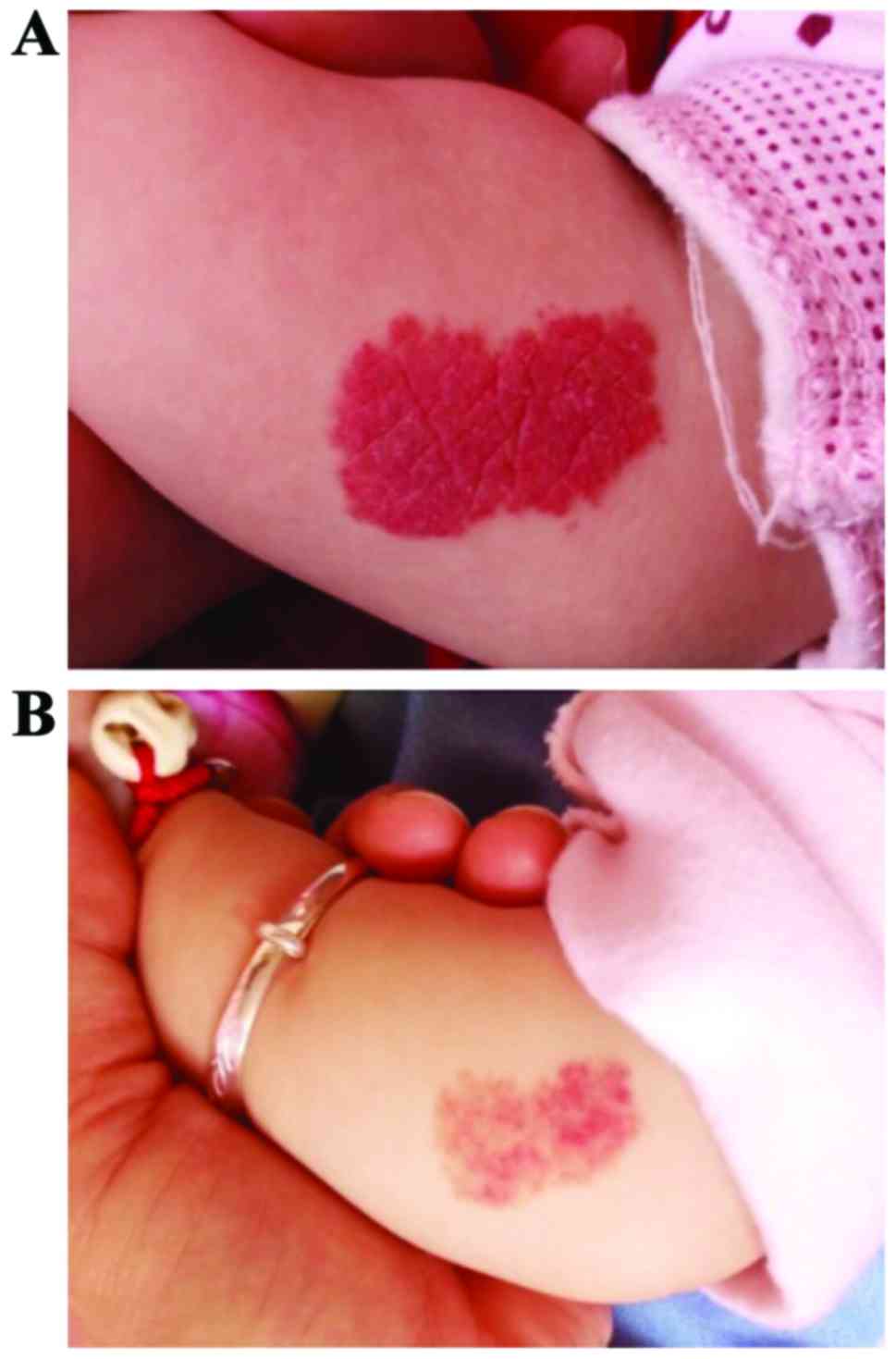
volume as the observation indexes: The total effective rate of the
clinical treatment in group A (combination therapy group with
propranolol and prednisone) was as high as 100%, which was
significantly higher than that in group B (monotherapy group with
propranolol; 81.82%). The statistical analysis showed that
differences between the two groups were significant and of
statistical significance (P<0.05) (Table II). The curative effect of group A
is shown in Fig. 1.
 | Table II.Comparisons of the tumor volume
shrinking status between two groups of children. |
Table II.
