Introduction
The incidence of viral myocarditis (VM) is gradually
increased in recent years, and attracts the attention of more and
more scholars (1). Coxsackievirus B3
(CVB3) is one of the most common viruses that cause VM (2). VM can develop into acute heart failure
(3,4). According to available clinical studies,
the mortality rate of VM in young people is as high as 21%, and
sudden deaths caused by VM or fatal ventricular arrhythmias in
children account for about 20% (5,6).
However, there have been few effective therapies for VM by now.
Current treatment for VM is still focused on the control of cardiac
arrhythmia and heart failure. Therefore, it is necessary to study
the molecular mechanism of VM.
VM is a common infectious disease in pediatric
clinical practice. It is caused by viral infection or disturbance
of autoimmune system, and characterized by localized or diffuse
acute or chronic inflammatory lesions of the myocardium (1,7). In
inflammation, cyclooxygenase (COX), especially COX-2 in the COX
family, plays an important role. COX-2 is produced after induction
by external stimuli (physical, chemical, biological, etc), and
catalyzes the synthesis of prostaglandin that is involved in
inflammatory responses (8). With the
study on genes entering microRNA (miRNA/miR) stage, some
researchers have found that up-regulation of miRNAs can inhibit the
expression of COX-2 (9). However,
basic and clinical research processes of VM and the regulation of
COX-2 by miRNAs are rarely reported. In the present study, we
determine the expression of COX-2 in the blood of children with VM,
and investigate the relationship between COX-2 and miR-381 in the
occurrence and development of the disease.
Materials and methods
Patients
A total of 26 children with VM (15 boys and 11
girls) admitted at our hospital between June 2014 and February 2017
were included in the present study. The age range of the children
was from 9 months to 16 years. Clinical diagnosis was made
according to the standards of VM established by the cardiovascular
research group of Chinese Medical Association in 2000 (10). All children with VM received large
doses of vitamin C, protection of important organ functions,
symptomatic treatments, myocardial nutrition, and anti-virus
treatment. Control group (33 children) included children with VM
who required regular follow-ups after recovery. Three types of
samples were collected and used in the experiments. Peripheral
blood was collected from all patient children and those in control
group at early morning fasting, and stored at −20°C in EDTA
anti-coagulant tubes. All procedures were approved by the Ethics
Committee of Hubei University of Medicine. Written informed
consents were obtained from all patients or their families.
Animals
A total of 60 male BALB/C mice (4 weeks old) were
obtained from Chongqing Tengxin Biotech Company (http://www.cqtx123.com/; Chongqing, China) with a
certificate numbered SCXK(Yu) 2016-0018. The weight of the mice
ranged between 18 and 22 g. During the week before experiments, the
mice had free access to food and water to adapt to the environment.
The Reduction, Replacement and Refinement animal welfare principle
was followed during the experiments. The mice were divided into
control group and infection (VM) group. Control group (n=30) was
not infected by any virus and intraperitoneally injected with
saline (0.2 ml). VM group (n=30) was intraperitoneally injected
with 100 TCID50 CVB3 (0.2 ml; Biopike, Beijing, China), and one
hour later, the mice were injected with saline (0.2 ml). The mice
in VM group were treated with CVB3 for three days, and sacrificed
on day 21. Peripheral blood was collected from the mice, and stored
at −20°C in EDTA anti-coagulant tubes. Then, myocardial tissues
were collected after the mice were sacrificed by decapitation, and
stored in liquid nitrogen.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Myocardial tissues (100 mg) were ground using liquid
nitrogen and mixed with 1 ml TRIzol (10606ES60; Yeasen, Shanghai,
China) for lysis. Serum samples (100 µl) were directly mixed with 1
ml TRIzol (10606ES60; Yeasen, Shanghai, China) for lysis. Then,
total RNA was extracted using phenol chloroform method. The
concentration and quality of RNA was examined using ultraviolet
spectrophotometry (Nanodrop ND2000; Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). Then, cDNA was obtained by reverse
transcription from 1 µg RNA and stored at −20°C. Reverse
transcription of mRNA was performed using TIANScript II cDNA First
Strand Synthesis kit (KR107; Tiangen, Beijing, China), and reverse
transcription of miRNA was carried out using miRcute miRNA cDNA
First Strand Synthesis Kit (KR201; Tiangen).
SuperReal PreMix (SYBR-Green) RT-qPCR kit (FP204;
Tiangen) was used to detect mRNA expression of COX-2, using β-actin
as internal reference. The sequences of human COX-2 were
5′-CAGCCATACAGCAAATCCTTG-3′ (upstream) and
5′-CAAATGTGATCTGGATGTCAAC-3′ (downstream), and those of β-actin
were 5′-CACCAGGGCGTGATGGT-3′ (upstream) and
5′-CTCAAACATGATCTGGGTCAT-3′ (downstream). PCR reaction system (20
µl) for human COX-2 determination was composed of 10 µl
RT-qPCR-Mix, 0.5 µl upstream primer, 0.5 µl downstream primer, 2 µl
cDNA and 7 µl ddH2O. PCR conditions for human COX-2
determination were: Initial denaturation at 95°C for 30 sec; 40
cycles of 95°C denaturation for 5 sec and annealing at 60°C for 34
sec (iQ5; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
sequences of mouse COX-2 were 5′-CAGCAAATCCTTGCTGTTCC-3′ (upstream)
and 5′-TGGGCAAAGAATGCAAACATC-3′ (downstream), and those of β-actin
were 5′-TCAGGAGGAGCAATGATCTTG-3′ (upstream) and
5′-TCCTCCCTGGAGAAGAGCTA-3′ (downstream). PCR reaction system (20
µl) for mouse COX-2 determination was composed of 10 µl
RT-qPCR-Mix, 0.5 µl upstream primer, 0.5 µl downstream primer, 2 µl
cDNA and 7 µl ddH2O. PCR conditions for mouse COX-2
determination were: Initial denaturation at 95°C for 2 min; 35
cycles of 94°C denaturation for 30 sec, annealing at 57°C for 30
sec and elongation at 72°C for 30 sec; final elongation at 72°C for
4 min (iQ5; Bio-Rad Laboratories, Inc.). The 2−ΔΔCq
method was used to calculate the relative expression of mRNA
against β-actin. Each sample was tested in triplicate.
The expression of miR-381 was determined by miRcute
miRNA RT-PCR kit (FP401; Tiangen), using U6 as internal reference.
The sequences of human miR-381 were
5′-ACACTCCAGCTGGGTATACAAGGGCAAGCT-3′ (upstream) and
5′-TGGTGTCGTGGAGTCG-3′ (downstream), and those of U6 were
5′-CTCGCTTCGGCAGCACA-3′ (upstream) and 5′-AACGCTTCACGAATTTGCGT-3′
(downstream). PCR reaction system (20 µl) for human miR-381
determination was composed of 10 µl RT-qPCR-Mix, 0.5 µl upstream
primer, 0.5 µl downstream primer, 2 µl cDNA and 7 µl
ddH2O. PCR conditions for human miR-381 determination
were: Initial denaturation at 95°C for 5 min; 40 cycles of 95°C
denaturation for 15 sec, annealing at 60°C for 30 sec, and
elongation at 72°C for 30 sec (iQ5; Bio-Rad Laboratories, Inc.).
