Introduction
Lung cancer is the most common cancer with the
highest mortality rate worldwide, and it is estimated that there
are 1.2 million new cases and 1.1 million deaths worldwide annually
(1,2). Non-small cell lung cancer accounts for
80% of lung cancer cases (3).
For patients with advanced (Stage IIIB or IV) lung
cancer who cannot undergo surgical resection, chemotherapy
effectively prolongs their overall survival (OS) despite poor
prognosis, in which the treatment with platinum-based compounds,
cisplatin (DDP) or carboplatin, combined with gemcitabine,
vinorelbine or taxane (paclitaxel or texotere) is the initial
therapy of clinical non-small cell lung cancer. The International
Adjuvant Lung Cancer Trial (IALT) showed that the 3-year survival
rate of patients treated with DDP adjuvant chemotherapy was 41%,
and the 5-year OS rate was 15% (4,5).
However, the resistance of tumor cells to platinum drugs is the
leading cause of clinical failure (6,7).
Therefore, how to overcome drug resistance in order to improve the
clinical efficacy of lung cancer patients is imperative.
Patients and methods
Collection of clinicopathological
data
Fourty eight paired non-small cell lung cancer and
cancer-adjacent tissues were selected from specimens receiving
biopsy or surgical resection in the Department of Pathology of
Peking Union Medical College Hospital (Beijing, China)from
September, 2012 to May, 2014, and all specimens were diagnosed and
confirmed by pathologists. The tested patients underwent
cisplatin-based neoadjuvant chemotherapy at least 4 times prior to
surgery as follows: PC (100 mg/m2 paclitaxel + 125
mg/m2 cisplatin; once every 3 weeks) or texotere + P (75
mg/m2 texotere + 125 mg/m2 cisplatin; once
every 3 weeks). The mean age of the patients was 61.43±12.3 years,
and all study subjects signed the informed consent. The study was
approved by the Ethics Committee of Peking Union Medical College
Hospital.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The total ribonucleic acid (RNA) was extracted using
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA), and 1 µg
RNA was reverse transcribed using the GenpCopoeia Reverse
Transcription kit (GeneCopoeia, Inc., Guangzhou, China). RT-PCR was
conducted at an annealing temperature of 55°C, and the
amplification was repeated for 45 cycles using an SYBR®
Premix Ex Taq™ II kit (Biomedical Technology Co., Ltd., Chendgu,
China). The relative quantification of each gene was analyzed by
2−∆∆Cq with glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) as the internal reference for correction. The relative
expression level of messenger RNA (mRNA) of each index was
calculated as: 2−∆∆Cq [∆∆Cq = Cq (target gene) - Cq
(GAPDH)]. Primer sequences used were, long non-coding ribonucleic
acid-homeobox transcript antisense ribonucleic acid
(lncRNA-HOTAIR): forward, 5′-CGGAGTGAGTTTATCGCAG-3′, reverse,
5′-GGCGACCGGAGCTCATCTTACC-3′; GAPDH: forward
5′-ATTGATGGATGCTAFGAGTATT-3′, reverse,
5′-AGTCTTCTGGGTGGCAGTGAT-3′.
Cell culture
Human lung adenocarcinoma NCI-H1299 cell line was
purchased from the Cell Bank, Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences. NCI-H1299/DPP was the
DPP-resistant cell line constructed by our team (DDP with the final
concentration of 100 µM maintained the drug resistance). The two
cell lines were cultured in Roswell Park Memorial Institute
(RPMI)-1640 medium containing 10% fetal bovine serum and 100 U/ml
penicillin and streptomycin. The cell culture flask was incubated
at 37°C with 5% CO2.
Detection of the sensitivity of cells
to DDP by Cell Counting Kit-8 (CCK-8)
Cells were seeded in 96-well plates at the density
of 1×104/ml, and the cells completely adhered to the
wall 24 h later. Each well was added with 100 µl DDP at a final
concentration of 0.1, 1, 10, 100 and 1,000 µM for incubation for 48
h, respectively. CCK-8 reagent (10 µl) (Biotool, Shanghai, China)
was added to each well and incubated for 1 h in an incubator at
37°C. The optical density (OD) of each well at 450 nm was then
measured using a microplate reader (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Target interference of small
interfering RNA (siRNA) on lncRNA-HOTAIR
HOTAIR siRNA and negative control siRNA sequences
were obtained from inernational high-scoring journals and were
produced by Qiagen (Shanghai, China). Cells were transfected with
Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols, and 48 h later, HOTAIR
expression levels were significantly inhibited. The sequence of
targeted interference with lncRNA-HOTAIR is as follows:
5′-UUUUCUACCAGGUCGGUAC-3′.
