Introduction
Over the last decade, there has been continuous
development of social productive forces as well as deterioration of
the environment. At the same time, living standards have shown
improvement, resulting in increased attention being given to
infertility, with diagnostic rates showing a notable increase
(1). Correlational studies have
shown that the reasons of infertility are complex. In an infertile
couple, approximately 40% of the time both the male and female have
an infertility issue, approximately 25% of the time the issue is
due to either the male or the female, or approximately 35% of the
time, the issue is unknown (2).
Currently, there is a lack of methods to diagnose male infertility
in clinic. One of the most direct and most certain methods to
diagnose males with infertility is to analyze azoospermatism in
clinic. In clinic, certain defects and shortages exist in the basic
assessment of male fertility by analyzing sperm density and
motility in routine sperm examination, as the assessment on male
fertility is not only limited to the above indices (3,4).
Currently, scientists have been studying the
correlation between sperm DNA damage and male infertility.
Correlational studies have reported that there is a great
difference in sperm DNA fragmentation indexes among the different
infertile patients, but total results indicate that sperm motility
of a mass of infertile patients presents an obvious negative
correlation with their sperm DNA fragmentation indexes (5,6). This
negative correlation shows that sperm DNA damage is one of the
important factors causing sperm motility decrease and male
infertility (5). By studying the
correlation between infertile male sperm DNA fragmentation index
and conventional semen parameters, blood microelements and seminal
plasma reactive oxygen species (ROS), the present study may provide
a theoretical basis for diagnosing and treating infertile
males.
Materials and methods
Sample selection
The present study included 80 infertile male
patients that presented at the Department of Andrology at the
Shiyan Taihe Hospital (Hubei, China) from February 2014 to February
2016. We also recruited 20 cases of healthy male volunteers who had
children as a positive control. The age range of all the study
subjects was 25–39 years. The diagnostic standard of male
infertility referred to the gold standard of the clinic which
included the inability to get pregnant for 1 year or more despite a
normal sexual life and no contraceptives. The study was approved by
the Ethics Committee of Shiyan Taihe Hospital and informed consents
were signed by the patients.
Routine inspection of sperm
The study subjects were asked to refrain from any
sexual activity for 3–7 days, and then ejaculate the sperm sample
through masturbation. The sperm samples were placed into a
disinfected plastic container. After being fully liquefied by the
constant temperature dry box, a routine inspection was conducted by
using the semen detecting instrument (Barui Medical Equipment Co.,
Beijing, China) at Huaian First People's Hospital. Each sample was
selectively observed from 6 fields of view, and parameters, such as
semen volume, sperm density and motility, were detected.
Detecting sperm fragmentation DNA
The sperm fragmentation DNA detection kit (Biosharp,
Hefei, China) and sperm chromatin diffusion method were used to
detect sperm fragmentation DNA. The fresh semen was diluted using
normal saline to the target concentration (5–10×106/ml),
and sperm fragmentation DNA detection was operated according to kit
instructions.
Detecting serum microelements and
seminal plasma ROS level
Blood (5 µl) was taken from each study subject
before breakfast. After the serum was separated, each microelement
in the serum was detected according to the ELISA kit instructions.
Within 30 min after the obtained semen sample was liquefied, the
seminal plasma was obtained and an ELISA kit was utilized to detect
seminal plasma ROS levels.
Statistical analysis
Experimental data were statistically analyzed using
statistical software, SPSS 19.0 (Chicago, IL, USA). The association
between the semen fragmentation index and conventional semen
parameters, blood microelements and seminal plasma ROS were
studied. Data were presented as mean ± standard deviation (mean ±
SD). P<0.05 was considered to indicate a statistically
significant difference.
