Introduction
Premature delivery is a common complication of
pregnancy. The World Health Organization reported in 2013 that ~15
million premature babies are born every year worldwide (1). Each year, >1 million premature
babies succumb to premature complications, and many surviving
children also suffer from defects in the nervous system,
respiratory tract, and digestive tract (2). It has previously been demonstrated that
infection is the most prevalent cause of premature delivery,
accounting for ~40% (3). The
placenta is the intermediary between the mother and the fetus, and
has an important barrier effect on the mother-fetus interface. Once
bacterial endotoxin lipopolysaccharide (LPS) enters the organism,
it can cause placental infection, and a number of pathological
changes occur in the placenta (4).
Under normal conditions, extracellular Ca2+ primarily
flows through calcium channels in the cell membrane, triggering the
release of Ca2+ via calcium release channels in the
sarcoplasmic reticulum (5–7). Thus, increased intracellular
Ca2+ concentrations may result in uterine contraction
and premature delivery. Taken together, calcium channels serve an
important role in regulating smooth muscle activity. Pyr3 is a
transient receptor protein 3 (TRPC3) channel blocker, which
potentiates dexamethasone sensitivity and apoptosis in acute
lymphoblastic leukemia cells by disturbing Ca2+
signaling, mitochondrial membrane potential changes and reactive
oxygen species production (8). TRPC
is a non-selective cation channel composed of four monomers with
six transmembrane helices, participates in many pathophysiological
processes, and can transport sodium, calcium and magnesium ions
(9,10). When cellular calcium stores are
depleted, the TRPC channel on the cell membrane is opened,
resulting in the influx of extracellular Ca2+ and
calcium stores are restored. This Ca2+ influx is known
as store-operated calcium entry (11). Protein kinase Cβ
(PKCβ)/C-kinase-activated protein phosphatase-1 inhibitor of 17 kDa
(CPI-17) is a Ca2+-activated phospholipid-dependent
protein kinase that regulates different cell functions, such as
gene expression, cell proliferation, apoptosis and cell migration
(12,13). Myosin is a basic constituent of a
thick filament and is a hexamer of macromolecular proteins
consisting of two myosin heavy chains and four myosin light chains
(MLC) (14). Ozaki et al
(15) previously demonstrated that a
PKC agonist could significantly increase the phosphorylation of MLC
and the contraction of vascular smooth muscle. However, PKC
agonists cannot increase the phosphorylation level of MLC20 and the
contractile function of vascular smooth muscle (16). PKCβ/CPI-17 serves a critical role in
the calcium sensitization mechanism of smooth muscle (15).
As an organic nutrient, if amino acids can be used
as therapeutic agents, it is relatively safe and reliable, has no
obvious side effects, and has no adverse effects on fetal
development, intelligence and heredity (17). Therefore, the use of amino acids is
of great clinical value. It has previously been demonstrated that
S-adenosyl methionine (SAMe) is an important metabolic intermediate
in methionine, a methyl donor for the catalytic reaction of >100
different methyltransferases in vivo, and serves an
important role in the metabolism of living cells (18). At present, SAMe is used in clinic
settings for intrahepatic cholestasis and fatty liver in pregnancy,
effectively improves the clinical symptoms and outcomes of pregnant
women, and reduces the rate of premature delivery (19). Furthermore, SAMe is also a
prescription drug for arthritis, has few side effects and has
exhibited greater efficacy than conventional clinical drugs, such
as indomethacin (20,21). Recent studies also demonstrated that
SAMe has antidepressant and antitumor effects (22,23).
The present study sought to establish rat models of
premature delivery induced by LPS, to investigate the effects of
SAMe on infectious premature inflammatory factors and uterine
contraction, and to further explore the underlying mechanism of the
TRPC3/PKCβ/CPI-17 signaling pathway following intervention with a
TRPC3 inhibitor.
Materials and methods
Animals and ethical approval
A total of 45 female and 30 male
specific-pathogen-free Sprague-Dawley rats (weight, 220–260 g; age,
7 weeks) were provided by the Experimental Animal Center of the
General Hospital of Shenyang Military Area Command [Shenyang,
China; license no. SCXK (JUN) 2012-0002]. Female and male rats were
placed in the same cage overnight at a ratio of 2:1 (3 rats per
cage). Animals were housed at a constant temperature (22±1°C) with
50% humidity in a 12 h light/dark cycle. The rats had access to
food and autoclaved water ad libitum. The next morning, the
detection of sperm on the vaginal plug or vaginal smear was
considered as the day 0 of pregnancy. All animal procedures were
approved by the Animal Experiments Ethics Committee of the General
Hospital of Shenyang Military Area Command. Pregnant rats were
randomly assigned to the control group (n=9), LPS group (model of
infectious premature delivery; n=12), SAMe group (SAMe + LPS;
n=12), and Pyr3 group (Pyr3 + SAMe + LPS; n=12).
Preparation of rat models of
infectious premature delivery and sample collection
LPS salt solution (100 µg/kg; cat. no. L8880;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China; saline solution was used as a solvent) was intraperitoneally
administered to pregnant rats in the LPS group at day 15 of
pregnancy. In the SAMe group, 30 mg/kg SAMe (physiological saline
was used as the solvent; cat. no. S9990; Beijing Solarbio Science
& Technology Co., Ltd.) was intraperitoneally injected at 0.5 h
following LPS injection. In the Pyr3 group, 30 mg/kg Pyr
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
intraperitoneally administered at 30 min following SAMe injection.
In the control group, an equal volume of physiological saline was
intraperitoneally administered concurrently with LPS administration
in the other groups. The occurrence of premature delivery (<19
days of pregnancy) was recorded. Following premature delivery, rats
were anesthetized with 2% sodium pentobarbital (40 mg/kg; Beijing
Huaye Huanyu Chemical Co., Ltd., Beijing, China). Arterial blood
was collected and ~5 ml serum was isolated by centrifugation at
1,000 × g for 5 min at 4°C. The blood plasma was isolated, packed
separately and stored at −80°C for ELISA. Rats were subsequently
sacrificed via anesthesia overdose with 2% sodium pentobarbital
(120 mg/kg). The route of sodium pentobarbital administration for
anesthesia and sacrifice was intraperitoneal injection. The uterus
and placenta was harvested and adipose tissue was removed. Blood
was washed away with physiological saline. A random section of each
sample was fixed in 10% paraformaldehyde at room temperature for 48
h and the other was stored in liquid nitrogen.
Determination of latency and live
birth rate in each group
Following the evaluation of premature delivery in
each group, latency (the time between LPS administration and the
first fetus delivery) and live birth rate in each group were
recorded.
Determination of muscle tension of
uterine muscle strips of pregnant rats
Uterus tissue samples (~1 cm longitudinal direction)
were washed with physiological saline, fixed on the specimen hook
of a multi-channel physiological signal acquisition system, and
placed in a tank filled with 8 ml Tyrode's solution. The sample was
also connected to a tension converter to record uterine
contraction.