Western blot analysis
The cells in the drug treatment group and the
control group were seeded in 6-well plates, and an appropriate
amount of protease inhibitors and protein lysate were added. The
cell lysate was aspirated and centrifuged at 12,000 × g at 4°C for
30 min. Total protein (40 µg) was electrophoresed in sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) membrane and
then transferred to a polyvinylidene difluoride (PVDF) membrane.
The respective bands were cut according to the marker and incubated
overnight with rabbit anti-human primary monoclonal antibodies
including multidrug resistance associated protein (anti-MRP1),
anti-Wnt3a, adenomatous polyposis coli (anti-APC), anti-β-catenin
and anti-GAPDH (dilution,1:800; cat. nos. ab180960; ab172612;
ab40778; ab32572 and ab181602, respectively; Abcam, Cambridge, UK)
and rabbit anti-human multidrug resistance 1 (MDR1) polyclonal
antibody (1:600; cat. no. 13978; Cell Signaling Technology,
Danvers, MA, USA). Then it was incubated with the secondary
antibody [goat anti-rabbit IgG (1:500; cat. no. ab6721, Abcam,
Cambridge, UK)]. The membrane was visualized with an enhanced
chemiluminescence (ECL) detection system (Bio-Rad lab, Hercules,
CA, USA), and the gray scale analysis was performed using a gel
analyzer. The relative content of the target protein was the ratio
of the target protein to the gray value of corresponding internal
reference bands.
Statistical analysis
The experimental results were analyzed using
GraphPad Prism software (version 5.01; GraphPad Software, San
Diego, CA, USA). The ANOVA test and Tukey post hoc test was used
for intergroup comparisons. The Kaplan-Meier method was used to
plot the patient's survival curve and examined using the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Detection of the expression level of
lncRNA-HOTAIR in cancer and cancer-adjacent tissues
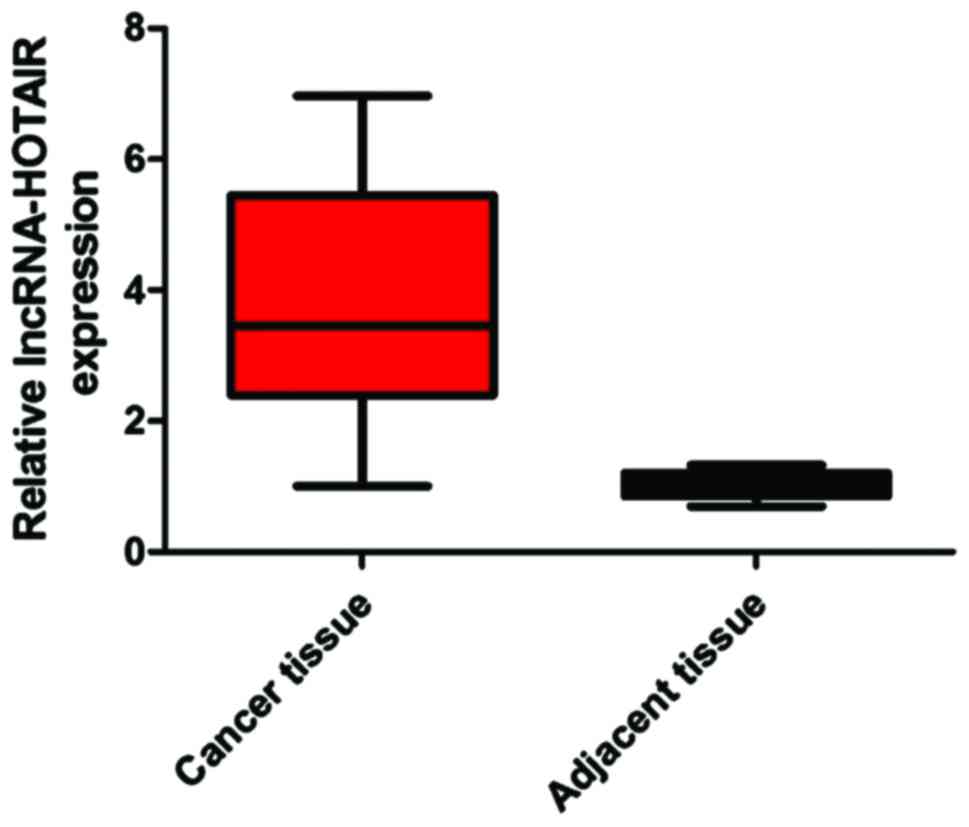
We extracted mRNAs from 48 pairs of lung cancer and
cancer-adjacent tissues of patients and detected the expression
level of lncRNA-HOTAIR. The results showed that the expression
level of lncRNA-HOTAIR in cancer tissues was significantly higher
than that in cancer-adjacent tissues (difference: 3.89-fold)
(P<0.05) (Fig. 1).