Comparisons of the tumor volume
shrinking status between two groups of children.
| Group | Case (n) | Grade IV
(excellent) | Grade III (good) | Grade II
(moderate) | Grade I (poor) | Total effective
rate |
|---|
| A | 22 | 15 | 3 | 4 | 0 | 100% |
| B | 22 | 8 | 5 | 5 | 4 | 81.82% |
Changes in the tumor surface
In addition, the following results were obtained
with the surface of IHs becoming flat as the observation index
after the treatment: The total effective rate of group A was
95.45%, and in the comparison with group B (86.36%), the results
were not significantly different between the two groups
(P>0.05), and of no statistical significance (Table III). The curative effect of group A
is shown in Fig. 2.
 | Table III.Comparisons of the tumor surface
becoming flat between two groups of children. |
Table III.
Comparisons of the tumor surface
becoming flat between two groups of children.
| Group | Case (n) | 2 points | 1 point | 0 point | Total effective
rate |
|---|
| A | 22 | 14 | 7 | 1 | 95.45% |
| B | 22 | 10 | 9 | 3 | 86.36% |
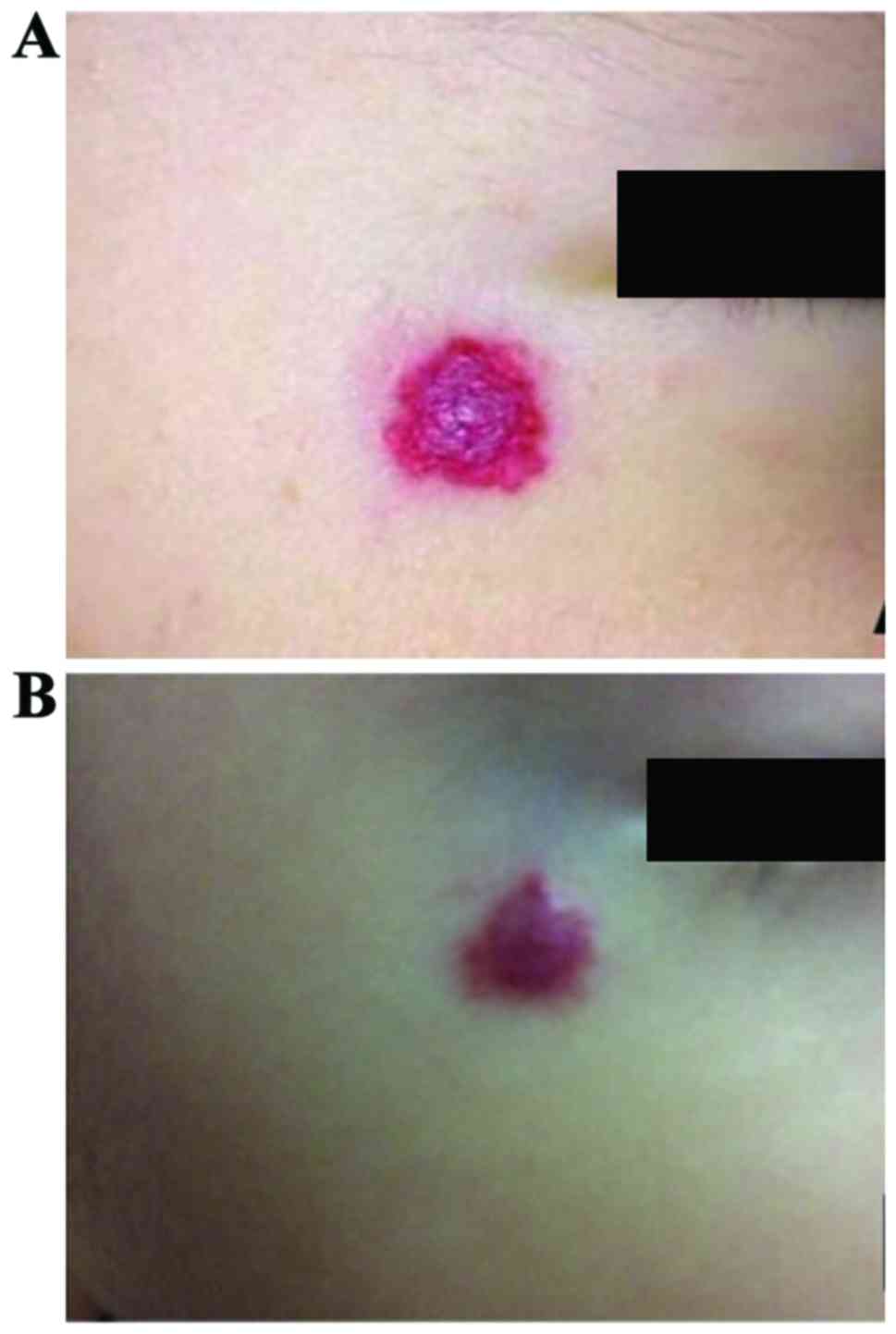
Comparison of the color shade of the
tumor
The following results were concluded with the tumor
color becoming light as the observation index: The total effective
rates of clinical treatment in group A and B were 100 and 77.27%,
respectively, and the comparison showed that the difference was
significant (P<0.05). It was found that the curative effect in
group A was significantly better than that in group B (Table IV), and the curative effect of group
A is shown in Fig. 3.
 | Table IV.Comparison of the color shade of the
tumor between two groups of children. |
Table IV.