Hematoxylin and eosin (HE)
staining
Rat uterus samples were dehydrated with a graded
alcohol series, permeabilized with xylene, immersed and embedded in
paraffin, and cut into 5-µm-thick sections. Sections were stained
with hematoxylin for 5 min at room temperature, washed with PBS,
differentiated with hydrochloric acid ethanol for 3 sec, stained
with eosin for 1 min at room temperature and mounted with neutral
resin. Changes in rat uterine tissue were observed under light
microscopy (magnification, ×12.6).
Immunohistochemistry
Paraffin-embedded 5-µm-thick sections were placed in
a 67°C oven for 2 h, dewaxed, hydrated in a descending alcohol
series and treated with citrate buffer (pH, 6.0; cat. no. C1010;
Beijing Solarbio Science & Technology Co., Ltd.) at 100°C for
10 min. Antigen retrieval was performed via microwave heating at
100°C. Each section was treated with ~30 µl 3% hydrogen peroxide
aqueous solution (cat. no. 10011208; Sinopharm Chemical Reagent
Co., Ltd., Shanghai, China) at room temperature for 10 min, and
blocked with 5% bovine serum albumin for 45 min at room
temperature. After abundant liquid was discarded, sections were
incubated with antibodies against TRPC3 (ab51560; rabbit; 97 kDa;
1:500; Abcam, Cambridge, UK); calcium channel, voltage-dependent, L
type, α 1C subunit (Cav1.2; ab58552; rabbit; 249 kDa; 1:1,000;
Abcam); calcium channel, voltage-dependent, T type, α 1G subunit
(Cav3.1; ab95092; rabbit; 262 kDa; 1:1,000; Abcam); and calcium
channel, voltage-dependent, T type, α 1H subunit (Cav3.2; ab95092;
rabbit; 262 kDa; 1:1,000 Abcam) at 4°C overnight. Sections were
subsequently incubated with horseradish peroxidase (HRP)-conjugated
anti-goat immunoglobulin G secondary antibodies (1:1,000; cat. no.
ab6721; Abcam) for 2 h at room temperature, washed with PBS,
visualized with 3,3′-diaminobenzidine, counterstained with
hematoxylin for 2 min at room temperature, dehydrated through a
graded alcohol series, permeabilized with xylene, mounted with
neutral resin and observed using a light microscope (magnification,
×25.2).
ELISA
The following ELISA kits were used to analyze rat
serum samples, according to the manufacturers' protocols:
Interleukin (IL)-1β (SEA563Ra; Uscn Life Sciences, Inc., Wuhan,
China), IL-8 (H008; Nanjing Jiancheng Bioengineering Institute,
Nanjing, China), tumor necrosis factor α (TNF-α; SEA133Ra; Uscn
Life Sciences, Inc.), IL-10 (SEA056Ra; Uscn Life Sciences, Inc.),
malondialdehyde (MDA; A003-2; Nanjing Jiancheng Bioengineering
Institute), superoxide dismutase (SOD; A001-3; Nanjing Jiancheng
Bioengineering Institute) and nitric oxide (NO; A013-2; Nanjing
Jiancheng Bioengineering Institute). Blank wells, standard wells
and detected wells were used. Blank wells contained 100 µl PBS; the
other wells contained 100 µl standard preparation (provided with
the kits) or rat serum samples. All were incubated at 37°C for 2 h.
Following removal of the liquid, the plate was washed three times
with the reagent provided by the kits. HRP-conjugated fluid (100
µl) was added to each well at 37°C for 1 h. Following removal of
the liquid, sections were dried, and 100 µl substrate solution was
added to each well and incubated at 37°C in the dark for ~15 min.
Stop buffer (50 µl) was subsequently added to each well. Optical
density values were measured in each well at 450 nm using a
microplate reader.
Western blot analysis
Rat placenta was lysed in radioimmunoprecipitation
assay lysate containing protease inhibitor (cat. no. R0010; Beijing
Solarbio Science & Technology Co., Ltd.) on ice for 30 min.
Following centrifugation at 4,000 × g for 20 min at 4°C,
supernatant was collected. Proteins were quantified using a
Bicinchoninc Acid Assay Protein Quantification kit. The protein
samples (30 µg/lane) were subjected to 10% SDS-PAGE, and
transferred onto polyvinylidene fluoride membranes. The membrane
was blocked with 5% skimmed milk for 2 h at room temperature, and
incubated with antibodies against Cav1.2 (1:1,000), Cav3.1
(1:1,000), Cav3.2 (1:1,000), PKCβ (ab227490; rabbit; 76 kDa;
1:1,000; Abcam) CPI-17 (ab32213; rabbit; 17 kDa; 1:1,000; Abcam),
phosphorylated (p-)CPI-17 (ab52174; rabbit; 17 kDa; 1:1,000; Abcam)
and GAPDH (ab181602; rabbit; 36kDa; 1:10,000; Abcam) at 4°C
overnight. Subsequently, the membrane was washed with TBST, and
incubated with HRP-conjugated secondary antibody (1:2,000; cat. no.
ab6721; Abcam) at room temperature for 2 h. Proteins were
visualized using an enhanced chemiluminescence kit and gel imaging
system. Absorbance values were analyzed using Image Tools (Image J
1.8.0; National Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Rat uterus samples were triturated and TRIzol
reagent (15596018; Life Technologies; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) was used to extract RNA, according to the
manufacturer's protocol. RNA was reverse-transcribed into cDNA
using a High-Capacity RNA-to-cDNA kit (4387406; Invitrogen; thermo
Fisher Scientific, Inc.). qPCR was performed using a QuantiFast
SYBR Green PCR kit (204057; Qiagen GmbH, Hilden, Germany) according
to the manufacturer's protocol. Relative gene expression data was
quantified using the 2−ΔΔCq method (24). GAPDH was used as the reference gene.
Primers used for qPCR are presented in Table I.
 | Table I.Gene primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Gene primers used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| Cav1.2 | Forward:
CGGATCTGGAAGCTCGGAT |
|
| Reverse:
GTGATTGCGGAGCCCGA |
| Cav3.1 | Forward:
TTAAGAGCTACCTGATCGAG |
|
| Reverse:
TGTATCCGCACCTTCTGCA |
| Cav3.2 | Forward:
GCCATTCTCTCCTTCCTGCA |
|
| Reverse:
CGCAGCAGCAAATTTATG |
| GAPDH | Forward:
GCATGATGCCGGCAGCTTT |
|
| Reverse:
CAGCAACTGAATGAGGCCA |
Statistical analysis
All data were statistically analyzed using SPSS 19.0
(IBM Corp., Armonk, NY, USA). Data are presented as the mean +
standard deviation. One-way analysis of variance followed by
Tukey's post-hoc test was used for comparison among groups. All
experiments were repeated in triplicate. P<0.05 was considered
to indicate a statistically significant difference.