The sequences of mouse miR-381 were 5′-ACGAGCGATACAAGGGCAAGC-3′
(upstream) and 5′-GCGAGCACAGAATAAATACGACTCACTA-3′ (downstream), and
those of U6 were 5′-CTCGCTTCGGCAGCACATATACT-3′ (upstream) and
5′-ACGCTTCACGAATTTGCGTGTC-3′ (downstream). PCR reaction system (25
µl) for mouse miR-381 determination was composed of 12.5 µl SYBR
Premix ExTaq™, 0.5 µl upstream primer, 0.5 µl downstream primer, 1
µl cDNA and 10.5 µl ddH2O. PCR conditions for mouse
miR-381 determination were: Initial denaturation at 95°C for 5 min;
40 cycles of 95°C denaturation for 10 sec, annealing at 60°C for 30
sec and elongation at 72°C for 15 sec (iQ5; Bio-Rad Laboratories,
Inc.). The 2−ΔΔCq method was used to calculate the
relative expression of miR-381 against U6. Each sample was tested
in triplicate.
Enzyme-linked immunosorbent assay
(ELISA)
Serum were tested using human COX-2 ELISA kit
(ab52237; Abcam, Cambridge, UK) and mouse COX-2 ELISA kit
(YQ-16-3537K; Imunbio, Beijing, China). In microplates, standards
(50 µl), samples (10 µl sample liquid and 40 µl diluent) and blank
were set into predefined wells. In the wells for standards and
samples, horseradish peroxidase-labelled conjugates (100 µl) were
added before sealing the plates for incubation at 37°C for 1 h.
After washing the plates for 5 times, substrates A (50 µl) and B
(50 µl) were added into each well. After incubation at 37°C for 15
min, stop solution (50 µl) was added into each well, and absorbance
of each well was measured at 450 nm within 15 min.
Bioinformatics
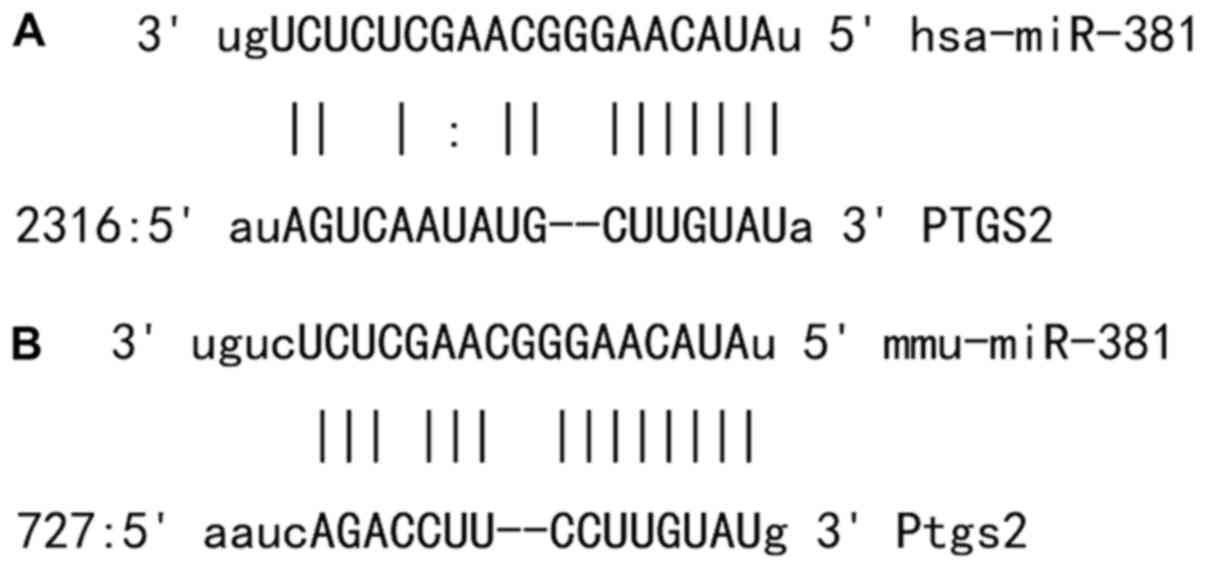
Bioinformatics prediction is a powerful tool for the
study of the functions of miRNAs. To understand the regulatory
mechanism of COX-2 in VM, we used miRanda (http://www.microrna.org/microrna/home.do), TargetScan
(http://www.targetscan.org), PiTa
(http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
and PICTA (http://pictar.mdc-berlin.de/) to predict miRNA
molecules that might regulate COX-2, and found that miR-381 was
able to potentially regulate COX-2 (Fig.
1).
Automatic biochemical analysis
The contents of creatine kinase (CK-MB) and lactate
dehydrogenase (LDH) were determined according to relative kits
(Beckman Coulter, Inc., Brea, CA, USA). Reagent I (250 µl) was
preincubated at 37°C in water bath for 3 min, and then liquid
samples (10 µl) were mixed with reagent I (250 µl) before
incubation at 37°C in water bath for 10 min. Then, reagent II (50
µl) was added. Within 5 min, absorbance was measured continuously.
Contents of CK-MB and LDH were determined by average absorbance
increase rate. The results were automatically calculated and given
by AU5800 automatic biochemical analyzer (Beckman Coulter,
Inc.).
Western blotting
Precooled Radio-Immunoprecipitation Assay (RIPA)
lysis buffer (600 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1%
sodium dodecyl sulfate, 1% Triton X-100, 1% sodium deoxycholate
(Beyotime Institute of Biotechnology, Shanghai, China) was used to
lyse the samples. After lysis for 50 min on ice, the mixture was
centrifuged at 12,000 × g at 4°C for 5 min. The supernatant was
used to determine protein concentration by bicinchoninic acid (BCA)
protein concentration determination kit (RTP7102, Real-Times
Biotechnology Co., Ltd., Beijing, China). Protein samples (20 µg)
were then mixed with sodium dodecyl sulfate loading buffer before
denaturation in boiling water bath for 5 min. Afterwards, the
samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis. The resolved proteins were transferred to
polyvinylidene difluoride membranes on ice (100 V, 2 h) and blocked
with 5% skimmed milk at room temperature for 1 h. Then, the
membranes were incubated with rabbit anti-mouse COX-2 polyclonal
primary antibody (1:1,000; ab15191; Abcam) and rabbit anti-mouse
β-actin primary antibody (1:5,000; ab8227; Abcam) at 4°C overnight.
