Introduction
Esophageal carcinoma is one of the most common
digestive tract-derived malignancies worldwide (1,2).
Esophageal carcinoma is comprised of squamous carcinoma and
adenocarcinoma (3). A high incidence
of esophageal squamous carcinoma (ESCC) has been reported in China
(4); in 2015, 429,200 cases of
cancer were reported in China, with 281,400 mortalities (5). Of these cases, 259,000 were ESCC,
making it the fifth most prevalent malignant tumor (6). Tumor recurrence and metastasis are
leading causes of mortality in patients with ESCC (7). It has previously been reported that
immunosuppression is associated with tumor recurrence and
metastasis (8,9), however the underlying mechanism remains
to be elucidated.
It is well established that immunosuppression is
associated with the recurrence and metastasis of multiple tumors
(10). The immune response of
infiltrating lymphocytes in tumor tissues is impaired by certain
components in the tumor microenvironment, which leads to tumor
recurrence and metastasis (11,12).
Furthermore, it has been reported that restoration of the immune
system may improve the survival of patients with ESCC (13). It has been demonstrated that various
anesthetic agents exert different but important effects on immune
cells in patients with tumors (14).
It may therefore be beneficial to select an anesthetic that
improves immunosuppression in patients with tumors.
Innate immunity is the body's first line of defense
against infection, tumors and virus invasion (15). Compromised innate immunity has been
reported in a number of tumor types and is associated with the
survival of patients with tumors, suggesting a crucial role of
innate immunity in the inhibition of tumors (16). Natural killer (NK) cells are an
important component of the innate immune system, they are typically
cluster of differentiation (CD)3−CD56+cells,
which and are primarily distributed in the peripheral blood, with
10–15% in lymphocytes (17,18). NK cells are responsible for immune
surveillance without antigen presentation and major
histocompatibility complex (MHC) restriction (19). Once activated, NK cells are able to
recognize target cells rapidly and release cytotoxic effector
molecules to trigger the immune response (20). The aim of the present study was to
evaluate the effects of propofol on NK cells derived from the
peripheral blood of Patients with ESCC. Propofol is one of the most
commonly used anesthetics and has been reported to exert analgesic
and anti-inflammatory effects (21).
Materials and methods
Patients and tissue sample
A total of 5 ml fresh peripheral blood was collected
from 15 patients with ESCC (age, 39–43 years; 10 male, 5 female)
prior to surgery and 15 healthy volunteers (age, 26–35 years; 11
male, 4 female) at the Affiliated Hospital of Southwest Medical
University (Luzhou, China) between January and May 2017. All
patients were diagnosed with primary ESCC and had not undergone
therapy. The exclusion criteria for patients with ESCC were as
follows: Previous chemotherapy or radiotherapy, chronic disease,
infectious disease or multi-primary cancer. Exclusion criteria for
the healthy patients included chronic, autoimmune or genetic
diseases. Patient clinical data is presented in Table I. The present study was approved by
the Ethics Committee of the Southwest Medical University and
informed consent was obtained from each patient.
 | Table I.Clinical data of the patients with
esophageal squamous cell carcinoma. |
Table I.
Clinical data of the patients with
esophageal squamous cell carcinoma.
| Index | Number of
patients |
|---|
| Sex |
|
|
Male | 3 |
|
Female | 12 |
| Age |
|
|
≤60 | 5 |
|
≥60 | 10 |
| Tumor size |
|
| ≤5
cm | 9 |
| ≥5
cm | 6 |
| Location |
|
|
Middle | 11 |
|
Lower | 4 |
|
Differentiation |
|
|
Well | 8 |
|
Moderate | 7 |
|
Poor | 0 |
| Invasion depth |
|
| Outer
layer | 2 |
| Inner
layer | 13 |
| Lymph node
metastases |
|
|
With | 7 |
|
Without | 8 |
Cell isolation
Fresh peripheral blood of patients with ESCC was
stored in anticoagulant tubes (BD Biosciences, Franklin Lakes, NJ,
USA) and used to isolate mononuclear lymphocytes and NK cells as
previously described (22). In
brief, mononuclear lymphocytes were isolated from 5 ml peripheral
blood using Ficoll separation medium (Tianjin Yanyang Biochemical
Co., Ltd., Tianjin, China). The cells were centrifuged at 2,000 × g
for 20 min at room temperature and the middle layer was mononuclear
lymphocytes used for the NK separation. NK cells were negatively
sorted using a human NK cell separation medium kit purchased from
BD Biosciences according to the manufacturer's protocol. NK cells
were negatively sorted using the MagniSort™ Human NK cell
Enrichment kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. Isolated NK cell
suspensions were then identified using flow cytometry analysis
characterized by CD3-CD56+.
Flow cytometry analysis
NK cells were incubated with the appropriate surface
antibodies for 15 min at room temperature. Cells were fixed with 4%
formaldehyde at room temperature for 10 min and subsequently
permeabilized for 40 min using Cytofix/Cytoperm (BD Biosciences)
according to the manufacturer's protocol. Permeabilized cells were
stained with antibodies against Ki67, granzyme B and perforin for
15 min at room temperature. For intracellular staining of
interferon (IFN)γ and tumor necrosis factor (TNF)α, NK cells were
stimulated for 4 h at 37°C in 5% CO2 with 50 ng/ml
phorbol myristate acetate and 1 mg/ml ionomycin in the presence of
Golgistop (BD Biosciences) prior to staining with fluorescence
labeling antibodies (Table II) for
15 min at room temperature. A flow cytometer was used for analysis
with FAC Suite version 1.0.3.2942 (BD Biosciences).
 | Table II.Antibodies used for flow
cytometry. |
Table II.
Antibodies used for flow
cytometry.
| Antibody | Company | Cat. no. | Conjugate | Dilution |
|---|
| NKG2D | BD Biosciences | 552364 | Percp-cy5.5 | 1:40 |
| NKp30 | BD Biosciences | 558407 | PE | 1:40 |
| NKp44 | BD Biosciences | 558563 | PE | 1:40 |
| NKG2A | R&D Systems,
Inc. | FAB1059C | Percp | 1:40 |
| CD3 | BD Biosciences | 555916 | Fluorescein
isothiocyanate | 1:40 |
| CD56 | BD Biosciences | 555518 |
Allophycocyanin | 1:40 |
| CD16 | BD Biosciences | 557744 | PE-cy7 | 1:40 |
| CD226 | BD Biosciences | 559789 | PE | 1:40 |
| CD158b | BD Biosciences | 559785 | PE | 1:40 |
| NKp46 | BD Biosciences | 557991 | PE | 1:40 |
| Ki67 | BD Biosciences | 561283 | PE-cy7 | 1:40 |
| CD107a | BD Biosciences | 555801 | PE | 1:40 |
| Interferon-γ | BD Biosciences | 559326 | PE | 1:40 |
| Granzyme B | BD Biosciences | 561142 | PE | 1:40 |
| Perforin | BD Biosciences | 563762 | Percp-cy5.5 | 1:40 |
| Tumor necrosis
factor α | BD Biosciences | 560679 | Percp-cy5.5 | 1:40 |
In vitro coculture of NK cells with
propofol
NK cells were separated from the peripheral blood
and cultured at 37°C in RPMI 1640 medium with 10% fetal bovine
serum (both BD Biosciences) in the presence of 100 U/ml interleukin
(IL)2, 100 U/ml IL12 and 100 U/ml IL15 for 48 h. Cells were
subsequently incubated in RPMI 1,640 medium with 50 µmol/l propofol
(Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for 24 h at 37°C.
The purity of NK cells was >90% as confirmed by flow cytometry.
