Introduction
Infertility is an important global health problem
that causes great distress to 15% of couples (1). Findings have shown that 50–60% of the
cases are due to male factors. Semen abnormalities including
oligozoospermia and asthenozoospermia or azoospermia have been
reported to be the main causes of infertility in men (2). Although the current test-tube baby
technique solves the fertility problems of some patients, the
technique has many shortcomings such as low success rate, high cost
and the proneness of fetus to disease (3).
At present, the important treatment methods for
oligozoospermia, asthenozoospermia and other symptoms caused by
different pathological conditions in males include endocrine
therapy, anti-inflammatory therapy, supplementation of trace
elements and arginine (4,5). However, there is still a lack of
clinically effective drug treatments for the above diseases.
Letrozole is a new generation of highly selective aromatase
inhibitor. It is a synthetic benzotriazole derivative that is used
mainly in the radiotherapy and chemotherapy for breast cancer
(6,7). A recent report also suggested the use
of the aromatase inhibitor letrozole for the successful treatment
of male infertility and human non-obstructive azoospermia (8). However, its molecular mechanism is not
yet clear. Thus, the relationship between letrozole and
mitogen-activated protein kinase (MAPK) signaling pathways and
their respective effects on the proliferation of mouse
spermatogonia were focus areas of the present study.
The primary aim of the present study was to explore
mechanism of action of letrozole that has been shown to promote the
MAPK pathway including RAS. APS-2-79 is an inhibitor, which was
utilized in the present study to confirm the stimulatory actions of
letrozole.
Materials and methods
Reagents
MAPK pathway inhibitor, APS-2-79, was procured from
Selleck Chemicals (cat. no. S8355; Houston, TX, USA). Letrozole in
the test was procured from MCE Corp. (cat. no. CGS-20267; Shanghai,
China).
Cell culture
Spermatogonia of GC-1 spg mice were purchased from
the Cell Bank, Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China) and cultured in Roswell Park
Memorial Institute (RPMI)-1640 medium containing 10% fetal bovine
serum as well as 100 U/ml penicillin and streptomycin. Cells in the
cell culture flask were incubated in the environment containing 5%
CO2 at 37°C. The Ethics Committee of Chongqing Three
Gorges Central Hospital approved the study.
Cell Counting Kit-8 (CCK-8)
detection
GC-1 spg cells were inoculated in three 96-well
plates at a density of 1×104/ml. The cells were
completely adherent to the wall 24 h later. Letrozole (100 µl) at a
final concentration of 0.1, 1, 10, 100, 1,000 nm was added and used
for incubation for 24, 48 and 72 h, respectively. Then, 10 µl CCK-8
reagent (Biotool, Shanghai, China) was added to each well and
incubated for 1 h in an incubator at 37°C. The optical density (OD)
of each well was measured at the wavelength of 450 nm using a
microplate reader (Bio-Rad, Hercules, CA, USA).
Cell plate clone
GC-1 spg cells in the logarithmic growth phase were
selected, and digested with 0.25% trypsin into single cells. Cells
were suspended in RPMI-1640 medium containing 10% fetal bovine
serum, and the cell concentration was adjusted to
1×103/ml. Then, 1 ml cell suspension and 3 ml complete
culture solution were placed in 6-well plates and gently rotated to
disperse the cells evenly. The 6-well plates were incubated in a
cell incubator with 5% CO2 at 37°C for 2–3 weeks. When a
clone visible to the naked eye appeared in the 6-well plates, the
culture was terminated. The supernatants were then discarded. This
was followed by two washings with phosphate-buffered saline (PBS) 5
ml of 4% paraformaldehyde was utilized for fixing cells for 15 min.
An appropriate amount of crystal violet staining solution was used
for staining for 30 min, and then washed with double distilled
water. Images were taken of the colonies with cell count more than
50.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The total ribonucleic acid (RNA) was extracted from
GC-1 spg cells in each group using the PureLink® RNA
Silica Column Extraction kit (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's
instructions. Complementary deoxyribonucleic acid (cDNA) was
synthesized with 1.0 µg total messenger RNA (mRNA) using a reverse
transcription kit (SuperScript® VILO cDNA Synthesis kit
and Master Mix; Invitrogen; Thermo Fisher Scientific, Inc.). The
annealing was performed at 55°C while extension was completed at
72°C. The expression of each index was detected by the gene
reactivation kit (Guangzhou, China) and quantitative PCR (Thermo
Fisher Scientific, Inc.). The calculation formula of the expression
level of mRNA as per kit: 2−ΔCq [ΔCq = Cq (target gene)
- Cq (glyceraldehyde 3-phosphate dehydrogenase)]. The corresponding
primer sequences are shown in Table
I.
 | Table I.Primer sequences of RT-PCR. |
Table I.