After extensive washing with phosphate-buffered saline with
Tween-20 for 3 times of 15 min, the membranes were incubated with
goat anti-rabbit horseradish peroxidase-conjugated secondary
antibody (1:3,000; ab6721; Abcam) for 1 h at room temperature
before washing with phosphate-buffered saline with Tween-20 for 3
times of 15 min. Then, the membrane was developed with enhanced
chemiluminescence detection kit (Abcam) for imaging. Image lab v3.0
software (Bio-Rad Laboratories, Inc.) was used to acquire and
analyze imaging signals. The relative content of COX-2 protein was
expressed as COX-2/β-actin ratio.
Dual luciferase reporter assay
According to bioinformatics results, wild-type (WT)
and mutant seed regions of miR-381 in the 3′-UTR of COX-2 gene were
chemically synthesized in vitro, added with Spe-1 and
HindIII restriction sites, and then cloned into pMIR-REPORT
luciferase reporter plasmids. Plasmids (0.8 µg) with WT or mutant
3′-UTR DNA sequences were co-transfected with agomiR-381 (100 nM;
Sangon Biotech, Shanghai, China) into 293T cells. After cultivation
for 24 h, the cells were lysed using dual luciferase reporter assay
kit (Promega Corporation, Madison, WI, USA) according to the
manufacturer's manual, and fluorescence intensity was measured
using GloMax 20/20 luminometer (Promega Corporation). Using renilla
fluorescence activity as internal reference, the fluorescence
values of each group of cells were measured.
Statistical analysis
The results were analyzed using SPSS 18.0