The markers of NK cells (NKG2D, NKp30, NKp44, NKG2A, CD3, CD56,
CD16, CD226, CD158b, NKp46, Ki67, CD107a, IFNγ, granzyme B,
perforin and TNFα) were detected using a flow cytometer.
NK cells cytotoxicity assay
Following coculture with propofol, the number of NK
cells was calculated. NK cells were subsequently mixed with K562
and Eca109 cells (Type Culture Collection of the Chinese Academy of
Sciences, Shanghai, China), each at a ratio of 1:5. Apoptosis was
evaluated using flow cytometry. NK cells were treated using the
fluorescein isothiocyanate Annexin V Apoptosis Detection kit I (BD
Biosciences) according to the manufacturer's protocol. Cells
positive for Annexin V alone were in early apoptosis, cells
positive for propidium iodide (PI) alone were necrotic and cells
positive for Annexin V and PI were in late apoptosis. Untreated NK
cells from patients with ESCC were used as the control for this
experiment.
Statistical analysis
Data are presented as the mean ± standard deviation.
Student's t-test was used to compare between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
The phenotype and function of NK cells
from the peripheral blood of patients with ESCC
NK cells have been reported to be important for
suppressing the recurrence and metastasis of tumor cells.
Furthermore, it has been reported that immunosuppression occurs in
the tumor microenvironment as well as the peripheral blood
(23). The results of flow cytometry
revealed that the percentage of NK cells gated by CD3-CD56+ in the
peripheral blood of patients with ESCC (21.6±0.89%) was
significantly increased compared with the control (11.8±0.54%;
Fig. 1). Furthermore, the percentage
of NKT cells gated by CD3+CD56+, another type of innate immune cell
(24), was significantly increased
in the peripheral blood of patients with ESCC compared with the
control group (3.99±0.43 vs. 1.15±0.10); Fig. 1), indicating that the innate immune
response activity was increased.
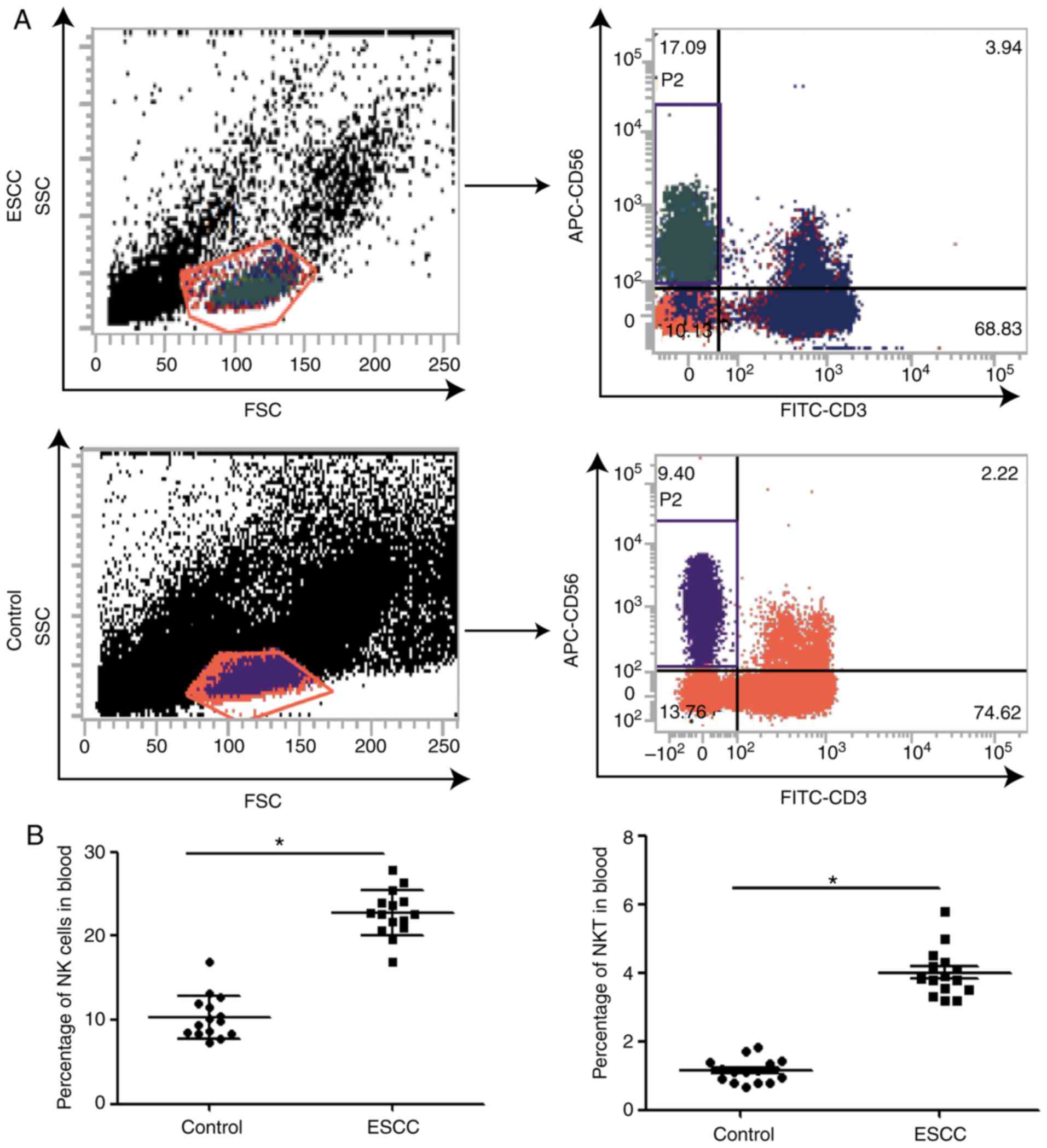 | Figure 1.Percentages of NK, NKT and T cells in
the peripheral blood of patients with ESCC and control subjects,
respectively. (A) Representative flow cytometry plots of NK, NKT
and T cells, gated by CD3-CD56+, CD3+CD56+ or CD3+, respectively.