Primer sequences of RT-PCR.
| Gene names | Primer sequences |
|---|
| Ras | 5′-3′
ACTGAATATAAACTTGTGGTAGTTGGACCT |
|
| 3′-5′
TCAAAGAATGGTCCTGGACC |
| ERK1 | 5′-3′
CTACACGCAGTTGCAGTACAT |
|
| 3′-5′
CAGCAGGATCTGGATCTCCC |
| c-Myc | 5′-3′
GGACTGCGCAGGGAGACCTACAGGGG |
|
| 3′-5′
GAGGGAGCCGGCTGAGAGAAGTTGGG |
| GAPDH | 5′-3′
AGGTCGGTGTGAACGGATTTG |
|
| 3′-5′
TGTAGACCATGTAGTTGAGGTCA |
Western blot analysis
Cells in each treatment group were inoculated in
6-well plates, and an appropriate amount of protease inhibitor and
protein lysate were added. The cell lysate was aspirated and
centrifuged at 12,000 × g at 4°C for 30 min. Total protein (40 µg)
was electrophoresed in sodium dodecyl sulfate (SDS)-polyacrylamide
gel, followed by transfer to the polyvinylidene difluoride (PVDF)
membrane. According to the instructions of the marker, the bands
containing the target proteins were cut and incubated overnight
with Ras (cat. no. ab52939), ERK1 (cat. no. ab17942), c-Myc (cat.
no. ab32072), Ki-67 (cat. no. ab156956), proliferating cell nuclear
antigen (PCNA) (cat. no. ab29) and GAPDH (cat. no. ab8245)
antibodies. All the antibodies were procured from Abcam (Cambridge,
UK). Dilutions were made with the blocking buffer (1:500 for all
antibodies) and the incubation time was 12 h at 4°C. The membranes
were visualized with an enhanced chemiluminescence (ECL) detection
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and gray
scale analysis was performed using a gel analyzer. The bands were
quantified by using ImageJ software.
Detection of cell proliferation by
bromodeoxyuridine (BrdU) staining
Cells were inoculated on a chamber slide (NEST
Science Co. Ltd., Shanghai, China) at a density of 10–20%. The
cells were incubated for 48 h according to the test requirements by
addition of letrozole and MAPK inhibitors. Before the termination
of cell culture, BrdU (final concentration: 30 µg/l; MCE Corp.) was
added, and the cells were further incubated at 37°C for 40 min. The
culture medium was then discarded, and the slide was washed 3 times
with PBS. Methanol was used for fixation for 10 min, after which
the slide was dried with air. Subsequently, 0.3%
H2O2-methanol was used for inactivating
endogenous oxidases for 30 min. Bovine serum albumin (BSA) (5%) was
used for sealing cells at room temperature for 1 h. Formamide was
used for denaturation of nucleic acid at 100°C for 5 min. After
cooling in the ice bath, PBS washing was performed, and the
anti-mouse BrdU monoclonal antibody (working concentration = 1:50)
was added. 4′,6-Diamidino-2-phenylindole (DAPI) was used for
staining nuclei for 2 min. Ten high-power fields were randomly
chosen to calculate the total number of cells in each field of
vision and the number of BrdU-positive cells.
Statistical analysis
The results were analyzed using GraphPad Prism
software (version 5.01; GraphPad Software, Inc., La Jolla, CA,
USA). The t-test was used to compare the differences in samples
between the two groups. One-way analysis of variance (ANOVA) was
used to compare the differences in the mean among multiple groups
and Tukey test was used as post hoc test. P<0.05 was considered
to indicate a statistically significant difference. The LSD post
hoc test was also performed for comparisons among groups.
Results
Detection of the effect of letrozole
on the proliferation of spermatogonia by CCK-8
Six gradient concentrations were set for letrozole
to treat mouse spermatogonia (GC-1 spg) for 24, 48 and 72 h,
respectively. CCK-8 assay showed that letrozole could promote the
proliferation of GC-1 spg cells and exhibited time and dose effects
(Fig. 1). In the follow-up
experiment, 100 nm letrozole was selected to treat cells for 72 h,
and then the detection was performed.
