Introduction
Gastric cancer has the highest incidence rate among
malignant tumors in China, and this rate is increasing year by
year, which is the leading threat to the health of people. At the
same time, the incidence rate of diabetes mellitus is increased
with the changes in lifestyles and the rising trend of the aging of
population (1). Surgery-based
comprehensive treatment is still the preferred effective treatment
for patients with opportunistic gastric cancer complicated with
diabetes mellitus. Most patients with gastric cancer complicated
with diabetes mellitus have cellular immune dysfunction,
malnutrition, poor cardiopulmonary function and decreased tissue
repair capacity and other symptoms because of preoperative tumor
consumption and intestinal preparation, postoperative fasting, and
the surgical trauma stress keeps the body at high metabolic state,
which further increases the patient's blood glucose level, thus
leading to poor control of blood glucose and aggravating
malnutrition (2). Surgical curative
effect will be affected by postoperative hyperglycemia and
malnutrition, and the incidence rate of postoperative complications
is increased, thus affecting the body's functional status of
patients (3). The nutrition support
is an important means to promote the rehabilitation of patients,
but how to better improve the postoperative nutritional status and
control the blood glucose level of patients with gastric cancer
complicated with diabetes mellitus is the focus and difficulty of
the current research. Therefore, in this study, patients with
gastric cancer complicated with diabetes mellitus were treated with
early enteral nutrition (EEN) or total parenteral nutrition (TPN)
support after radical gastrectomy, thus helping explore the effects
of different nutrition supports on the nutritional status and blood
glucose of patients, which is expected to provide a certain
guidance for clinical operations.
Materials and methods
Clinical data
One hundred and twenty-nine patients with complete
clinical data and pathologically confirmed as gastric cancer
complicated with diabetes mellitus after operation who were
admitted to the First People's Hospital of Jinan and received
radical gastrectomy from June 2012 to June 2016 were selected into
the study. According to the diagnostic criteria for diabetes
mellitus type 2 issued by the American Diabetes Association (ADA)
in 2006, the patients had diabetes mellitus type 2 (4). Inclusion criteria: Patients with
primary gastric cancer and diabetes mellitus received no
preoperative treatment and were complicated with no other
malignancies. Exclusion criteria: Patients with severe liver or
renal function impairment. According to different nutritional
pathways, patients were randomly divided into the EEN group (n=66)
and the TPN group (n=63). In the EEN group, there were 34 males and
32 females at the mean age of 48.07±7.45 years. The preoperative
complications included hypertension (n=12), coronary heart disease
(n=8) and peripheral neuropathy (n=3). Pathological features of
patients: Stage I (n=9), Stage II (n=21) and Stage III (n=36);
histological grading: G1 (n=23), G2 (n=29) and G3 (n=14); operation
methods: Billroth I (n=10), Billroth II (n=45) and total
gastrectomy (n=11). In the TPN group, there were 32 males and 31
females at the mean age of 48.21±6.78 years. Preoperative
complications included hypertension (n=11), coronary heart disease
(n=7) and peripheral neuropathy (n=2). Pathological features of
patients: Stage I (n=8), Stage II (n=20) and Stage III (n=35);
histological grading: G1 (n=23), G2 (n=28) and G3 (n=12); operation
methods: Billroth I operation (n=9), Billroth II operation (n=44)
and total gastrectomy (n=10). There were no significant differences
between the two groups in general clinical data (P>0.05), and
the baseline data were relatively consistent and comparable. This
study was approved by the Ethics Committee of the First People's
Hospital of Jinan, (Jinan, China). Patients were informed of the
condition and signed informed consent. Preoperative preparation was
actively conducted (Table I).
 | Table I.General information of patients in the
two groups. |
Table I.