Results
Therapeutic effect of SAMe on
infectious premature delivery
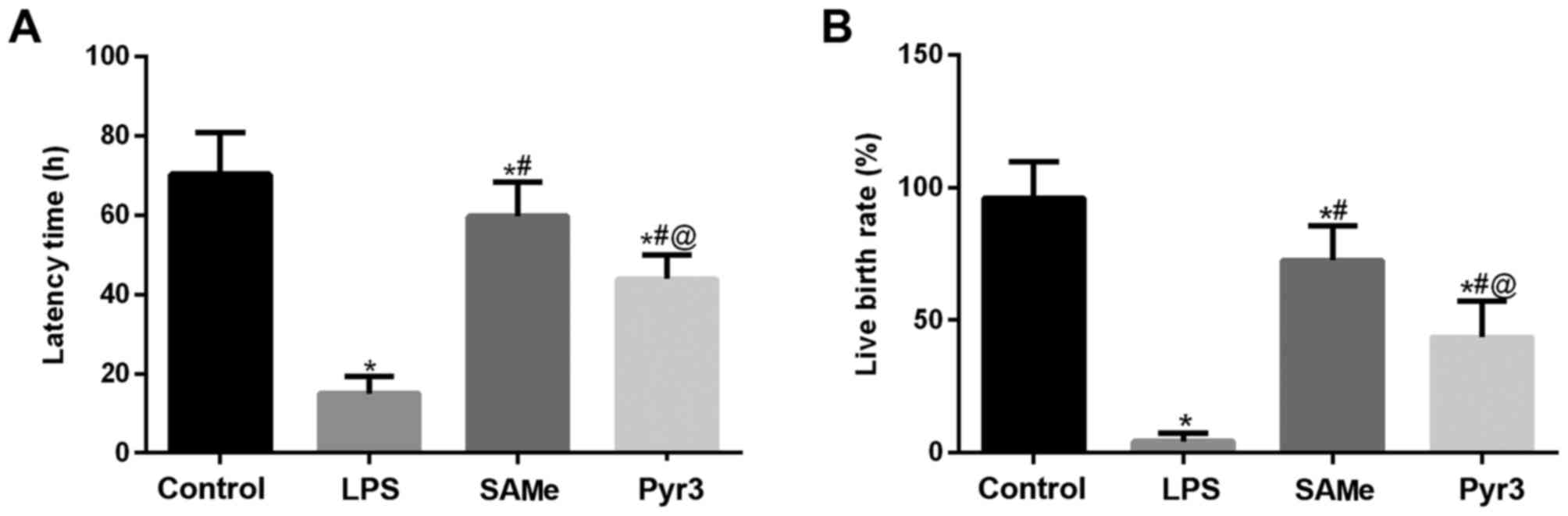
The effect of SAMe on rats with infectious premature
delivery was assessed as latency and live birth rate (Fig. 1A and B). In the control group,
latency time was ~70.4 h, or ~19 days of pregnancy, with a live
birth rate of 96%. In the LPS group, the fetus was delivered within
24 h, ~15 h, with a live birth rate of 4.3%. These results
indicated the model of infectious premature delivery was
established successfully. In the SAMe group the latency time was
59.7 h and the live birth rate was 72.5%. However, the therapeutic
effect of SAMe was significantly reduced following addition of
Pyr3. Compared with the LPS group, latency was prolonged by ~28.9
h, and the live birth rate was 43.5%.
SAMe suppresses inflammatory factors
IL-1β, IL-8, TNF-a, IL-10, MDA, SOD and NO serum content
To verify the effect of SAMe against inflammatory
reaction and oxidative stress, rat serum was collected for ELISA
(Fig. 2A-D). Results demonstrated
that IL-1β, IL-8 and TNF-α expression was significantly increased
in the LPS group (P<0.05 vs. control), whereas IL-10 expression
was significantly reduced (P<0.05 vs. control). Following
treatment with SAMe, IL-1β, IL-8 and TNF-α expression was
significantly diminished (P<0.05 vs. LPS), whereas IL-10
expression significantly increased (P<0.05 vs. LPS). Following
treatment with Pyr3, the anti-inflammatory effect of SAMe was
significantly reduced; IL-1β, IL-8 and TNF-α expression
significantly increased (P<0.05 vs. SAMe) and IL-10 expression
significantly decreased (P<0.05 vs. SAMe). These data suggest
that SAMe treatment significantly reduces the inflammatory reaction
induced by LPS.
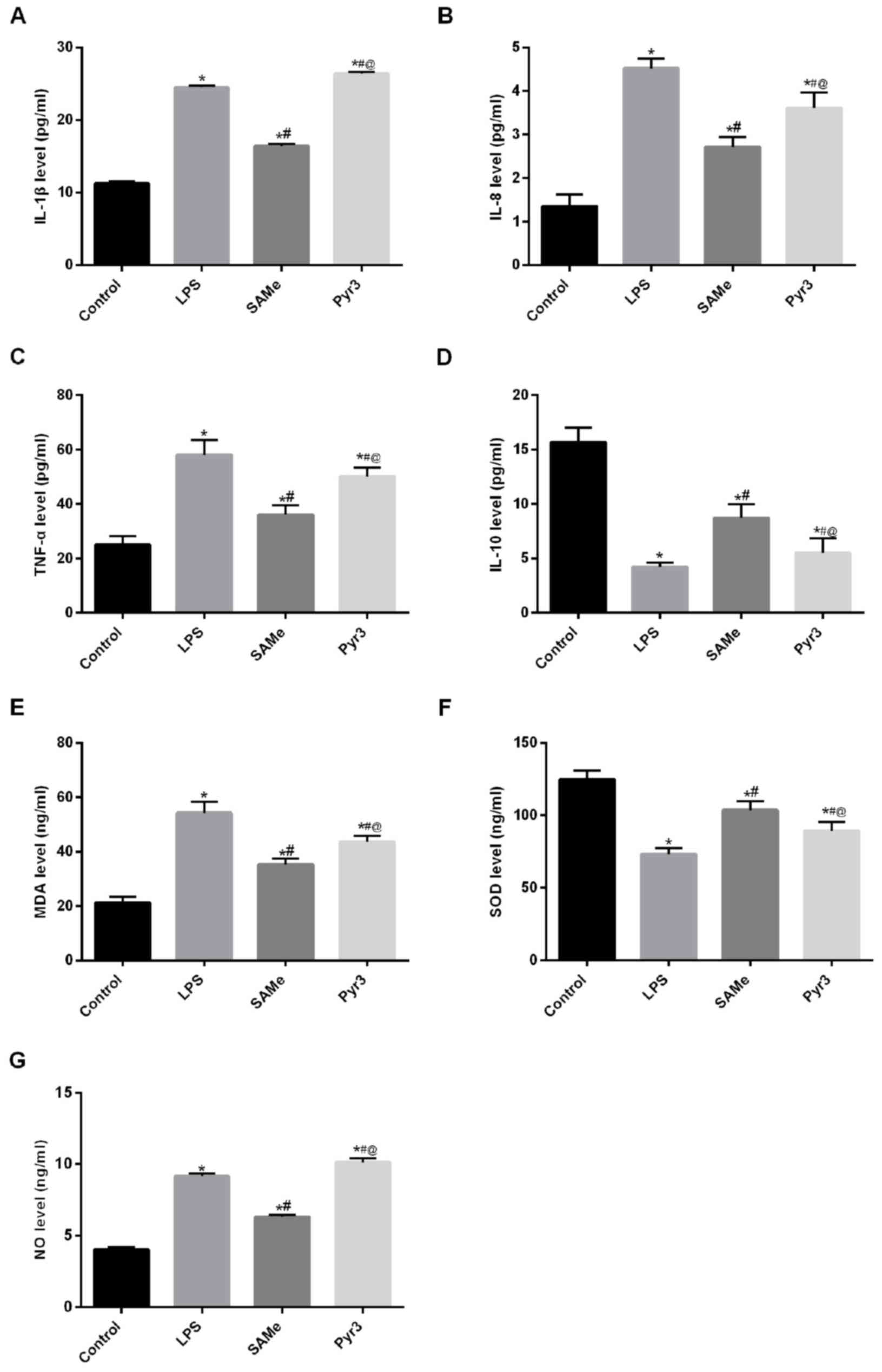 | Figure 2.SAMe suppresses inflammatory factors
IL-1β, IL-8, TNF-α, IL-10, MDA, SOD and NO. (A) IL-1β, (B) IL-8,