Detection of the effect of letrozole
on the proliferation of spermatogonia by the plate clone formation
assay
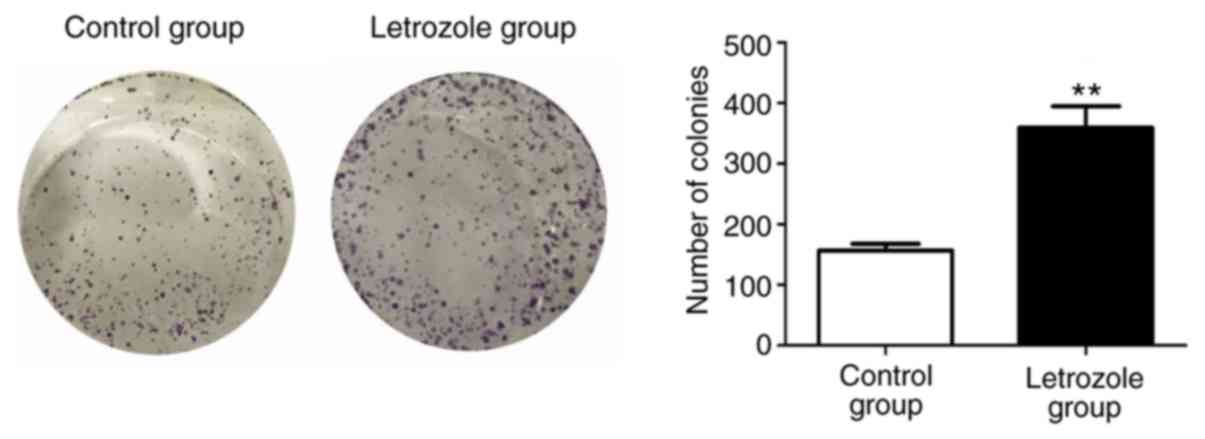
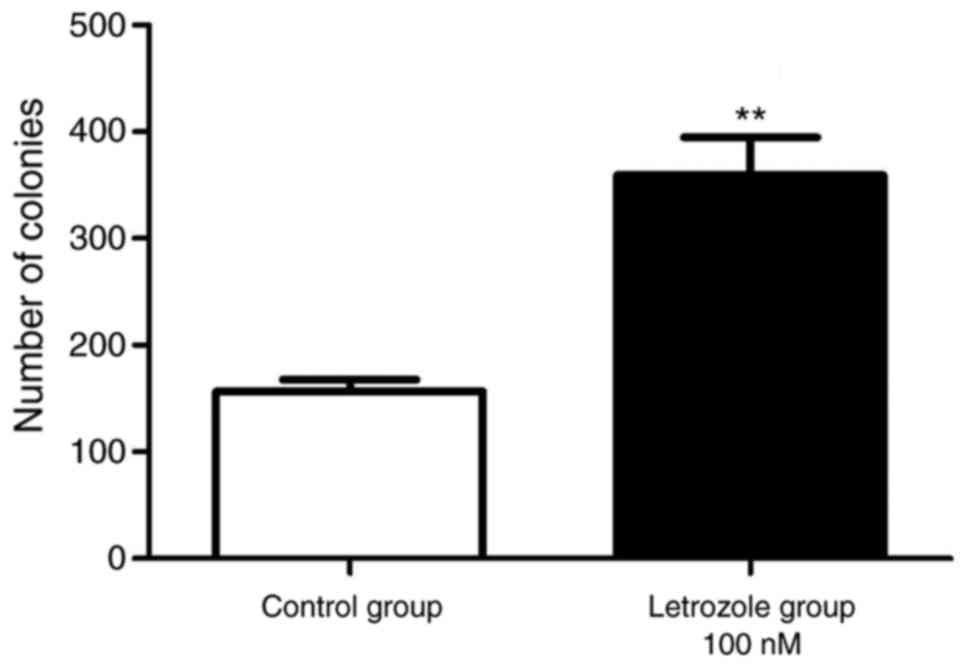
The plate clone formation assay was used to further
verify the effect of letrozole on the proliferation of GC-1 spg
cells. As shown in Fig. 2, the
number of cell colonies in the letrozole treatment was
significantly higher than the control group (p<0.05).