General information of patients in the
two groups.
| Characteristics | EEN group (n=66) | TPN group (n=63) | t value | P-value |
|---|
| Sex (n) |
|
| 1.769 | 0.168 |
| Male | 34 | 32 |
|
|
|
Female | 32 | 31 |
|
|
| Age (years) | 48.07±7.45 | 48.21±6.78 | 2.126 | 0.073 |
| Preoperative
complications (n) |
|
| 1.912 | 0.089 |
|
Hypertension | 12 | 11 |
|
|
| Coronary
heart disease | 8 | 7 |
|
|
|
Peripheral neuropathy | 3 | 2 |
|
|
| Tumor, node and
metastasis (TNM) staging |
|
| 1.901 | 0.094 |
| Stage
I | 9 | 8 |
|
|
| Stage
II | 21 | 20 |
|
|
| Stage
III | 36 | 35 |
|
|
| Histological
grading |
|
| 2.313 | 0.065 |
| G1 | 23 | 23 |
|
|
| G2 | 29 | 28 |
|
|
| G3 | 14 | 12 |
|
|
| Operation methods
(n) |
|
| 1.834 | 0.152 |
| Billroth
I operation | 10 | 9 |
|
|
| Billroth
II operation | 45 | 44 |
|
|
| Total
gastrectomy | 11 | 10 |
|
|
General clinical data records
General clinical data included sex, age, weight
(patients were weighed in fasting state, wearing unlined dress and
no hat or shoes), past medical history (hypertension, coronary
heart and disease), family history, operation methods, the
operation of the International Federation of Gynecology and
Obstetrics (FIGO) in 2012, histological grading, postoperative
evacuation time, complications.
Methods
Nitrogen (0.2 g/kg) and 125.5 kJ/(kg/day) calorie
were given to the EEN group and TPN group. The blood glucose of two
groups of patients was maintained using the insulin pump, which was
monitored once every 6 h, and the pumping quantity was adjusted
according to the condition of blood glucose. According to the
evacuation condition of patients, the nutrition input was
correspondingly decreased. In supplying nutrition, the nutrition
was supplied in a slow-fast speed, from a small amount to a large
amount and from a low concentration to a high concentration, so as
to avoid patient discomforts. a) Patients in the EEN group received
EEN (nutrition was given through intestinal canals within 24 h
after operation). When the anastomotic stoma of the digestive tract
was reconstructed, the nutrition tube was inserted into the
jejunum. The nutrition tube was inserted at 30–50 cm of the flexor
ligament in patients receiving Billroth I operation; it was
inserted at 30–50 cm of the distal anastomotic stoma of the gastric
jejunum in patients receiving Billroth II operation, and the length
of the tube in the jejunum was above 40 cm; it was inserted at
30–50 cm of the distal anastomotic stoma of the oesophagus jejunum
in patients receiving total gastrectomy with one end buried in the
tube along the intestinal wall in the tunnel type with the length
of approximately 10 cm and the other end fixed by the parietal
peritoneum and skin. At 24 h after operation, at the first place,
patients with gastrointestinal symptoms in the nutrition tube after
instilling 300 ml saline were excluded, and those without this
symptom further received nutrition support in the jejunum. b)
Patients in the TPN group were supported by parenteral nutrition,
including amino acids, fat emulsion, carbohydrates, vitamins,
electrolytes and other nutrients needed by the body, which were
provided by parenteral pathways, and these nutrient solutions were
intravenously input after operation.
Observation indexes
Venous blood (3–5 ml) before the elbow was extracted
from patients under the fasting state in the early morning, which
was measured after the anticoagulation centrifugation and
refrigeration. The levels of liver function indexes [total
bilirubin (TBL), alanine aminotransferase (ALT) and aspartate
transaminase (AST)] and nutritional indexes [total protein (TP),
prealbumin (PAB) and hemoglobin (HGB)] were measured before
operation and on the 4th day and 8th day after operation. The blood
glucose levels of patients at fasting and 2 h after a meal on the
1st day before operation, the date of operation and the 1st-8th day
after operation were dynamically measured.