(C) TNF-α, (D) IL-10, (E) MDA, (F) SOD and (G) NO serum levels were
detected by ELISA. *P<0.05 vs. control group;
#P<0.05 vs. LPS group; @P<0.05 vs. SAMe
group. SAMe, S-adenosyl methionine; IL, interleukin; TNF-α, tumor
necrosis factor α; MDA, malondialdehyde; SOD, superoxide dismutase;
NO, nitric oxide; LPS, lipopolysaccharide. Data are provided as the
mean ± standard deviation (n=45). |
Further detection of serum oxidative stress-related
factors (Fig. 2E-G) revealed that
MDA and NO expression was significantly increased in the LPS group
(P<0.05 vs. control), and SOD expression was significantly
diminished (P<0.05 vs. control). Following SAMe treatment, MDA
and NO expression was significantly reduced (P<0.05 vs. LPS),
and SOD expression was significantly increased (P<0.05 vs. LPS).
Following treatment with Pyr3, MDA and NO expression was
significantly increased (P<0.05 vs. SAMe), while SOD expression
was significantly reduced (P<0.05 vs. SAMe). These data indicate
that SAMe can significantly inhibit LPS-induced inflammatory
reaction and oxidative stress. Following inhibiting TRPC3, the
effect of SAMe was significantly weakened. These findings suggest
that SAMe exerted its effect through the TRPC3 signaling
pathway.
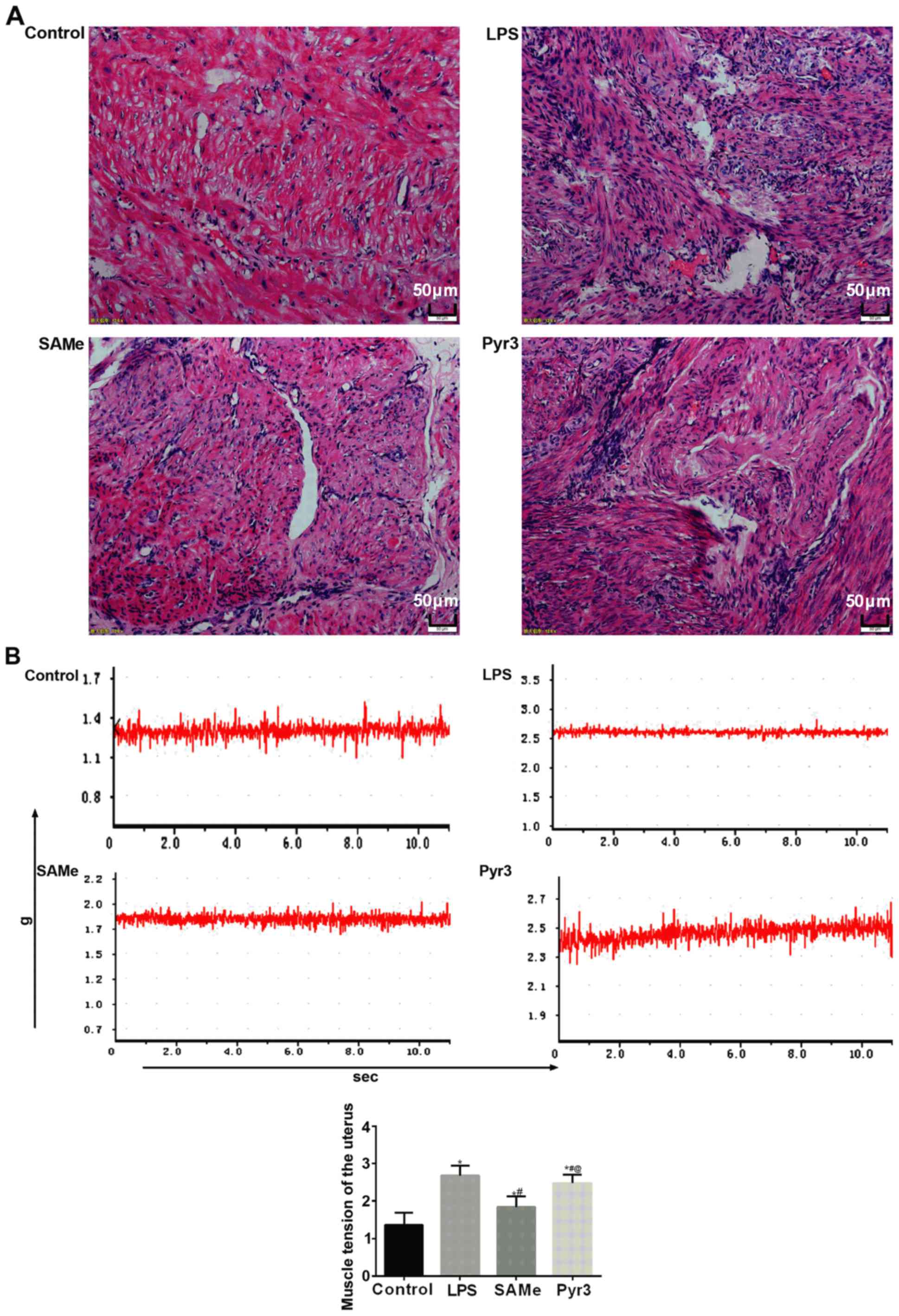
SAMe improves muscle tension of the
uterus
H&E staining was utilized to observe the
pathological changes in rat uterus (Fig.
3A). Results indicated that following LPS-induced premature
delivery, in the LPS group, a large number of inflammatory cells
infiltrated into the smooth muscle layer of uterus in rats, and
cells were irregularly distributed. Following SAMe treatment, this
inflammatory cell infiltration was markedly reduced. Following
suppressing TRPC3 expression with Pyr3, inflammatory cell
infiltration markedly increased in the smooth muscle layer of rat
uterus.
