Introduction
Bronchial asthma is a chronic airway inflammatory
disease mediated by a variety of cell types and factors (1). A key hallmark associated with bronchial
asthma is marked airway remodeling, which at tissue and cellular
levels primarily includes airway wall thickening, airway epithelial
fibrosis, airway smooth muscle cell hyperplasia and hypertrophy and
myofibroblast proliferation and activation (2). At a molecular level, the pathological
changes of airway remodeling are known to be associated with
increased deposition of extracellular matrix (ECM) components,
including type I, III and IV collagen fibers and fibronectin
(3–5). Although the underlying mechanism of
airway remodeling is not fully understood, it is generally
acknowledged that airway remodeling is predominantly a result of
the imbalance of airway injury and repair following repeated airway
injury from chronic exposure to hazardous environmental factors and
abnormal repair due to chronic airway inflammation (2). This association between airway
remodeling and environmental hazards may be a contributing factor
to the rising incidence of bronchial asthma, particularly in
industrial countries worldwide during recent decades; the highest
prevalence of clinical asthma worldwide were reported in Australia,
Sweden, UK, Netherlands and Brazil were 21.5, 20.2, 18.2, 15.3 and
13.0%, respectively (6).
Among the environmental hazardous factors known to
cause adverse effects on health, ozone (O3) is a major
factor in air pollution, which is widely recognized as a
particularly harmful risk to airway health (7,8). In
human and animal studies, it has been demonstrated that early
exposure at post-natal stage or long-term exposure at later stages
to O3 may result in pathological changes in the
respiratory system, including modifications of time and mode of
alveolar growth and development (9,10). It
has been reported that O3 exposure may also cause
inflammation in the airways and the lungs and structural
reorganization of smooth muscle cells (11,12).
Previous studies indicated that O3 induced oxidative
stress to airway epithelial cells cultured in vitro,
resulting in the release of nitric oxide and epithelial cell injury
(13), and that exposure to
O3 of the bronchial epithelium resulted in airway
inflammation and airway hyperresponsiveness (14,15).
Notably, there is little evidence to support whether
O3 exposure may result in airway remodeling. The limited
reports regarding this may partly be due to the fact that
O3 directly targets bronchial epithelial cells (BECs)
but not the fibroblasts that reside beneath these cells, which
serve a key role in airway remodeling (16).
In order to explore the association between
O3 exposure and airway remodeling, it was hypothesized
that BECs exposed to O3 will release inflammatory growth
factors, including transforming growth factor (TGF)-β1, which may
subsequently enhance the secretion and activity of collagen from
adjacent fibroblasts in the airway wall and ultimately result in
airway remodeling. To investigate this hypothesis in the present
study, a co-culture model of human lung fibroblasts (HLFs) and
human bronchial epithelial cells (HBECs) was constructed.
O3 stress was applied to the HBECs and cell
proliferation and secretion of cytokines in the HLFs were assessed.
The present study aimed to elucidate the mechanism of
O3-induced airway remodeling from a novel perspective of
maintenance/loss of steady-state function of the airway
epithelium.
Materials and methods
HBE and HLF culture
HBECs from a cell line were provided by Professor
Gruenent of University of California, San Francisco (San Francisco,
USA) (17). Embryonic diploid HLF-02
HLFs were purchased from the Cell Center of Central South
University (Changsha, China). HBECs were seeded in Dulbecco's
modified Eagle medium (DMEM)/F12 (1:1 ratio; Hyclone, Logan, UT,
USA) supplemented with 15% newborn calf serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
U/ml streptomycin in a 100-ml culture flask. Cells grew adherently
at 37°C until 80% confluence was reached. Subsequently, the culture
medium was displaced and the cells were rinsed with
phosphate-buffered saline one to two times. Following this, HBECs
were trypsinized with 1 ml 0.25% trypsin with 0.05% EDTA and
scattered gently through a pipette to create the cell suspension.
HBECs were divided in 1:2 or 1:3 proportions for subculture. When
cells in subculture grew to 80% confluence with a healthy, cuboid
morphology, they were seeded on culture plates for subsequent
experiments. HLFs were cultured and prepared for experiments as
described above.
Co-culture of HLFs and
O3-stimulated HBECs
Prior to co-culture, confluent HBECs in subculture
flasks were harvested by trypsinization and then counted manually
using trypan blue (0.4%) staining to detect the live cells. HBECs
were seeded onto coverslips that had been placed in 24-well culture
plates (Thermo Fisher Scientific, Inc.) at 1×105
cells/ml (1 ml per well). Subsequently, the cells were cultured at
37°C and 5% CO2 for 48 h in DMEM medium supplemented
with 15% newborn calf serum to allow the cells to adhere to the
coverslip. After 48 h, the medium was replaced with DMEM
supplemented with 1% newborn calf serum (Gibco; Thermo Fisher
Scientific, Inc.).
To establish the O3 stimulated HBECs
group, HBECs were prepared as above and were incubated in DMEM
supplemented with 1% newborn calf serum for 8 h. Subsequently,
HBECs were stimulated with O3 (2 ppm) for 30 min and
incubated for 1 h prior to co-culture with HLFs. For the control
(HBECs) group, the HBECs followed the same time course and
procedure, with the exception that the HBECs were not exposed to
O3 for 30 min.
HLFs were also harvested and counted as described
above for HBECs. HLFs were seeded into the 24-well plates at
2×104 cells/ml (1 ml per well). HLFs were cultured in
DMEM supplemented with 15% newborn calf serum for 12 h to allow the
cells to adhere to the culture dish. The medium was replaced with
DMEM supplemented with 1% newborn calf serum and cells were
incubated for a further 12 h. Subsequently, a sterile stainless
steel screen mesh was inserted into each well containing the HLFs
and a coverslip with HBECs that had been stimulated or not
simulated with O3 was placed on top of the stainless
steel screen mesh that provided separation between the HBECs and
HLFs in the same well. HBECs and HLFs were co-cultured in the same
well for 24 h. following this, the coverslip and the mesh were
removed from the well, respectively, and the proliferation of HLFs
was assessed by MTT colorimetric method.
MTT colorimetry for evaluation of cell
proliferation
The proliferation of HLFs was evaluated using the
MTT colorimetric method. HLFs were divided into the following three
groups: Fibro group, which contained HLFs that had been cultured
alone; co-culture group, which contained HLFs co-cultured with
HBECs without O3 stimulation; and O3
co-culture group, which contained HLFs co-cultured with
O3-stimulated HBECs. Cells were seeded at
2×104 cells/ml (1 ml/well) in 24-well plates and treated
with 100 µl of 0.5% MTT solution. Cells were incubated at 37°C for
4 h and the supernatant was removed. A total of 800 µl dimethyl
sulfoxide was added to each well followed by 10 min steady
oscillation of the 24-well plates. The cell suspension was
aliquoted into 96-well plates and was analyzed using an automatic
microplate reader (Elx800, Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 570 nm. Culture medium without cells served as a blank
control. Each experiment was repeated six times.
The proliferation of HBECs in the O3
stimulated and control groups was assessed as described above. The
proliferation of HBECs was measured at 1, 12 and 24 h. Each
measurement was repeated four times.