Expression level of lncRNA-HOTAIR and
the OS of patients
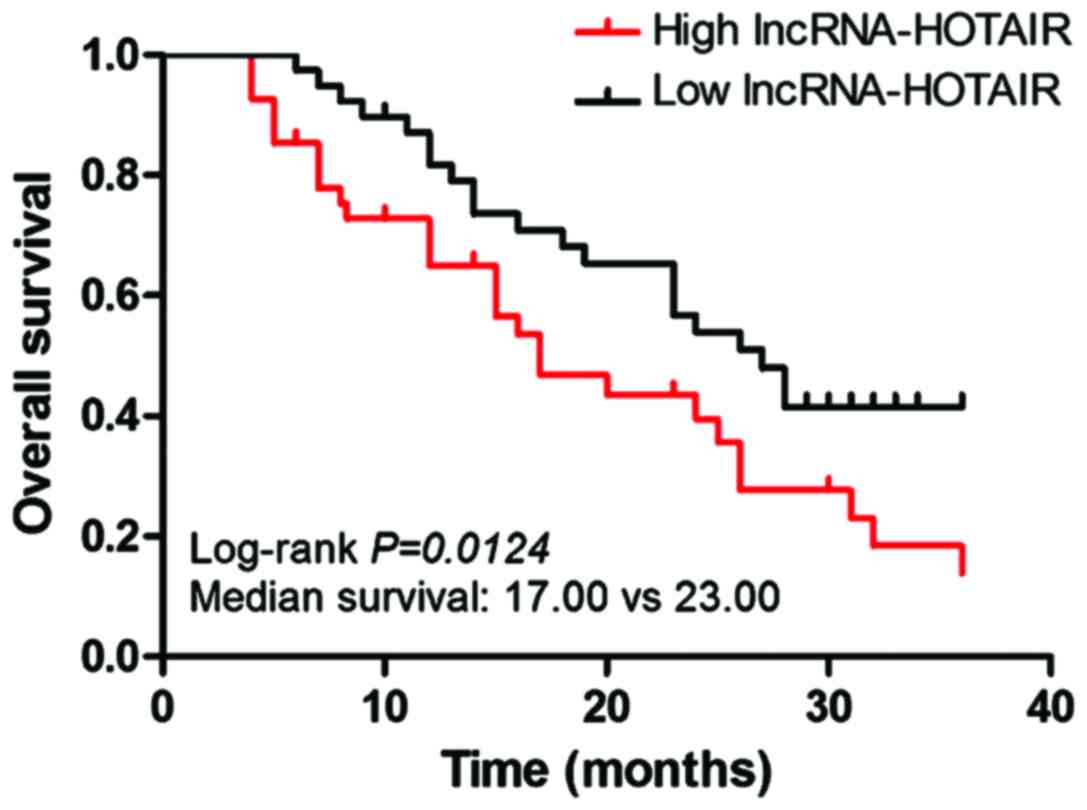
The correlation curve of the expression of
lncRNA-HOTAIR with the OS of patients was plotted using the
Kaplan-Meier method. The results indicated that compared with the
lncRNA-HOTAIR low expression group, the high expression represented
shorter OS of patients (Fig. 2).
Median survival time: lncRNA-HOTAIR low expression group vs.
lncRNA-HOTAIR high expression group = 23 months vs. 17 months
(log-rank test, P=0.0124).