(B) Percentages of NK and NKT cells in patients with ESCC and
control subjects. *P<0.05. NK, natural killer; NKT, natural
killer T; ESCC, esophageal squamous cell carcinoma; CD, cluster of
differentiation; SSC, side-scattered light; FSC, forward scatter;
APC, allophycocyanin; FITC, fluorescein isothiocyanate. |
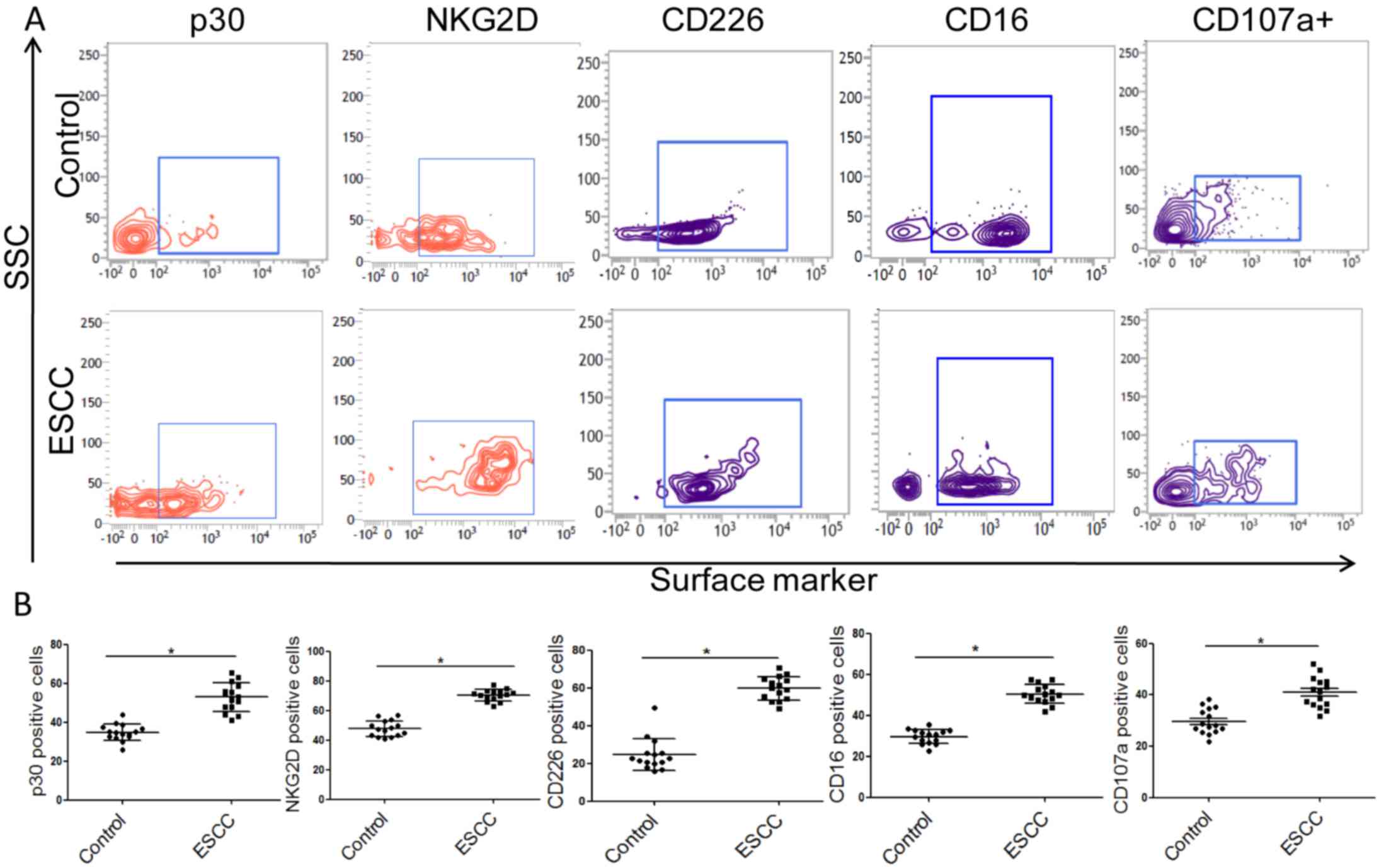
NK cell activation is known to be controlled by
activating and inhibitory receptors (25). In the present study, the expression
of activating and inhibitory receptors was assessed to evaluate the
activity and function of NK cells. The results revealed that the
expression of activating receptors of CD16, NKG2D, CD226 and p30
were significantly increased in compared with the control group
(Fig. 2). However, no significant
difference in p44, p46, CD158b, G2A, granzyme B, perforin, IFNγ and
TNFα expression was observed (Fig.
3). The cytotoxicity of NK cells to K562 was assessed by
detecting the expression of CD107a in NK cells. It was demonstrated
that the number of CD107a+ NK cells was significantly increased in
patients with ESCC compared with the healthy controls, suggesting
that the cytotoxicity of NK cells is reduced in patients with ESCC
(Fig. 2). Ki67+ cells were also
counted to evaluate the proliferation potential of NK cells. No
significant difference in proliferation was observed between groups
(Fig. 3). These data suggest that
the function of NK cells in the peripheral blood of patients with
ESCC is impaired compared with those from healthy subjects.
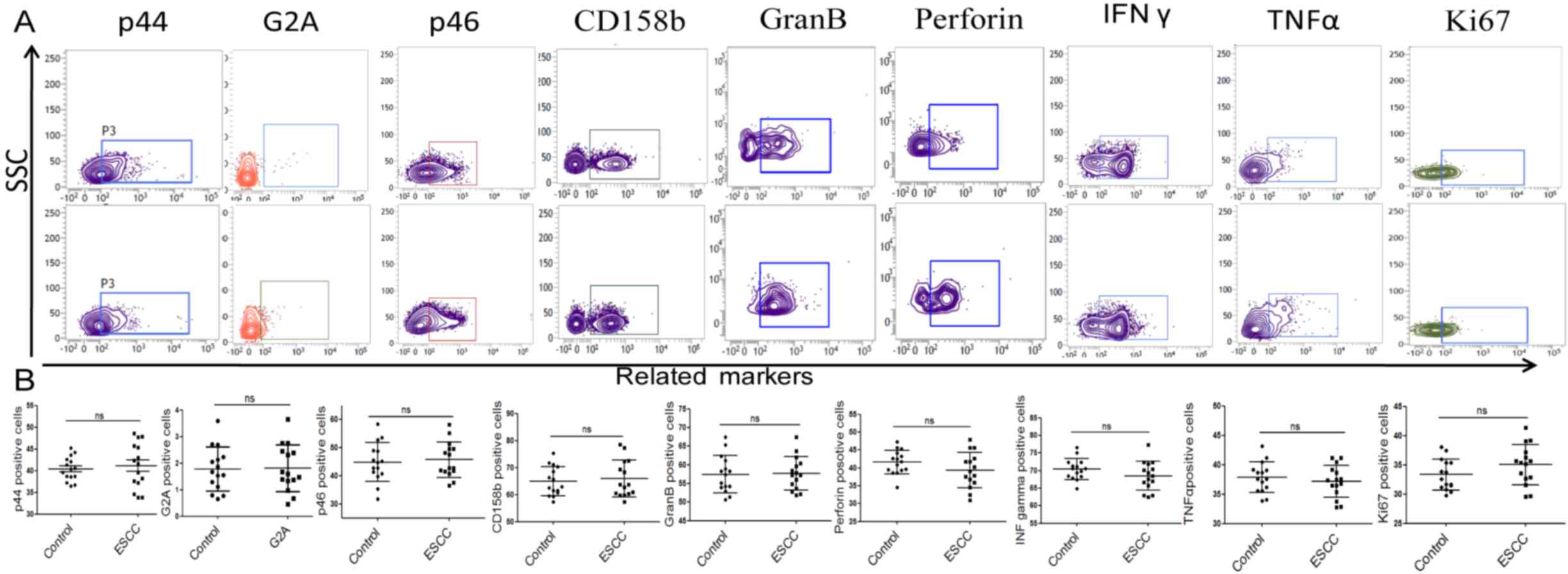 | Figure 3.The nonsense phenotype of NK cells
from the peripheral blood of patients with ESCC and control
subjects. (A) Representative flow cytometry images and (B)
quantitative analysis of activating and inhibitory receptors and
effect molecules in NK cells. (B) The column showed that the
expression of p44, G2A, p46, CD158b, granzyme B, perforin, TNFα,
IFNγ and Ki67. NK, natural killer; ESCC, esophageal squamous cell
carcinoma; G2A, G-protein coupled receptor 132; CD, cluster of