Assessment of HLF collagen
synthesis
Collagen synthesis in HLFs was determined by
assessing the hydroxyproline levels in HLF culture medium
supernatant using a hydroxyproline kit (cat. no. A030-1; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) according to
the manufacturer's instructions. Hydroxyproline is a major
component of collagen (18).
Therefore, measurement of hydroxyproline levels in cell culture
medium indicates the ability of the cell to produce collagen
(18–21). To Pleameasure the hydroxyproline
levels in the culture medium supernatant, 0.5 ml of culture medium
was combined with 1 ml hydrolysate and hydrolyzed for 20 min in a
boiling water bath (pH 6.0–6.8). A blank tube with distilled water,
a standard tube with 5 µg/ml standard solution and a measurement
tube with the hydrolyzed supernatant of culture medium were
prepared, respectively. Subsequently, a 1-ml sample was taken from
each tube and mixed with reagent One. Following 10 min standing at
room temperature, each sample was mixed with reagent Two and left
to stand for 5 min at room temperature. Following this, the sample
was mixed with reagent Three, incubated at 60°C for 15 min, cooled
and centrifuged at 4°C, 2,054 × g for 10 min. The absorbance of the
supernatant was measured at 550 nm. Each tube was repeatedly
measured four times. Total protein was assayed using the Lowry
protein assay (22). Hydroxyproline
levels were calculated as follows: Hydroxyproline (µg/mg
protein)=[optical density (OD) of test tube/OD of standard sample
tube]x(concentration of standard sample/total protein).
Detection of TGF-β1, TNF-α and PGE2
concentration in the supernatant of the co-cultured system and
HBECs cultured alone
The concentration of inflammatory mediators TGF-β1
and TNF-α in the supernatant of O3-stimulated HBECs
co-cultured with HLFs was assessed at 6, 12, 18 and 24 h following
co-culture using ELISA kits (cat. no. MM-0090H1 and MM-0122H1,
respectively; Jingmei Bioengineering Co., Ltd., Yancheng, China)
according to the manufacturer's protocol. The OD value measured at
450 nm was directly proportional to the concentration of the
indicator. By constructing a standard curve, the concentration of
TGF-β1 and TNF-α in the specimen could be determined accordingly.
The concentration of the inflammatory mediator PGE2 in the
supernatant of the co-cultured system was measured using a PGE2
radioimmunoassay kit (cat. no. ADI-905-025-1; Enzo Life Sciences,
Inc., Farmingdale, NY, USA) according to the manufacturer's
protocol. The concentration of PGE2 in the sample was directly
calculated using an automatic γ immuno-counter. The TGF-β1, TNF-α
and PGE2 concentration was detected in the supernatant of
O3-stimulated HBECs and HBECs cultured alone at 24 h as
described above.
Detection of TGF-β1 protein expression
levels in HBECs alone or co-cultured with HLFs
The protein expression levels of TGF-β1 in HBECs
alone or co-cultured with HLFs were detected using an
immunocytochemistry method. Cells were labeled for TGF-β1
expression using an immunocytochemistry staining kit (cat. no.
SA1022; Wuhan Boster Biological Technology Ltd., Wuhan, China)
according to the manufacturer's instructions. Cells were fixed with