Results
Analysis on sperm fragmentation DNA of
infertile male patients
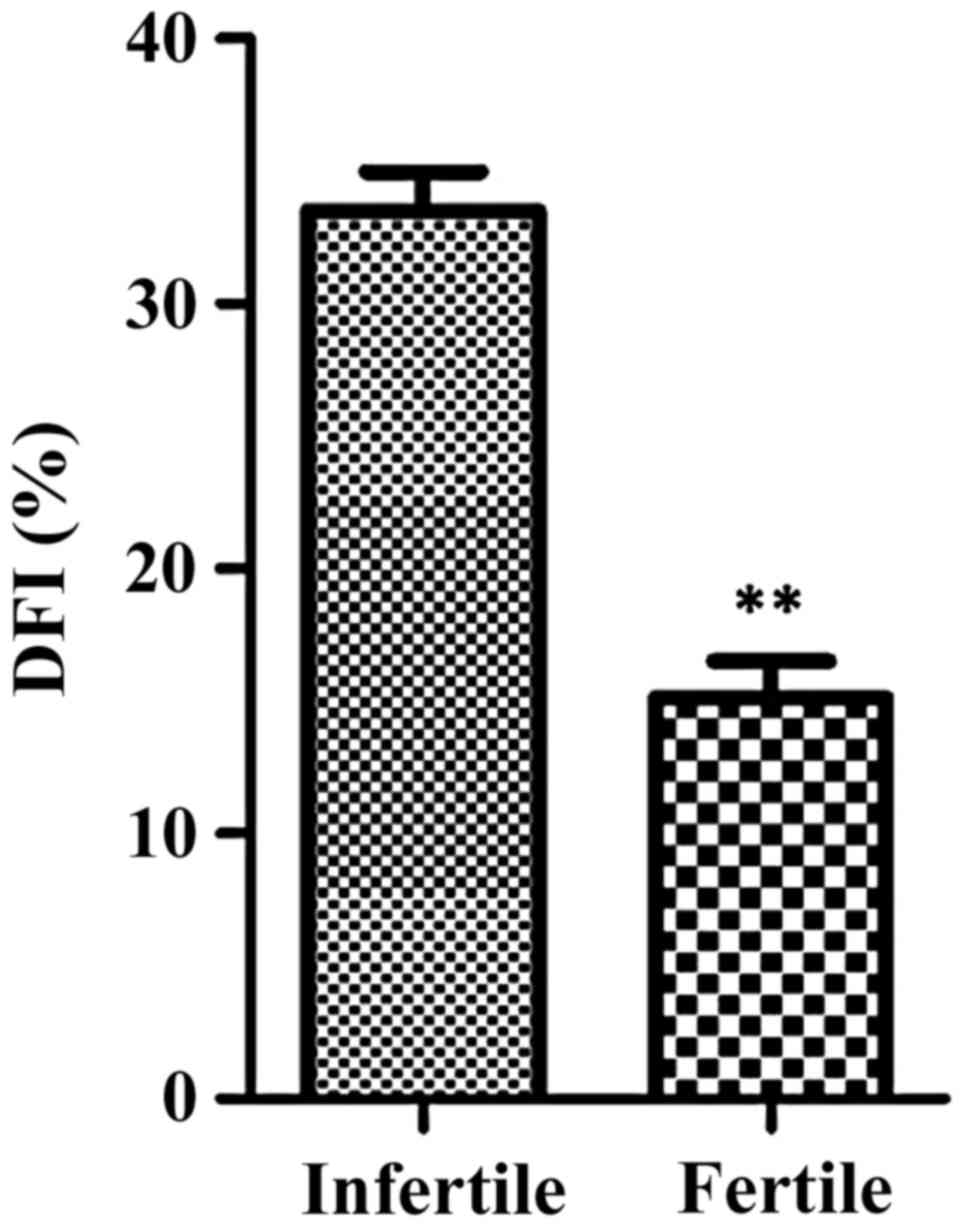
There were 80 male infertile patients and 20 males
with children in this study. After their sperm DNA fragmentation
indexes were analyzed, it was found that the sperm DNA
fragmentation indexes of infertile patients were higher than the
control subjects (p=0.008). Data information is shown in Table I and Fig.
1.
 | Table I.Sperm fragmentation DNA indexes of
infertile males and males who had children. |
Table I.