Muscle tension of the rat uterus (Fig. 3B) indicated that following
LPS-induced premature delivery, muscle tension of in vitro
uterine muscle strips significantly increased (P<0.05 vs.
control). The muscle tension in the SAMe group significantly
diminished (P<0.05 vs. LPS). Following suppressing TRPC3, muscle
tension significantly increased (P<0.05 vs. SAMe).
SAMe reduces the expression of calcium
channel in rats with infectious premature delivery
Western blot analysis (Fig. 4A) was used to measure Cav1.2, Cav3.1
and Cav3.2 expression in the rat uterus. Data suggested that
Cav1.2, Cav3.1 and Cav3.2 expression increased in rats with
premature delivery induced by LPS (all, P<0.05 vs. control).
Following treatment with SAMe, Cav1.2, Cav3.1 and Cav3.2 expression
significantly decreased (all, P<0.05 vs. LPS). Following
inhibition of TRPC3, Cav1.2, Cav3.1 and Cav3.2 expression
significantly increased (all, P<0.05 vs. SAMe). RT-qPCR
(Fig. 4B) results were consistent
with western blot assay results. Immunohistochemical analysis
(Fig. 4C) indicated that compared
with the control group, Cav1.2, Cav3.1 and Cav3.2 expression
notably increased in rats with premature delivery induced by LPS.
Following treatment with SAMe, Cav1.2, Cav3.1 and Cav3.2 expression
significantly decreased compared with the LPS group. Following
inhibition of TRPC3, Cav1.2, Cav3.1 and Cav3.2 expression
significantly increased compared with the SAMe group.
Immunostaining was indicated with a blue color. These findings
suggest that L-type calcium channels and T-type calcium channels
participate in the occurrence of premature delivery. SAMe can
effectively weaken the expression of L-type and T-type calcium
channel-related proteins and uterine contraction.
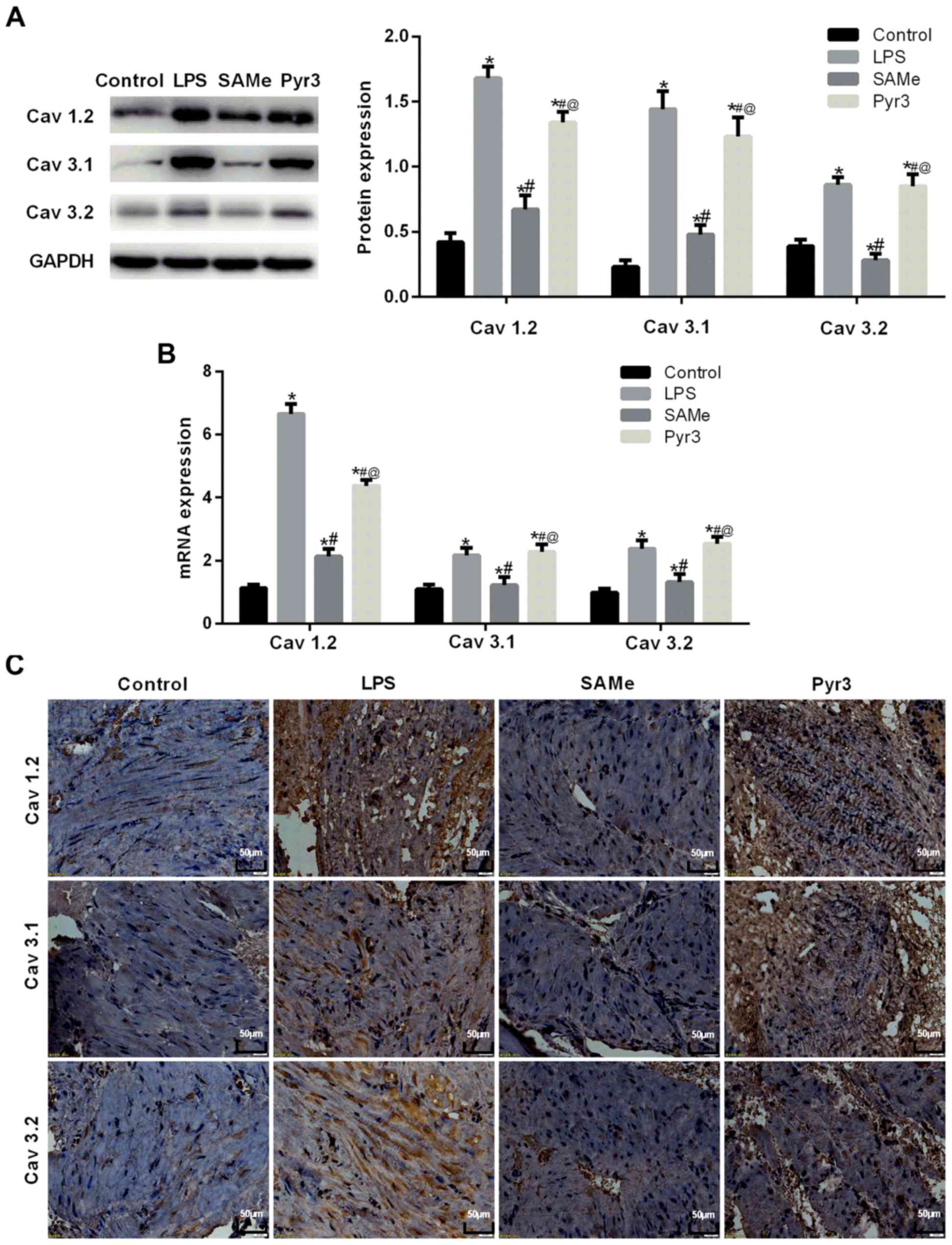 | Figure 4.SAMe reduces the expression of
calcium channels in rats with infectious premature delivery.