Statistical analysis
Data were recorded using Statistical Product and
Service Solutions (SPSS) 20.0 software (IBM Corp., Armonk, NY,
USA). Measurement data were described as [mean ± standard deviation
(SD)] and compared using the t-test and the analysis of variance.
Count data were presented as percentage and compared using Fisher's
exact test or Chi-square test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Comparison of the improvement of
nutritional indexes between the two groups of patients
There were no significant differences in
preoperative indexes of patients between the two groups, and the
differences were not statistically significant (P>0.05). On the
8th day after operation, there were no statistically significant
differences between the two groups in terms of TBL, ALT, AST, TP,
PAB, HGB and weight (Wt) (P<0.05). On the 4th day after
operation, the levels of TBL, ALT, AST, TP, PAB, HGB and Wt in the
EEN group were significantly higher than those in the conventional
group (P<0.05) (Table II).
 | Table II.Comparison of the improvement of
nutritional indexes between the two groups of patients (mean ±
SD). |
Table II.
Comparison of the improvement of
nutritional indexes between the two groups of patients (mean ±
SD).
|
| EEN group | TPN group |
|---|
|
|
|
|
|---|
| Index | Before operation | On the 4th day after
operation | On the 8th day after
operation | Before operation | On the 4th day after
operation | On the 8th day after
operation |
|---|
| TBL (U/mmol) | 10.65±3.63 |
12.34±4.61b | 11.82±2.38 | 11.07±3.46 | 26.43±4.69 | 10.56±3.72 |
| ALT (U/mmol) | 26.12±4.34 |
29.05±4.39a,b | 25.64±3.84 | 25.78±4.26 |
41.27±5.39a | 27.21±4.37 |
| AST (U/mmol) | 26.57±3.67 |
35.45±4.36a,b | 26.64±4.52 | 27.35±3.51 |
54.81±5.45a | 28.62±4.33 |
| TP (g/l) | 63.64±4.52 |
59.09±5.64a,b | 65.48±4.65 | 63.84±4.58 |
43.26±4.07a | 64.21±5.18 |
| PAB (g/l) | 180.34±19.73 |
191.43±18.62a,b | 202.31±20.04 | 189.35±19.82 |
181.05±18.47a | 193.10±19.58 |
| HGB (g/l) | 99.67±4.12 |
92.32±4.23a,b | 97.76±5.15 | 99.32±3.96 |
80.92±4.15a | 96.39±5.02 |
| Wt (kg) | 52.21±9.27 |
54.13±7.16b | 52.18±8.75 | 52.24±9.19 |
50.43±7.54a | 52.59±8.71 |
Comparison of postoperative
complications, gastrointestinal function recovery and other
conditions between the two groups of patients
In the TPN group, there were a total of 29 patients
with adverse reactions, including pulmonary infection (n=5),
anastomotic fistula (n=6), wound infection (n=7), liver function
impairment (n=3) and vein catheter infection (n=8); in the EEN
group, there was no patient with adverse reactions. The incidence
rate of complications in the EEN group was significantly lower than
that in the TPN group, and the difference was statistically
significant (P<0.05). The postoperative evacuation time of
patients in the TPN group was later than that in the EEN group, and
the hospitalization time and cost were higher than those in the EEN
group (P<0.05) (Table III).
 | Table III.Comparison of postoperative
complications and gastrointestinal function recovery between the
two groups of patients. |
Table III.
Comparison of postoperative
complications and gastrointestinal function recovery between the
two groups of patients.
