Introduction
Thyroid cancer, which represents 2% of all malignant
disease cases and almost 90% of neuroendocrine cancer cases, is the
most common endocrine malignant tumor (1,2).
Papillary thyroid carcinoma (PTC) is the most common thyroid
malignancy, with a faster increase in incidence over the past three
decades (3,4). The survival rate of patients with PTC
is considered to be relatively favorable following complete
thyroidectomy and treatment with levothyroxine and radioactive
iodine (5). However, 10–15% of
patients with PTC develop recurrence and distant metastases
(6,7). Therefore, there is a need for improved
therapeutic strategies for PTC. Previous studies have implicated
genetics in the pathogenesis of PTC, including activation of
oncogenes and silencing of tumor-suppressor genes (8,9).
However, more efforts are required to identify key molecules that
may be involved in the development of PTC and that may serve as
potential therapeutic targets.
MicroRNAs (miRNAs) are a series of small non-coding
RNAs, 19–25 nucleotides in length (10), that regulate post-transcriptional
gene expression by binding to the 3′-untranslated region (3′-UTR)
of target mRNAs (11). miRNAs serve
key functions in cell proliferation, apoptosis and other biological
processes in cancer (12). Aberrant
expression and dysregulation of miRNAs has been observed in
patients with pancreatic cancer (PC), as well as in cancer cell
lines (8,13–15),
indicating the potential value of miRNA profiles as cancer
biomarkers and in the development of novel drugs.
miRNA (miR)-150 was first identified due to its
crucial regulatory role in normal hematopoiesis (16). However, in recent years, researchers
have demonstrated that aberrant expression and dysregulation of
miR-150 is closely associated with various types of hematological
malignancy (17) and solid tumor
(18). miR-150 may act as an oncomiR
or a tumor suppressor, depending on the targeted mRNA (17). Srivastava et al (19) observed downregulation of miR-150 in
malignant pancreatic tissues and demonstrated the role of miR-150
in the regulation of mucin (MUC)4 and tumor suppression in PC. The
authors hypothesized that restoring miR-150 levels may be of
therapeutic value in PC. Wu et al (20) revealed that miR-150 accelerated the
spread of gastric cancer by downregulating the pro-apoptotic gene,
early growth response 2. In addition, Wang et al (21) highlighted a novel function for
cyclin-dependent kinase 3 (CDK3) in myoblast cell proliferation and
confirmed CDK3 as a key target that further enhances the tumor
suppressor function of miR-150. However, the expression profile of
miR-150 and its direct target in PTC remain elusive.
Based on previous reports (19–21), it
was hypothesized that miR-150 may be differentially expressed in
PTC and associated with the biological functions of PTC cells.
Therefore, in the present study, the miR-150 expression profile was
evaluated in PTC tissues and cell lines through reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting. Through bioinformatics analysis, the potential
targets of miR-150 were identified and the results were further
confirmed by luciferase reporter assay. Cell viability, migration
and invasion rates were also investigated in PTC cell lines.
Materials and methods
Cell lines and thyroid tissue
specimens
The human PTC cell line TPC-1 and the normal thyroid
cell line Nthy-ori 3-1 were purchased from (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). The cells were cultured and maintained
in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and penicillin-streptomycin (1:100;
Sigma-Aldrich; Merck KGaA) according to a previous study (22) in an incubator with 5% CO2
at 37°C.
Thyroid tumor tissue and adjacent normal thyroid
tissue samples were obtained from 30 patients (age range, 34–65
years; median age, 46; 12 males and 18 females) with PTC from May
2015 to July 2016 at Wujin Affiliated Hospital of Jiangsu
University (Changzhou, China). All experiments involving human
tissues were reviewed and approved by the Committee for Ethical
Review of Research Involving Human Subjects at Wujin Affiliated
Hospital of Jiangsu University. All patients provided written
informed consent for the use of their tissues.
Cell transfection
miR-150 mimics (5′-UCUCCCAACCCUUGUACCAGUG-3′) and
negative control miR sequences (5′-CCGAAACCUCGGUUGAUUGCGG-3′) were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China).
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was
used to perform TPC-1 cell transfection, according to the
manufacturer's protocol. The cells were then cultured for 24 h at
37°C and 5% CO2 for further analysis.