Construction and detection of
drug-resistant cell lines
NCI-H1299 cells in the logarithmic growth phase were
taken, and after the reaction with DDP for 48 h, the culture medium
and dead cells were discarded. The cells were incubated in the
normal cell culture medium until they grew to the logarithmic
growth phase, and then the cells were incubated for 48 h after the
drug concentration was elevated. The initial drug concentration was
0.1 µM, gradually increased to 100 µM with 10 µM each time, and
then gradually increased to 1,000 µM with 100 µM each time. Through
the progressive increase of the drug concentration and repeated
intermittent induction, 4 months later, the human non-small cell
lung cancer DDP-resistant cell line, named NCI-H1299/DDP, was
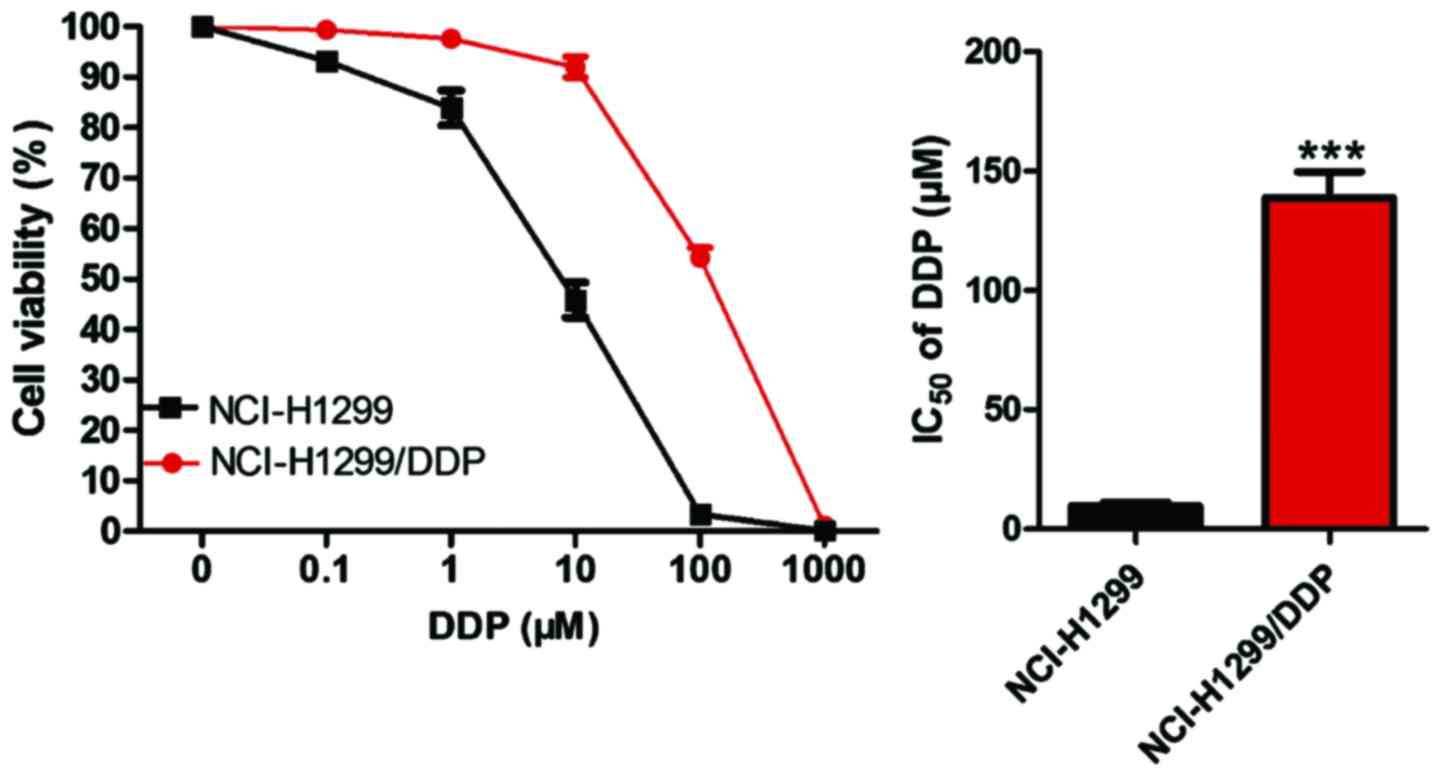
obtained. The sensitivity of the NCI-H1299/DDP drug-resistant cell
line and NCI-H1299 parental cells to cisplatin was detected by
CCK-8 assay. As shown in Fig. 3, the
half maximal inhibitory concentration (IC50) of
NCI-H1299 parental cells was 8.40 µM, while that of NCI-H1299/DDP
cells was 127.82 µM, which was significantly higher than that of
parental cells (P<0.05).