95% alcohol at room temperature for 15 min and dipped in 30%
H2O2 combined with 100% methanol (1:50 ratio)
at room temperature for 30 min to block endogenous peroxidase
activity. Cells were immersed in 5% bovine serum albumin blocking
reagent at room temperature for 20 min. Following this, cells were
treated with TGF-β1 antibodies (1:1,000; cat. no. BA0290) at 37°C
for 1–2 h and biotinylated goat anti-rabbit IgG antibodies (1:500;
cat. no. BA1003; both Wuhan Boster Biological Technology Ltd.) at
room temperature for 20 min. Reagent SABC (Wuhan Boster Biological
Technology Ltd.) was added at room temperature for 20 min. Cells
were subsequently counterstained with hematoxylin at room
temperature for 30 sec, dehydrated and observed under an optic
microscope. For quantification of TGF-β1 expression in HBECs,
images were captured of five randomly selected fields of view and
analyzed using a medical image analysis and management system (MIAS
version 4.1; Image Processing Center of Beihang University,
Beijing, China) to assess the photodensity.
Statistical analysis
Data was presented as the mean ± standard deviation
from the representative experiment. Each experiment was repeated
four to six times. All data were processed using SPSS 21.0 software
(IBM Corp., Armonk, NY, USA). An unpaired Student's t-test was
performed to determine the statistical difference between two
groups and multiple comparisons were determined using one-way
analysis of variance followed by Dunnett's t-test. Correlations
were analyzed using Pearson's correlation analysis method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
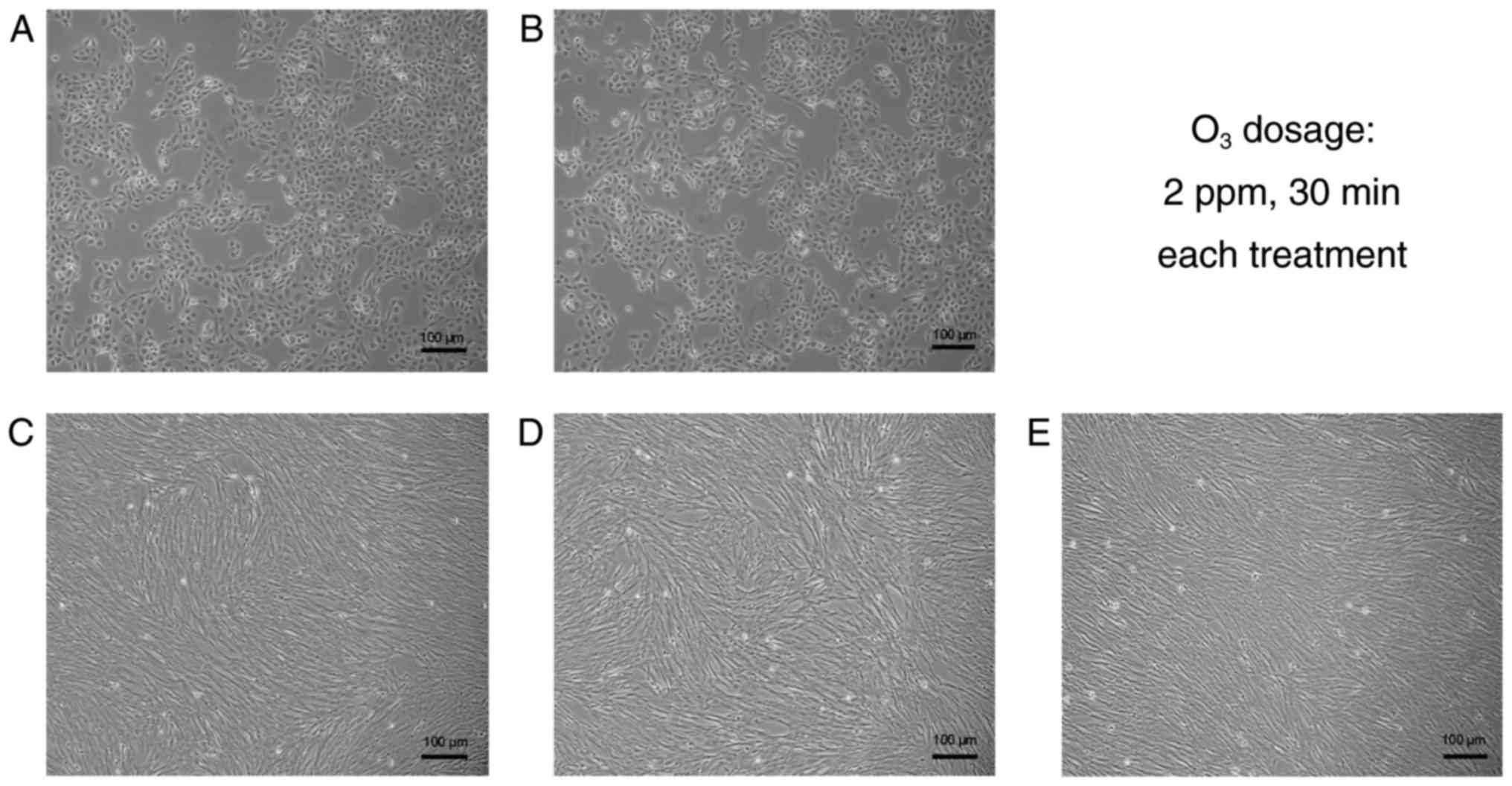
Effect of O3 stimulation on
cell morphology and proliferation
The morphology of HBECs and HLFs cultured alone or
co-cultured with or without O3 stimulation was
determined (Fig. 1). Results
indicated the cells in culture demonstrated a normal shape and size
individually (HBECs were cuboidal-like shape and HLFs were
spindle-like shape) and were organized in usual structures
collectively (HBECs were in a relatively uniform monolayer and HLFs
were in a hill-valley-like formation), respectively. When the
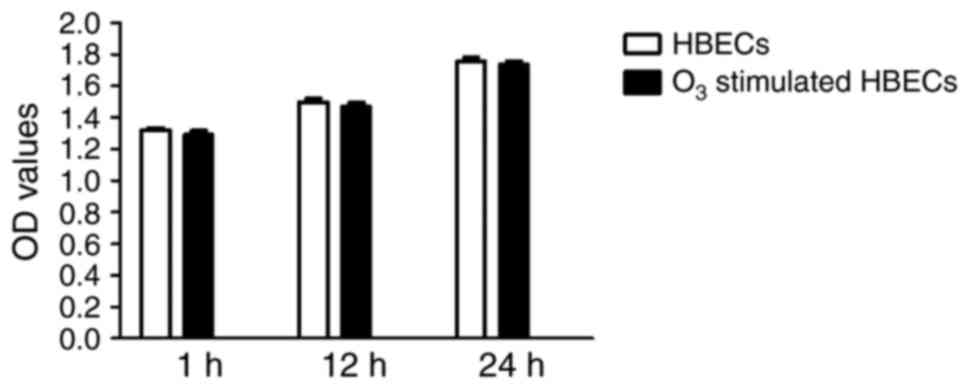
proliferation of HBECs was evaluated using the MTT method, the OD
values were not significantly different between
O3-stimulated HBECs and their non-stimulated
counterparts when cultured for up to 24 h (P>0.05; Fig. 2). These results indicated that
O3 stimulation had no marked effect on cell morphology
regardless of the cell type and culture approach; furthermore,
there was no significant effect on the cell proliferation of
HBECs.
Co-culture with
O3-stimulated HBECs enhances cell proliferation of
HLFs
To investigate the cell proliferation of Fibro,
Co-culture and O3 co-culture groups, the MTT assay was
performed (Fig. 3). In comparison
with the Fibro group, proliferation was significantly decreased in
the Co-culture group (0.252±0.018 vs. 0.241±0.017, respectively;
P<0.05), which indicated that co-culture conditions without
O3 stimulation inhibited the proliferation of HLFs.
However, cell proliferation of the O3 co-culture group
was significantly increased compared with the Fibro group