Sperm fragmentation DNA indexes of
infertile males and males who had children.
| Group | DFI (%) |
|---|
| Infertile male
patients | 36.5±3.87 |
| Males who had
children | 16.23±2.65 |
Analysis of routine data of semen
After an analysis of routine data of the semen of
infertile patients, it was found that the differences between sperm
DNA fragmentation indexes and each conventional semen parameter had
no statistical significance (p>0.05) (Table II). The study on the correlation
between sperm DNA fragmentation index and each conventional semen
parameter showed that the sperm DNA fragmentation index was not
correlated with semen volume, sperm density, sperm motility and
normal sperm formation rate (p>0.05) (Table III).
 | Table II.Association between sperm
fragmentation DNA and each parameter of semen. |
Table II.
Association between sperm
fragmentation DNA and each parameter of semen.
| Group | Semen volume
(ml) | Sperm motility | Normal form of sperm
(%) | Sperm density
(106/ml) | DFI (%) |
|---|
| Infertile male
patients | 2.85±1.33 | 28.21±12.03 | 3.82±3.5 | 32.3±17.6 | 36.5±3.87 |
| Males who had
children | 3.02±1.21 | 32.01±11.89 | 3.55±2.8 | 38.6±12.8 | 16.23±2.65 |
 | Table III.Study on the correlation between the
sperm DNA fragmentation index and each conventional semen
parameter. |
Table III.
Study on the correlation between the
sperm DNA fragmentation index and each conventional semen
parameter.
| Detection index | r | P-value |
|---|
| Semen volume | 0.067 | 0.106 |
| Sperm density | 0.815 | 0.533 |
| Sperm motility | −0.114 | 0.068 |
| Normal form rate of
sperm | −0.089 | 0.093 |
Analysis on microelements in blood
plasma
After the sperm DNA fragmentation index and
microelements in blood plasma were analyzed, it was found that zinc
and lead in blood plasma were positively correlated with sperm DNA
fragmentation index (r=0.187, r=0.078, p<0.05). Magnesium was
also negatively correlated with sperm DNA fragmentation index
(r=0.263, p<0.05). Results are shown in Table IV.
 | Table IV.Association between sperm DNA
fragmentation index, conventional parameters and microelements in
blood plasma. |
Table IV.
Association between sperm DNA