Detection of the expression level of
lncRNA-HOTAIR in cells by RT-PCR
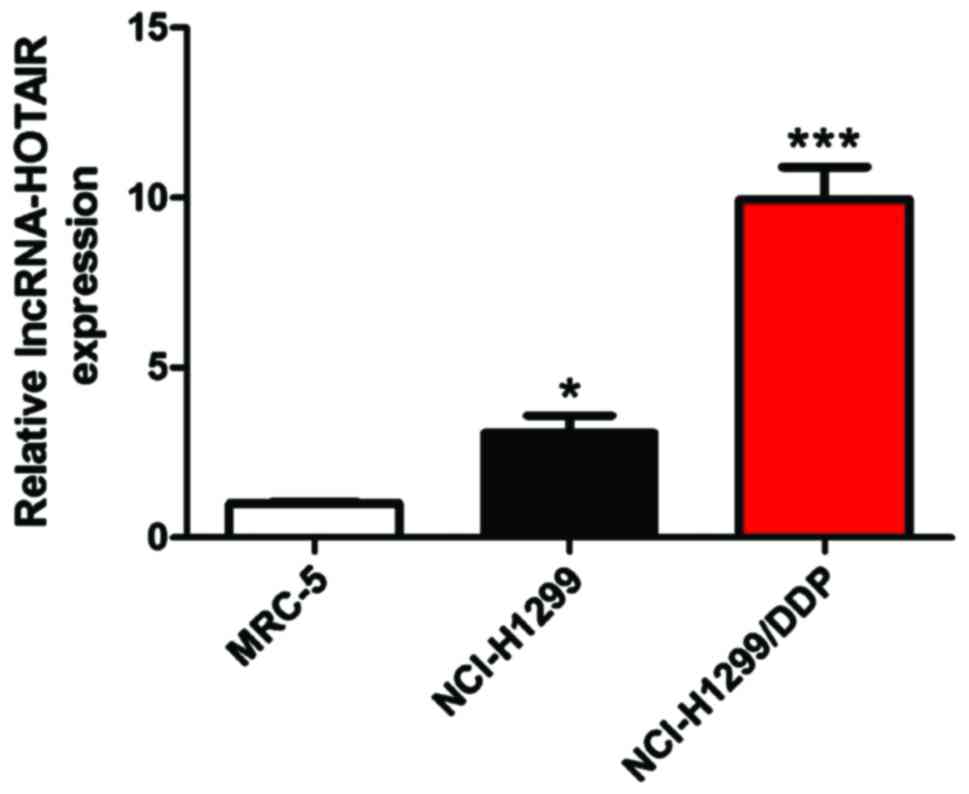
We used RT-PCR to detect the mRNA levels of
lncRNA-HOTAIR in normal lung fibroblast MRC-5, lung cancer parental
NCl-H1299 and DDP-resistant NCl-H1299/DDP cells (Fig. 4).
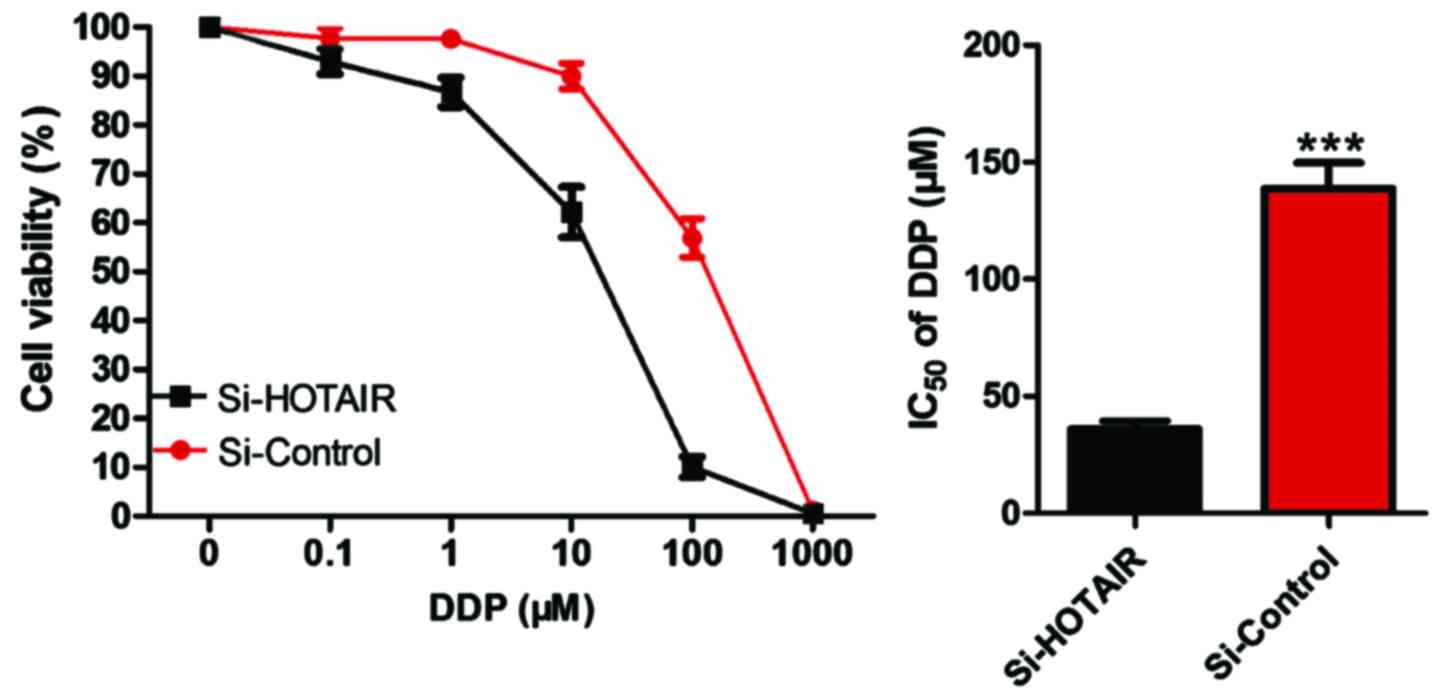
Detection of the effect of si-HOTAIR
on IC50 of drug-resistant cell lines by CCK-8
After NCI-H1299/DDP cells were transfected by
si-HOTAIR, CCK-8 was used to determine whether the sensitivity of
cells to DDP was altered. The results (Fig. 5) revealed that compared with that in
the control group, si-HOTAIR significantly decreased the
IC50 value of NCI-H1299/DDP to DDP, and si-HOTAIR vs.
si-control, i.e., 44.34 µM vs. 131.85 µM (P<0.05).