Cav1.2, Cav3.1 and Cav3.2 expression were detected by (A) western
blot analysis, (B) reverse transcription-quantitative polymerase
chain reaction and (C) immunohistochemistry. *P<0.05 vs. control
group; #P<0.05 vs. LPS group; @P<0.05
vs. SAMe group. SAMe, S-adenosyl methionine; Cav1.2, calcium
channel, voltage-dependent, L type, α 1C subunit; Cav3.1, calcium
channel, voltage-dependent, T type, α 1G subunit; Cav3.2, calcium
channel, voltage-dependent, T type, α 1H subunit; LPS,
lipopolysaccharide. Data are provided as the mean ± standard
deviation (n=45). |
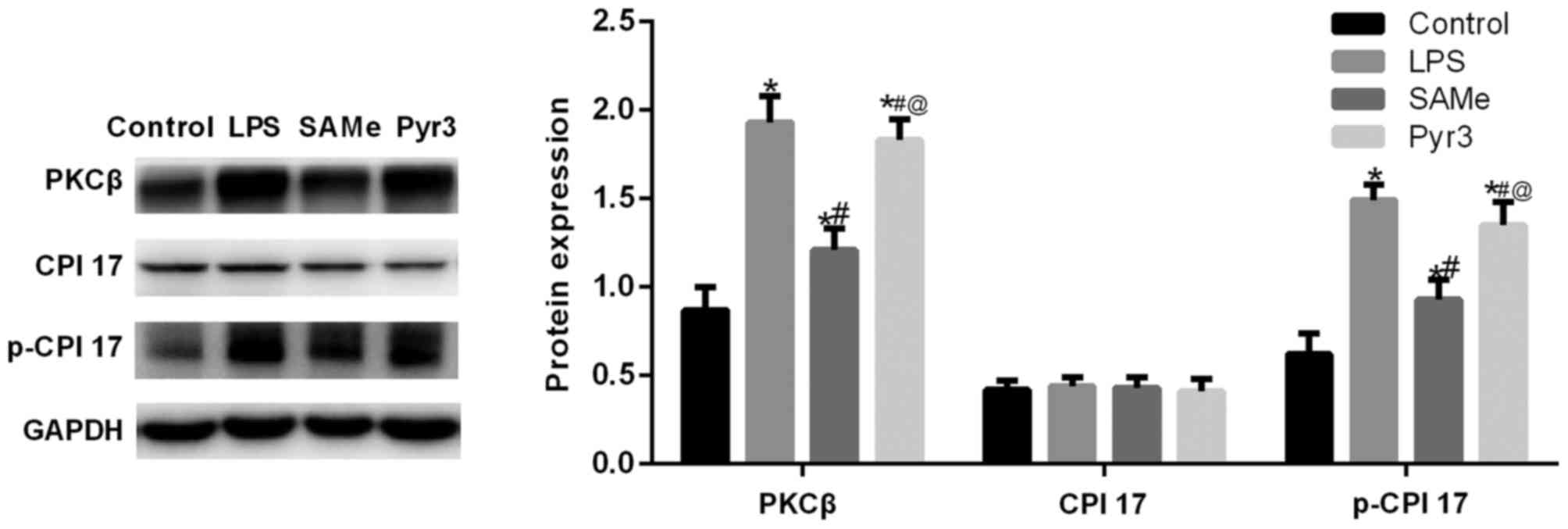
SAMe promotes uterine contraction and
delivery via the TRPC3/PKCβ/CPI-17 signaling pathway
Western blot analysis was utilized to measure the
expression of TRPC3/PKCβ/CPI-17 signaling pathway-related proteins
in the rat uterus (Fig. 5). Data
suggested that following premature delivery induced by LPS, PKCβ
and p-CPI-17 expression significantly increased (P<0.05 vs.
control). Following treatment with SAMe, PKCβ and p-CPI-17
expression significantly diminished (P<0.05 vs. LPS). These data
indicate that the TRPC3/PKCβ/CPI-17 signaling pathway participated
in the occurrence and development of premature delivery. Following
the application of Pyr3, inflammatory factor expression increased,
and PKCβ and p-CPI-17 expression significantly increased (P<0.05
vs. SAMe). These results indicate that the anti-inflammatory effect
of SAMe is exerted via the TRPC3/PKCβ/CPI-17 signaling pathway.
Discussion
Infection has been demonstrated to be associated
with premature delivery. Histological evidence of intrauterine
infection is exhibited in 19–74% preterm infants (25). At present, it is believed that
infectious preterm delivery is associated with Gram-negative
bacteria endotoxin LPS that stimulates an increase in local
cytokines, and subsequently stimulates abnormal uterine contraction
(26). In the present study,
pregnant rat models of premature delivery were successfully
established via LPS administration. SAMe treatment revealed that
SAMe could reduce the rate of premature delivery, elevate survival
rate of fetal rats, improve muscle tension, and increase
calcium-specific protein expression, indicating that SAMe has a
therapeutic effect on infectious premature delivery. Administration
of the TRPC3 protein inhibitor Pyr3 demonstrated that the
therapeutic effect of SAMe was suppressed, and that the
TRPC3/PKCβ/CPI-17 signaling pathway has an important regulatory
effect.
SAMe is a physiologically active substance found in
all tissues and fluids, and is converted from methionine under the
action of adenosylmethionase (18).
As a methyl donor and a physiological sulfur compound, SAMe can
participate in important biochemical reactions in the human body
and transform thioxo into taurine (18). Previous studies have also verified
that SAMe could regulate IL-10 production in monocytes, and
increase IL-6 synthesis in monocytes and liver Kupffer cells
following LPS stimulation (27,28). Its
metabolite, 5-methylthioadenosine, has an important
immunomodulatory effect on the inflammatory response of hepatocytes
(29). Simultaneously, SAMe is able
to reduce serum TNF-α and TGF-β contents (30,31). In
the present study, SAMe treatment increased IL-1β, IL-8, TNF-α and
IL-10 contents in rats with infectious premature delivery, and had
an inhibitory effect on oxidative stress factors. These findings
indicated that SAMe could suppress inflammatory reaction and the
resulting stress response in rats with infectious premature
delivery.
The mechanism of infectious premature delivery is
complex and is the result of a number of factors. There are many
theories about the causes of premature childbirth, including
neurotransmitter theory, mechanical theory, hormone control theory
and immunity theory (32–34). These theories share a common final
path; the induction of the contraction of uterine smooth muscle
(35). The contraction of the uterus
is determined by the concentration of free calcium in the muscle
cells (36). Prior to uterine
contraction, cytoplasmatic free calcium concentration increases
significantly. Mark et al (37) recently presented a mechanism of
intracellular calcium signaling that leads to cyclic uterine
contractions. Normally, extracellular calcium ions flow through the
calcium channel of the cell membrane, triggering Ca2+
release from the sarcoplasmic reticulum calcium release channel.
Therefore, elevated intracellular calcium concentration results in
uterine contraction and premature delivery. It can be observed that
the calcium channel serves an important role in regulating smooth
muscle activity. The present study detected the expression of
L-type calcium channel Cav1.2 and T-type calcium channel Cav3.1 and
Cav3.2 expression in the rat uterus (38,39).
Results indicated that each protein expression increased in
premature delivery in rats of the LPS group, and confirmed that
L-type and T-type calcium channels participated in the occurrence
of premature delivery. The addition of SAMe revealed that calcium
channel protein expression decreased in each group, and muscle
tension test verified this conclusion. It is thus clear that SAMe
could effectively weaken L-type and T-type calcium channel-related
protein expression, and weaken uterine contraction.
TRPC channel has an extensive physiological role in
addition to mediating extracellular calcium influx and inducing
uterine contraction, which has been confirmed in previous studies
of the nervous system, vascular smooth muscle, myocardium, and skin
(40–43). A variety of physiological phenomena
depend on the opening of TRPC channels. A previous study verified
that TRPC channel was expressed in the pregnant uterus (44). The upregulation of TRPC3 channel, and
L-type and T-type calcium channel functions enhances the
contractility of uterine spiral arteries during pregnancy, which is
associated with labor onset. In the present study, on the basis of
SAMe treatment, Pyr3 was administered, so that the inhibitory
effect of SAMe on inflammation and regulatory effect on calcium
channels were suppressed, indicating that the therapeutic effect of
SAMe on infectious premature delivery is associated with TRPC
channel. It has previously been demonstrated that TRPC channels are
expressed in mammalian uterine muscle tissue (45). Mechanical traction of human uterine
smooth muscle ex vivo can increase the expression of TRPC 3
channel (46).