fragmentation index, conventional parameters and microelements in
blood plasma.
| Parameters | Zinc | Iron | Calcium | Magnesium | Lead | Copper |
|---|
| DFI (%) | 0.187a | −0.037 | 0.016 | −0.263a | 0.078a | −0.132 |
| Sperm | −0.013 | −0.021 | −0.042 | 0.053a | 0.079 | 0.018 |
| density |
|
|
|
|
|
|
| Sperm | −0.223a | −0.178 | −0.087 | 0.285a | −0.045 | −0.057 |
| motility |
|
|
|
|
|
|
| Sperm | −0.029a | −0.022 | −0.027 | 0.267a | −0.028 | 0.124 |
| motility |
|
|
|
|
|
|
| rate |
|
|
|
|
|
|
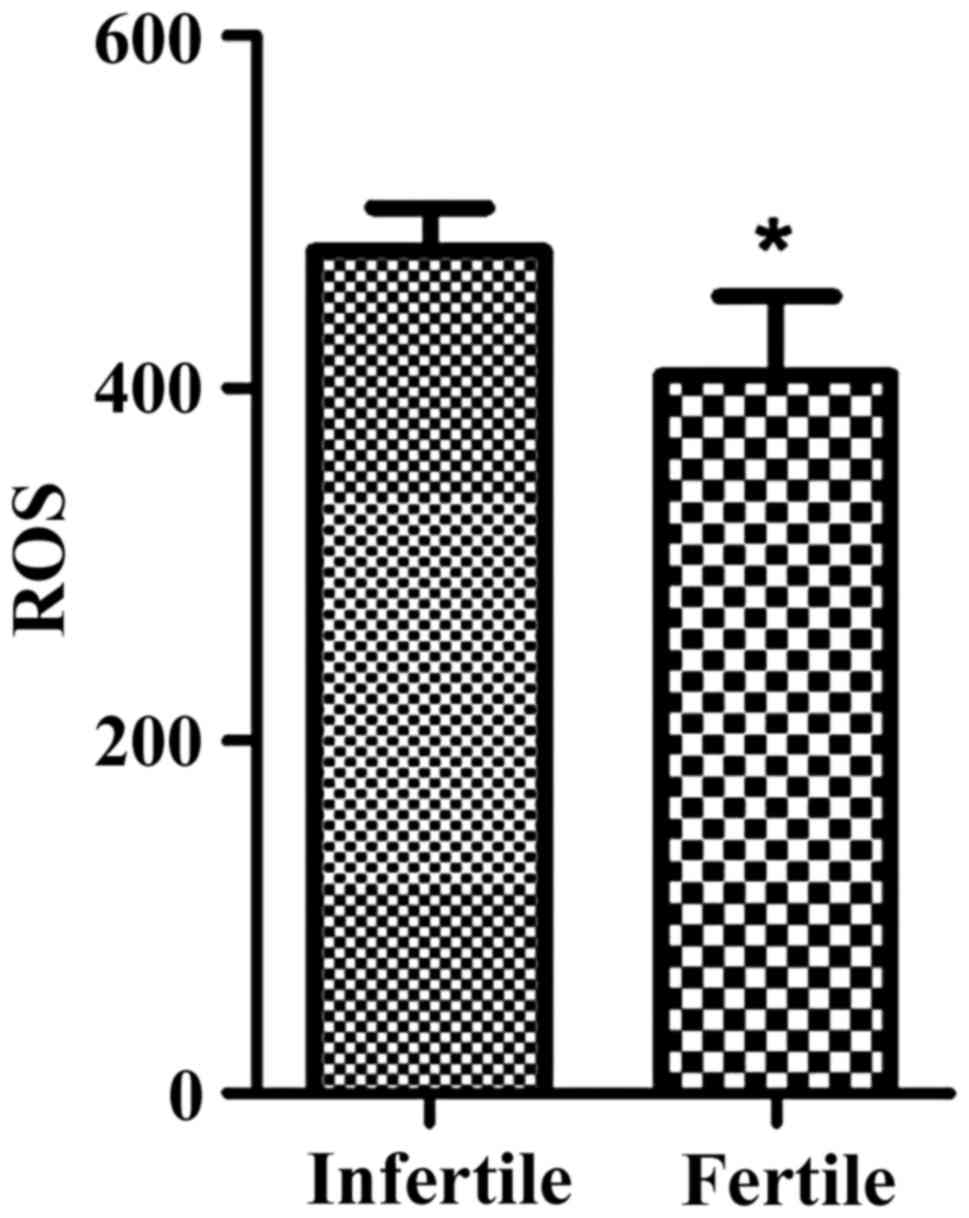
Analysis on seminal plasma ROS
After an analysis of the sperm DNA fragmentation
index and seminal plasma ROS, it was found that the sperm DNA
fragmentation index in the infertility group was higher than that
of control group. In addition, seminal plasma ROS levels were
higher than those of males who had children (p<0.05). Results
were shown in Table V and Fig. 2.
 | Table V.Association between sperm DNA
fragmentation index and seminal plasma ROS level. |
Table V.
Association between sperm DNA
fragmentation index and seminal plasma ROS level.
| Group | DFI (%) | ROS level |
|---|
| Infertile male
patients | 36.5±3.87 | 548.9±108.2 |
| Males who had
children | 16.23±2.65 | 416.3±95.5 |
Discussion
Genetic information of the parental generation is
passed onto the offspring through fertilization of the egg by the
sperm, and therefore, the integrity of sperm DNA is important to
correctly pass on genetic material to offspring (7). Correlational studies have shown that
the failures of natural childbirth and assisted reproductive
technology are associated with sperm DNA damage. Although the exact
causes of sperm DNA damage remain to be determined, it has been
suggested that there are numerous reasons that cause it.
Abnormities of environmental factors can lead to variation of genes
and chromosomes, and because the DNA damage leads to the
destruction of the integrity of genetic information, this may
result in the occurrence of infertility. Additionally, leukocytes
in human tissue may produce excessive ROS because of an increase in
infectious diseases in the reproductive system, which causes
further damage to sperm DNA (8).