statistical software (IBM Corp., Armonk, NY, USA). The data were
expressed as means ± standard deviations. Data were tested for
normality. Multigroup measurement data were analyzed using one-way
ANOVA. In case of homogeneity of variance, Least Significant
Difference and Student-Newman-Keuls methods were used; in case of
heterogeneity of variance, Tamhane's T2 or Dunnett's T3 method was
used. P<0.05 was considered to indicate a statistically
significant difference.
Results
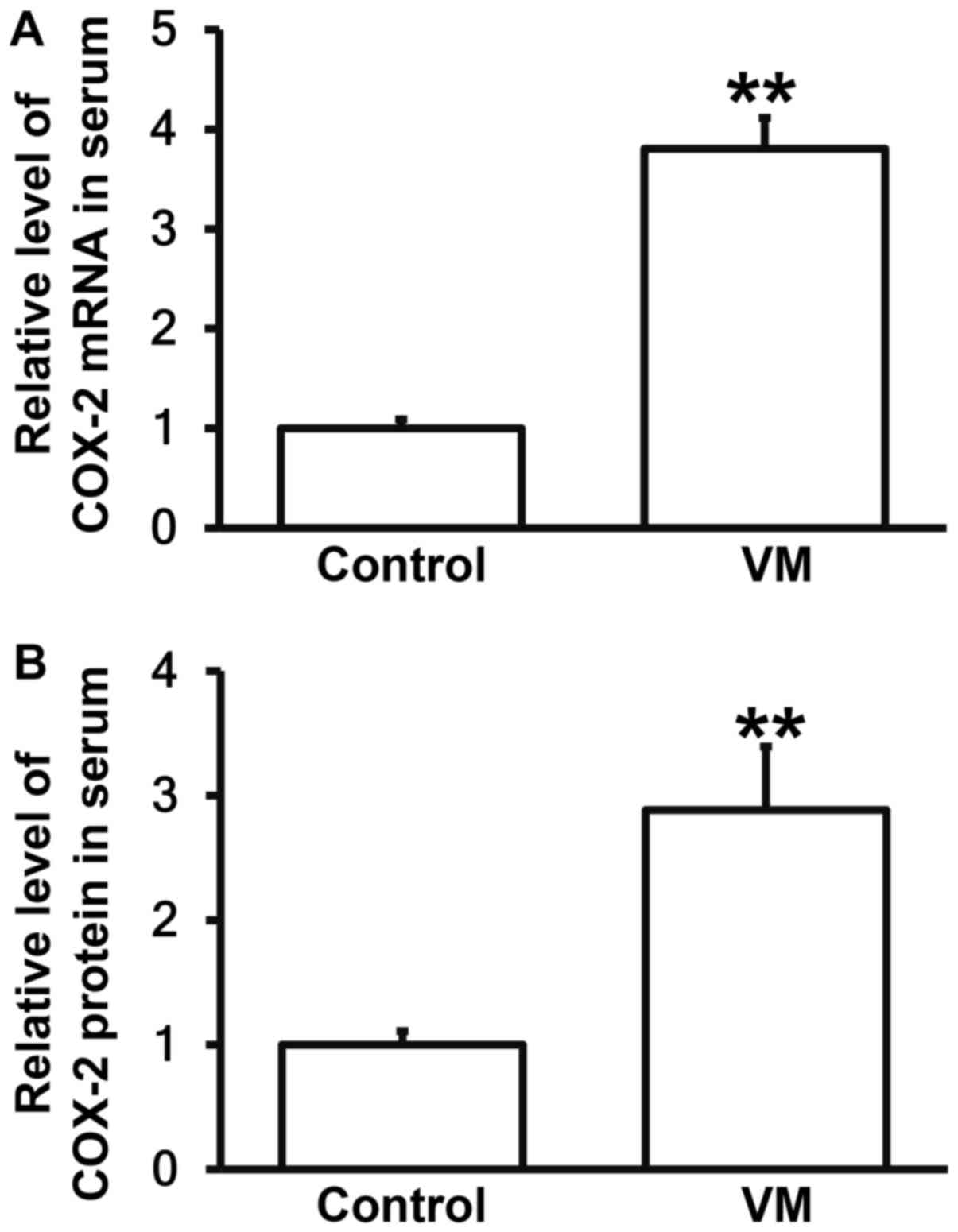
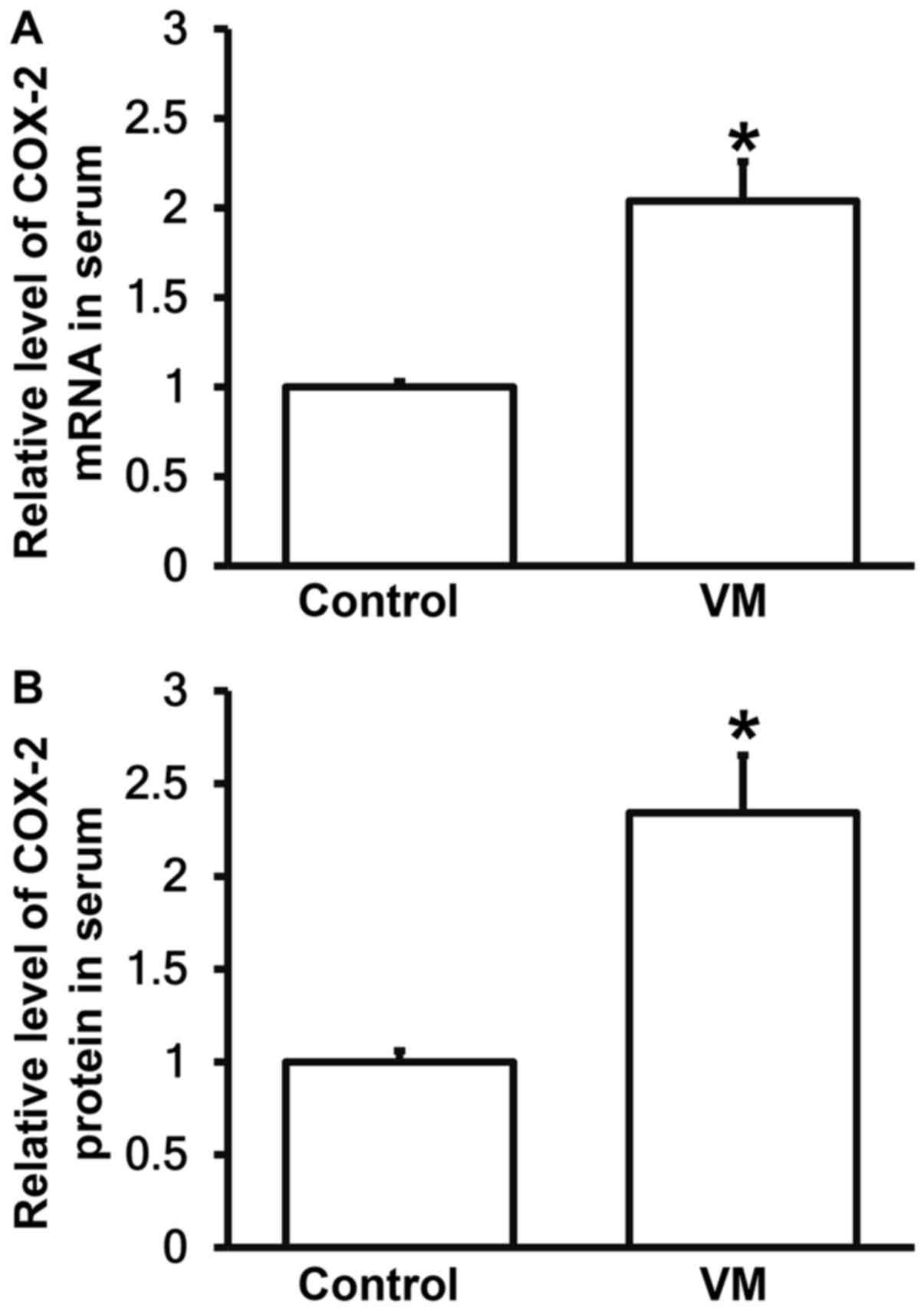
Children with VM have higher COX-2 levels in
peripheral blood than those who have recovered from VM. To measure
the expression of COX-2 mRNA and protein in serum, RT-qPCR and
ELISA were carried out. The data showed that the expression of
COX-2 mRNA and protein in serum from children with VM were
significantly higher than those in control group, respectively
(P<0.05; Fig. 2A and B). The
result suggests that children with VM have higher COX-2 levels in
peripheral blood than those who have recovered from VM.
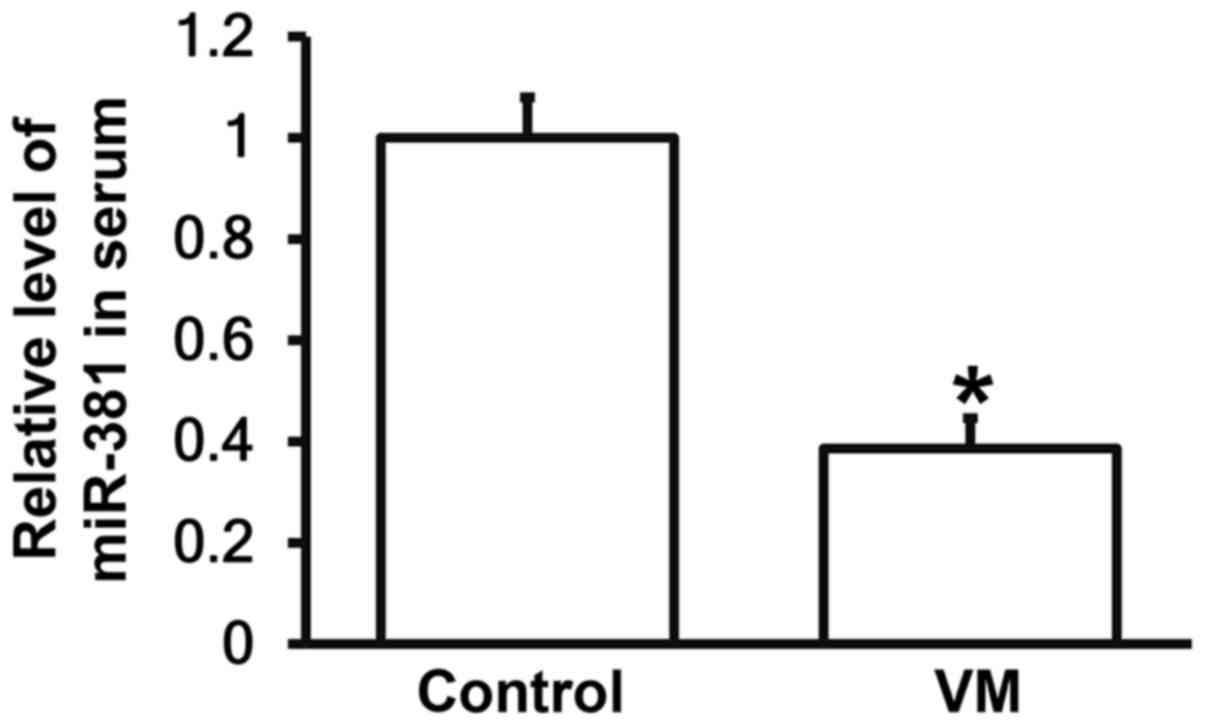
Children with VM have lower miR-381 levels in
peripheral blood than those who have recovered from VM. To
investigate the level of miR-381 in peripheral blood, RT-qPCR was
employed. The data showed that expression of miR-381 in serum from
children with VM were significantly lower than those from control
group (P<0.05; Fig. 3). The
result indicates that children with VM have lower miR-381 levels in
peripheral blood than those who have recovered from VM.