Comparison of the color shade of the
tumor between two groups of children.
| Group | Case (n) | 2 points | 1 point | 0 point | Total effective
rate |
|---|
| A | 22 | 14 | 8 | 0 | 100% |
| B | 22 | 11 | 6 | 5 | 77.27% |
Comparisons of curative effects on
children based on age
In children younger than 6 months, those with
excellent and good curative effects accounted for 100% in group A
and 78.6% in group B, indicating that the curative effect of group
A was significantly better than that of group B (P<0.05).
However, in children older than 6 months, those with excellent and
good curative effects in the two groups accounted for 50% and
37.5%, respectively, and the comparison revealed that the
difference was not significant (P>0.05) (Table V). At the same time, at the end of
the treatment, all children were followed up for 3 months, and the
recurrence of IHs was not found in any case.
 | Table V.Comparisons of curative effects on
children of different age. |
Table V.
Comparisons of curative effects on
children of different age.
|
| Younger than 6
months | Older than 6
months |
|---|
|
|
|
|
|---|
|
| A | B | A | B |
|---|
| Case (n) | 14 | 14 | 8 | 8 |
|---|
| Excellent | 12 | 8 | 3 | 0 |
| Good | 2 | 2 | 1 | 3 |
| Moderate | 0 | 1 | 4 | 4 |
| Poor | 0 | 3 | 0 | 1 |
Comparisons of effective rates of the
treatment of different types of IHs
The effective rates of the combination therapy group
(group A) was higher than those in monotherapy group with
propranolol (group B) in the treatment of strawberry hemangiomas
and cavernous hemangiomas, and the differences were statistically
significant (P<0.05) (Table
VI).
 | Table VI.Comparisons of effective rates of the
treatment of different types of IHs between the two groups n
(%). |
Table VI.
Comparisons of effective rates of the
treatment of different types of IHs between the two groups n
(%).
| Type of IHs | Group | Case (n) | Recovery | Effective | Ineffective | Total effective | P-value |
|---|
| Strawberry
hemangiomas | A | 10 | 6 (60.0%) | 4 (40.0%) | 0 (0) | 100% | 0 |
|
| B | 10 | 4 (40.0%) | 4 (40.0%) | 2 (20.0%) |
80% |
| Spider
angiomas | A | 5 | 5
(100.0%) | 0 (0) | 0 (0) | 100% | 1 |
|
| B | 5 | 3 (60.0%) | 2 (40.0%) | 0 (0) | 100% |
| Cavernous
hemangiomas | A | 7 | 4 (57.1%) | 2 (28.6%) | 1 (14.3%) | 83.70% | 0.037 |
|
| B | 7 | 1 (14.3%) | 4 (57.1%) | 2 (28.6%) | 71.40% |
Adverse reactions
In the course of each follow-up, adverse reactions
of two groups of IH children were observed closely. In group A,
adverse reactions included loss of appetite (n=1) and bronchial and
upper respiratory tract infections (n=1); in group B, adverse
reactions included crying at night (n=1), reduced heart rate (n=1)
and loss of appetite (n=2). These adverse reactions were not
severe, and became naturally alleviated after the treatment.
Adverse reactions such as hypotension, hypoglycemia and facial
edema were not found. The incidence rate of adverse reactions was
compared between the two groups, and the difference was not
significant (P>0.05), indicating that the administration method
in group A did not increase the incidence rate of adverse reactions
(Table VII).
 | Table VII.Comparison of the total incidence
rate of adverse reactions between two groups of children. |
Table VII.
Comparison of the total incidence
rate of adverse reactions between two groups of children.
| Group | Case (n) | Crying at
night | Reduced heart
rate | Loss of
appetite | Bronchial and upper
respiratory tract infections | Total incidence
rate of adverse reactions |
|---|
| A | 22 | 0 | 0 | 1 | 1 | 9.1% |
| B | 22 | 1 | 1 | 2 | 0 | 18.2% |
Discussion
An infantile hemangioma (IH) is a recognized type of
hemangioma worldwide (12), and is
the most common type in many benign tumors occurring in infants and
young children. Its pathogenesis and unique regression process have
not been completely explained. IH often occur in females on the
head and face and grows rapidly at the proliferative phase.