MTT assay
An MTT assay kit (Beyotime Institute of
Biotechnology, Shanghai, China) was used to measure TPC-1 cell
viability at 24, 48 and 72 h after transfection, according to the
manufacturer's protocol. TPC-1 cells (5×104 per well)
were cultured in 96-well plates and incubated for 24, 48 and 72 h
at 37°C. A total of 10 µl MTT in PBS (5 mg/ml) was then added to
each well and incubated at 37°C for 4 h. Subsequently, the medium
was removed and formazan crystals were dissolved using dimethyl
sulfoxide (150 µl/well) for 30 min at 37°C. The absorbance was
measured at a wavelength of 450 nm, using a Bio-Rad iMark plate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell migration and invasion
assays
Wound healing and Transwell invasion experiments
were used to evaluate cell migration and invasion, respectively.
For the wound-healing assay, confluent monolayers of TPC-1 cells
cultured in 24-well plates were mechanically wounded using a 10-µl
pipette tip. The wells were washed to remove cellular debris and
the cells were allowed to migrate for 24 h. Representative images
were captured at ×100 magnification under an inverted microscope
(Olympus Corporation, Tokyo, Japan). The experiments were repeated
at least three times. This assay was performed 24 h after
transfection.
For Transwell invasion experiments, TPC-1 cells were
cultured in 200 µl RPMI-1640 medium in suspension (5×105
cells/ml) and seeded into the upper chamber of a Transwell insert
with an 8-mm pore size membrane and a Matrigel-coated membrane
matrix. RPMI-1640 medium with 10% FBS was added to the lower
chamber as a chemoattractant. After incubation for 24 h,
non-migrated cells were removed with a cotton swab in the upper
chamber of the Transwell insert and the migrated cells on the
underside of the filter membrane were fixed in 100% methanol for 15
min at room temperature and stained with 0.1% crystal violet for 30
min at 37°C (Sigma-Aldrich; Merck KGaA) The migrated cells were
counted and photographed (×100 magnification) in five randomly
selected microscopic fields under an inverted microscope.
RNA isolation and RT-qPCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from TPC-1 cells
and thyroid tumor tissue and was then converted into cDNA using a
Reverse Transcription kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The expression of miR-150
was determined with the bulge-loop™ miRNA qPCR Primer Set
(Guangzhou RiboBio Co., Ltd., Guangzhou, China) and a SYBR-Green
qPCR kit (Takara Biotechnology Co., Ltd., Dalian, China). U6 served
as an internal control. The expression of MUC4 mRNA was analyzed by
qPCR with the SYBR Premix Ex Taq™ kit (Takara Biotechnology, Co.,
Ltd.). GAPDH was used as an endogenous control. The primers for
qPCR were obtained from GenScript (Piscataway, NJ, USA). The primer
sequences as follows: MUC4, forward: 5′-GGACCAGAGCGAAAGCATTTGCC-3′,
reverse: 5′-TCAATCTCGGGTGGCTGAACGC-3′; GAPDH, forward:
5′-CTGGGCTACACTGAGCACC-3′, reverse: 5′-AAGTGGTCGTTGAGGGCAATG-3′;
miR-150, forward:
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCACTGGTA-3′, reverse:
5′-ACACTCCAGCTGGGTCTCCCAACCCTTGTA-3′; U6, forward:
5′-CTCGCTTCGGCAGCACA-3′, Reverse: 5′-AACGCTTCACGAATTTGCGT-3′. All
experiments were performed according to the manufacturer's
protocols. The thermocycling conditions for qPCR were as follows:
95°C for 5 min, followed by 40 cycles of denaturation at 95°C for
15 sec and annealing/elongation at 60°C for 30 sec. The
2−ΔΔCq method (23) was
used to calculate the relative quantities of each gene. Data were
obtained from three independent experiments.