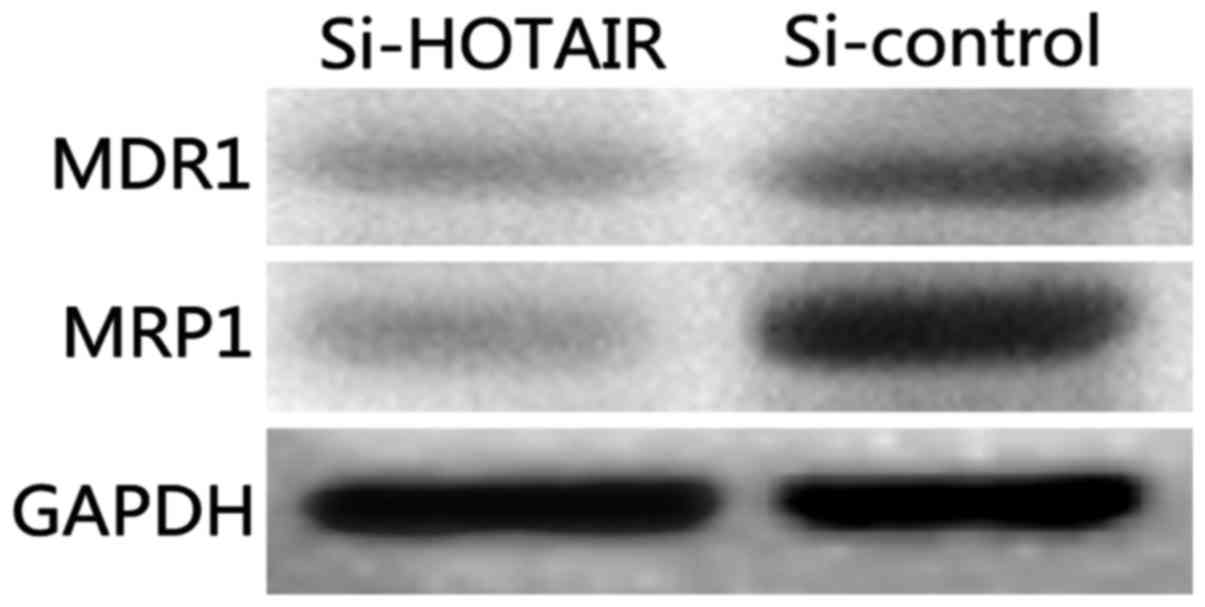
Detection of the effect of si-HOTAIR
on drug-resistant proteins and proliferation indexes by western
blot analysis
Classical multidrug-resistant proteins, MDR1 and
MRP1, were selected as the detection indexes to analyze the effect
of lncRNA-HOTAIR on the drug resistance of NCI-H1299/DDP cells. The
results (Fig. 6) show that the
protein levels of MDR1 and MRP1 in Si-HOTAIR cells were
significantly decreased compared with those in the si-control group
(P<0.05).