| Index | EEN group
(n=66) | TPN group
(n=63) | t/χ2
value | P-value |
|---|
| Pulmonary
infection | 0 | 5 (7.94) | 3.912 | 0.006 |
| Anastomotic
fistula | 0 | 6 (9.52) | 4.235 | 0.004 |
| Wound
infection | 0 | 7 (11.11) | 5.214 | 0.002 |
| Liver function
impairment | 0 | 3 (4.76) | 3.126 | 0.009 |
| Vein catheter
infection | 0 | 8 (12.70) | 5.769 | <0.001 |
| Postoperative
evacuation time (h) | 38.65±6.21 | 53.08±5.34 | 2.658 | 0.012 |
| Hospitalization
time (days) | 12.3±4.5 | 18.1±3.7 | 7.253 | <0.001 |
| Postoperative
hospitalization cost (ten thousand yuan) |
2.4±1.9 | 3.3±0.8 | 9.561 | <0.001 |
Comparison of the hospitalization
costs between the two groups of patients
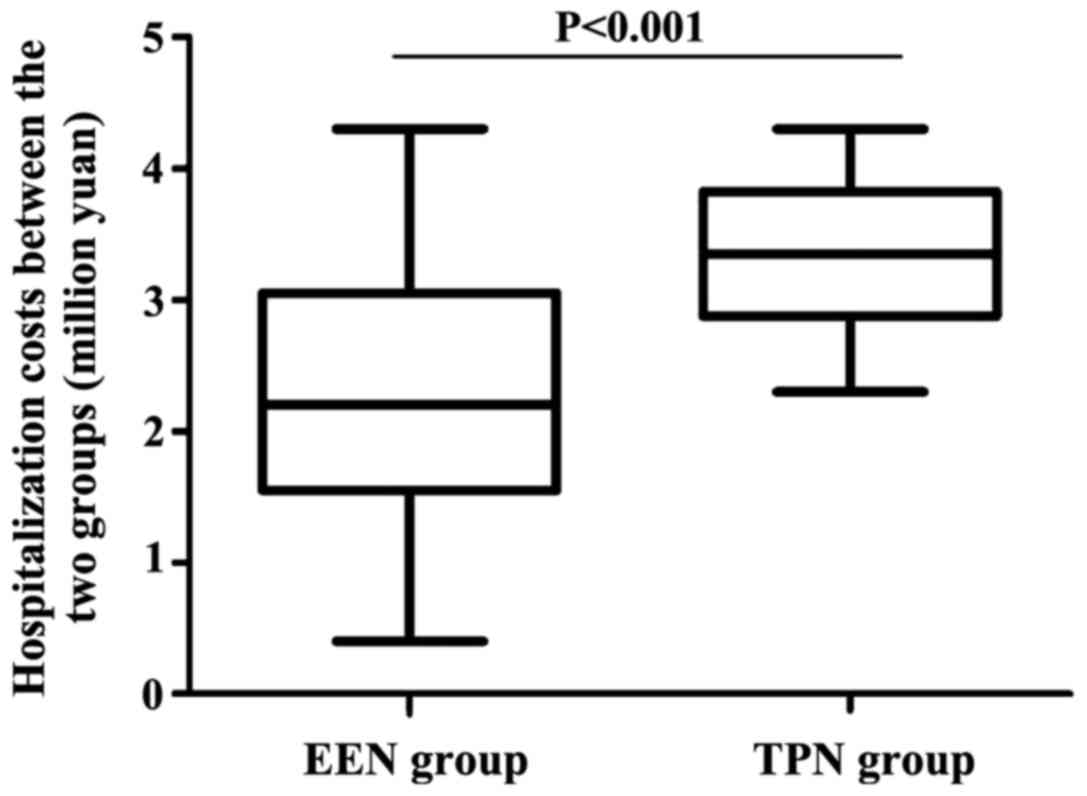
The hospitalization cost in the EEN group was
significantly lower than that in the TPN group (2.4±1.9 vs.
3.3±0.8), and the difference was statistically significant
(P<0.001) (Fig. 1).