Western blot analysis
Total protein was extracted from TPC-1 cells using
RIPA lysis buffer (Thermo Fisher Scientific, Inc.) and then
quantified using a BCA protein assay kit (Beyotime Institute of
Biotechnology) Total protein (50 µg/lane) was then separated on 10%
SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were then blocked
with 5% skimmed milk for 2 h and incubated overnight at 4°C with
the following primary antibodies at 1:1,000 dilution: Anti-MUC4
(catalog no. ab60720), anti-human epidermal growth factor receptor
2 (HER2; catalog no. ab16901), anti-p-HER2 (catalog no. ab53290),
anti-focal adhesion kinase (FAK; catalog no. ab40794), anti-p-FAK
(catalog no. ab81298), anti-extracellular signal-regulated kinase
(ERK; catalog no. ab196883), anti-p-ERK (catalog no. ab4819),
anti-GAPDH (catalog no. ab8245) (all Abcam, Cambridge, UK). The
membranes were then washed and incubated with horseradish
peroxidase-conjugated secondary antibodies (catalog nos. ab191866
and ab218695; dilution rate, 1:1,000; Abcam) for 1 h at room
temperature. Immunoreactive bands were detected using the ChemiDoc
XRS+ system (Bio-Rad Laboratories, Inc.) and an ECL Plus kit
(Beyotime Institute of Biotechnology). Data were analyzed by
densitometry using Image Pro Plus v.6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). GAPDH served as an internal
control for all experiments.
Bioinformatics prediction and