Detection of the effects of letrozole
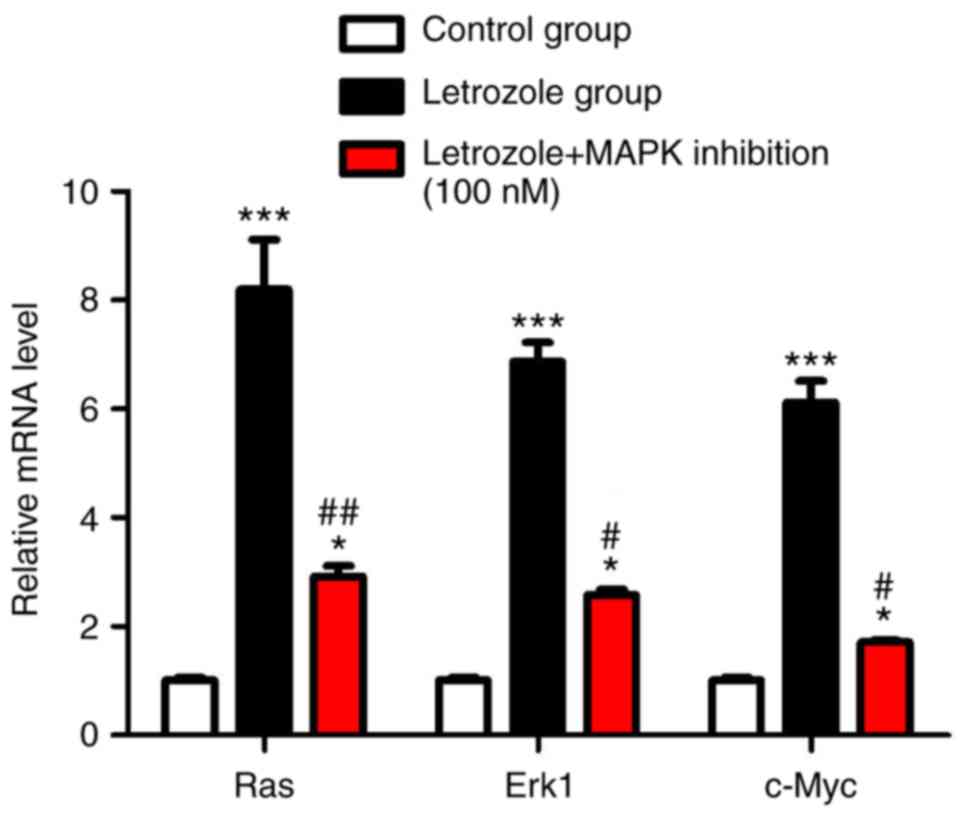
on the MAPK pathway by RT-PCR
As MAPK is an important signaling pathway for cell
proliferation, letrozole and letrozole + MAPK inhibitors were set
to explore the relationship between letrozole and MAPK pathways.
RT-PCR results showed that, letrozole significantly increased the
mRNA levels of Ras/ERK1/c-Myc in the classical MAPK pathway
(p<0.05) as compared to those of control group. Further,
letrozole + MAPK inhibition significantly decreased the mRNA levels
of Ras/ERK/c-Myc (p<0.05) (Fig.
3).
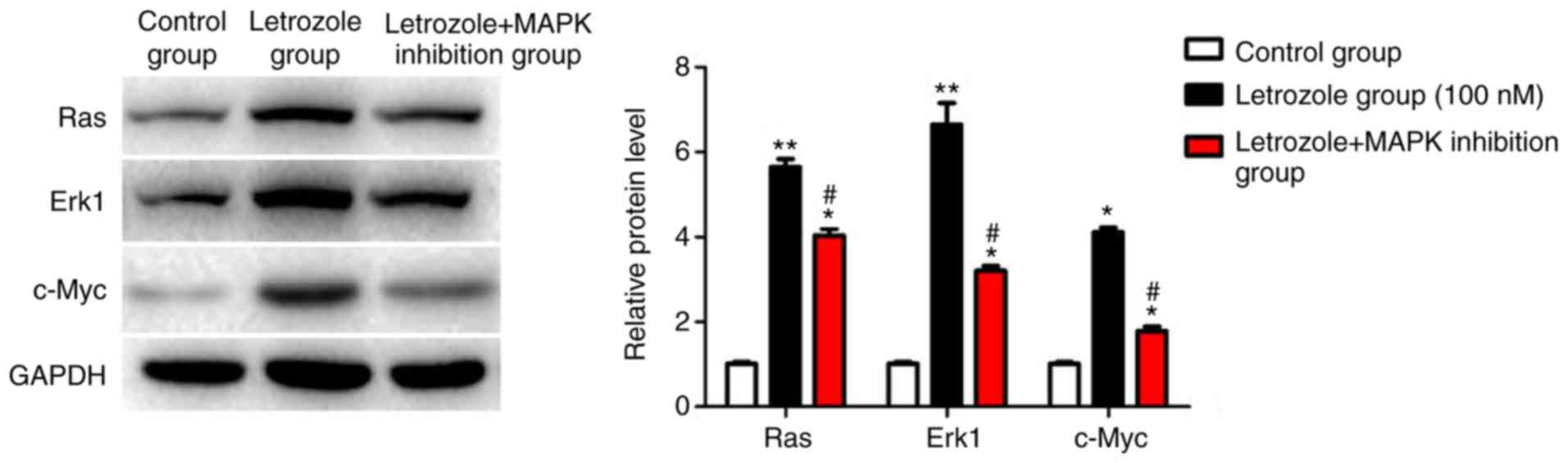
Detection of effects of letrozole on
the MAPK pathway by western blot analysis
The results of western blot analysis were consistent
with those of RT-PCR, which further verified that compared with
those in the control group, letrozole significantly increased the
protein levels of Ras/ERK1/c-Myc in the classical MAPK pathway
(p<0.05). On the other hand, letrozole + MAPK inhibition
significantly decreased the protein levels of Ras/ERK/c-Myc
(p<0.05) (Fig. 4).