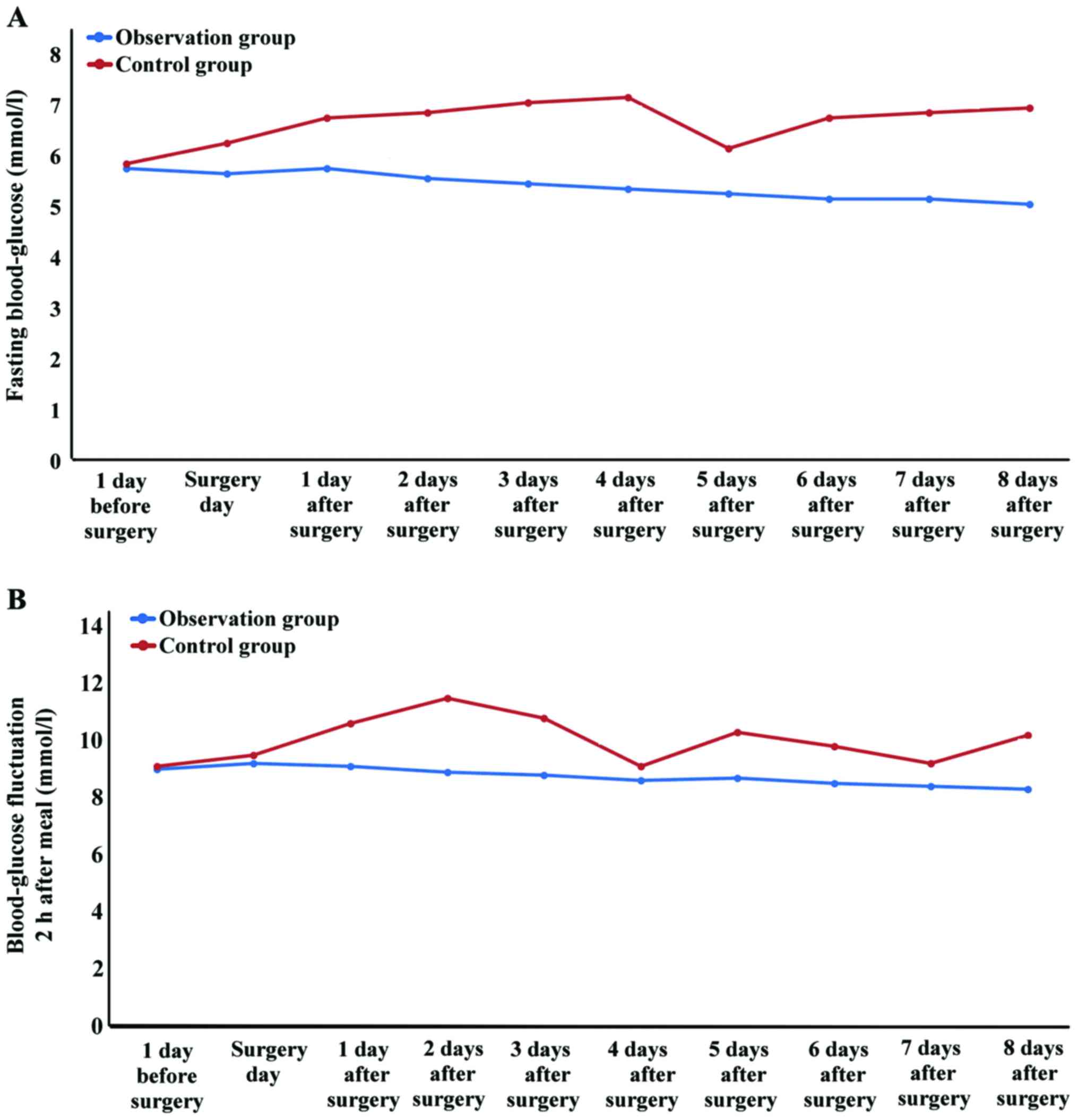
Comparison of perioperative blood
glucose fluctuation between the two groups of patients
There was no significant difference in preoperative
blood glucose between the two groups of patients (P>0.05), and
the preoperative blood glucose in the two groups was comparable. In
the comparison of the blood glucose fluctuation level at fasting
and 2 h after a meal, the blood glucose fluctuation values in the
TPN group were higher than those in the EEN group, and the
differences were statistically significant (χ2=13.219,
P=0.002; χ2=20.527, P<0.001), indicating that the
blood glucose fluctuation was more obvious in the TPN group, and
the blood glucose in the EEN group was stable (Fig. 2).