Besides, the regression period is long, which not only seriously
affects the appearance, but also oppresses the lesion, thus causing
local dysfunction, so early intervention treatment is conducive to
restricting the growth of tumor body, speeding up its regression
process, reducing complications and improving the physical and
mental health of children.
Propranolol (also known as inderal), is a
traditional non-selective β-blocker that has been used for the
treatment of arrhythmia and hypertension for nearly 50 years, and
used for the treatment of IHs since 2008 when Storch and Hoeger
accidentally found that propranolol can inhibit the growth of IHs.
The mechanism of propranolol in the treatment of IHs is still not
very clear, which may be to inhibit β receptor resulting in
vasoconstriction at the lesion sites, thus inhibiting angiogenesis
and inducing IH endothelial cell apoptosis (13); and the mechanism may also be to
regulate the mitogen-activated protein kinase (MAPK) pathway, thus
reducing the expression of basic fibroblast growth factors and
vascular endothelial growth factor genes (14). In view of the cardiovascular side
effects of propranolol, there are still many uncertainties about
using propranolol as the best method to treat IH children,
including dose, frequency of medication, time of treatment, optimal
timing of treatment and reduction methods. Clinically, the
‘stepped-care treatment program’ proposed by Siegfried et al
(15) is widely used. In the
existing studies, the dose range of propranolol was 1–3 mg/kg/day
in two divided doses; in this study, the dose of propranolol was 2
mg/kg/day in three divided doses, which is the most commonly used
dose in the current literature. Twice a day of medication can
simplify the number of medication times and reduce the risk of
nocturnal hypoglycemia, but in this study, it was increased to
three times a day in view of the relatively shorter half-life of
propranolol (3–6 h) (16). The
treatment time was set at 6 months because the time period covered
most of the proliferative phase (17).
Prednisone is a corticosteroid hormone, which has
been used as a first-line drug in the treatment of IHs, and its
mechanism of action is not fully defined. Some studies suggest that
the mechanism may be associated with the inhibition of the activity
of IH stem cells, nuclear factor kappa-light-chain-enhancer of
activated B cells (NF-κB), thus, reducing the expression levels of
vascular endothelial growth factor A (VEGF-A) and other angiogenic
cytokines, including monocyte chemotactic protein 1 (MCP-1), matrix
metalloproteinase-1 (MMP-1), urokinase-type plasminogen activator
and receptor (uPAR) and interleukin 6 (IL-6) (18). Some other studies indicated that the
mechanism may be that the competitive binding of prednisone to IH
estrogen receptors inhibits the growth of tumors by acting as
antagonists. Although prednisone has certain curative effect in the
treatment of IHs, it causes many adverse reactions, and the
long-term use will lead to dysplasia or immune disorders of
children (19), so it is currently
used as an auxiliary drug in the clinical treatment of IHs.
Previously, Koay et al (20)
tried to use prednisone combined with propranolol in the treatment
of a child with IHs on the orbital region aged 3 months, and the
curative effect was good without any adverse reactions. Although it
is an individual case, this treatment program is a new attempt for
drug therapy of IHs. The dose of prednisone was 2 mg/kg/day in two
divided doses in this study, and as it was within the usual dose
range, the relevant adverse reactions were relatively lighter and
became naturally alleviated at the end of the treatment.
In this study, the comparison of the clinical
curative effect between group A and group B revealed that the
clinical curative effect in group A was significantly better than
that in group B, and that on children younger than 6 months was
better, which further confirmed that the best timing for IH
children was at the age of less than 6 months at the proliferative
stage. The comparison of adverse reactions between the two groups
showed that the difference was not significant, and the total
incidence rate of adverse reactions in group A was lower.
In summary, the combination therapy with propranolol
and prednisone in the treatment of IHs can significantly reduce the
tumor volume at the proliferative phase and significantly improve
the tumor color with a low incidence rate of adverse reactions in a
mild degree. Children have high tolerance to this treatment method,
and the treatment method is highly safe and of great significance
in clinical practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL conceived and designed the study. RH and GX were
responsible for the collection and analysis of the patient data. HL
interpreted the data and drafted the manuscript. HL revised the
manuscript critically for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics
Committee of Jinan Center Hospital (Jinan, China). Signed written
informed consents were obtained from the patients' guardians.