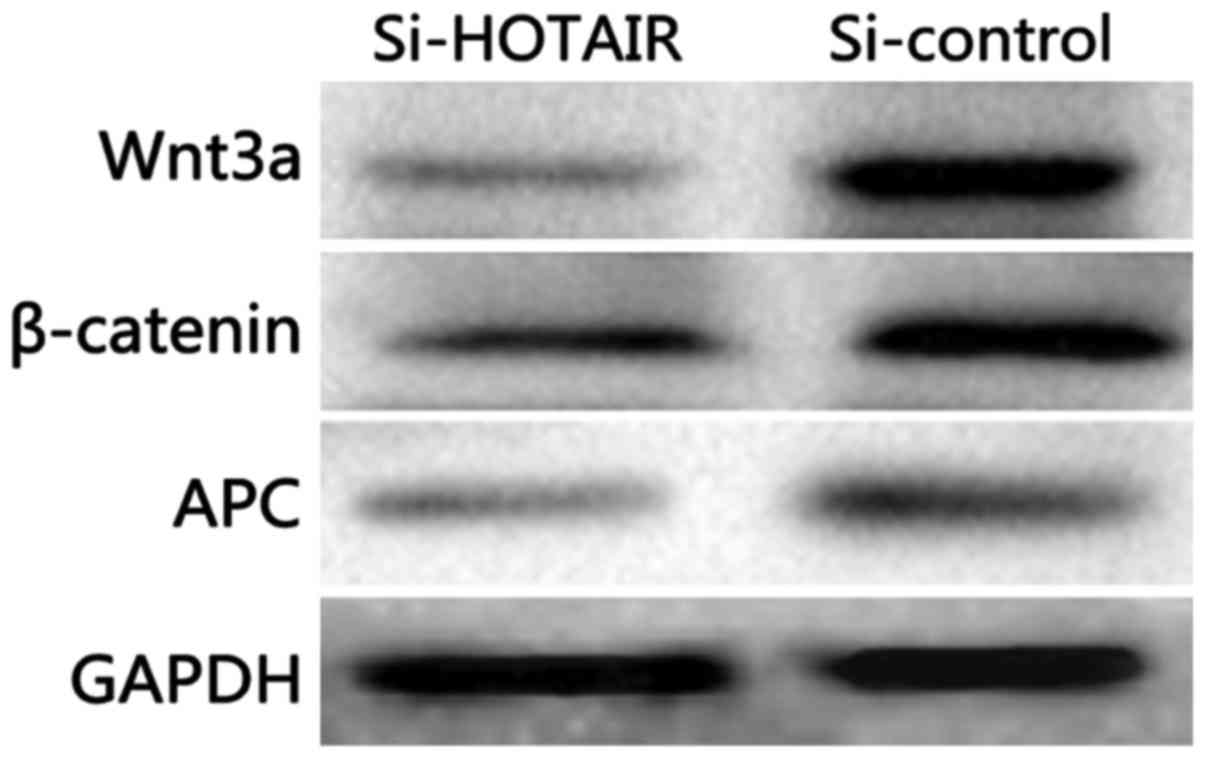
Detection of the effect of si-HOTAIR
on the Wnt signaling pathway by western blot analysis
As shown in Fig. 7,
compared with those in the si-control group, si-HOTAIR
significantly decreased the protein expression levels of Wnt3a,
β-actin and APC in NCI-H1299/DDP cells (P<0.05) and inhibited
the activation of Wnt/β-catenin signaling pathway.
Discussion
Drug resistance is caused by genetic variation in
patient's tumor cells (8). Previous
reports have shown that the dormancy of cancer stem cells and
epithelial-mesenchymal transition are associated with chemotherapy
resistance (9); a common mechanism
for obtaining resistance is the energy-dependent translocator
expression, accelerated cell excretion of anticancer drugs,
induction of drug detoxification and reduced susceptibility to
apoptosis signals (10). Although
the design of cancer chemotherapy becomes more and more complex,
few drugs can avoid the emergence of drug resistance. Recent
evidence suggests that lncRNA plays a key role in tumor resistance
(11).
lncRNAs regulate gene expression and chromatin
structure in the following ways: i) Bait effect: Altering its
function with other RNAs and proteins (12); ii) Scaffold effect: Modifying
chromatin so as to regulate protein and DNA junctions to form
signals (13); and iii)
post-transcriptional effect: Forming RNA dimers with mRNA sequences
to block transcription-related sites, thus regulating the
stability, cleavage and translation of protein-coding genes
(14). lncRNA can silence or
activate a gene or gene family by altering the chromatin structure.
According to recent studies, lnc-HOTAIR, as a modular scaffold,
assembles molecules and specific enzymes, which can regulate the
combination of target genes. Further studies have shown that the
chromatin of recombined HOTAIR enhances the invasion of pancreatic
cancer cells, inhibits cell growth, regulates cell cycle
progression and induces apoptosis in vitro. In addition, it
enhances the transfer potential of liver, breast and gastric cancer
cells (15,16).
The classical Wnt signaling pathway is involved in
the development of the human body and various tumors (17). Among them, the release of a key
protein of the Wnt signaling pathway, β-catenin is usually degraded
by phosphorylation and targeted destruction of the
Axin-APC-GSK3β-β-catenin complex. Subsequently, β-catenin migrates
into the nucleus and binds to the T-cell factor/lymphoid
enhancer-binding factor (TCF/LEF) transcription site to stimulate
the transcription of the Wnt target gene, thus affecting tumor cell
proliferation, invasion and metastasis as well as drug sensitivity
(18,19).