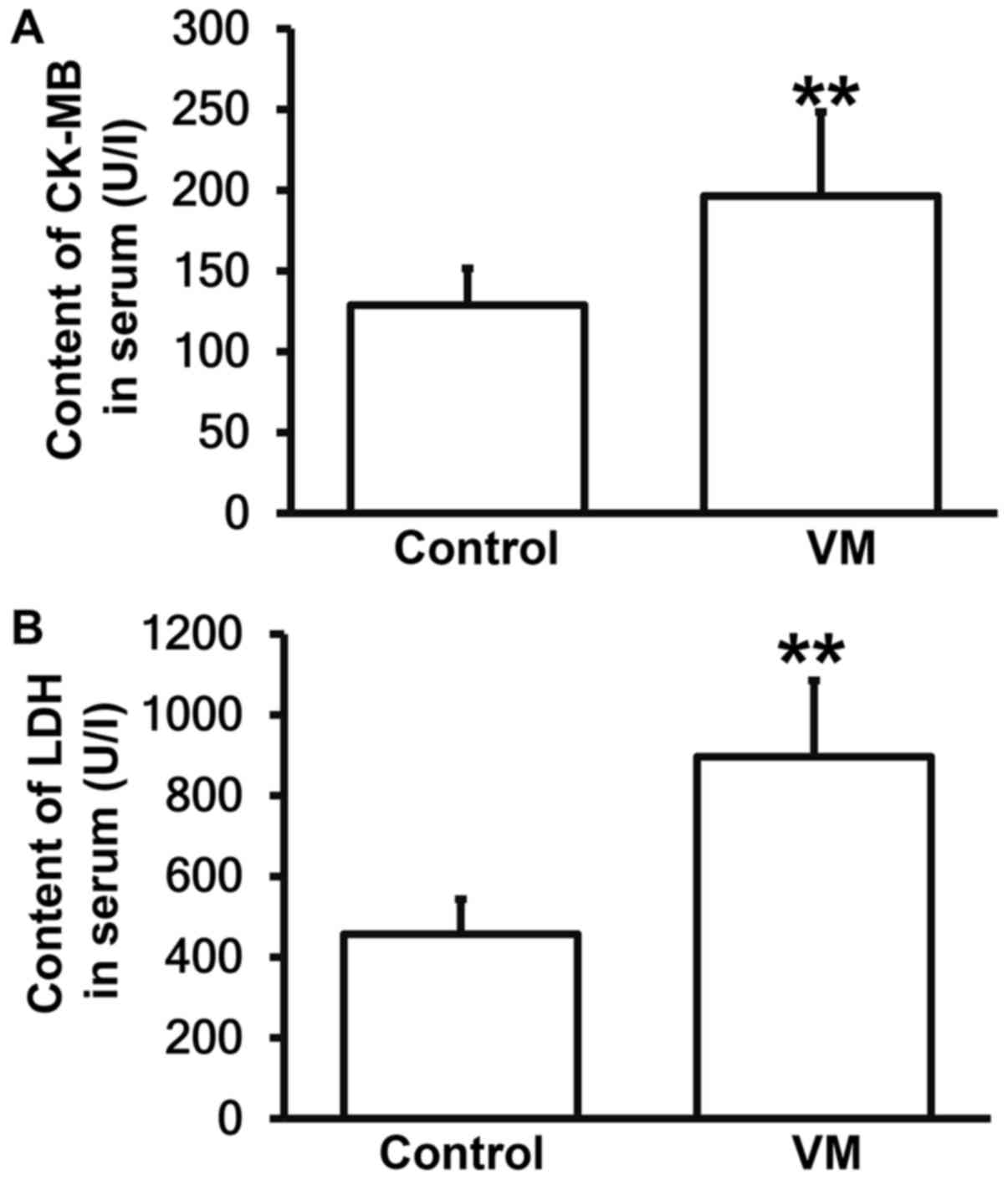
CVB3 infection results in damages in
myocardium of mice, and elevates CK-MB and LDH contents
To determine the contents of CK-MB and LDH,
automatic biochemical analysis was performed. The data showed that
the concentrations of CK-MB and LDH in peripheral blood from mice
infected by CVB3 were significantly higher than those from mice in
control group, respectively (P<0.05; Fig. 4A and B). The result suggests that
CVB3 infection results in damages in myocardium of mice, and
elevates CK-MB and LDH contents.
VM model mice have increased COX-2
levels in peripheral blood than normal mice
To measure the expression of COX-2 mRNA and protein
in serum, RT-qPCR and ELISA were carried out. The data showed that
the levels of COX-2 mRNA and protein in serum from VM model mice
were significantly higher than those in control group, respectively
(P<0.05; Fig. 5A and B). The
result suggests that VM model mice have increased COX-2 levels in
peripheral blood than normal mice.
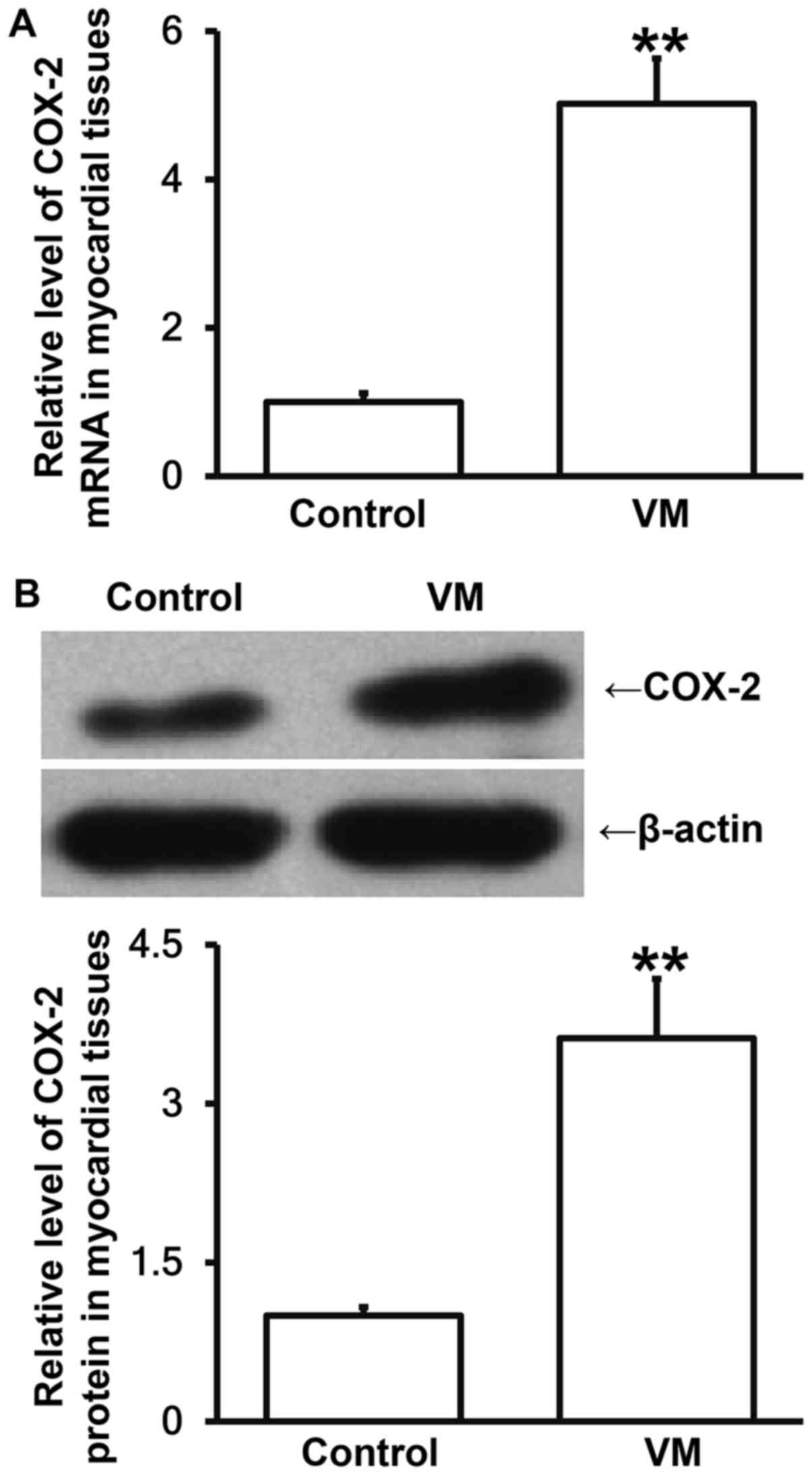
VM model mice have elevated COX-2
expression in myocardial tissues than normal mice
To measure the levels of COX-2 mRNA and protein in
myocardial tissues, RT-qPCR and Western blotting were employed. The
data showed that the levels of COX-2 mRNA and protein in myocardial
tissues from VM model mice were significantly higher than those in
control group, respectively (P<0.05; Fig. 6A and B). The result indicates that VM
model mice have elevated COX-2 expression in myocardial tissues
than normal mice.