(0.263±0.017 vs. 0.252±0.018, respectively; P<0.05) and
Co-culture group (0.263±0.017 vs. 0.241±0.017, respectively;
P<0.01), respectively. These findings suggested that co-culture
with O3-stimulated HBECs significantly enhanced the
proliferation of HLFs when compared with HLFs cultured alone.
Furthermore, co-culture with O3-stimulated HBECs
significantly reversed the inhibition of HLF proliferation when
co-cultured with HBECs that were not stimulated with
O3.
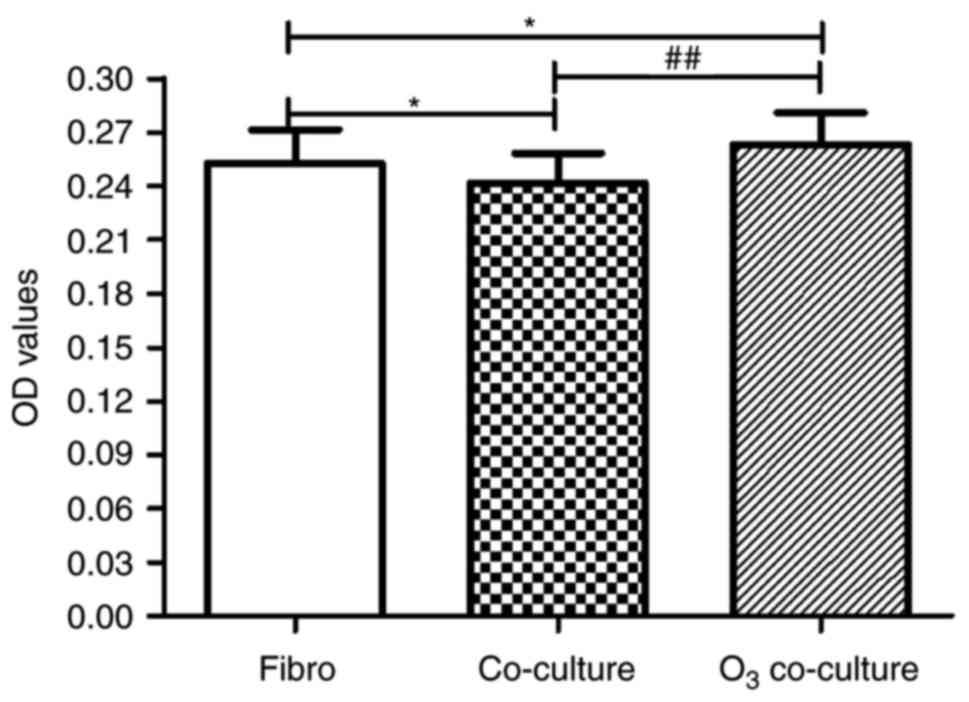 | Figure 3.Effect of O3-stressed
HBECs on HLF proliferation. HLF proliferation was assessed using
the MTT method. Compared with the Fibro group, the proliferation of
Co-culture group was significantly inhibited. However, in the
O3 co-culture group, the proliferation was significantly
increased compared with the Fibro group and Co-culture group. Data
are presented as the mean ± standard deviation (n=18). *P<0.05,
##P<0.01 as indicated. HBECs, human bronchial
epithelial cells; HLFs, human lung fibroblasts; O3,
ozone; OD, optical density; Fibro group, HLFs cultured alone;
Co-culture group, HLFs co-cultured with HBECs without
O3-stimulation; O3 co-culture group, HLFs
were co-cultured with O3-stimulated HBECs. |
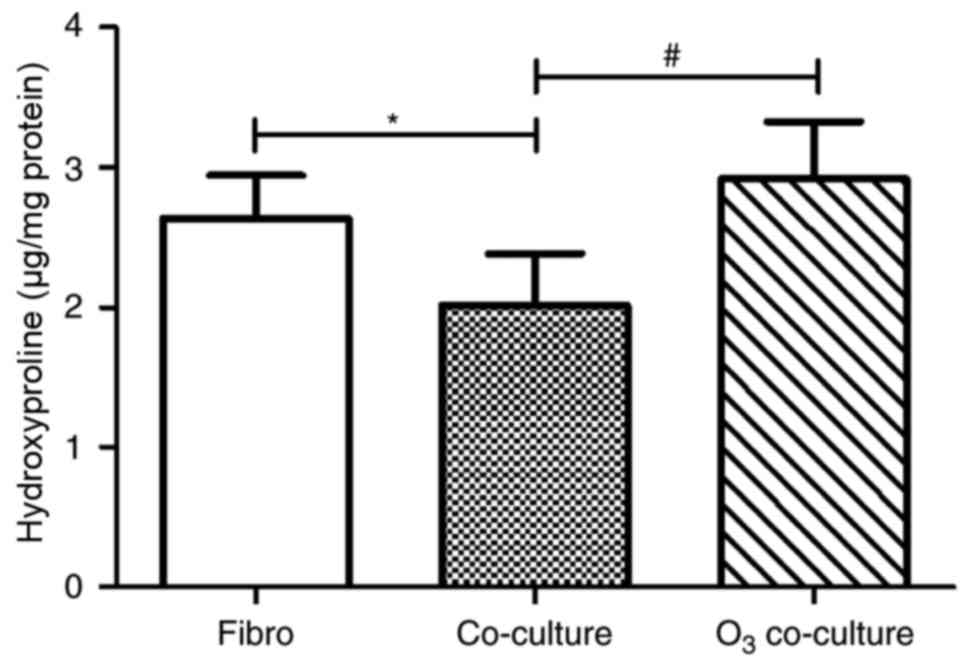
Co-culture with
O3-stimulated HBECs enhances collagen synthesis of
HLFs
As indicated in Fig.
4, the effects on collagen synthesis of HLFs co-cultured with
O3-stimulated HBECs were similar to those indicated for
cell proliferation. Notably, the Co-culture group exhibited
significantly decreased collagen synthesis-associated
hydroxyproline levels compared with the Fibro group (2.014±0.366
vs. 2.634±0.310, respectively; P<0.05), which indicated that
co-culture conditions without O3 stimulation reduced the
collagen synthesis capacity of HLFs. However, the levels of
hydroxyproline from the O3 co-culture group were
significantly increased compared with the Co-culture group
(2.919±0.407 vs. 2.014±0.366, respectively; P<0.05). These
results also suggested that co-culture with
O3-stimulated HBECs may reverse the inhibition of HLF
collagen synthesis due to co-culture with HBECs that were not
stimulated with O3.
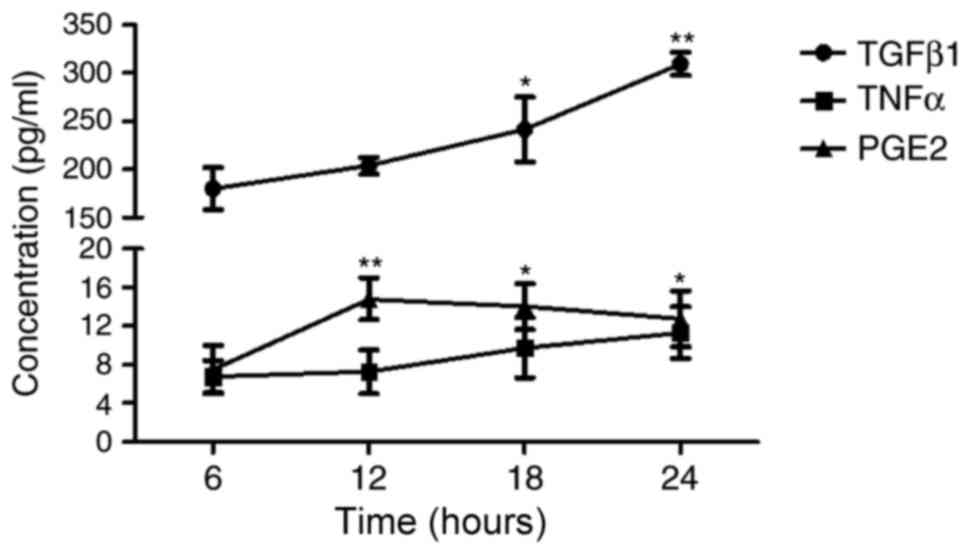
Co-culture with
O3-stimulated HBECs influences cytokine secretion in the
supernatant of the co-culture system
The concentration of major cytokines, including
TGF-β1, TNF-α and PGE2, in the supernatant of the co-culture system
with O3-stimulated HBECs were assessed (Fig. 5). Results indicated that the
concentration of TGF-β1 remained high and continuously increased
over the 24 h after co-culture. TNF-α concentration remained low
and did not significantly vary over the 24-h time period. Unlike
TGF-β1, the concentration of PGE2 was significantly increased to
the maximum at 12 h compared with 6 h (P<0.01) and plateaued
thereafter. PGE2 concentration was significantly increased at 18
and 24 h compared with 6 h (P<0.05), but no significant
difference was identified when compared with 12 h.
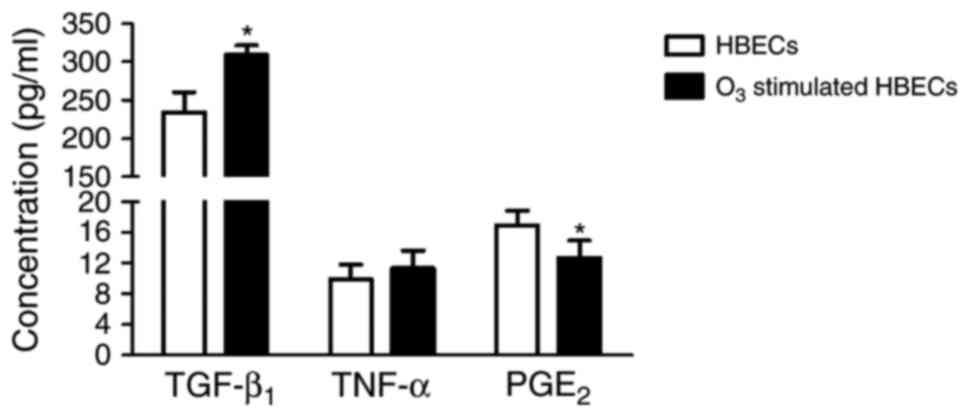
When compared with HBECs alone, the
O3-stimulated HBECs cultured for 24 h exhibited
significantly increased TGF-β1 concentration (234.80±25.55 vs.