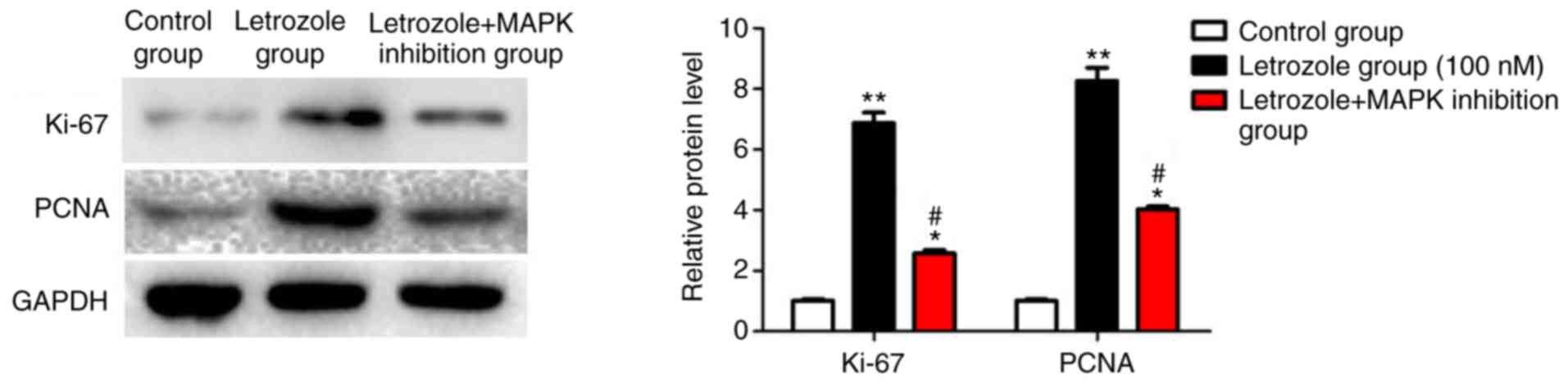
Detection of the expression of
proliferation indexes
The protein expression levels of Ki-67 and PCNA (two
classical proliferation indexes), were examined to further
investigate the effects of letrozole on the proliferation of GC-1
spg cells. Letrozole significantly increased the protein levels of
Ki-67 and PCNA (p<0.05) (Fig. 5),
in comparison to the control group. The protein levels of Ki-67 and
PCNA in the letrozole + MAPK inhibition group showed a significant
declining trend as compared to the letrozole group (p<0.05).