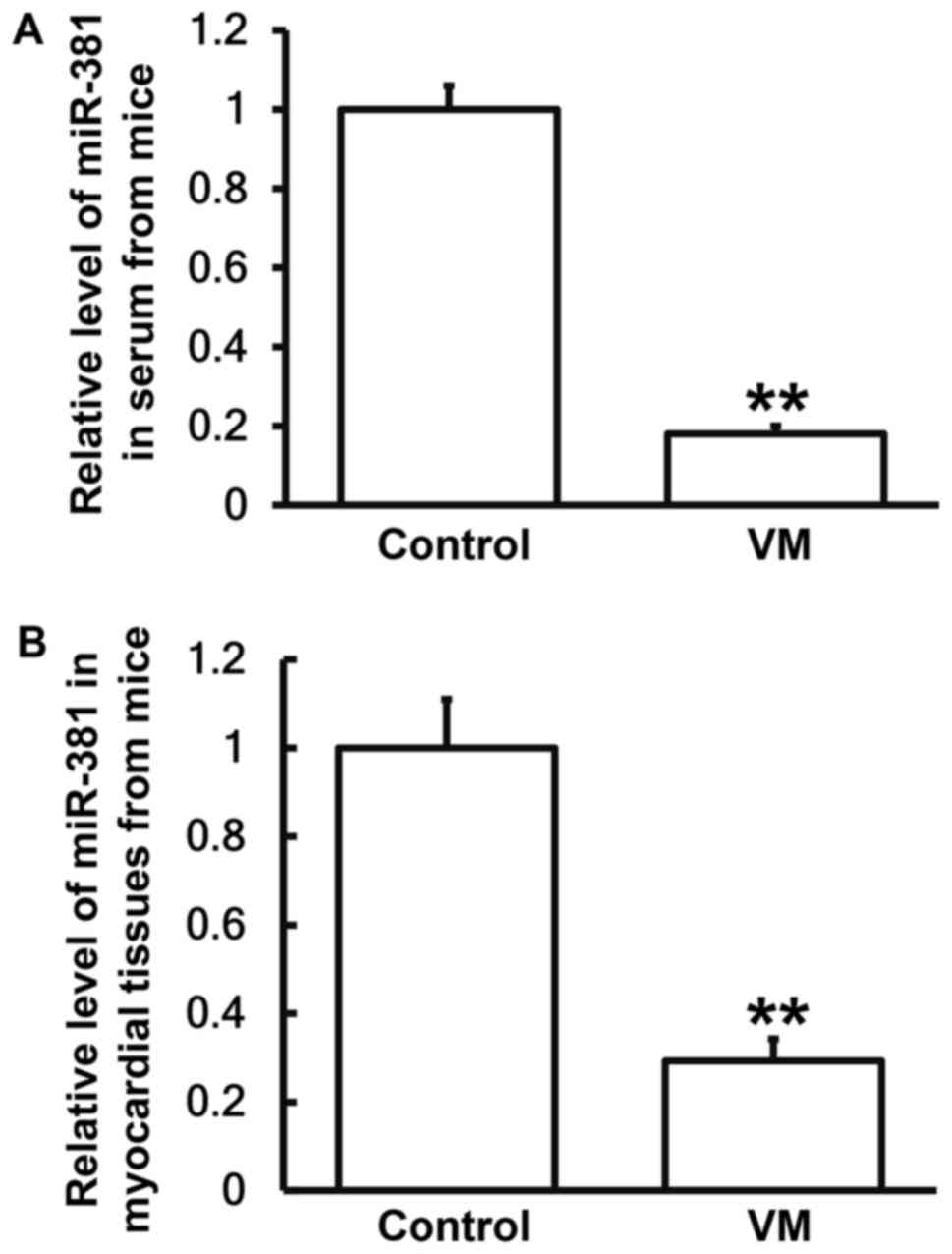
VM model mice have lower miR-381
levels in peripheral blood and myocardial tissues than normal
mice
To determine the expression of miR-381 in peripheral
blood and myocardial tissues from mice, RT-qPCR was used. The data
showed that expression of miR-381 in serum and myocardial tissues
from VM model mice were significantly reduced than those from
normal mice, respectively (P<0.05; Fig. 7A and B). The result suggests that VM
model mice have lower miR-381 levels in peripheral blood and
myocardial tissues than normal mice.