differentiation; TNF, tumor necrosis factor; IFN, interferon; SSC,
side-scattered light; ns, no significance. |
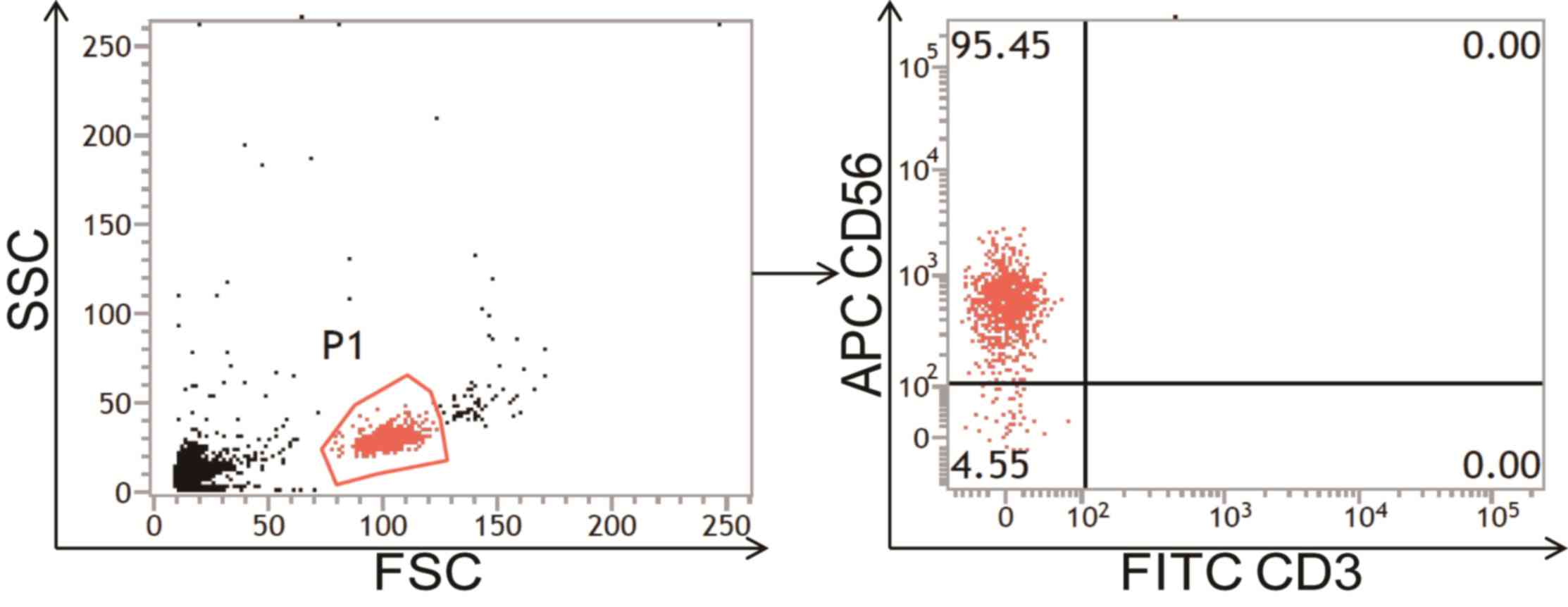
Purification and identification of NK
cells
To further investigate the potential influence of
propofol on NK cells in ESCC, NK cells were negatively isolated
using magnetic beads sorting (26).
CD3-CD56+ cells associated with the lymphocyte gate by forward
scatter (FSC) and side scatter (SSC) on a dot-plot were defined as
NK cells. The results demonstrated that the number of sorted NK
cells from the peripheral blood of patients with ESCC or healthy
volunteers was 3.5±0.25×105 or 2.7±0.11×105,
respectively, while the purity of NK cells was >90% and the
percentage of CD3+ T cells was <1%, suggesting that the sorting
of NK cells was successful and suitable for subsequent experiments
(Fig. 4).
Surface markers of NK cells as
detected by flow cytometry
To investigate the impact of propofol on NK cells,
the phenotype of isolated NK cells cocultured with propofol was
assessed. A concentration of 50 µmol/l propofol was selected as
this has been reported to be an appropriate concentration for
studying its function (27). The
expression of activating and inhibitory receptors on the surface of
NK cells was determined using flow cytometry. The results revealed
that the percentage of several activating receptors was
significantly increased in NK cells cocultured with propofol
compared with the control group, including CD16, NKp30, NKp44 and
NKG2D (Fig. 5). Conversely, the
expression of inhibitory receptors CD158b and NKG2A was
significantly decreased in NK cells treated with propofol compared
with the control group (Fig. 5). No
significant differences in NKp44, NKp46 and CD226 expression was
observed (data not shown). These data suggest that propofol may
enhance the activity of NK cells in ESCC by regulating the
equilibrium between activating and inhibitory receptors.
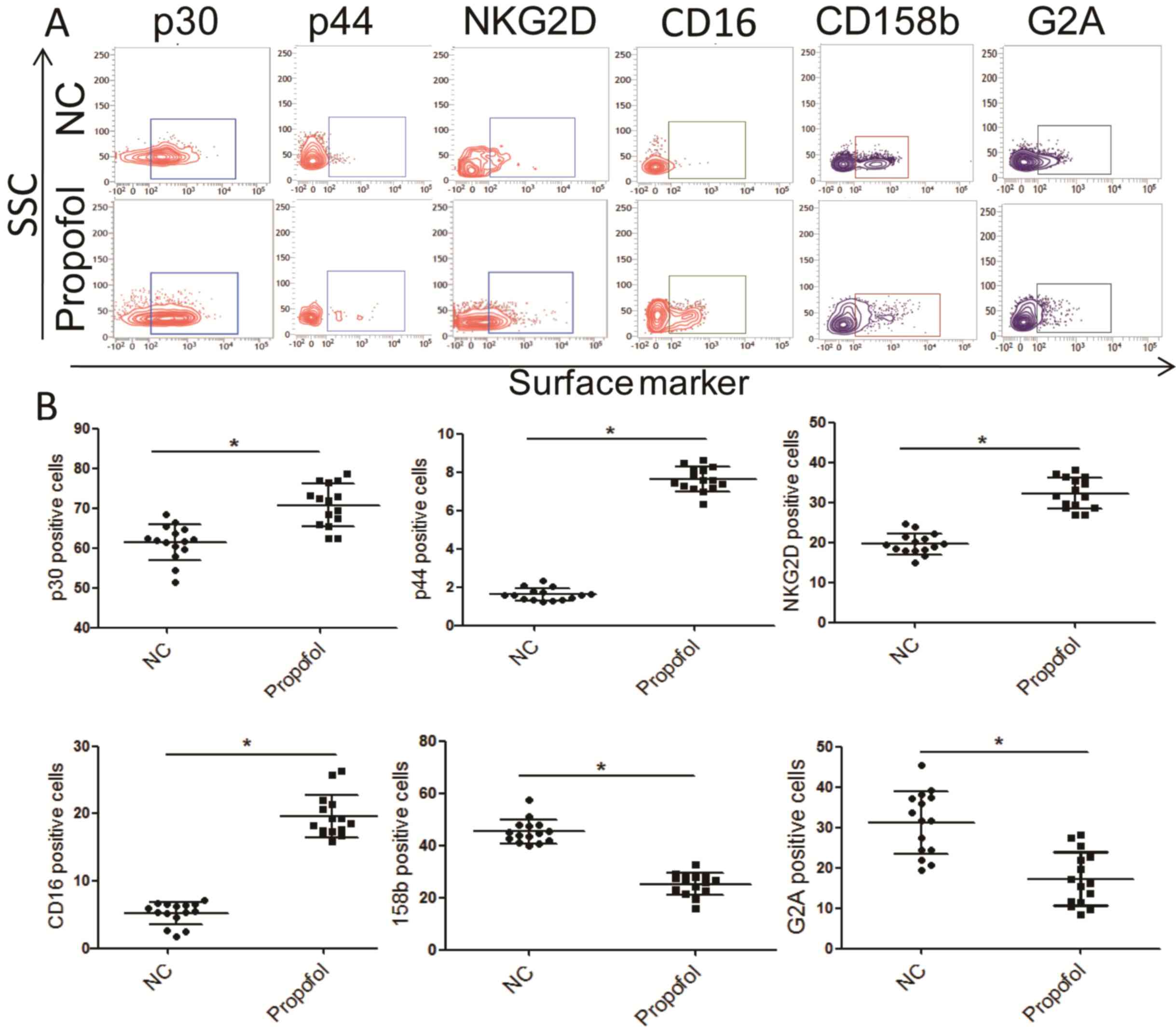 | Figure 5.Effect of propofol on the expression
of activating and inhibitory receptors of NK cells in vitro.