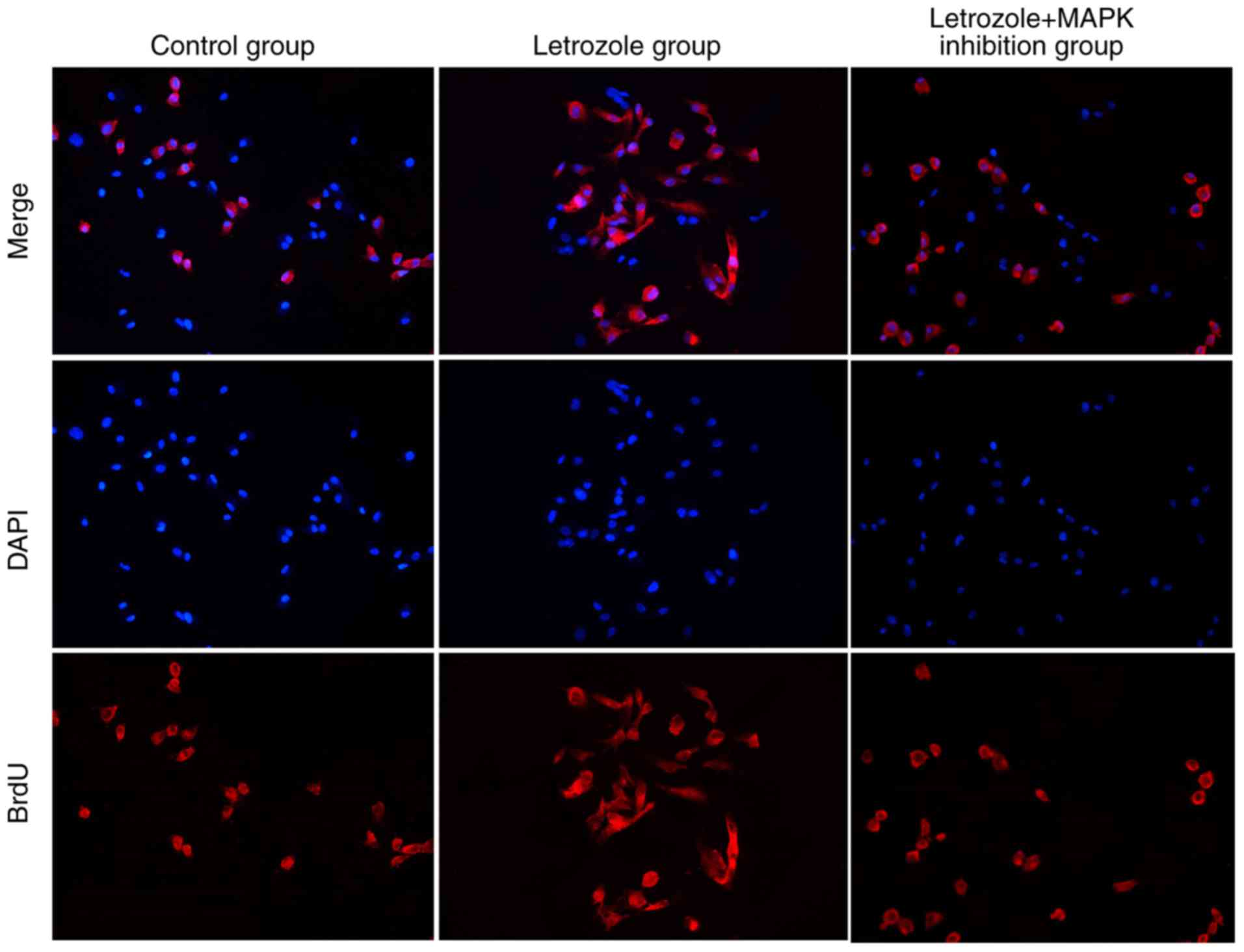
Detection of effects of letrozole on
cell proliferation by BrdU
The BrdU assay was used to detect the effects of
letrozole and the MAPK pathway on the proliferation of GC-1 spg
cells. The results (Fig. 6)
indicated that the number of BrdU-stained cells in the letrozole
group was higher than that in the control group, while that in the
letrozole + MAPK inhibition group was significantly less in
comparison to letrozole group.
Discussion
Oligozoospermia is a complex disease, which is
caused by the combination of genetic factors and acquired
conditions (9). The occurrence of
spermatozoa is also a complex cell process, which is divided into
the division stage (proliferation and differentiation of
spermatogonia), the meiosis stage (meiosis of spermatogonia) and
the haploid stage (from sperm cells to sperm differentiation)
(10). In this process, the number
of spermatogonia directly affects the production of sperm.
Abnormalities in spermatogonia often lead to oligozoospermia,
asthenozoospermia and sperm abnormalities (11). Therefore, improvement in the number
of spermatogonia is crucial during male oligozoospermia.
Letrozole is considered as a safe as well as an
effective aromatase inhibitor that could be orally taken. It has
the ability to act as an aromatase inhibitor to decrease the
estrogen level. In this way, it eliminates the stimulation of
estrogen on tumor growth. Besides, no potential toxicity to the
normal systemic system has been reported. Moreover, target organs
showed good tolerance and strong pharmacological effects (12,13). The
recent 3-phase clinical trials utilized letrozole as a chemotherapy
drug for postmenopausal metastatic breast cancer (14). In addition, the literature also
suggested that the main source of estrogen in men is the process
that showed aromatase-catalyzed testosterone into estradiol. So, it
has a strong negative feedback on the hypothalamic-pituitary axis
so as to reduce the production and the release of follicle
stimulating hormone (FSH). Aromatase inhibitors could inhibit the
conversion of testosterone into estradiol; thereby reduced the
concentration of serum estradiol. Thus, the negative feedback on
the hypothalamic-pituitary axis increased the levels of serum FSH
(15). FSH regulates the
development, growth, adolescent sexual maturity and a series of
physiological processes related to reproduction, which acts on
seminiferous tubules of the testis and promotes sperm formation.