Under normal physiological status, ROS participates in processes
such as regulating immunological surveillance, signal transduction
and cell growth, and is therefore an important medium of signal
transduction. ROS plays an important role in regulating body
function and increasing probability of fertilization (9,10). The
main components of ROS are free radicals, peroxide, oxygen ions and
are mainly produced by leukocyte and sperm in semen. The reactivity
of the oxygen in ROS may cause damage to the sperm of the
cytomembrane, thus resulting in the damage of sperm DNA and
impacting sperm motility, which may finally affect fertility
(11).
By analyzing the infertile male sperm DNA
fragmentation index and conventional semen parameters, this study
has shown that the conventional semen parameters were not
correlated with sperm DNA fragmentation index, and therefore, sperm
DNA detection can be added to the diagnosis and treatment of
infertile patients in clinic in addition to the routine semen
analysis, which may further completely reflect male fertility
(12). After an infertile male's
sperm DNA fragmentation index and blood microelements were
analyzed, it was found that zinc and lead in serum were positively
correlated with the sperm DNA fragmentation index. The function of
zinc is that it mainly has an effect on the thalamus-pituitary
gland-testis axis (13), and through
the regulation of the sex hormone secretion and controlling gonad
development, it may have a great effect on semen quality and cause
an issue of spermatogenesis, which may lead to male infertility
(14). An excessive lead ion
concentration in blood can produce toxic effects on sperm (15), and impact sperm motility, which may
cause the decrease of male sperm quality and result in infertility.
Magnesium in serum was found to be negatively correlated with sperm
DNA fragmentation index. Study results also show that when the
content of magnesium ion in the serum rises, sperm motility rates
and rectilinear motion sperm motility rates notably increase, at
the same time, sperm density also increases. The results of the
present study on the association between infertile male sperm DNA
fragmentation index and seminal plasma ROS levels have shown that
the ROS levels in the infertility group were apparently higher than
those of the normal childbirth group (p<0.05). The main reason
of an increase in seminal plasma ROS levels casing sperm damage and
thus resulting in infertility is that the sperm cytomembrane is
damaged to varying degrees by the existence of a superoxide anion
and oxygen free radicals (16–18).
Additionally, correlational research data have shown that seminal
plasma ROS levels have an effect on both sperm forward movement and
sperm activity rate, which may cause a decrease in its activity
rate (19). Therefore, in clinic,
male fertility ability can be improved by reducing seminal plasma
ROS levels. Sperm DNA chain break causes sperm DNA damage, leading
to chromatin crosslinking, which is the main mechanism causing an
increase in ROS levels and subsequently causing infertility. At the
same time, it can reduce the production of the spermatoblast ATP,
reduce the sperm motility rate, damage sperm cytomembrane and cause
infertility (20–22).
In conclusion, infertile male's sperm DNA
fragmentation index is not correlated with conventional semen
parameters but is positively correlated with blood microelements
zinc, lead and seminal plasma ROS, and is negatively correlated
with magnesium. The results above provide enough theoretical bases
for diagnosing and treating infertile males in clinic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are
not publicly available due to the protection of patient privacy but
are available from the corresponding author on reasonable
request.
Authors' contributions
DX and XJ designed and conducted the study and
analyzed the data. CL detected the sperm parameters like density
and motility. YZ detected the sperm fragmentation DNA. SZ and EJY
detected the serum microelements and seminal plasma ROS level. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shiyan Taihe Hospital (Shiyan, China) and informed consents were
signed by the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bungum M, Bungum L and Giwercman A: Sperm
chromatin structure assay (SCSA): A tool in diagnosis and treatment
of infertility. Asian J Androl. 13:69–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
González-Marín C, Gosálvez J and Roy R:
Types, causes, detection and repair of DNA fragmentation in animal
and human sperm cells. Int J Mol Sci. 13:14026–14052. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aitken RJ and De Iuliis GN: On the
possible origins of DNA damage in human spermatozoa. Mol Hum
Reprod. 16:3–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakkas D, Moffatt O, Manicardi GC,
Mariethoz E, Tarozzi N and Bizzaro D: Nature of DNA damage in
ejaculated human spermatozoa and the possible involvement of
apoptosis. Biol Reprod. 66:1061–1067. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Erenpreiss J, Spano M, Erenpreisa J,
Bungum M and Giwercman A: Sperm chromatin structure and male
fertility: Biological and clinical aspects. Asian J Androl.