309.47±12.22 pg/ml, respectively; P<0.05; Fig. 6). However, the concentration of PGE2
was significantly decreased in O3-stimulated HBECs
compared with HBECs alone (12.74±2.19 vs. 16.97±1.92 pg/ml,
respectively; P<0.05) and no significant difference was
indicated in the concentration of TNF-α between the experimental
groups (P>0.05).
Correlation between cytokine secretion
and cell proliferation and collagen synthesis in HLFs
In order to investigate whether cell proliferation
or collagen synthesis was associated with the secretion of
cytokines when HLFs were co-cultured with O3-stimulated
HBECs, correlation analysis between HLF proliferation and
hydroxyproline levels and cytokine secretion was performed
(Fig. 7). Results indicated a
significant positive correlation between the secretion of TGF-β1
from the co-cultured system with O3-stimulated HBECs and
cell proliferation (r=0.772, P=0.015) and collagen synthesis
(r=0.758, P=0.018, Fig. 7A and B).
Notably, no correlation was indicated between the cytokine
secretion of TNF-α with cell proliferation or collagen synthesis of
HLFs when co-cultured with O3-stimulated HBECs (Fig. 7C and D). However, a significant
negative correlation was indicated between the cytokine secretion
of PGE2 and cell proliferation (r=−0.783, P=0.013) and collagen
synthesis (r=−0.817, P=0.007) of HLFs co-cultured with
O3-stimulated HBECs.
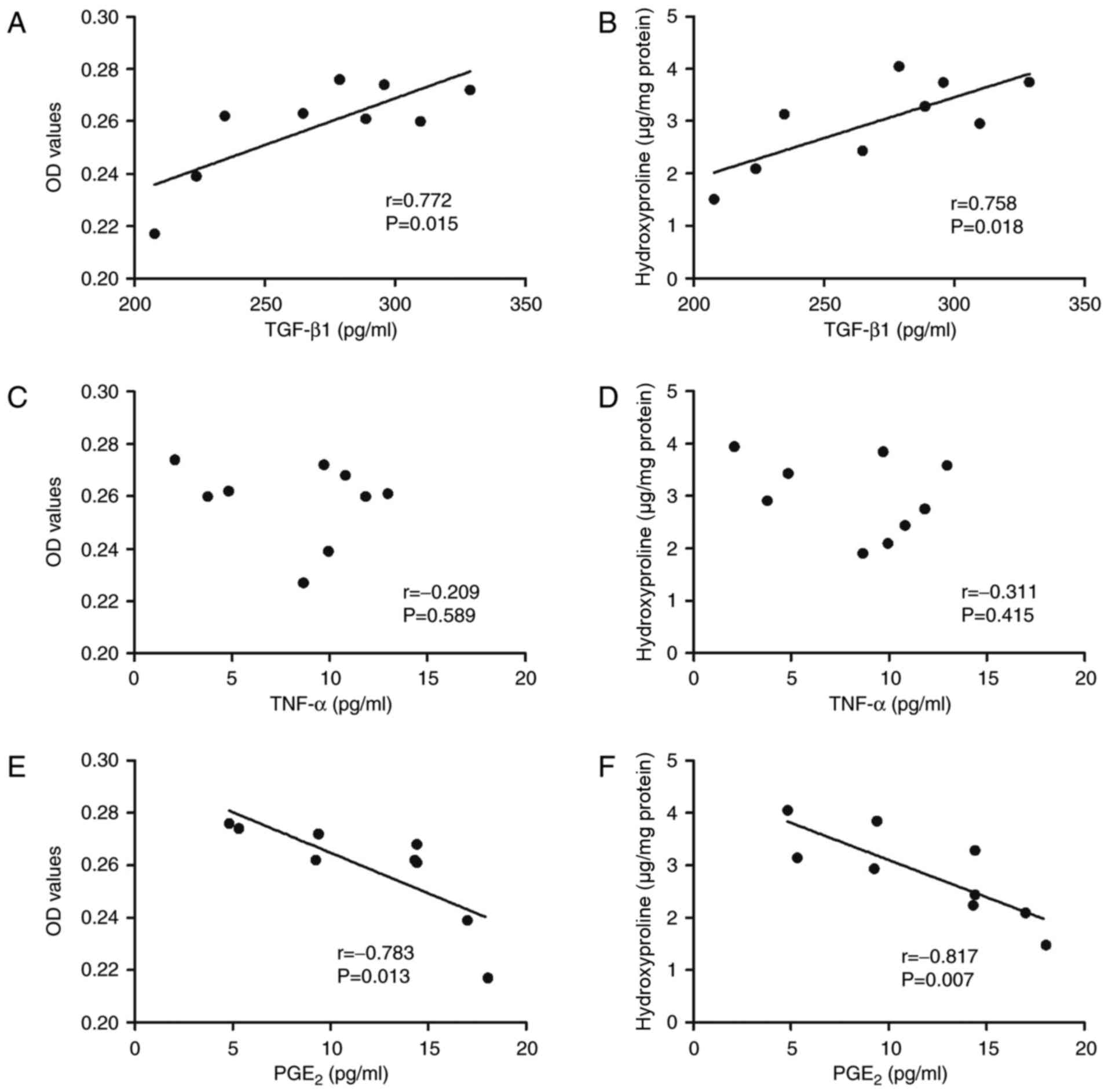 | Figure 7.Correlation between cytokine
concentration and cell proliferation and collagen synthesis in HLFs
co-cultured with O3-stressed HBECs. (A and B) A positive
linear correlation between proliferation of HLFs and the
concentration of TGF-β1 in the co-culture supernatant. Collagen
synthesis capacity in the culture supernatant was also positively
correlated with TGF-β1 concentration (r=0.758, P=0.018). (C and D)
There was no correlation between the proliferation and TNF-α
concentration (r=0.209, P=0.589), nor between collagen synthesis
and TNF-α concentration (r=0.311, P=0.415). (E and F) A negative
linear correlation between the proliferation and PGE2 concentration
of HLFs (r=0.783, P=0.013) and between collagen synthesis and PGE2
concentration (r=0.817, P=0.007) was indicated. TGF-β1,
transforming growth factor-β1; TNF-α, tumor necrosis factor-α;
PGE2, prostaglandin E2; O3, ozone; HBECs, human
bronchial epithelial cells; HLFs, human lung fibroblasts; OD,
optical density. |
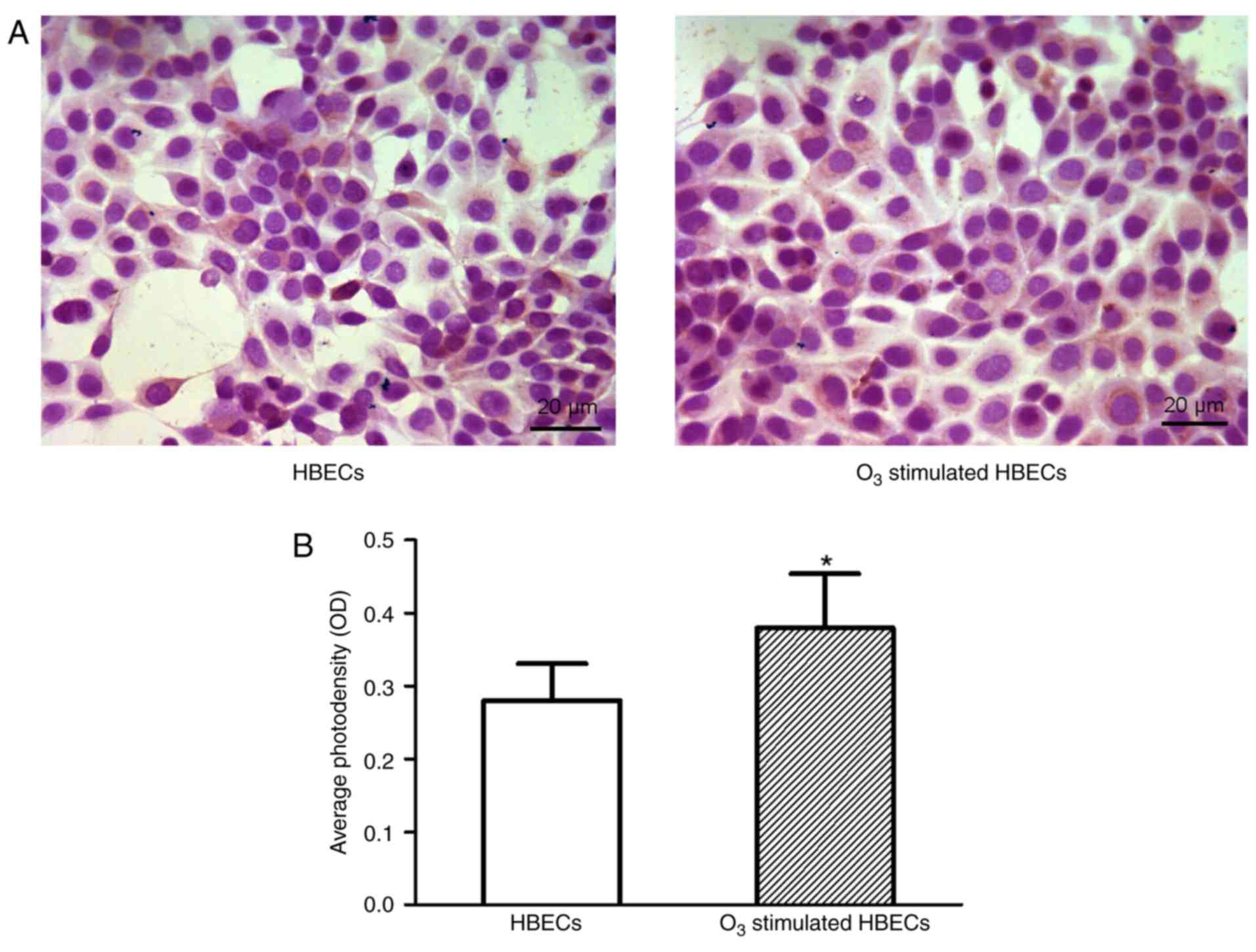
Effect of O3 stimulation on
TGF-β1 expression in HBECs
Immunocytochemistry was conducted to investigate
TGF-β1 expression levels in HBECs and quantitative expression
analysis was performed (Fig. 8).
Results indicated that the expression level of TGF-β1 in
O3-stimulated HBECs cultured for 24 h was increased
compared with HBECs alone (Fig. 8A).
This difference was statistically significant (P<0.05; Fig. 8B). In the co-culture system of HBECs
and HLFs, the expression level of TGF-β1 was similarly enhanced in
the presence of O3-stimulated HBECs (data not
shown).
Discussion
Damage to the bronchial epithelium destroys the
integrity of airway structure and function, leading to instability
of the local microenvironment and dysfunction of the airways. These
are initiative events of multiple airway diseases, including asthma
and bronchitis (23). Repeated
airway injury and abnormal repair may cause chronic airway
inflammation, which eventually results in airway remodeling, as
characterized by the activation and proliferation of fibroblasts
and accumulation of the ECM (24).
As one of the major structural cells of the airway submucosa, lung