(A) Representative flow cytometry images and (B) quantitative
analysis of p30, p44, NKG2D, CD16, CD158b and G2A expression.
*P<0.05. NK, natural killer; NKG2D, NK group 2, member D; CD,
cluster of differentiation; G2A, G-protein coupled receptor 132;
NC, negative control; SSC, side-scattered light. |
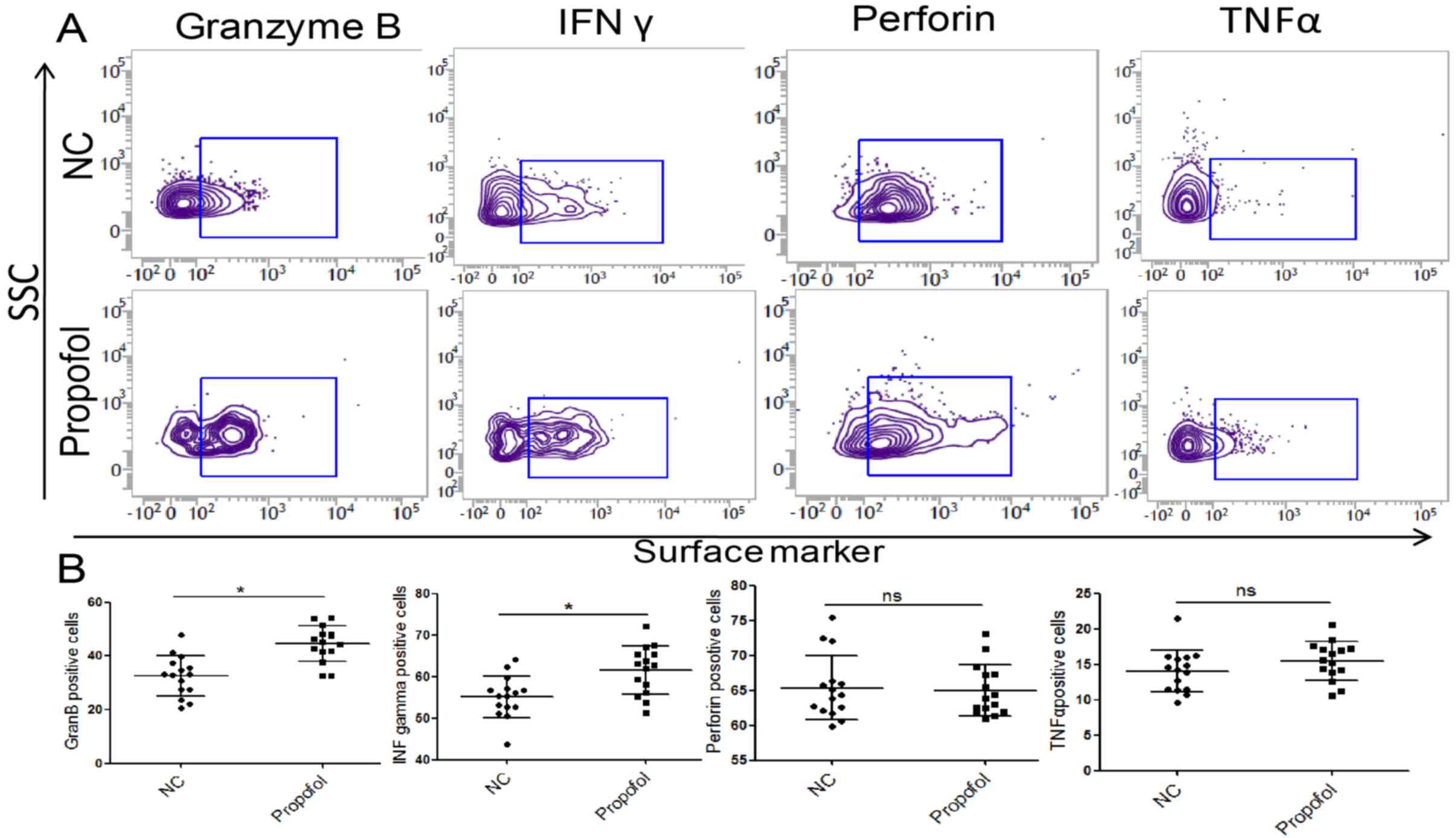
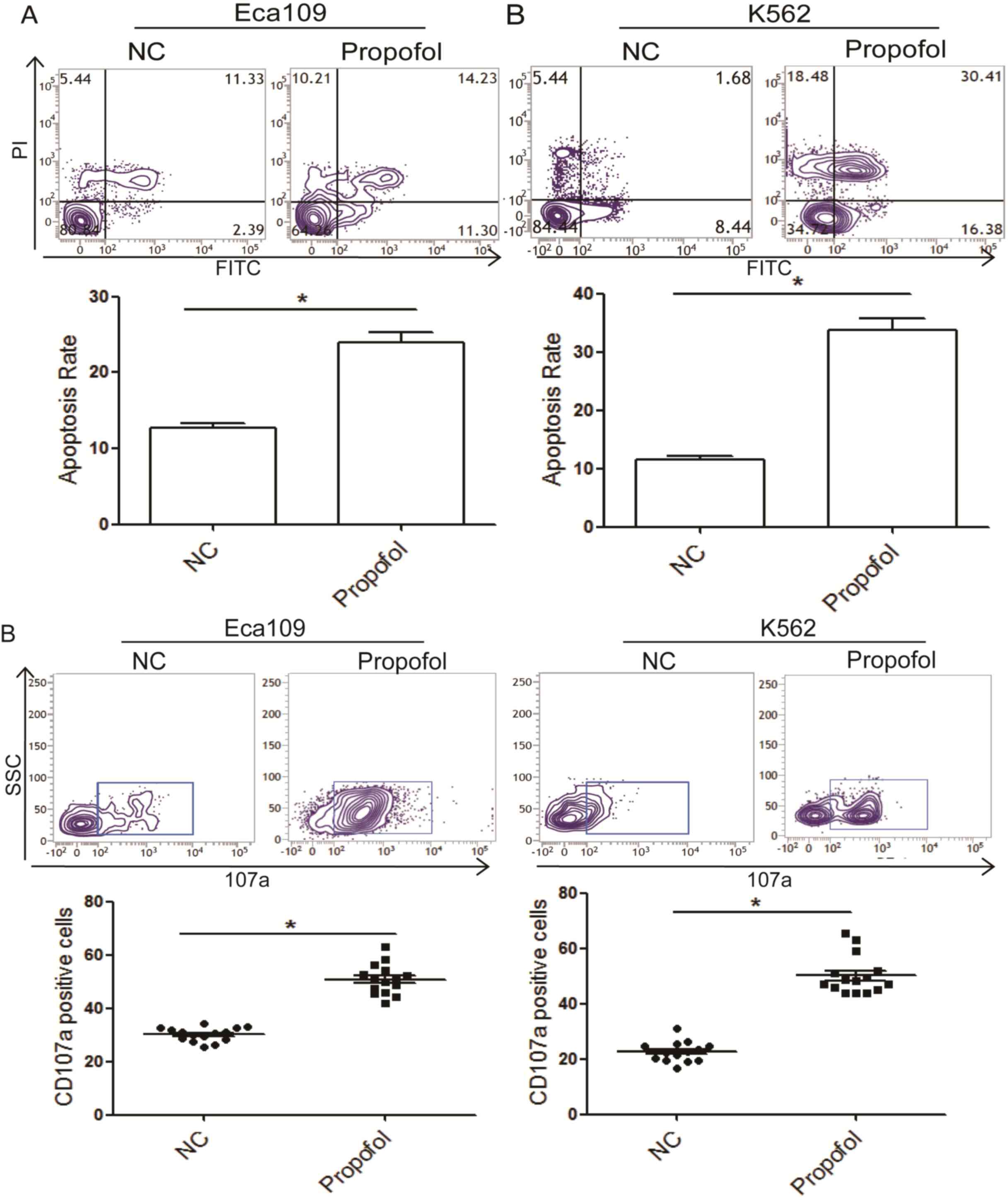
Propofol enhances the expression of
granzyme B and IFN-γin NK cells
It is well established that activated NK cells
destroy tumor cells mainly via cytotoxicity effector molecules,
including granzyme B, perforin, IFN-γ and TNF-α (28). The results of flow cytometry revealed
that the expression of granzyme B and IFN-γ was significantly
increased following incubation with propofol (Fig. 6), suggesting that NK cell
cytotoxicity was increased. These data suggest that propofol may
promote the cytotoxicity of NK cells from patients with ESCC.