Discussion
Patients with malignant tumors often suffer
malnutrition, and some of them are manifested as cachexia (5). Poor nutritional status and high blood
glucose levels of patients with gastric cancer complicated with
diabetes mellitus type 2 are bottlenecks of postoperative recovery.
For patients with gastric cancer complicated with diabetes mellitus
type 2, surgical trauma stress can increase blood glucose (6), and postoperative fasting intestinal
mucosa will shrink, become necrosis and change permeability
(7). Nutrition support is divided
into enteral nutrition and parenteral nutrition, which is an
important means to promote patients' rehabilitation. EEN refers to
giving the enteral nutrition support within postoperative 6–24 h
(8). Parenteral nutrition is to
provide the body with an appropriate amount of three major
nutrients, vitamins, electrolytes and water through parenteral
pathways (usually through veins), but how to better improve the
postoperative nutritional status and control blood glucose level of
patients with gastric cancer complicated with diabetes mellitus is
the focus and difficulty of the current research.
Studies have shown that after an abdominal
operation, the function recovery of the stomach and colon is slow,
and small intestine function generally returns to normal within
6–12 h after operation, which is the theoretical basis for EEN
(9). EEN is the preferred nutrition
support method for patients receiving gastrointestinal operation
currently recognized by most scholars (10–12). It
is more in line with the physiological state so as to
comprehensively provide nutrition with less complications, which is
a safe and effective method to effectively improve the visceral
function (13,14). It is more convenient to regulate
blood glucose by EEN as on the one hand, EEN maintains intestinal
mucosal integrity and increases intestinal permeability so as to
release glucose-dependent insulinotropic hormones (15), and on the other hand, the addition of
dietary fiber in EEN preparations can delay the digestion and
absorption rates of carbohydrates (16). This study revealed that in the
comparison of the blood glucose fluctuation level of the two groups
of patients at fasting and 2 h after a meal within 8 days after
operation, the blood glucose fluctuation values in the TPN group
were higher than those in the EEN group (P<0.05), indicating
that it is more convenient to regulate postoperative blood glucose
of patients with gastric cancer complicated with diabetes mellitus
type 2 by EEN.
EEN is conducive to the growth of intestinal
epithelial cells, the prevention of mucosal atrophy and the
maintenance of mechanical barrier function. It contributes to the
secretion of immunoglobulin A (IgA) from intestinal cells so as to
maintain immunological barriers, avoids the flora shift, which is
beneficial to the growth of normal intestinal bacteria and
maintains the biological barriers of the intestine. It can also
promote the secretion of the stomach (gastrin and stomach acid) to
maintain the mucosal chemical barriers, thereby promoting
gastrointestinal function recovery. At the same time, EEN
stimulates the secretion of digestive juice, hormones and enzymes,
promotes gastrointestinal motility and gallbladder contraction and
increases visceral blood flow, thus effectively reducing the
incidence of hepatobiliary complications (17).
Visceral protein is the most important nutritional
monitoring index, including albumin, transferrin, prealbumin and
fibronectin. Albumin cannot be the index rapidly reflecting the
body's nutritional status as it is easily affected by the intake of
protein and energy and liver functions due to its long half-life;
transferrin is also rarely applied clinically due to its complex
metabolism and susceptibility to many factors; fibronectin with
short half-life can only act as a short-term nutrition support
index; prealbumin has received much attention in recent years among
visceral proteins due to its short half-life, good specificity and
the close relation to patient's nutritional status and prognosis
(18). Therefore, it is a reliable
index to determine the nutritional status of patients, and is one
of the indexes selected in this study to determine nutritional
status.
Nutritional pathways can cause relevant
complications. For example, parenteral nutrition is given mainly by
intravenous pathways, whose major complications are
catheter-related complications (pneumothorax, peripheral vascular
injury, venous thrombosis and air embolism), catheter-induced
infections or septicemia, which may even threaten patient lives.