Consent for publication
The patients' guardians have provided written
informed consent for the publication of any associated data and
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fu Y, Yang ZG and Zhao LY: Angiogenesis
characteristics of infantile hemangioma and feasibility observation
of transplantation model of human hemangioma on mice. Eur Rev Med
Pharmacol Sci. 21:1276–1280. 2017.PubMed/NCBI
|
|
2
|
de Graaf M, Breur JM, Raphaël MF, Vos M,
Breugem CC and Pasmans SG: Adverse effects of propranolol when used
in the treatment of hemangiomas: A case series of 28 infants. J Am
Acad Dermatol. 65:320–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eivazi B, Ardelean M, Bäumler W, Berlien
HP, Cremer H, Elluru R, Koltai P, Olofsson J, Richter G, Schick B,
et al: Update on hemangiomas and vascular malformations of the head
and neck. Eur Arch Otorhinolaryngol. 266:187–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chinnadurai S, Snyder K, Sathe N,
Fonnesbeck C, Morad A, Likis FE, Surawicz T, Ness G, Ficzere C and
McPheeters ML: Diagnosis and Management of Infantile Hemangioma
[Internet]Agency for Healthcare Research and Quality. Rockville,
MD: 2016
|
|
5
|
Sethuraman G, Yenamandra VK and Gupta V:
Management of infantile hemangiomas: Current trends. J Cutan
Aesthet Surg. 7:75–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Izadpanah A, Izadpanah A, Kanevsky J,
Belzile E and Schwarz K: Propranolol versus corticosteroids in the
treatment of infantile hemangioma: A systematic review and
meta-analysis. Plast Reconstr Surg. 131:601–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Léauté-Labrèze C, de la Roque Dumas E,
Hubiche T, Boralevi F, Thambo JB and Taïeb A: Propranolol for
severe hemangiomas of infancy. N Engl J Med. 358:2649–2651. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruckner AL and Frieden IJ: Hemangiomas of
infancy. J Am Acad Dermatol. 48:477–493; quiz 494–496. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsang MW, Garzon MC and Frieden IJ: How to
measure a growing hemangioma and assess response to therapy.
Pediatr Dermatol. 23:187–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Achauer BM, Chang CJ and Vander Kam VM:
Management of hemangioma of infancy: Review of 245 patients. Plast
Reconstr Surg. 99:1301–1308. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hogeling M, Adams S and Wargon O: A
randomized controlled trial of propranolol for infantile
hemangiomas. Pediatrics. 128:e259–e266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang LC, Haggstrom AN, Drolet BA, Baselga
E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW,
et al: Hemangioma Investigator Group: Growth characteristics of
infantile hemangiomas: Implications for management. Pediatrics.
122:360–367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Storch CH and Hoeger PH: Propranolol for
infantile haemangiomas: Insights into the molecular mechanisms of
action. Br J Dermatol. 163:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou HX, Jia J, Zhang WF, Sun ZJ and Zhao
YF: Propranolol inhibits endothelial progenitor cell homing: A
possible treatment mechanism of infantile hemangioma. Cardiovasc
Pathol. 22:203–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siegfried EC, Keenan WJ and Al-Jureidini
S: More on propranolol for hemangiomas of infancy. N Engl J Med.
359:2846–2847. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sánchez-Carpintero I, Ruiz-Rodriguez R and
López-Gutiérrez JC: Propranolol in the treatment of infantile
hemangioma: Clinical effectiveness, risks, and recommendations.
Actas Dermosifiliogr. 102:766–779. 2011.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sans V, de la Roque ED, Berge J, Grenier
N, Boralevi F, Mazereeuw-Hautier J, Lipsker D, Dupuis E, Ezzedine
K, Vergnes P, et al: Propranolol for severe infantile hemangiomas:
Follow-up report. Pediatrics. 124:e423–e431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang W, Chen G, Wang FQ, Ren JG, Zhu JY,
Cai Y, Zhao JH, Jia J and Zhao YF: Macrophages contribute to the
progression of infantile hemangioma by regulating the proliferation
and differentiation of hemangioma stem cells. J Invest Dermatol.
135:3163–3172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rössler J, Wehl G and Niemeyer CM:
Evaluating systemic prednisone therapy for proliferating
haemangioma in infancy. Eur J Pediatr. 167:813–815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koay AC, Choo MM, Nathan AM, Omar A and
Lim CT: Combined low-dose oral propranolol and oral prednisolone as
first-line treatment in periocular infantile hemangiomas. J Ocul
Pharmacol Ther. 27:309–311. 2011. View Article : Google Scholar : PubMed/NCBI
|