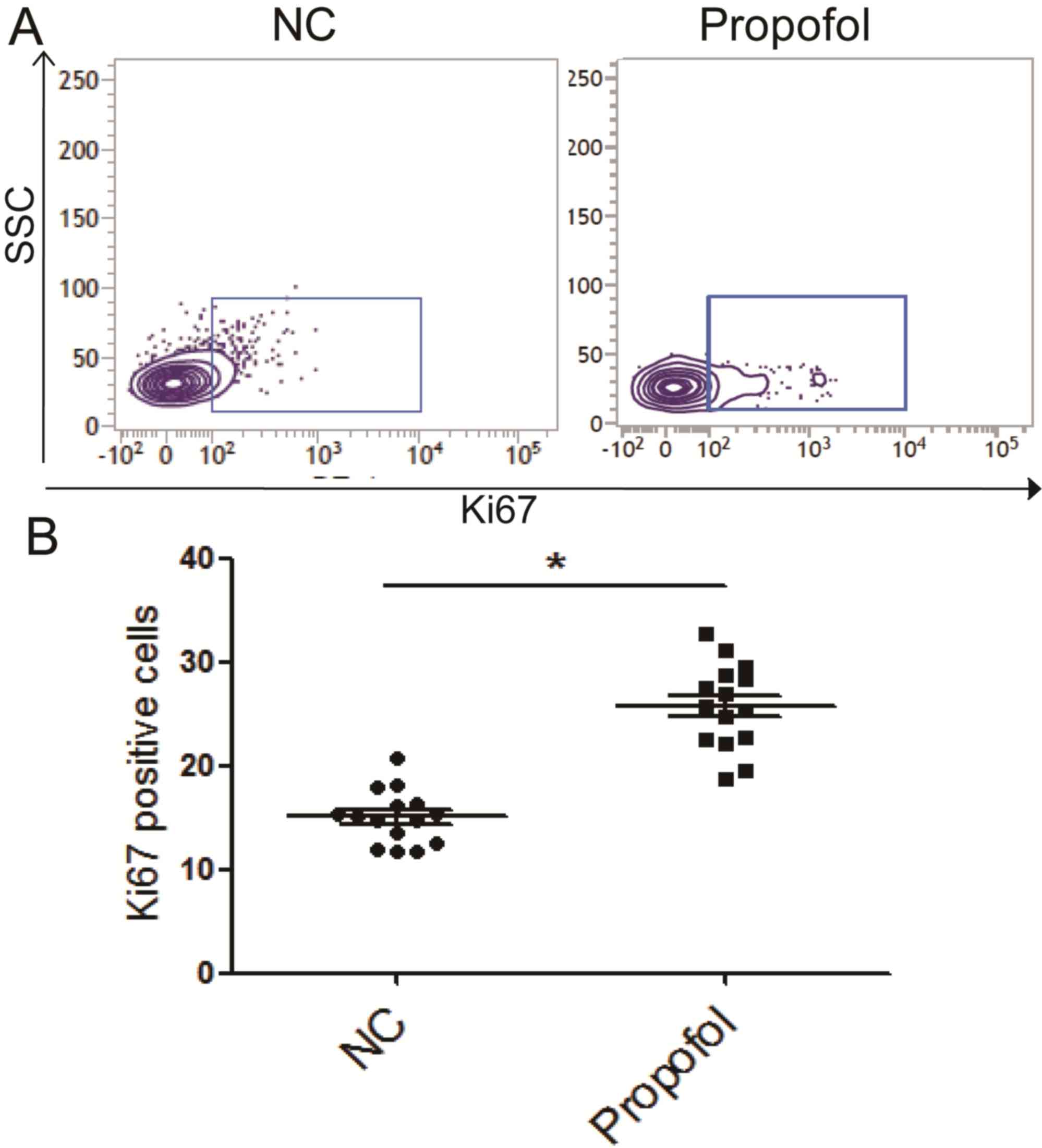
Propofol promotes the proliferation
potential of isolated NK cells
The influence of propofol on the proliferation
potential of isolated NK cells was assessed by detecting expression
of Ki67 through flow cytometry. The results demonstrated that the
number of Ki67+ NK cells was significantly higher following
propofol stimulation compared with control cells (Fig. 7). These data suggest that propofol
may promote the proliferative potential of NK cells in patients
with ESCC.
Propofol enhances the function of NK
cells in vitro
Based on previous findings, the cytotoxicity of NK
cells cocultured with propofol to tumor cells was assessed in
vitro using apoptosis analysis. K562 was selected as the target
cell line as it does not express MHCI molecules (29). The apoptosis rate of K562 cells
cocultured with NK cells stimulated by propofol was significantly
higher compared with the control group (Fig. 8). To further investigate the
cytotoxicity of NK cells to ESCC cells, the apoptosis rate of
Eca109 cells incubated with NK cells was assessed. Consistent with
K562, NK cells cultured with propofol exerted a greater cytotoxic
effect on Eca109 cells compared with the control (Fig. 8). These data suggest that propofol
may enhance the cytotoxicity of NK cells from the peripheral blood
of patients with ESCC.
Discussion
Elucidating the effect of anesthesia on immune
inhibition during the postoperative period is essential for
preventing tumor metastasis and improving the prognosis of patients
with ESCC (30). Although anesthetic
agents have been demonstrated to affect tumor recurrence and
metastasis (31), the impact of
anesthetics on anti-tumor immune cells is not well understood. In
the present study, NK cells were successfully isolated from the
peripheral blood of patients with ESCC and it was confirmed that
propofol is able to increase the activity of NK cells by regulating
the expression of receptors and cytotoxicity effect molecules.
Furthermore, propofol enhances the cytotoxicity of NK cells to ESCC
cells in vitro, indicating that it serves as a promotion
effector for the postoperative immune system in ESCC.