In this study, it was found that the expression of
lnc-HOTAIR in lung cancer tissues of patients receiving neoadjuvant
chemotherapy was significantly higher than that in cancer-adjacent
tissues, and the high expression of lnc-HOTAIR was significantly
correlated with poor prognosis. The result was consistent with the
study of Bhan and Mandal, indicating that the, high expression of
HOTAIR is closely related to breast cancer metastasis (20). Therefore, DDP-resistant cell lines
were constructed, and the comparison between normal lung fibroblast
cells and parental cells showed that the expression level of
lnc-HOTAIR in drug-resistant cell lines was the highest. Then we
used siRNA interference technology to silence lnc-HOTAIR
expression, and interestingly NCI-H1299/DDP drug sensitivity was
significantly improved, while the Wnt signaling pathway and
resistance indexes were decreased significantly. In summary, we
originally analyzed the expression of lnc-HOTAIR in cells of
patients with lung cancer DDP resistance and found that the Wnt
signaling pathway played a key role. Although the mechanism of
lnc-HOTAIR specific control of chromosomes and targets has not yet
been clarified, our experiments provide a potential treatment for
lung cancer patients with a certain reference value.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FG wrote the manuscript and performed the cell
culture. ZC performed and analyzed RT-PCR. HG analyzed and
interpreted CCK-8 test. SL helped with western blot analysis and
the conception of the study. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Peking Union Medical College Hospital (Beijing, China). All
patients signed the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hill C: Cancer prevention and screening.
Bull Cancer. 100:547–554. 2013.(In French). PubMed/NCBI
|
|
2
|
Dearing KR, Sangal A and Weiss GJ:
Maintaining clarity: Review of maintenance therapy in non-small
cell lung cancer. World J Clin Oncol. 5:103–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK,
Govindan R, et al: NCCN (National Comprehensive Cancer Network):
Non-small cell lung cancer. J Natl Compr Cancer Netw. 10:1236–1271.
2012. View Article : Google Scholar
|
|
4
|
Ettinger DS, Akerley W and Borghaei H:
Elemene increases autophagic apoptosis and drug sensitivity in
human cisplatin (DDP)-resistant lung cancer cell line SPC-A-1/DDP
by inducing Beclin-1 expression. Oncol Res Featuring Preclinical
Clin Cancer Ther. 4:502–510. 2017.
|
|
5
|
Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu
Y and Feng J: Exosomes: Decreased sensitivity of lung cancer A549
cells to cisplatin. PLoS One. 9:e895342014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen W, Liang B, Yin J, Li X and Cheng J:
Noscapine increases the sensitivity of drug-resistant ovarian
cancer cell line SKOV3/DDP to cisplatin by regulating cell cycle
and activating apoptotic pathways. Cell Biochem Biophys.
72:203–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang T, Song X, Liu YF and Wang WY: PEITC
reverse multi-drug resistance of human gastric cancer SGC7901/DDP
cell line. Cell Biol Int. 38:502–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pastan I and Gottesman M: Multiple-drug
resistance in human cancer. N Engl J Med. 316:1388–1393. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: an evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng H, Zhang J, Shi J, Guo Z, He C, Ding
L, Tang JH and Hou Y: Role of long non-coding RNA in tumor drug
resistance. Tumour Biol. 37:11623–11631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Yu B, Li J, Su L, Yan M, Zhu Z and
Liu B: Overexpression of lncRNA H19 enhances carcinogenesis and
metastasis of gastric cancer. Oncotarget. 5:2318–2329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Engreitz JM, Pandya-Jones A, McDonel P,
Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander
ES, et al: The Xist lncRNA exploits three-dimensional genome
architecture to spread across the X chromosome. Science.
341:12379732013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang F, Zhou X and Xu Y: Assessment of
varied long noncoding RNA and messenger RNA expression levels in
adolescent idiopathic scoliosis. Int J Clin Exp Med. 5:8031–8038.
2016.
|
|
16
|
Furió-Tarí P, Tarazona S, Gabaldón T,
Enright AJ and Conesa A: spongeScan: A web for detecting microRNA
binding elements in lncRNA sequences. Nucleic Acids Res.
44:W176–W180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clevers H: Wnt/β-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clevers H, Loh KM and Nusse R: Stem cell
signaling. An integral program for tissue renewal and regeneration:
Wnt signaling and stem cell control. Science. 346:12480122014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fifis T, Tran BM, Schwab RHM, Johanson TM,
Warner N, Barker N and Vincan E: Wnt signaling regulation of tissue
architecture (EMT and MET) and morphogenesisWnt Signaling in
Development and Disease: Molecular Mechanisms and Biological
Functions. Hoppler S and Moon RT: John Wiley & Sons, Inc.; pp.
315–328. 2014, View Article : Google Scholar
|
|
20
|
Bhan A and Mandal SS: LncRNA HOTAIR: A
master regulator of chromatin dynamics and cancer. Biochim Biophys
Acta. 1856:151–164. 2015.PubMed/NCBI
|