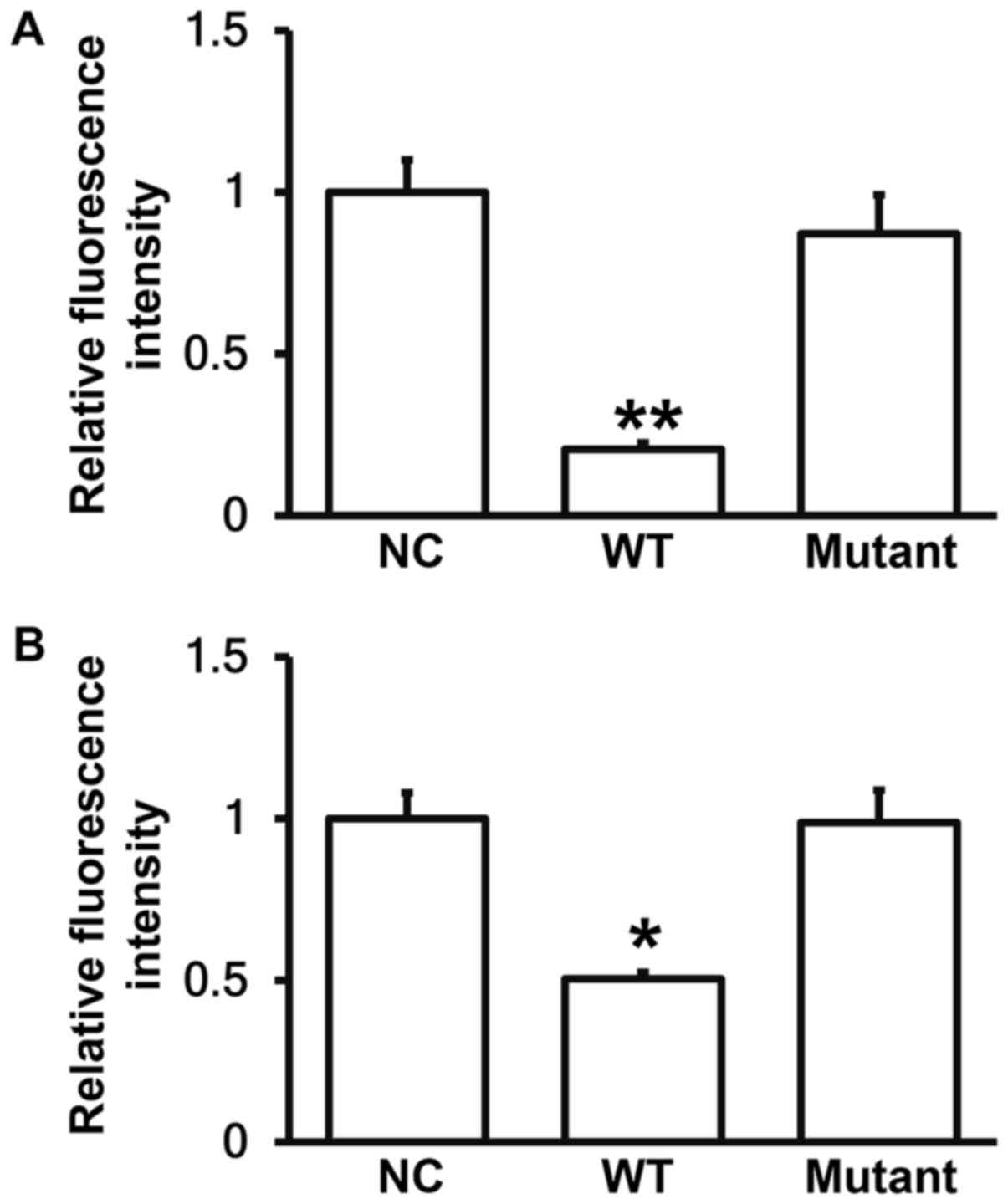
miR-381 can bind with the 3′-UTR seed
regions of both human and mouse COX-2 mRNA to regulate their
expression
To identify the interaction between miR-381 and the
3′-UTR of human and mouse COX-2 mRNA, dual luciferase reporter
assay was performed. The fluorescence value of cells co-transfected
with miR-381 mimics and pMIR-REPORT-WT luciferase reporter plasmids
was significantly lower than that in negative control group
(P<0.05). By contrast, the fluorescence value of cells
co-transfected with miR-381 mimics and pMIR-REPORT-mutant
luciferase reporter plasmids was not significantly different from
that in negative control group (P>0.05; Fig. 8A and B). The result indicates that
miR-381 can bind with the 3′-UTR seed regions of both human and
mouse COX-2 mRNA to regulate their expression.
Discussion
VM is a clinically common disease in children. It is
reported that the incidence of VM in children is increasing
(11). Because the clinical
manifestations of VM are atypical, the prognosis of the children is
seriously affected if the diagnosis and treatment are not timely
(11). The pathological changes of
myocardial cells are the main pathological changes in VM, and they
include direct damages to cardiac muscle cells by virus and immune
damages (12). Patients with
persistent VM have poor prognosis, with an average 5-year survival
rate of about 50%. Persistent chronic inflammation can lead to
myocardial cell necrosis, myocardial hypertrophy and cell
apoptosis, which can lead to myocardial fibrosis, dilated
cardiomyopathy (DCM) and heart failure (13).
In the present study, we have first detected the
related indexes in peripheral blood from children with VM, and then
constructed mouse model of VM by infecting the mice with CVB3. CVB3
can cause extensive damages to myocardium in weaning and adult
animals (14), leading to similar
progression and pathological changes of the disease (15). Our results on the peripheral blood of
children with VM show that expression of COX-2 in peripheral blood
is significantly up-regulated, suggesting that inflammatory
responses exist in VM. Similar results have also been observed in
our mouse model. The expression of CK-MB and LDH in the blood of
the mice with pathological myocarditis is significantly
up-regulated, suggesting the occurrence of myocardial injury. In
addition, COX-2 expression in peripheral blood and myocardial
tissues from the mice is also up-regulated, further suggesting that
COX-2 plays a role in VM.
miRNA is an important class of posttranscriptional
regulator. It is reported that miRNA is widely involved in the
regulation of cardiac development, cardiac hypertrophy, heart
failure, and vascular proliferation (16). For example, Wang et al report
that miR-142-3p inhibits myocardial cell injury induced by
hypoxia/reoxygenation by targeting high mobility group box 1 gene
(17). Singh et al discover
that miR-200c affects MAPK signaling pathway and promotes
cardiomyocyte hypertrophy by targeting DUSP-1 gene expression
(18). Our bioinformatics prediction
shows that miRNA-381 is closely related with COX-2, and may be an
upstream miRNA that regulates COX-2. Intracellular, small,
non-coding miRNAs may cut mRNA of COX-2 and inhibit its translation
(19). miRNAs exert regulatory
actions in a way of cutting, and promote the up-regulation or
down-regulation of mRNA expression to mediate the regulation of
protein-coding genes, playing important roles in the occurrence and
development of diseases (20,21). It
is discovered that down-regulation of miR-381 in colon cancer
tissues leads to up-regulation of LRH-1 and induces proliferation
and invasion of colon cancer cells (22). In addition, miR-381, which is present
in the p53/PTTG1 negative feedback loop, can inhibit the growth of
pituitary adenomas (23). miR-381 is
closely related to MDR1 gene and plays an important role in
multidrug resistance (24). Together
with miR-424, miR-381 can target WEE1 gene to inhibit the activity
of renal cancer Cdc2 cells (25).
Moreover, miR-381 is closely related to lung adenocarcinoma
(26). These studies demonstrate
that miR-381 is closely related to the occurrence and development
of human diseases. Our data show that miR-381 expression is reduced
in peripheral blood of children with VM. Considering the abnormally
high expression of COX-2 in the blood of these children, we
hypothesize that down-regulation of miR-381 is a reason for the
up-regulation of COX-2. Consistently, we have observed similar
trends in peripheral blood and myocardial tissues from VM model
mice. Our observation further demonstrates that negative regulatory
relationship exists between miR-381 and COX-2 in various species.