NK cells are a group of innate lymphoid cells, which
serve a crucial role in the inhibition of multiple tumors (32). A novel tumor therapy technique,
adoptive immunotherapy, based on NK cells has been investigated in
human trials (33). The activation
or inhibition of NK cells has been reported to depend on the
recognition of altered tumor cells by the activation or inhibition
of receptors on the NK cell surface. Therefore, screening factors
that influence the expression of NK cell surface receptors is a
critical area of immunotherapy for tumors (34). Anesthetics have been reported to be
associated with innate immune system regulation, providing a novel
perspective that anesthesia was implicated with tumor recurrence
and metastasis by regulation of immune response (35). The cytotoxicity of NK cells from
patients with breast cancer receiving propofol-remifentanil
anesthesia with postoperative ketorolac analgesia was clearly lower
compared with those receiving sevoflurane-remifentanil anesthesia
with postoperative fentanyl analgesia (36); Inada et al (37) reported that propofol promotes the
expression of IFNγ in NK cells by suppressing prostaglandin E2
(37). This suggests that propofol
is associated with the regulation of NK cytotoxicity; however, its
impact on the expression of activation and inhibitory receptors
remains unclear. Consistent with previous studies (38), the percentage of NK cells from
patients with ESCC was increased compared with the control, which
may be a response to tumorigenesis. The phenotype and cytotoxicity
of NK cells was investigated and the results demonstrated that NK
cells from patients with ESCC had a higher expression of activating
receptors (p30, NKG2D, CD226 and CD16) compared with the control,
suggesting that NK cells from the peripheral blood of patients with
ESCC were activated. Conversely, it has previously been reported
that NK cells patients with tumors had impaired function (39,40).
These contradictory results may be because some crucial signaling
pathway downstream of activating receptors also serves a role in
the regulation of NK cells,.
To further evaluate the effect of propofol on NK
cells, isolated NK cells from patients with ESCC were incubated
with propofol followed by analysis via flow cytometry. The results
revealed that propofol increased the expression of activating
receptors (p30, NKG2D, p44, CD16) expression and suppressed
inhibitory receptors (CD158b, NKG2A). The cytotoxicity of NK cells
from patients with ESCC was also enhanced, as indicated by the
increased expression of IFNγ and granzyme B. Ki67 was also
upregulated in NK cells stimulated with propofol, indicating that
propofol improves the proliferation potential of NK cells. Although
data within the present study indicated that propofol probably
promoted the activation of NK cells from patients with ESCC, the
cytotoxicity of NK cells still needs to be confirmed. Blocking the
activating interaction between activating receptors and matched
ligands led to impaired NK cell function in the presence of high
expression of activating receptors. The cytotoxic effects of NK
cells on K562 and Eca109 were investigated and it was revealed that
propofol-stimulated NK cells increased apoptosis in K562 and Eca109
cells. Furthermore, a significant increase in CD107a + NK cells,
which are characteristic of degranulation, was observed following
propofol stimulation. These data suggest that propofol is able to
enhance the cytotoxic effects of NK cells from the peripheral blood
of patients with ESCC in vitro. In the present study, the
number of patients recruited was insufficient to analyze the
association between NK cell markers and clinicopathological
characteristics, including lymph node metastasis and TNM stage.
Furthermore, the function of propofol on NK cells should be
verified using an in vivo mouse model in the future.
In conclusion, the results of the present study
demonstrated that propofol is able to enhance the function of NK
cells from the peripheral blood of patients with ESCC. Based on
these results, propofol may have the potential to improve
postoperative immunosuppression in patients with ESCC.
Acknowledgements
The authors would like to thank Miss XueMei He and
Dr Yang Long (The Affiliated Hospital of South West Medical
University, Luzhou, China) for providing valuable technical
supports and writing assistance.
Funding
This study was supported by National Natural Science
Foundation of China (grant no. 81271478).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
XW designed the study and wrote the paper. MZ and
JCD performed the experiments. YZ, JW, TX and DZ collected the
samples and performed flow cytometry. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants in the present study. The study was approved by the
Ethics Review Board at Southwest Medical University (Luzhou,
China).
Consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that there were no competing
interests.
References
|
1
|
Lin C, Zhang N, Wang Y, Wang Y, Nice E,
Guo C, Zhang E, Yu L, Li M, Liu C, et al: Functional role of a
novel long noncoding RNA TTN-AS1 in esophageal squamous cell
carcinoma progression and metastasis. Clin Cancer Res. 24:486–498.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou X, Qiu GQ, Bao WA and Zhang DH: The
prognostic role of nutrition risk score (NRS) in patients with
metastatic or recurrent esophageal squamous cell carcinoma (ESCC).
Oncotarget. 8:77465–77473. 2017.PubMed/NCBI
|
|
3
|
Kurimoto K, Hayashi M, Guerrero-Preston R,
Koike M, Kanda M, Hirabayashi S, Tanabe H, Takano N, Iwata N, Niwa
Y, et al: PAX5 gene as a novel methylation marker that predicts
both clinical outcome and cisplatin sensitivity in esophageal
squamous cell carcinoma. Epigenetics. 12:865–874. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu X, Dinglin X, Wang X, Luo W, Shen Q, Li
Y, Gu L, Zhou Q, Zhu H, Li Y, et al: Long noncoding RNA XIST
promotes malignancies of esophageal squamous cell carcinoma via
regulation of miR-101/EZH2. Oncotarget. 8:76015–76028.
2017.PubMed/NCBI
|
|
5
|
Cai W, Lu JJ, Xu R, Xin P, Xin J, Chen Y,
Gao B, Chen J and Yang X: Survival based radiographic-grouping for
esophageal squamous cell carcinoma may impact clinical T stage.
Oncotarget. 9:9512–9530. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Feng Z, Wang W, Dong J, Gong X,
Pu H and Chen X: Expression of heat shock protein-27 (Hsp27) and
P38MAPK in esophageal squamous cell carcinoma. Med Sci Monit.
23:5246–5253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kunizaki M, Hamasaki K, Wakata K, Tobinaga
S, Sumida Y, Hidaka S, Yasutake T, Miyazaki T, Matsumoto K,
Yamasaki T, et al: Clinical value of serum p53 antibody in the
diagnosis and prognosis of esophageal squamous cell carcinoma.
Anticancer Res. 38:1807–1813. 2018.PubMed/NCBI
|
|
8
|
Chen K, Zhu Z, Zhang N, Cheng G, Zhang F,
Jin J, Wu J, Ying L, Mao W and Su D: Tumor-infiltrating CD4+
lymphocytes predict a favorable survival in patients with operable
esophageal squamous cell carcinoma. Med Sci Monit. 23:4619–4632.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jesinghaus M, Steiger K, Slotta-Huspenina
J, Drecoll E, Pfarr N, Meyer P, Konukiewitz B, Bettstetter M,
Wieczorek K, Ott K, et al: Increased intraepithelial CD3+
T-lymphocytes and high PD-L1 expression on tumor cells are
associated with a favorable prognosis in esophageal squamous cell
carcinoma and allow prognostic immunogenic subgrouping. Oncotarget.
8:46756–46768. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yankuzo HM, Baraya YS, Mustapha Z, Wong KK
and Yaacob NS: Immunomodulatory effects of a bioactive fraction of
Strobilanthes crispus in NMU-induced rat mammary tumor model. J
Ethnopharmacol. 213:31–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuang PY, Zhang KW, Wang JD, Zhou XP, Liu
YB, Quan ZW and Shen J: Effect of TALEN-mediated IL-6 knockout on
cell proliferation, apoptosis, invasion and anti-cancer therapy in
hepatocellular carcinoma (HCC-LM3) cells. Oncotarget.
8:77915–77927. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maj T, Wang W, Crespo J, Zhang H, Wang W,
Wei S, Zhao L, Vatan L, Shao I, Szeliga W, et al: Oxidative stress
controls regulatory T cell apoptosis and suppressor activity and
PD-L1-blockade resistance in tumor. Nat Immunol. 18:1332–1341.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Wang C, Wang J, Huang X and Cheng
Y: A novel systemic immune-inflammation index predicts survival and
quality of life of patients after curative resection for esophageal
squamous cell carcinoma. J Cancer Res Clin Oncol. 143:2077–2086.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pérez-González O, Cuéllar-Guzmán LF, Soliz
J and Cata JP: Impact of regional anesthesia on recurrence,
metastasis, and immune response in breast cancer surgery: A
Systematic review of the literature. Reg Anesth Pain Med.