Dual-luciferase reporter assay
Targetscan (www.targetscan.org/vert_71) and miRWalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/micrornapredictedtarget.html)
were used to predicate the putative target genes of miR-150. To
investigate the direct effect of miR-150 on the expression of MUC4,
a miRNA target luciferase reporter assay was performed using a
pEZX-MT01 target reporter plasmid containing the MUC4 3′-UTR region
(GeneCopoeia, Inc., Rockville, MD, USA). Additionally, a mutant
MUC4 3′ UTR (MUT-MUC4 3′ UTR) reporter construct was generated by
site-directed mutagenesis in the putative target site of miR-150 in
the wild-type (WT) MUC4 3′-UTR using the Quickchange XL
site-directed mutagenesis kit (Agilent Technologies, Inc., Santa
Clara, CA, USA). The reporter plasmids were co-transfected into
cells with miR-150 mimics or the control vector using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) in 24-well
plates. The Dual-Luciferase Reporter Assay system (Promega
Corporation, Madison, WI, USA) was used to perform luciferase
activity 48 h following transfection, according to the
manufacturer's protocol. Renilla luciferase activity was normalized
to firefly luciferase activity. The results were obtained from
three independent experiments.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 software (IBM Corp., Armonk, NY, USA). Data are presented as
the mean ± standard deviation. Differences between groups were
analyzed using the two-tailed Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Downregulation of miR-150 and
upregulation of MUC4 in PTC
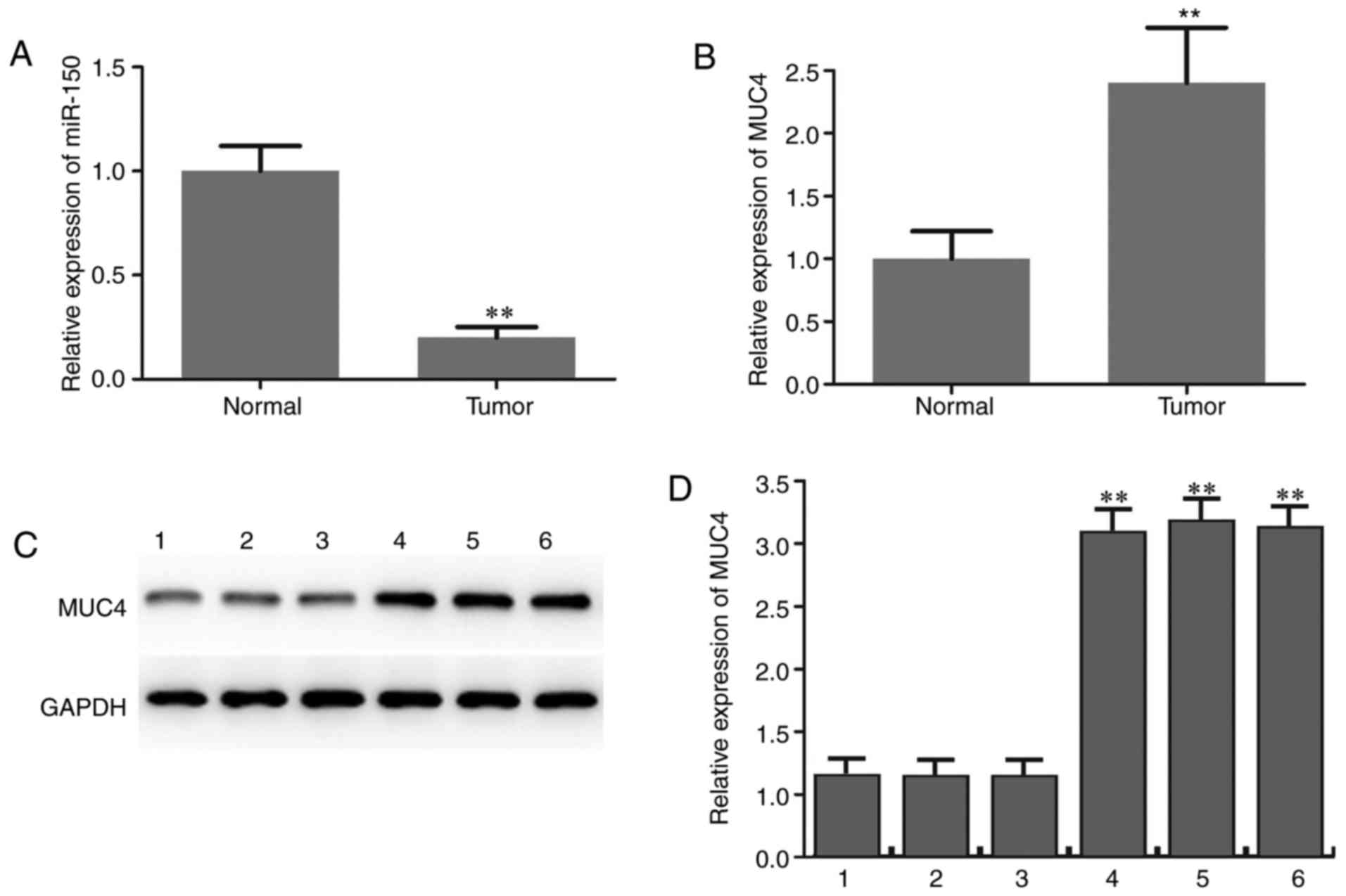
The expression level of miR-150 in PTC specimens and
adjacent normal thyroid tissues was initially determined by RT-qPCR
analysis. The results revealed that miR-150 levels in PTC were
significantly lower compared with those in matched normal tissues
(Fig. 1A). The expression level of
MUC4 in PTC specimens and adjacent normal thyroid tissues was also
determined and the results suggested that MUC4 was significantly
upregulated in PTC compared with adjacent normal tissues (Fig. 1B-D). The expression patterns of
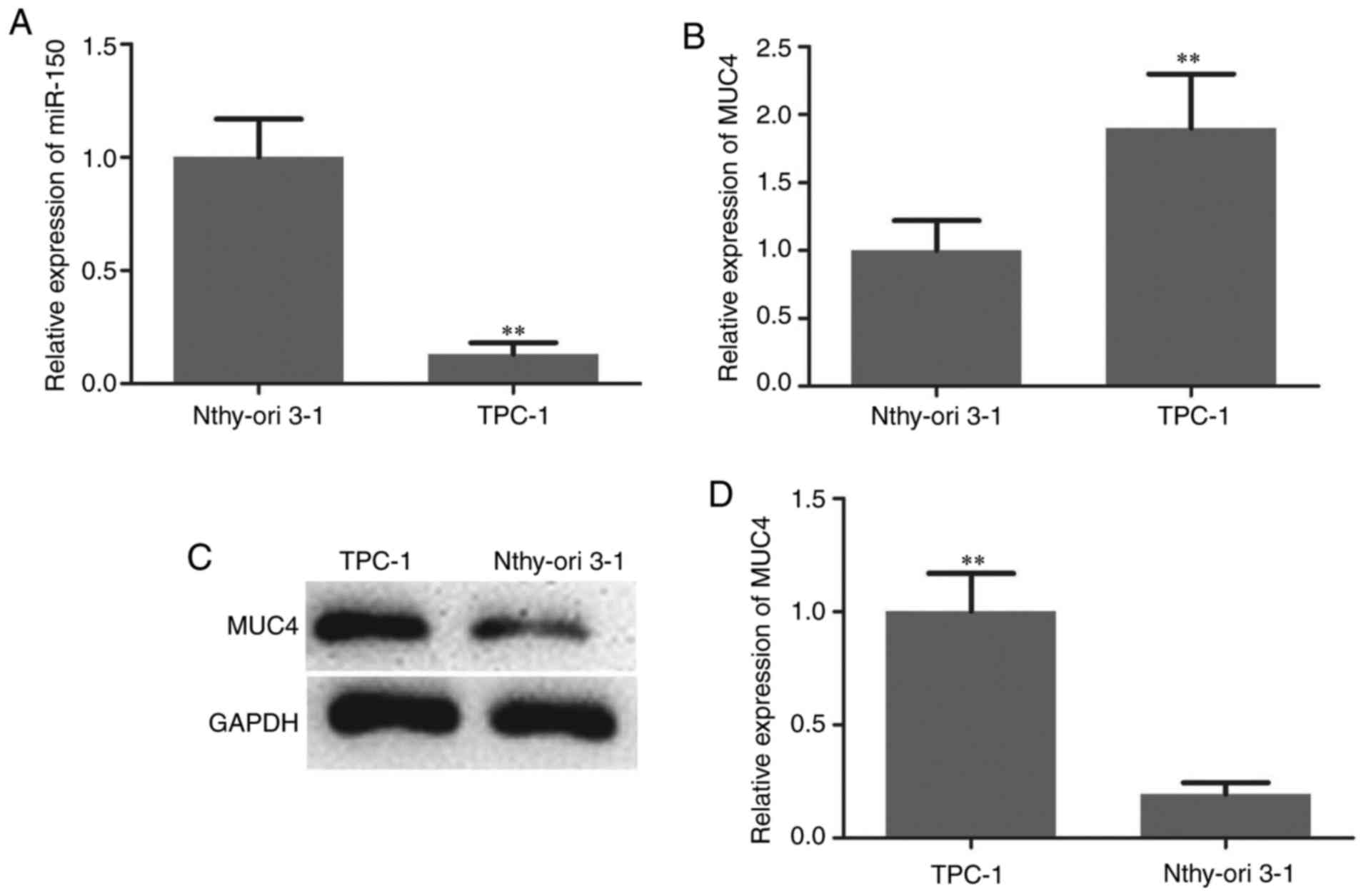
miRNA-150 in TPC-1 human PTC cells and the normal thyroid cell line
Nthy-ori 3-1 were analyzed to confirm the aforementioned findings.
As indicated in Fig. 2A, the
expression of miR-150 was significantly downregulated in PTC cells.
The expression level of MUC4 in PTC cells and normal thyroid cells
was then measured by RT-qPCR and western blotting. The mRNA and
protein levels of MUC4 were significantly higher in PTC cells
compared with normal thyroid cells (Fig.
2B-D).
Upregulation of miR-150 suppresses
human PTC cell proliferation and metastasis
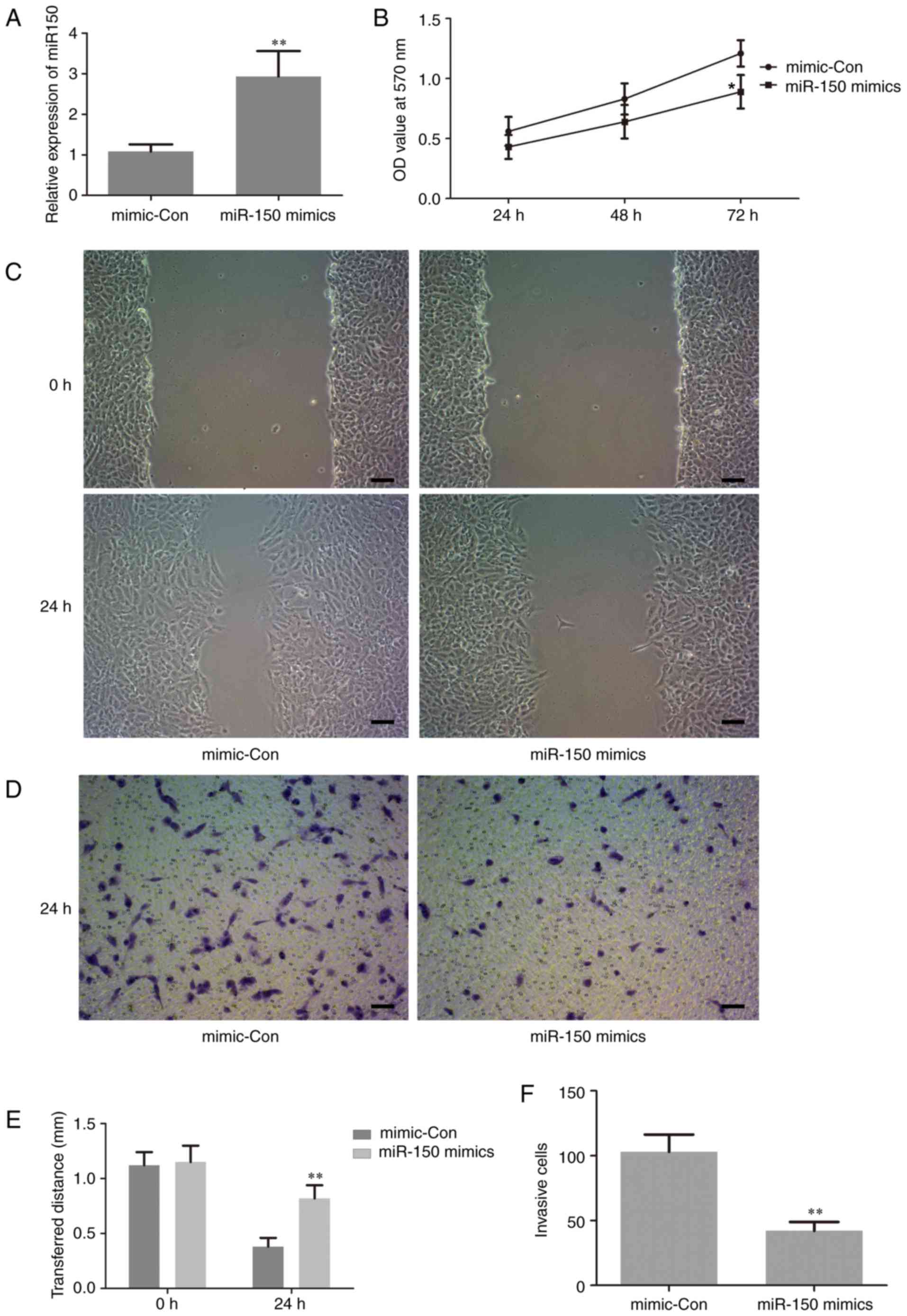
To investigate the biological functions of miR-150
in the proliferation and invasion of human PTC cells, which are
closely associated with tumorigenesis, gain-of function experiments
were performed by transfection of miR-150 mimics in TPC-1 cells.
The level of miR-150 in the treated cells was assessed by RT-qPCR.
The results revealed that the expression level of miR-150 was
significantly increased in cells transfected with miR-150 mimics
compared with the negative control (Fig.
3A). This result suggested that the transfection of miR-150
mimics in TPC-1 cells was successful. Thus, in the following
experiments, the level of miR-150 was manipulated by transfection
with miR-150 mimics, in order to investigate the potential role of
miR-150 in the proliferation and invasion of human PTC cells.
The effect of miR-150 on cell proliferation was
examined using an MTT assay. The results demonstrated that
upregulation of miR-150 significantly inhibited cell proliferation
at 72 h following transfection with the miR-150 mimic compared with
the control group (Fig. 3B). This is
important, since metastasis in late-stage disease adds to the
difficulties of cancer treatment (24). Thus, wound-healing and Transwell
invasion assays were performed to evaluate the effect of miR-150 on
cell migration and invasion, respectively. As indicated in Fig. 3C-F, upregulation of miR-150
significantly suppressed cancer cell migration and invasion.