This, in turn, might exert positive effects on sperms (16). However, concerning the molecular
mechanism, the main role of letrozolein in the signaling pathway
has not yet been reported.
MAPK pathway plays important roles in the
differentiation, proliferation, survival and migration of mammalian
cells (17). The four major MAPK
cascade reactions [ERK1/2, c-Jun N-terminal kinase 1/3 (JNK1/3),
p38 and ERK5] in mammalian cells have been well studied. The action
mechanism of cascade reactions includes activation of the Ras
protein-coupled receptor of the Ras family [such as Ras, cell
division control protein 42 homolog (Cdc42) and Rac], followed by
the activation of mitogen activated protein kinase kinases (MAPKKs)
and MAPKs (18). A study of Jaldety
and Breitbart showed that ERK1 and 2 were expressed at all stages
of mouse germ cells, but the activation of ERK1/2 peaks in mouse
spermatogonia (19). In addition,
ERK1/2 actively participates in the process of mouse spermatogonial
stem cell factor (SCF) accelerating the cell cycle progression by
inducing the transient activation of MAPK cascades (20). Besides, c-Myc (as the target gene of
the MAPK pathway), is involved in the process of cells from G0 to S
phases, and its increased expression positively regulates cell
proliferation (21).
In the present study, in vitro cell
experiments showed that letrozole significantly increased the
proliferation of mouse GC-1 spg cells accompanied by the activation
of the MAPK signaling pathway. However, the exploration of RAF is
also crucial for concrete conclusion. So, it could be one of the
prime limitations of the study but we will include this in our
future studies. We did not include APS-2-79 group in the
present study, which is the second limitation of the study.
Experiments using MAPK inhibitors found that MAPK signaling pathway
played an important role in promoting the proliferation ability of
spermatogonia. Based on the above experimental results, we
speculated that letrozole might have a good clinical effects on
infertility caused by male oligozoospermia and asthenozoospermia.
Thus, letrozole has strong potential for its clinical application
against oligozoospermia. However, further studies are essential for
concrete conclusions.
Acknowledgements
Not applicable.
Funding
This study was funded by the Medical Research
Project of Chongqing Health and Family Planning Commission
(2016MSXM114).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ShundeW wrote the manuscript and was responsible for
cell culture. ShuhongW and HL performed CCK-8 assay, XL was devoted
to PCR. MX and JW helped with western blot analysis, ML and TL
contributed to BrdU staining. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Chongqing Three Gorges
Central Hospital (Chongqing, China) approved the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glazer CH, Bonde JP, Eisenberg ML,
Giwercman A, Hærvig KK, Rimborg S, Vassard D, Pinborg A, Schmidt L
and Bräuner EV: Male infertility and risk of nonmalignant chronic
diseases: A systematic review of the epidemiological evidence.
Semin Reprod Med. 35:282–290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bridges N, Trofimenko V, Fields S, Carrell
D, Aston K and Hotaling J: Male factor infertility and clomiphene
citrate: A meta-analysis - the effect of clomiphene citrate on
oligospermia. Urol Pract. 2:199–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chirputkar R and Vaidya A: Understanding
infertility and the potential role of stem cells in infertility
treatment: A short communication. Int J Reprod Fertil Sex Health.
1:37–40. 2015.
|
|
4
|
Sami MA, Nazar H and Usmanghani K:
Idiopathic oligospermia treatment: An alternate approach. Int J Med
Med Sci. 7:74–79. 2015. View Article : Google Scholar
|
|
5
|
Hezelgrave N, Abbott D and Shennan AH:
Pre-implantation genetic diagnosis in oligozoospermiaChallenging
Concepts Obstet Gynaecol. Oxford University Press; Oxford: pp.
49–59. 2014
|
|
6
|
Legro RS, Brzyski RG, Diamond MP,
Coutifaris C, Schlaff WD, Casson P, Christman GM, Huang H, Yan Q,
Alvero R, et al: NICHD reproductive medicine network: Letrozole
versus clomiphene for infertility in the polycystic ovary syndrome.