Lastly, we have identified that miR-381 directly binds with the
3′-UTR of COX-2 mRNA, and regulates its expression.
In conclusion, the present study demonstrates that
miR-381 expression in peripheral blood is decreased in children
with VM, being negatively correlated with COX-2 expression. In
addition, miR-381 alleviates myocardial cell injury by inhibiting
myocardial cell inflammatory responses via the regulation of COX-2.
Therefore, miR-381 may be a potential diagnostic and therapeutic
biomarker for VM in children.
Acknowledgements
We would like to thank Dr Shuzhen Deng from Wuhan
Children's Hospital.
References
|
1
|
Wang C, Dong C and Xiong S: IL-33 enhances
macrophage M2 polarization and protects mice from CVB3-induced
viral myocarditis. J Mol Cell Cardiol. 103:22–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen VK, Klawonn F, Mikolajczyk R and
Hernandez-Vargas EA: Analysis of practical identifiability of a
viral infection model. PLoS One. 11:e01675682016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rienks M, Papageorgiou A, Wouters K,
Verhesen W, Leeuwen RV, Carai P, Summer G, Westermann D and Heymans
S: A novel 72-kDa leukocyte-derived osteoglycin enhances the
activation of toll-like receptor 4 and exacerbates cardiac
inflammation during viral myocarditis. Cell Mol Life Sci.
74:1511–1525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yue-Chun L, Guang-Yi C, Li-Sha G, Chao X,
Xinqiao T, Cong L, Xiao-Ya D and Xiangjun Y: The protective effects
of ivabradine in preventing progression from viral myocarditis to
dilated cardiomyopathy. Front Pharmacol. 7:4082016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Márquez-González H, López-Gallegos D,
González-Espinosa AM, Zamudio-López JO and Yáñez-Gutiérrez L:
Effect of immune therapy in the prognosis of viral myocarditis in
pediatric patients. Rev Med Inst Mex Seguro Soc. 54 Suppl
3:S296–S301. 2016.PubMed/NCBI
|
|
6
|
Yu M, Long Q, Li HH, Liang W, Liao YH,
Yuan J and Cheng X: IL-9 inhibits viral replication in
coxsackievirus B3-induced myocarditis. Front Immunol. 7:4092016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
An B, Liu X, Li G and Yuan H:
Interleukin-37 ameliorates coxsackievirus B3-induced viral
myocarditis by modulating the Th17/regulatory T cell immune
response. J Cardiovasc Pharmacol. 69:305–313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alhouayek M and Muccioli GG: COX-2-derived
endocannabinoid metabolites as novel inflammatory mediators. Trends
Pharmacol Sci. 35:284–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hao Y, Gu X, Zhao Y, Greene S, Sha W,
Smoot DT, Califano J, Wu TC and Pang X: Enforced expression of
miR-101 inhibits prostate cancer cell growth by modulating the
COX-2 pathway in vivo. Cancer Prev Res (Phila). 4:1073–1083. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu T: Diagnostic criteria for viral
myocarditis (Revised Draft). Chin J Prac Pediat. 5:3152000.(In
Chinese).
|
|
11
|
Casadonte JR, Mazwi ML, Gambetta KE, Palac
HL, McBride ME, Eltayeb OM, Monge MC, Backer CL and Costello JM:
Risk factors for cardiac arrest or mechanical circulatory support
in children with fulminant myocarditis. Pediatr Cardiol.
38:128–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simpson KE, Storch GA, Lee CK, Ward KE,
Danon S, Simon CM, Delaney JW, Tong A and Canter CE: High frequency
of detection by pcr of viral nucleic acid in the blood of infants
presenting with clinical myocarditis. Pediatr Cardiol. 37:399–404.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tse G, Yeo JM, Chan YW, Lai ET and Yan BP:
What is the arrhythmic substrate in viral myocarditis? Insights
from clinical and animal studies. Front Physiol. 7:3082016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grodums EI and Dempster G: The
pathogenesis of Coxsackie group B viruses in experimental
infection. Can J Microbiol. 8:105–113. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Webb SR, Loria RM, Madge GE and Kibrick S:
Susceptibility of mice to group B coxsackie virus is influenced by
the diabetic gene. J Exp Med. 143:1239–1248. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bardooli F, McAlindon E, Littlejohns B,
Suleiman MS, Chiara BD and Baumbach A: TCT-184 Early changes in
circulating miRNA 133a are indicative of cardiac remodelling after
3 months in patients presenting with acute ST elevation myocardial
infarction. J Am Coll Cardiol. 68:B75–B76. 2016. View Article : Google Scholar
|
|
17
|
Wang Y, Ouyang M, Wang Q and Jian Z:
MicroRNA-142-3p inhibits hypoxia/reoxygenationinduced apoptosis and
fibrosis of cardiomyocytes by targeting high mobility group box 1.
Int J Mol Med. 38:1377–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh GB, Raut SK, Khanna S, Kumar A,
Sharma S, Prasad R and Khullar M: MicroRNA-200c modulates DUSP-1
expression in diabetes-induced cardiac hypertrophy. Mol Cell
Biochem. 424:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu D, Wang D, Xu Z, Gao J, Liu M, Liu Y,
Jiang M and Zheng D: Dysregulated expression of miR-101b and
miR-26b lead to age-associated increase in LPS-induced COX-2
expression in murine macrophage. Age (Dordr). 37:972015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang Y, Zhao Q, Fan L, Zhang Z, Tan B,
Liu Y and Li Y: Down-regulation of MicroRNA-381 promotes cell
proliferation and invasion in colon cancer through up-regulation of
LRH-1. Biomed Pharmacother. 75:137–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang HQ, Wang RJ, Diao CF, Li JW, Su JL
and Zhang S: The PTTG1-targeting miRNAs miR-329, miR-300, miR-381
and miR-655 inhibit pituitary tumor cell tumorigenesis and are
involved in a p53/PTTG1 regulation feedback loop. Oncotarget.
6:29413–29427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Y, Ohms SJ, Li Z, Wang Q, Gong G, Hu Y,
Mao Z, Shannon MF and Fan JY: Changes in the expression of miR-381
and miR-495 are inversely associated with the expression of the
MDR1 gene and development of multi-drug resistance. PLoS One.
8:e820622013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen B, Duan L, Yin G, Tan J and Jiang X:
Simultaneously expressed miR-424 and miR-381 synergistically
suppress the proliferation and survival of renal cancer cells-Cdc2
activity is up-regulated by targeting WEE1. Clinics (Sao Paulo).
68:825–833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rothschild SI, Tschan MP, Jaggi R, Fey MF,
Gugger M and Gautschi O: MicroRNA-381 represses ID1 and is
deregulated in lung adenocarcinoma. J Thorac Oncol. 7:1069–1077.
2012. View Article : Google Scholar : PubMed/NCBI
|