8:11–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sergerie M, Laforest G, Boulanger K,
Bissonnette F and Bleau G: Longitudinal study of sperm DNA
fragmentation as measured by terminal uridine nick end-labelling
assay. Hum Reprod. 20:1921–1927. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rasool EA, Abdul-Rasheed OF and AL-Hashimi
AF: Comparison between different DNA and conventional sperm
parameters in infertile men. Al-Kindy Coll Med J. 8:40–47.
2012.
|
|
8
|
Brahem S, Mehdi M, Landolsi H, Mougou S,
Elghezal H and Saad A: Semen parameters and sperm DNA fragmentation
as causes of recurrent pregnancy loss. Urology. 78:792–796. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ilma SY, Zergeroglu AD and Yilmaz E:
Effects of sperm DNA fragmentation on semen parameters and ICSI
outcome determined byan improved SCD testhalosperm. Int J Fertil
Steril. 4:73–78. 2010.
|
|
10
|
Schulte RT, Ohl DA, Sigman M and Smith GD:
Sperm DNA damage in male infertility: Etiologies, assays, and
outcomes. J Assist Reprod Genet. 27:3–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zini A, Phillips S, Courchesne A, Boman
JM, Baazeem A, Bissonnette F, Kadoch IJ and Gabriel San M: Sperm
head morphology is related to high deoxyribonucleic acid
stainability assessed by sperm chromatin structure assay. Fertil
Steril. 91:2495–2500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar K, Deka D, Singh A, Mitra DK,
Vanitha BR and Dada R: Predictive value of DNA integrity analysis
in idiopathic recurrent pregnancy loss following spontaneous
conception. J Assist Reprod Genet. 29:861–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Colacurci N, Monti MG, Fornaro F, Izzo G,
Izzo P, Trotta C, Mele D and De Franciscis P: Recombinant human FSH
reduces sperm DNA fragmentation in men with idiopathic
oligoasthenoteratozoospermia. J Androl. 33:588–593. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
La Maestra S, De Flora S and Micale RT:
Effect of cigarette smoke on DNA damage, oxidative stress, and
morphological alterations in mouse testis and spermatozoa. Int J
Hyg Environ Health. 218:117–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hammiche F, Laven JS, Boxmeer JC, Dohle
GR, Steegers EA and Steegers-Theunissen RP: Sperm quality decline
among men below 60 years of age undergoing IVF or ICSI treatment. J
Androl. 32:70–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shome D and Esmaeli B: Targeted monoclonal
antibody therapy and radioimmunotherapy for lymphoproliferative
disorders of the ocular adnexa. Curr Opin Ophthalmol. 19:414–421.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanders RD and Maze M: Neuroinflammation
and postoperative cognitive dysfunction: Can anaesthesia be
therapeutic? Eur J Anaesthesiol. 27:3–5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Z, Zhu L, Jiang H, Chen H, Chen Y
and Dai Y: Sperm DNA fragmentation index and pregnancy outcome
after IVF or ICSI: A meta-analysis. J Assist Reprod Genet.
32:17–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao J, Zhang Q, Wang Y and Li Y: Whether
sperm deoxyribonucleic acid fragmentation has an effect on
pregnancy and miscarriage after in vitro
fertilization/intracytoplasmic sperm injection: A systematic review
and meta-analysis. Fertil Steril. 102:998–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stevanato J, Bertolla RP, Barradas V,
Spaine DM, Cedenho AP and Ortiz V: Semen processing by density
gradient centrifugation does not improve sperm apoptotic
deoxyribonucleic acid fragmentation rates. Fertil Steril.
90:889–890. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nabi A, Khalili MA, Halvaei I and Roodbari
F: Prolonged incubation of processed human spermatozoa will
increase DNA fragmentation. Andrologia. 46:374–379. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lewis SE, Aitken John R, Conner SJ, Iuliis
GD, Evenson DP, Henkel R, Giwercman A and Gharagozloo P: The impact
of sperm DNA damage in assisted conception and beyond: Recent
advances in diagnosis and treatment. Reprod Biomed Online.
27:325–337. 2013. View Article : Google Scholar : PubMed/NCBI
|