The PKCβ/CPI-17 pathway serves an important role in
the contraction of vascular smooth muscle and the mechanism of
calcium sensitization. Su et al (47) previously induced CPI-17 gene
silencing in bronchial smooth muscle using RNA interference
technology, and observed that calcium sensitization, contraction
frequency and contractility of bronchial smooth muscle decreased,
which suggested that CPI-17 has an effect on the contraction of
smooth muscle. Ozaki et al (15) previously demonstrated that PKC
agonist can significantly increase the phosphorylation of MLC and
the contraction of vascular smooth muscle. In the presence of the
specific MLCP activity inhibitor, Rho-associated kinase, exogenous
phosphorylated CPI-17 can increase the contraction of vascular
smooth muscle in a dose-dependent manner (48). The present study detected proteins in
the TRPC3/PKCβ/CPI-17 signaling pathway, and demonstrated that SAMe
could downregulate the increased expression of TRPC3, PKCβ and
p-CPI-17 induced by infectious premature delivery. When TRPC3
inhibitor was added, the effect of SAMe was no longer apparent.
These data indicate that SAMe improves muscle tension, suppresses
the influx of exogenous calcium ions, and inhibits inflammatory
reaction through the TRPC3/PKCβ/CPI-17 pathway, thereby
ameliorating infectious premature delivery. The present study is a
preliminary exploration of the mechanism of action of SAMe, and the
underlying regulatory mechanism in improving infectious premature
delivery requires further investigations.
In conclusion, the present study successfully
established a rat model of infectious premature delivery. SAMe was
able to prolong the delivery time, decrease the mortality rate,
inhibit the inflammatory reaction and oxidative stress, and
regulate the calcium channel expression in this model. Its
regulatory mechanism is possibly associated with the
PKC/PLCβ/CPI-17 signaling pathway. These findings provide a basis
for the clinical prevention and treatment of infectious premature
delivery and targeted therapy of novel drugs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Startup
Foundation for Liaoning Province of China (grant no. 201601392) and
the Startup Foundation for China (grant no. 2017M613440).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG, TH, XL, JW and MH conceived and designed the
study, acquired the data, interpreted the results and drafted the
manuscript. MH contributed to the acquisition of funding and
support. JG, TH, XL, LS and JZ performed the experiments. YH and YX
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Animal
Experiments Ethics Committee of the General Hospital of Shenyang
Military (Shenyang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vogel JP, Chawanpaiboon S, Watananirun K,
Lumbiganon P, Petzold M, Moller AB, Thinkhamrop J, Laopaiboon M,
Seuc AH, Hogan D, et al: Global, regional and national levels and
trends of preterm birth rates for 1990 to 2014: Protocol for
development of World Health Organization estimates. Reprod Health.
13:762016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryan JG and Dogbey E: Preterm births: A
global health problem. MCN Am J Matern Child Nurs. 40:278–283.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rours GI, Duijts L, Moll HA, Arends LR, de
Groot R, Jaddoe VW, Hofman A, Steegers EA, Mackenbach JP, Ott A, et
al: Chlamydia trachomatis infection during pregnancy associated
with preterm delivery: A population-based prospective cohort study.
Eur J Epidemiol. 26:493–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dowling O, Chatterjee PK, Gupta M, Tam Tam
HB, Xue X, Lewis D, Rochelson B and Metz CN: Magnesium sulfate
reduces bacterial LPS-induced inflammation at the maternal-fetal
interface. Placenta. 33:392–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bagur R and Hajnóczky G: Intracellular
Ca2+ sensing: Its role in calcium homeostasis and
signaling. Mol Cell. 66:780–788. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaneko S: Cognitive Function and Calcium.
Structures and functions of Ca2+-permeable channels. Clin Calcium.
25:181–187. 2015.(In Japanese). PubMed/NCBI
|
|
7
|
Reddish FN, Miller CL, Gorkhali R and Yang
JJ: Calcium dynamics mediated by the endoplasmic/sarcoplasmic
reticulum and related diseases. Int J Mol Sci. 18:pii: E1024.
2017.PubMed/NCBI
|
|
8
|
Abdoul-Azize S, Buquet C, Vannier JP and
Dubus I: Pyr3, a TRPC3 channel blocker, potentiates dexamethasone
sensitivity and apoptosis in acute lymphoblastic leukemia cells by
disturbing Ca(2+) signaling, mitochondrial membrane potential
changes and reactive oxygen species production. Eur J Pharmacol.
784:90–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eder P, Poteser M, Romanin C and Groschner
K: Na(+) entry and modulation of Na(+)/Ca(2+) exchange as a key
mechanism of TRPC signaling. Pflugers Arch. 451:99–104. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pedersen SF, Owsianik G and Nilius B: TRP
channels: An overview. Cell Calcium. 38:233–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hogan PG and Rao A: Store-operated calcium
entry: Mechanisms and modulation. Biochem Biophys Res Commun.
460:40–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pagiatakis C, Gordon JW, Ehyai S and
McDermott JC: A novel RhoA/ROCK-CPI-17-MEF2C signaling pathway
regulates vascular smooth muscle cell gene expression. J Biol Chem.
287:8361–8370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eto M, Kirkbride JA, Chugh R, Karikari NK
and Kim JI: Nuclear localization of CPI-17, a protein phosphatase-1
inhibitor protein, affects histone H3 phosphorylation and
corresponds to proliferation of cancer and smooth muscle cells.
Biochem Biophys Res Commun. 434:137–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaki N and Sutoh K: Structure-function
relationship of myosin. Tanpakushitsu Kakusan Koso. 46 11
Suppl:S1740–S1749. 2001.(In Japanese).
|
|
15
|
Ozaki H, Yasuda K, Kim YS, Egawa M,
Kanzaki H, Nakazawa H, Hori M, Seto M and Karaki H: Possible role
of the protein kinase C/CPI-17 pathway in the augmented contraction
of human myometrium after gestation. Br J Pharmacol. 140:1303–1312.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Lv J, Wu J, Zhou X, Jiang L, Zhu X,
Tu Q, Tang J, Liu Y, He A, et al: Maternal high-salt diet altered
PKC/MLC20 pathway and increased ANG II receptor-mediated
vasoconstriction in adult male rat offspring. Mol Nutr Food Res.
60:1684–1694. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uneyama H and Takeuchi K: New therapeutic
strategy for amino acid medicine: Preface. J Pharmacol Sci.
118:129–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Struck AW, Thompson ML, Wong LS and
Micklefield J: S-adenosyl-methionine-dependent methyltransferases:
highly versatile enzymes in biocatalysis, biosynthesis and other
biotechnological applications. Chembiochem. 13:2642–2655. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frezza M, Centini G, Cammareri G, Le
Grazie C and Di Padova C: S-adenosylmethionine for the treatment of
intrahepatic cholestasis of pregnancy. Results of a controlled
clinical trial. Hepatogastroenterology. 37 Suppl 2:S122–S125.