N Engl J Med. 371:119–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
André F, Stemmer SM, Hortobagyi GN, Burris
HA, Paluch-Shimon S, Campone M, Villanueva C, Chan A, Wist E,
Marschner N, et al: Ribociclib + letrozole for first-line treatment
of HR+, HER2− ABC: Efficacy, safety, and
pharmacokinetics. Eur J Cancer. 69:S72016. View Article : Google Scholar
|
|
8
|
Diamond MP, Legro RS, Coutifaris C, Alvero
R, Robinson RD, Casson P, Christman GM, Ager J, Huang H, Hansen KR,
et al: NICHD Reproductive Medicine Network: Coutifaris. Letrozole,
gonadotropin, or clomiphene for unexplained infertility. N Engl J
Med. 373:1230–1240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Milardi D, Luca G, Grande G, Ghezzi M,
Caretta N, Brusco G, De Filpo G, Marana R, Pontecorvi A, Calafiore
R, et al: Prednisone treatment in infertile patients with
oligozoospermia and accessory gland inflammatory alterations.
Andrology. 5:268–273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nozawa YI, Yao E, Gacayan R, Xu SM and
Chuang PT: Mammalian Fused is essential for sperm head shaping and
periaxonemal structure formation during spermatogenesis. Dev Biol.
388:170–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Y, Wang N, Yu Y, Li Y, Li YB, Yu YB,
Zhou XQ and Sun ZW: Exposure to silica nanoparticles causes
reversible damage of the spermatogenic process in mice. PLoS One.
9:e1015722014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Finn RS, Crown JP, Lang I, Boer K,
Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et
al: The cyclin-dependent kinase 4/6 inhibitor palbociclib in
combination with letrozole versus letrozole alone as first-line
treatment of oestrogen receptor-positive, HER2-negative, advanced
breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study.
Lancet Oncol. 16:25–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dickler MN, Barry WT, Cirrincione CT,
Ellis MJ, Moynahan ME, Innocenti F, Hurria A, Rugo HS, Lake DE,
Hahn O, et al: Phase III trial evaluating letrozole as first-line
endocrine therapy with or without bevacizumab for the treatment of
postmenopausal women with hormone receptor-positive advanced-stage
breast cancer: CALGB 40503 (Alliance). J Clin Oncol. 34:2602–2609.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noorafshan A, Ahmadi M, Mesbah SF and
Karbalay-Doust S: Stereological study of the effects of letrozole
and estradiol valerate treatment on the ovary of rats. Clin Exp
Reprod Med. 40:115–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simoni M, Santi D, Negri L, Hoffmann I,
Muratori M, Baldi E, Cambi M, Marcou M, Greither T, Baraldi E, et
al: Treatment with human, recombinant FSH improves sperm DNA
fragmentation in idiopathic infertile men depending on the FSH
receptor polymorphism p.N680S: A pharmacogenetic study. Hum Reprod.
31:1960–1969. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bernet JD, Doles JD, Hall JK, Tanaka Kelly
K, Carter TA and Olwin BB: p38 MAPK signaling underlies a
cell-autonomous loss of stem cell self-renewal in skeletal muscle
of aged mice. Nat Med. 20:265–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loi S, Dushyanthen S, Beavis PA, Salgado
R, Denkert C, Savas P, Combs S, Rimm DL, Giltnane JM, Estrada MV,
et al: RAS/MAPK activation is associated with reduced
tumor-infiltrating lymphocytes in triple-negative breast cancer:
Therapeutic cooperation between MEK and PD-1/PD-L1 immune
checkpoint inhibitors. Clin Cancer Res. 22:1499–1509. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jaldety Y and Breitbart H: ERK1/2 mediates
sperm acrosome reaction through elevation of intracellular calcium
concentration. Zygote. 23:652–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu X, Tang Z, Li Y, Liu W, Zhang S, Wang
B, Tian Y, Zhao Y, Ran H, Liu W, et al: Deletion of the tyrosine
phosphatase Shp2 in Sertoli cells causes infertility in mice. Sci
Rep. 5:129822015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tögel L, Nightingale R, Chueh AC,
Jayachandran A, Tran H, Phesse T, Wu R, Sieber OM, Arango D,
Dhillon AS, et al: Dual targeting of bromodomain and extraterminal
domain proteins, and WNT or MAPK signaling, inhibits c-MYC
expression and proliferation of colorectal cancer cells. Mol Cancer
Ther. 15:1217–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|