There are some problems in the intestinal nutrition clinically,
such as discomforts after intubation, nausea, vomiting and
diarrhea. However, recent studies have shown that placing a
nasogastric tube or a nasointestinal tube for enteral nutrition
after operation is safe as it does not increase postoperative
complications and mortality rates compared with the parenteral
nutrition (19).
The incidence of postoperative complications in
patients with gastric cancer is affected by different nutritional
pathways. A meta-analysis (20)
showed that the enteral nutrition can significantly reduce the
incidence rate of postoperative complications in patients with
gastric cancer, especially in the anastomotic fistula, abdominal
abscess and mortality rate, and significantly shorten the
hospitalization time. It was also found in this study that there
were a total of 29 patients with adverse reactions in the TPN
group, but no patient in the EEN group, indicating that the
incidence rate of complications in the EEN group was significantly
lower than in the TPN group (P<0.05). EEN group had shorter
evacuation time, shorter hospitalization time and less
hospitalization cost (P<0.05), which was consistent with the
literature. Different nutrition support pathways affect liver
function in different degrees due to the different forms,
concentrations and rates of nutrients into the liver. In the
enteral nutrition support, the liver function state when patients
are eating is not able to be reached, especially for patients
receiving long-term TPN as long-term high energy and no foods
containing fats passing through the intestine easily cause liver
injuries. Liver enzyme abnormalities will occur in 20–40% of TPN
patients 2 weeks after operation or even cholestatic liver
dysfunction in severe cases (21).
Different nutrition support pathways exert different
effects on glucose metabolism. Generally, it is believed that TPN
is more likely to cause metabolic disorders of the body such as
glucose metabolism disorder, electrolyte imbalance, acid-base
disturbance and azotemia. Among them, the glucose metabolism
disorder is the most common one, which may cause hyperosmolar coma
for severely ill patients. The reason may be that the intake of
glucose per unit time is too high and rapid so as to cause a
transient hyperglycemia, while stresses increase gluconeogenesis
and the emergence of insulin resistance. If the patient suffers
from diabetes mellitus or liver diseases before operation, the
applied glucose in vivo is more likely to be limited, thus
promoting the increase of blood glucose, and the elevated blood
glucose increases the incidence rate of postoperative incision
infections (22).
This is a prospective study on patients with gastric
cancer complicated with diabetes mellitus type 2. The EEN pathway
was used to provide nutrition with such advantages as less
complications, early postoperative evacuation time, short
hospitalization time, low cost and stable control of blood glucose.
It helps patients with gastric cancer with diabetes mellitus pull
through the perioperative period safely, which provides a certain
guidance for clinical practice. However, this study is limited to a
single-center and small sample size, and it is expected that
multi-center study with a large sample size may lead to more
meaningful results.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and JZ collected and analyzed the general data of
patients. YZ helped with liver function indexes. JW and CL were
responsible for nutritional indexes. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First People's Hospital of Jinan (Jinan, China). Patients were
informed of the conditions and signed informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith U and Gale EA: Cancer and diabetes:
Are we ready for prime time? Diabetologia. 53:1541–1544. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bower M, Jones W, Vessels B, Scoggins C
and Martin R: Nutritional support with endoluminal stenting during
neoadjuvant therapy for esophageal malignancy. Ann Surg Oncol.
16:3161–3168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernández-Sordo JO, Konda VJ, Chennat J,
Madrigal-Hoyos E, Posner MC, Ferguson MK and Waxman I: Is
endoscopic ultrasound (EUS) necessary in the pre-therapeutic
assessment of Barrett's esophagus with early neoplasia? J
Gastrointest Oncol. 3:314–321. 2012.PubMed/NCBI
|
|
4
|
American Diabetes Association, . Diagnosis
and classification of diabetes mellitus. Diabetes Care. 30 Suppl
1:S42–S47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Makeeva TK and Galkin AA: Nutritional
support in the treatment of gastric cancer. Vopr Onkol. 55:237–240.