1990.
|
|
20
|
De Silva V, El-Metwally A, Ernst E, Lewith
G and Macfarlane GJ: Arthritis Research UK Working Group on
Complementary and Alternative Medicines: Evidence for the efficacy
of complementary and alternative medicines in the management of
osteoarthritis: A systematic review. Rheumatology (Oxford).
50:911–920. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polli E, Cortellaro M, Parrini L, Tessari
L and Chériè Lignière G: Pharmacological and clinical aspects of
S-adenosylmethionine (SAMe) in primary degenerative arthropathy
(osteoarthrosis). Minerva Med. 66:4443–4459. 1975.(In Italian).
PubMed/NCBI
|
|
22
|
Galizia I, Oldani L, Macritchie K, Amari
E, Dougall D, Jones TN, Lam RW, Massei GJ, Yatham LN and Young AH:
S-adenosyl methionine (SAMe) for depression in adults. Cochrane
Database Syst Rev. 10:CD0112862016.PubMed/NCBI
|
|
23
|
Zhao Y, Li JS, Guo MZ, Feng BS and Zhang
JP: Inhibitory effect of S-adenosylmethionine on the growth of
human gastric cancer cells in vivo and in vitro. Chin J Cancer.
29:752–760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agrawal V and Hirsch E: Intrauterine
infection and preterm labor. Semin Fetal Neonatal Med. 17:12–19.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bondarenko KM and Bondarenko VM: Bacterial
lipopolsaccharides in pathogenesis of gynecological diseases and
obstetric complications. Zh Mikrobiol Epidemiol Immunobiol. 80–86.
2014.(In Russian). PubMed/NCBI
|
|
27
|
Song Z, Chen T, Deaciuc IV, Uriarte S,
Hill D, Barve S and McClain CJ: Modulation of endotoxin stimulated
interleukin-6 production in monocytes and Kupffer cells by
S-adenosylmethionine (SAMe). Cytokine. 28:214–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song Z, Barve S, Chen T, Nelson W, Uriarte
S, Hill D and McClain C: S-adenosylmethionine (AdoMet) modulates
endotoxin stimulated interleukin-10 production in monocytes. Am J
Physiol Gastrointest Liver Physiol. 284:G949–G955. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hevia H, Varela-Rey M, Corrales FJ,
Berasain C, Martínez-Chantar ML, Latasa MU, Lu SC, Mato JM,
García-Trevijano ER and Avila MA: 5′-methylthioadenosine modulates
the inflammatory response to endotoxin in mice and in rat
hepatocytes. Hepatology. 39:1088–1098. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song Z, Zhou Z, Song M, Uriarte S, Chen T,
Deaciuc I and McClain CJ: Alcohol-induced S-adenosylhomocysteine
accumulation in the liver sensitizes to TNF hepatotoxicity:
Possible involvement of mitochondrial S-adenosylmethionine
transport. Biochem Pharmacol. 74:521–531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang Y, Chu H, Cao G, Du X, Min X and Wan
C: S-Adenosylmethionine attenuates bile duct early warm ischemia
reperfusion injury after rat liver transplantation. Mol Immunol.
95:83–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zurabishvili S, Mamamtavrishvili I,
Apridonidze K and Shanidze L: Role of intracellular infections in
premature childbirth. Georgian Med News. 126:58–60. 2005.
|
|
33
|
Papiernik E: Premature childbirth and its
prevention. Arch Fr Pediatr. 34:488–491. 1977.(In French).
PubMed/NCBI
|
|
34
|
Penev I: Etiopathogenesis of premature
childbirth. Akush Ginekol (Sofiia). 23:193–200. 1984.(In
Bulgarian). PubMed/NCBI
|
|
35
|
Renthal NE, Williams KC, Montalbano AP,
Chen CC, Gao L and Mendelson CR: Molecular regulation of
parturition: A myometrial perspective. Cold Spring Harb Perspect
Med. 5:pii: a023069. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sanborn BM: Hormones and calcium:
Mechanisms controlling uterine smooth muscle contractile activity.
The litchfield lecture. Exp Physiol. 86:223–237. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mark MD, Schwitalla JC, Groemmke M and
Herlitze S: Keeping our calcium in balance to maintain our balance.
Biochem Biophys Res Commun. 483:1040–1050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blanks AM, Zhao ZH, Shmygol A, Bru-Mercier
G, Astle S and Thornton S: Characterization of the molecular and
electrophysiological properties of the T-type calcium channel in
human myometrium. J Physiol. 581:915–926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Collins PL, Moore JJ, Lundgren DW,
Choobineh E, Chang SM and Chang AS: Gestational changes in uterine
L-type calcium channel function and expression in guinea pig. Biol
Reprod. 63:1262–1270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang R, Luo H, Wang S, Chen W, Chen Z,
Wang HW, Chen Y, Yang J, Zhang X, Wu W, et al: MicroRNA-377
inhibited proliferation and invasion of human glioblastoma cells by
directly targeting specificity protein 1. Neuro Oncol.
16:1510–1522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi J, Miralles F, Birnbaumer L, Large WA
and Albert AP: Store depletion induces Gαq-mediated PLCβ1 activity
to stimulate TRPC1 channels in vascular smooth muscle cells. FASEB
J. 30:702–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ho JC and Lee CH: TRP channels in skin:
From physiological implications to clinical significances.
Biophysics (Nagoya-shi). 11:17–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zeng C, Tian F and Xiao B: TRPC channels:
Prominent candidates of underlying mechanism in neuropsychiatric
diseases. Mol Neurobiol. 53:631–647. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kawarabayashi Y, Hai L, Honda A, Horiuchi
S, Tsujioka H, Ichikawa J and Inoue R: Critical role of
TRPC1-mediated Ca2+ entry in decidualization of human
endometrial stromal cells. Mol Endocrinol. 26:846–858. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ku CY, Babich L, Word RA, Zhong M, Ulloa
A, Monga M and Sanborn BM: Expression of transient receptor channel
proteins in human fundal myometrium in pregnancy. J Soc Gynecol
Investig. 13:217–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng D, Zhang L, Na Q, Liu S, Zhuang Y,
Lv Y and Liu C: Enhanced expression of transient receptor potential
channel 3 in uterine smooth muscle tissues of
lipopolysaccharide-induced preterm delivery mice. Iran J Basic Med
Sci. 19:567–572. 2016.PubMed/NCBI
|
|
47
|
Su W, Xie Z, Liu S, Calderon LE, Guo Z and
Gong MC: Smooth muscle-selective CPI-17 expression increases
vascular smooth muscle contraction and blood pressure. Am J Physiol
Heart Circ Physiol. 305:H104–H113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Swärd K, Mita M, Wilson DP, Deng JT,
Susnjar M and Walsh MP: The role of RhoA and Rho-associated kinase
in vascular smooth muscle contraction. Curr Hypertens Rep. 5:66–72.
2003. View Article : Google Scholar : PubMed/NCBI
|