fibroblasts are known to contribute to the formation of
subepithelial fibrosis, increase airway responsiveness and promote
airway remodeling (25–27). Furthermore, lung fibroblasts have
been acknowledged as the primary cells that produce ECM proteins to
form collagen fibers, elastic fibers and reticular fibers, which
are downstream components of the airway remodeling process
(28). Although the function of lung
fibroblasts has been extensively studied, it is not clear whether
stress induced on the bronchial epithelium from the external
environment, including air pollutants, may influence the submucosal
lung fibroblasts to contribute to pathological airway remodeling. A
useful model to explore this effect is the use of O3 to
stimulate the bronchial epithelium prior to examination of the
cellular functions of adjacent fibroblasts not in direct contact of
O3 (29). O3
is a common major component of air pollution and has been revealed
in various studies to cause airway epithelial shedding, airway
inflammation and airway hyperresponsiveness (16,30).
Therefore, the aim of the present study was to co-culture HLFs with
airway epithelial cells pre-stimulated with O3 and
subsequently measure the functional changes, including collagen
synthesis, of the fibroblasts. Results of the present study
indicated that, although O3 stimulation seemed to cause
no noticeable changes in cell morphology or proliferation of HBECs
cultured for up to 24 h, functional changes in the co-culture
system of O3-stimulated HBECs and HLFs were indicated.
Specifically, in the co-culture group without O3
stimulation, the proliferation of HLFs was significantly inhibited.
However, co-culture of HLFs with O3-stressed HBECs
resulted in significantly enhanced HLF proliferation, indicating
that O3 stimulation reversed co-culture-induced
inhibition of cell proliferation. O3 stimulation on
HBECs had a similar effect on the collagen synthesis capacity of
the HLFs in the in vitro co-culture model. These results
suggest that O3 stress on the airway epithelium may be
associated with the pathogenesis of airway remodeling via its
indirect effect on the submucosal structural cells, including the
HLFs.
The bronchial epithelium is an important source of
complex inflammatory mediators, including cytokines and chemokines
(31). By secreting inflammatory
mediators, HBECs may interact with interstitial fibroblasts and
mediate the process of epithelial-mesenchymal transition or
fibroblast-myofibroblast transition, therefore actively
participating in asthmatic airway remodeling (32–34). In
the present study, O3 exposure upregulated the
concentration of TGF-β1 in the supernatant of the co-culture system
persistently for up to 24 h. Furthermore, O3 stress also
promoted TGF-β1 secretion in the HBEC supernatant and TGF-β1
expression in HBECs labeled by immunocytochemistry. In addition,
correlation analysis suggested that O3-induced
upregulation of TGF-β1 secretion was positively and linearly
correlated with cell proliferation and collagen synthesis of HLFs
co-cultured with O3-stimulated HBECs. These results
indicated that the increased TGF-β1 content may serve a key role in
the proliferation and collagen synthesis of HLFs in the co-culture
system. Furthermore, it is acknowledged that TGF-β1 may promote
differentiation of fibroblasts into myofibroblasts, which secrete
collagen and growth factors, including endothelin 1 and vascular
endothelial growth factor, and then further promote proliferation
of smooth muscle cells and vascular endothelial cells (35,36).