2009.(In Russian). PubMed/NCBI
|
|
6
|
Finney SJ, Zekveld C, Elia A and Evans TW:
Glucose control and mortality in critically ill patients. JAMA.
290:2041–2047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu G, Chen G, Huang B, Shao W and Zeng G:
Effect of early enteral nutrition on postoperative nutritional
status and immune function in elderly patients with esophageal
cancer or cardiac cancer. Chin J Cancer Res. 25:299–305.
2013.PubMed/NCBI
|
|
8
|
Mahmoodzadeh H, Shoar S, Sirati F and
Khorgami Z: Early initiation of oral feeding following upper
gastrointestinal tumor surgery: A randomized controlled trial. Surg
Today. 45:203–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kirby DF and Teran JC: Enteral feeding in
critical care, gastrointestinal diseases, and cancer. Gastrointest
Endosc Clin N Am. 8:623–643. 1998.PubMed/NCBI
|
|
10
|
Moskovitz DN and Kim YI: Does
perioperative immunonutrition reduce postoperative complications in
patients with gastrointestinal cancer undergoing operations? Nutr
Rev. 62:443–447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farreras N, Artigas V, Cardona D, Rius X,
Trias M and González JA: Effect of early postoperative enteral
immunonutrition on wound healing in patients undergoing surgery for
gastric cancer. Clin Nutr. 24:55–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang K, Cheng L, Wang JJ, Li JS and Nie
J: Fast track clinical pathway implications in esophagogastrectomy.
World J Gastroenterol. 15:496–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou XP, Chen M, Wei W, Cao J, Chen L and
Tian M: Effects of enteral immunonutrition on the maintenance of
gut barrier function and immune function in pigs with severe acute
pancreatitis. JPEN J Parenter Enteral Nutr. 34:554–566. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barlow R, Price P, Reid TD, Hunt S, Clark
GW, Havard TJ, Puntis MC and Lewis WG: Prospective multicentre
randomised controlled trial of early enteral nutrition for patients
undergoing major upper gastrointestinal surgical resection. Clin
Nutr. 30:560–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lidder P, Flanagan D, Fleming S, Russell
M, Morgan N, Wheatley T, Rahamin J, Shaw S and Lewis S: Combining
enteral with parenteral nutrition to improve postoperative glucose
control. Br J Nutr. 103:1635–1641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang H: Reconsideration on nutritional
treatment and nutritional support in patients with diabete mellitus
during perioperative period. Chin J Colorec Dis. 2:14–19. 2013.
|
|
17
|
Bower RH, Talamini MA, Sax HC, Hamilton F
and Fischer JE: Postoperative enteral vs parenteral nutrition. A
randomized controlled trial. Arch Surg. 121:1040–1045. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen DW, Fei Wei Z, Zhang YC, Ou JM and Xu
J: Role of enteral immunonutrition in patients with gastric
carcinoma undergoing major surgery. Asian J Surg. 28:121–124. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doglietto GB, Papa V, Tortorelli AP,
Bossola M, Covino M and Pacelli F: Italian Total Gastrectomy Study
Group: Nasojejunal tube placement after total gastrectomy: A
multicenter prospective randomized trial. Arch Surg. 139:1309–1313.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mazaki T and Ebisawa K: Enteral versus
parenteral nutrition after gastrointestinal surgery: A systematic
review and meta-analysis of randomized controlled trials in the
English literature. J Gastrointest Surg. 12:739–755. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marik PE and Zaloga GP: Early enteral
nutrition in acutely ill patients: A systematic review. Crit Care
Med. 29:2264–2270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu QG and Zheng QC: The influence of
enteral nutrition in postoperative patients with poor liver
function. World J Gastroenterol. 9:843–846. 2003. View Article : Google Scholar : PubMed/NCBI
|