42:751–756. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eroglu T, Bozkurt M, Kapi E, Selcuk CT,
Kuvat SV, Tufek A, Isik FB, Bozarslan BH, Firat U and Satici O: A
study on the effects of the use of propofol in experimental model
inferior epigastric island flap on ischemia-reperfusion injury. J
Craniofac Surg. 28:2193–2198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim R, Kawai A, Wakisaka M, Funaoka Y,
Ohtani S, Ito M, Kadoya T and Okada M: Differences in immune
response to anesthetics used for day surgery versus hospitalization
surgery for breast cancer patients. Clin Transl Med. 6:342017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Javaheri A, Wang AR, Prak Luning E, Lal P,
Goldberg LR and Kamoun M: Fatal accelerated rejection with a
prominent natural killer cell infiltrate in a heart transplant
recipient with peripartum cardiomyopathy. Transpl Immunol.
47:49–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pollmann J, Götz JJ, Rupp D, Strauss O,
Granzin M, Grünvogel O, Mutz P, Kramer C, Lasitschka F, Lohmann V,
et al: Hepatitis C virus-induced natural killer cell proliferation
involves monocyte-derived cells and the OX40/OX40L axis. J Hepatol.
68:421–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baltner K, Kübler A, Pal M, Balvociute M,
Mezger M, Handgretinger R and André MC: Expression of KIR2DS1 does
not significantly contribute to NK cell cytotoxicity in HLA-C1/C2
heterozygous haplotype B donors. Int Immunol. 29:423–429. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dansako H, Imai H, Ueda Y, Satoh S, Wakita
T and Kato N: ULBP1 is induced by hepatitis C virus infection and
is the target of the NK cell-mediated innate immune response in
human hepatocytes. FEBS Open Bio. 8:361–371. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Z, Cheng F, Yan G, Xiong L and Liu H:
Propofol protects against endotoxin-induced myocardial injury by
inhibiting NF-κB-mediated inflammation. Exp Ther Med. 15:2032–2036.
2018.PubMed/NCBI
|
|
22
|
Klöß S, Oberschmidt O, Morgan M, Dahlke J,
Arseniev L, Huppert V, Granzin M, Gardlowski T, Matthies N,
Soltenborn S, et al: Optimization of human NK cell manufacturing:
Fully automated separation, improved ex vivo expansion using IL-21
with autologous feeder cells, and generation of
anti-CD123-CAR-expressing effector cells. Hum Gene Ther.
28:897–913. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voutsadakis IA: Expression and function of
immune ligand-receptor pairs in NK cells and cancer stem cells:
Therapeutic implications. Cell Oncol (Dordr). 41:107–121. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cameron G and Godfrey DI: Differential
surface phenotype and context-dependent reactivity of functionally
diverse NKT cells. Immunol Cell Biol. Mar 5–2018.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nham T, Poznanski SM, Fan IY, Shenouda MM,
Chew MV, Lee AJ, Vahedi F, Karimi Y, Butcher M, Lee DA, et al: Ex
vivo-expanded NK cells from blood and ascites of ovarian cancer
patients are cytotoxic against autologous primary ovarian cancer
cells. Cancer Immunol Immunother. 67:575–587. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakashima Y, Deie M, Yanada S, Sharman P
and Ochi M: Magnetically labeled human natural killer cells,
accumulated in vitro by an external magnetic force, are
effective against HOS osteosarcoma cells. Int J Oncol. 27:965–971.
2005.PubMed/NCBI
|
|
27
|
Liu S, Gu X, Zhu L, Wu G, Zhou H, Song Y
and Wu C: Effects of propofol and sevoflurane on perioperative
immune response in patients undergoing laparoscopic radical
hysterectomy for cervical cancer. Medicine (Baltimore).
95:e54792016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Glasner A, Isaacson B, Viukov S, Neuman T,
Friedman N, Mandelboim M, Sexl V, Hanna JH and Mandelboim O:
Increased NK cell immunity in a transgenic mouse model of NKp46
overexpression. Sci Rep. 7:130902017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Erokhina SA, Streltsova MA, Kanevskiy LM,
Telford WG, Sapozhnikov AM and Kovalenko EI: HLA-DR+ NK cells are
mostly characterized by less mature phenotype and high functional
activity. Immunol Cell Biol. 96:212–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsota P, Kostopanagiotou G, Kalimeris K,
Pandazi A, Kotsaki A, Kontogiannopoulou S and
Giamarellos-Bourboulis EJ: Transient effects of anesthesia on
leukocyte apoptosis and monocyte cytokine stimulation: A clinical
study. Immunol Invest. 1–8. 2018.
|
|
31
|
Rossaint J and Zarbock A: Perioperative
inflammation and its modulation by anesthetics. Anesth Analg.
126:1058–1067. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Acuff NV, Li X, Latha K, Nagy T and
Watford WT: Tpl2 promotes innate cell recruitment and effector T
cell differentiation to limit citrobacter rodentium burden and
dissemination. Infect Immun. 85:pii: e00193. –17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li L, Li W, Wang C, Yan X, Wang Y, Niu C,
Zhang X, Li M, Tian H, Yao C, et al: Adoptive transfer of natural
killer cells in combination with chemotherapy improves outcomes of
patients with locally advanced colon carcinoma. Cytotherapy.
20:134–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gumbleton M, Sudan R, Fernandes S,
Engelman RW, Russo CM, Chisholm JD and Kerr WG: Dual enhancement of
T and NK cell function by pulsatile inhibition of SHIP1 improves
antitumor immunity and survival. Sci Signal. 10:pii: eaam5353.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sedghi S, Kutscher HL, Davidson BA and
Knight PR: Volatile anesthetics and immunity. Immunol Invest.
46:793–804. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cho JS, Lee MH, Kim SI, Park S, Park HS,
Oh E, Lee JH and Koo BN: The effects of perioperative anesthesia
and analgesia on immune function in patients undergoing breast
cancer resection: A prospective randomized stud. Int J Med Sci.
14:970–976. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Inada T, Hirota K and Shingu K:
Intravenous anesthetic propofol suppresses prostaglandin E2 and
cysteinyl leukotriene production and reduces edema formation in
arachidonic acid-induced ear inflammation. J Immunotoxicol.
12:261–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dai K, Huang Y, Chen Z, Sun X, Yang L and
Jiang Y: Kbtbd2 inhibits the cytotoxic activity of immortalized NK
cells through down-regulating mTOR signaling in a mouse
hepatocellular carcinoma model. Eur J Immunol. Jan 13–2018.(Epub
ahead of print). View Article : Google Scholar
|
|
39
|
Hoshikawa M, Aoki T, Matsushita H,
Karasaki T, Hosoi A, Odaira K, Fujieda N, Kobayashi Y, Kambara K,
Ohara O, et al: NK cell and IFN signatures are positive prognostic
biomarkers for resectable pancreatic cancer. Biochem Biophys Res
Commun. 495:2058–2065. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stangl S, Tontcheva N, Sievert W, Shevtsov
M, Niu M, Schmid TE, Pigorsch S, Combs SE, Haller B, Balermpas P,
et al: Heat shock protein 70 and tumor-infiltrating NK cells as
prognostic indicators for patients with squamous cell carcinoma of
the head and neck after radiochemotherapy: A multicentre
retrospective study of the German Cancer Consortium Radiation
Oncology Group (DKTK-ROG). Int J Cancer. 142:1911–1925. 2018.
View Article : Google Scholar : PubMed/NCBI
|