Notably, it has also been reported that TGF-β1 induces mesenchymal
transition of bronchial epithelial cells and thus promotes airway
remodeling in asthma (37).
Additionally, damaged epithelial cells may extend and amplify
remodeling signals into a deeper layer of the mucous membrane
(38). Such communication between
epithelial cells and mesenchymal cells during asthma has been
termed as epithelial mesenchymal trophic unit reactivation
(38).
The present study indicated a low concentration of
TNF-α was identified in the supernatant of the co-culture system
with or without O3 stimulation. TNF-α content was also
not significantly correlated with HLF proliferation and collagen
synthesis. However, the present study demonstrated that the
synthesis of PGE2 in the co-culture system with
O3-stressed HBECs was significantly increased during the
early stage and plateaued from 12–24 h. However, the secretion of
PGE2 in O3-stimulated HBECs was significantly reduced
compared with HBECs alone. In addition, correlation analysis
indicated a negative linear correlation of PGE2 content with HLF
proliferation and collagen synthesis. Therefore, the present
findings suggested the regulatory effect of O3-stressed
HBECs on HLF activity was associated with reduced PGE2 content in
the airway tissues. Notably, PGE2 is a lipid mediator that may be
derived from cell membrane phospholipids via the cyclooxygenase
signaling pathway of arachidonic acid metabolism (39). PGE2 released from HBECs has been
reported to relax airway smooth muscles, reduce the sensitivity of
smooth muscles to contractile medium, inhibit the proliferation of
smooth muscle cells and fibroblast activity and enhance the
tolerance of lung epithelial cells to injury factors (23). These findings suggest that PGE2 may
act as a negative feedback factor to counteract
O3-induced proliferation and airway remodeling
processes.
In the present study, the fact that the HBECs
without O3 stimulation in the co-culture system
inhibited the proliferation of HLFs is conducive to maintaining the
normal structure and function of the bronchial airways. However,
co-culture with O3-stimulated HBECs with HLFs resulted
in increased cell proliferation and collagen synthesis of the HLFs
and increased secretion of TGF-β1. Considering the pro- and
anti-inflammatory properties of TGF-β1 and PGE2 respectively, the
present findings suggest that the possible mechanism involved in
the effect of O3 stimulation on the co-culture system
may lie in the system's ability to maintain balance between pro-
and anti-inflammatory factors. Under physiological conditions, the
expression of anti-inflammatory factors, including PGE2, may
prevail in the system to inhibit the proliferation and collagen
synthesis of fibroblasts via PGE2 receptor EP2 subtype binding and
cAMP production, therefore maintaining the airway homeostasis
(40,41). However, under a stressed state, such
as with O3 stimulation, the expression of
pro-inflammatory factors, including TGF-β1, may be increased in the
system and result in enhanced cell proliferation and collagen
synthesis of fibroblasts, which may primarily occur through the
regulation of p21/Waf1 gene transcription factors and the cyclin
E-associated kinase (42). This may
ultimately result to chronic and permanent airway remodeling in
vivo.
In conclusion, the present findings suggest that
O3 stimulation of HBECs is a likely factor in promoting
cell proliferation and collagen synthesis activity of HLFs via the
regulation of pro- and/or anti-inflammatory cytokines, including
TGF-β1 and PGE2. Such cellular and molecular functional
associations between HBECs and HLFs in response to O3
stimulation may provide an important connection between air
pollution and airway remodeling in diseases such as asthma.
Acknowledgements
The authors would like to thank Professor Xiaoqun
Qin of Central South University (Changsha, China) for his valuable
advice to the data analysis and manuscript drafting.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 11532003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW, HO and LD designed the study. YW and MT
performed the experiments. YW, HO and LD wrote the manuscript.
Ethics approval and consent to
participate
All experiments using in vitro cultured human
bronchial epithelial cells and lung fibroblasts were approved by
the Medical Ethics Committee of Changzhou University (Changzhou,
China) according to Ethics Examination Methods on Human Related
Biomedical Research issued by China's Health Ministry (approval
ref. no. CCZUBME20150516YW).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Busse WW: Inflammation in asthma: The
cornerstone of the disease and target of therapy. J Allergy Clin
Immunol. 102:S17–S22. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boulet LP: Airway remodeling in asthma:
Update on mechanisms and therapeutic approaches. Curr Opin Pulm
Med. 24:56–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dekkers BG, Maarsingh H, Meurs H and
Gosens R: Airway structural components drive airway smooth muscle
remodeling in asthma. Proc Am Thorac Soc. 6:683–692. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burgess JK, Ceresa C, Johnson SR, Kanabar
V, Moir LM, Nguyen TT, Oliver BG, Schuliga M and Ward J: Tissue and
matrix influences on airway smooth muscle function. Pulm Pharmacol
Ther. 22:379–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogel ER, Britt RD Jr, Faksh A, Kuipers I,
Pandya H, Prakash YS, Martin RJ and Pabelick CM: Moderate hyperoxia
induces extracellular matrix remodeling by human fetal airway
smooth muscle cells. Pediatr Res. 81:376–383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
To T, Stanojevic S, Moores G, Gershon AS,
Bateman ED, Cruz AA and Boulet LP: Global asthma prevalence in
adults: Findings from the cross-sectional world health survey. BMC
Public Health. 12:2042012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turner MC, Jerrett M, Pope CA III, Krewski
D, Gapstur SM, Diver WR, Beckerman BS, Marshall JD, Su J, Crouse DL
and Burnett RT: Long-term ozone exposure and mortality in a large
prospective study. Am J Respir Crit Care Med. 193:1134–1142. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berman JD, Fann N, Hollingsworth JW,
Pinkerton KE, Rom WN, Szema AM, Breysse PN, White RH and Curriero
FC: Health benefits from large-scale ozone reduction in the United
States. Environ Health Perspect. 120:1404–1410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller DB, Snow SJ, Henriquez A,
Schladweiler MC, Ledbetter AD, Richards JE, Andrews DL and
Kodavanti UP: Systemic metabolic derangement, pulmonary effects,
and insulin insufficiency following subchronicozone exposure in
rats. Toxicol Appl Pharmacol. 306:47–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Avdalovic MV, Tyler NK, Putney L, Nishio
SJ, Quesenberry S, Singh PJ, Miller LA, Schelegle ES, Plopper CG,
Vu T and Hyde DM: Ozone exposure during the early postnatal period
alters the timing and pattern of alveolar growth and development in
nonhuman primates. Anat Rec (Hoboken). 295:1707–1716. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carey SA, Ballinger CA, Plopper CG,
McDonald RJ, Bartolucci AA, Postlethwait EM and Harkema JR:
Persistent rhinitis and epithelial remodeling induced by cyclic
ozone exposure in the nasal airways of infant monkeys. Am J Physiol
Lung Cell Mol Physiol. 300:L242–L254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wiegman CH, Michaeloudes C, Haji G, Narang
P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J,
et al: Oxidative stress-induced mitochondrial dysfunction drives
inflammation and airway smooth muscle remodeling in patients with
chronic obstructive pulmonary disease. J Allergy Clin Immunol.
136:769–780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin XQ, Xiang Y, Luo ZQ, Zhang CQ and Sun
XH: Fibronectin or RGD peptide promotes nitric oxide synthesis of
rabbit bronchial epithelial cells. Sheng Li Xue Bao. 52:519–521.
2000.(In Chinese). PubMed/NCBI
|
|
14
|
Ren YH, Qin XQ, Guan CX, Luo ZQ, Zhang CQ
and Sun XH: Temporal and spatial distribution of VIP, CGRP and
their receptors in the development of airway hyperresponsiveness in
the lungs. Sheng Li Xue Bao. 56:137–146. 2004.PubMed/NCBI
|
|
15
|
Tan YR, Qi MM, Qin XQ, Xiang Y, Li X, Wang
Y, Qu F, Liu HJ and Zhang JS: Wound repair and proliferation of
bronchial epithelial cells enhanced by bombesin receptor subtype 3
activation. Peptides. 27:1852–1858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leroy P, Tham A, Wong H, Tenney R, Chen C,
Stiner R, Balmes JR, Paquet AC and Arjomandi M: Inflammatory and
repair pathways induced in human bronchoalveolar lavage cells with
ozone inhalation. PLoS One. 10:e01272832015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gruenert DC, Finkbeiner WE and Widdicombe
JH: Culture and transformation of human airway epithelial cells. Am
J Physiol. 268:L347–L360. 1995.PubMed/NCBI
|
|
18
|
Chiariello M, Ambrosio G, Cappelli-Bigazzi
M, Perrone-Filardi P, Brigante F and Sifola C: A biochemical method
for the quantitation of myocardial scarring after experimental
coronary artery occlusion. J Mol Cell Cardiol. 18:283–290. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woessner JF Jr: The determination of
hydroxyproline in tissue and protein samples containing small
proportions of this amino acid. Arch Biochem Biophys. 93:440–447.
1961. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laato M, Kähäri VM, Niinikoski J and
Vuorio E: Epidermal growth factor increases collagen production in
granulation tissue by stimulation of fibroblast proliferation and
not by activation of procollagen genes. Biochem J. 247:385–388.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rhaleb NE, Peng H, Harding P, Tayeh M,
LaPointe MC and Carretero OA: Effect of
N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis
in rat cardiac fibroblasts. Hypertension. 37:827–832. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Upreti GC, Wang Y, Finn A, Sharrock A,
Feisst N, Davy M and Jordan RB: U-2012: An improved Lowry protein
assay, insensitive to sample color, offering reagent stability and
enhanced sensitivity. Biotechniques. 52:159–166. 2012.PubMed/NCBI
|
|
23
|
Hirota N and Martin JG: Mechanisms of
airway remodeling. Chest. 144:1026–1032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fehrenbach H, Wagner C and Wegmann M:
Airway remodeling in asthma: What really matters. Cell Tissue Res.
367:551–569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roche WR: Fibroblasts and asthma. Clin Exp
Allergy. 21:545–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sarna M, Wojcik KA, Hermanowicz P, Wnuk D,
Burda K, Sanak M, Czyż J and Michalik M: Undifferentiated bronchial
fibroblasts derived from asthmatic patients display higher elastic
modulus than their non-asthmatic counterparts. PLoS One.
10:e01168402015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ball SL, Mann DA, Wilson JA and Fisher AJ:
The role of the fibroblast in inflammatory upper airway conditions.
Am J Pathol. 186:225–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al-Muhsen S, Johnson JR and Hamid Q:
Remodeling in asthma. J Allergy Clin Immunol. 128:451–462. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang CQ, Tan YR and Qin XQ: Stimulation
of ozone stress on the adhesion of inflammatory cells to bronchial
epithelial cells. Hunan Yi Ke Da Xue Xue Bao. 27:192–194. 2002.(In
Chinese). PubMed/NCBI
|
|
30
|
Lambert JA and Song W: Ozone-induced
airway hyperresponsiveness: Roles of ROCK isoforms. Am J Physiol
Lung Cell Mol Physiol. 309:L1394–L1397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Polito AJ and Proud D: Epithelia cells as
regulators of airway inflammation. J Allergy Clin Immunol.
102:714–718. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Minor DM and Proud D: Role of human
rhinovirus in triggering human airway epithelial-mesenchymal
transition. Respir Res. 18:1102017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pain M, Bermudez O, Lacoste P, Royer PJ,
Botturi K, Tissot A, Brouard S, Eickelberg O and Magnan A: Tissue
remodeling in chronic bronchial diseases: From the epithelial to
mesenchymal phenotype. Eur Respir Rev. 23:118–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reeves SR, Kolstad T, Lien TY,
Herrington-Shaner S and Debley JS: Fibroblast-myofibroblast
transition is differentially regulated by bronchial epithelial
cells from asthmatic children. Respir Res. 16:212015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ojiaku CA, Yoo EJ and Panettieri RA Jr:
Transforming growth factor β1 function in airway remodeling and
hyperresponsiveness. The missing link? J Respir Cell Mol Biol.
56:432–442. 2017. View Article : Google Scholar
|
|
36
|
Yang YC, Zhang N, Van Crombruggen K, Hu
GH, Hong SL and Bachert C: Transforming growth factor-beta1 in
inflammatory airway disease: A key for understanding inflammation
and remodeling. Allergy. 67:1193–1202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang ZC, Yi MJ, Ran N, Wang C, Fu P, Feng
XY, Xu L and Qu ZH: Transforming growth factor-β1 induces bronchial
epithelial cells to mesenchymal transition by activating the Snail
pathway and promotes airway remodeling in asthma. Mol Med Rep.
8:1663–1668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Holgate ST, Davies DE, Puddicombe S,
Richter A, Lackie P, Lordan J and Howarth P: Mechanisms of airway
epithelial damage: Epithelial-mesenchymal interactions in the
pathogenesis of asthma. Eur Respir J. 22 Suppl 44:S24–S29. 2003.
View Article : Google Scholar
|
|
39
|
Wang J, Liu M, Zhang X, Yang G and Chen L:
Physiological and pathophysiological implications of PGE2 and the
PGE2 synthases in the kidney. Prostaglandins Other Lipid Mediat.
134:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang S, Wettlaufer SH, Hogaboam C,
Aronoff DM and Peters-Golden M: Prostaglandin E(2) inhibits
collagen expression and proliferation in patient-derived normal
lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am
J Physiol Lung Cell Mol Physiol. 292:L405–L413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Zhang M, Tan Y, Xiang Y, Liu H, Qu
F, Qin L and Qin X: BRS-3 activation transforms the effect of human
bronchial epithelial cells from PGE2 mediated inhibition to
TGF-beta1 dependent promotion on proliferation and collagen
synthesis of lung fibroblasts. Cell Biol Int. 31:1495–1500. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miyazaki M, Ohashi R, Tsuji T, Mihara K,
Gohda E and Namba M: Transforming growth factor-beta 1 stimulates
or inhibits cell growth via down- or up-regulation of p21/Waf1.
Biochem Biophys Res Commun. 246:873–80. 1998. View Article : Google Scholar : PubMed/NCBI
|