MUC4 is a direct target of miR-150 in
PTC cells
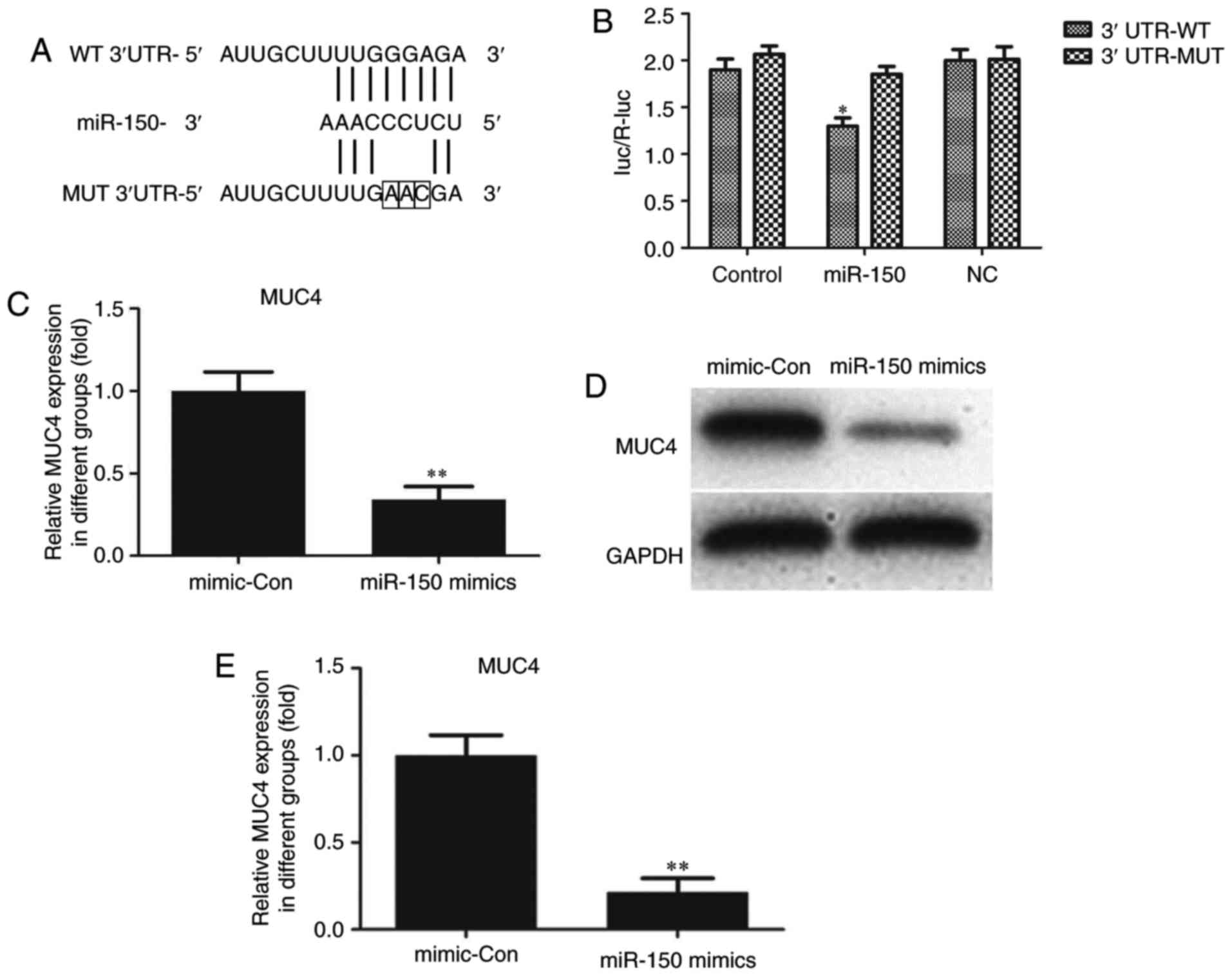
Through bioinformatics analysis via the Targetscan
(www.targetscan.org/vert_71) and
miRWalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/micrornapredictedtarget.html)
websites, MUC4 was identified to be a potential target of miR-150.
As indicated in Fig. 4A, a putative
binding site for miR-150 was identified in MUC4 3′-UTR. To
determine whether MUC4 expression was mediated by miR-150, a
dual-luciferase reporter assay was performed. The 3′-UTR of MUC4
mRNA, including the putative miR-150 binding sequence (WT 3′-UTR)
or the mutant sequence (MUT 3′-UTR), was subcloned into luciferase
reporter plasmids. TPC-1 cells were then co-transfected with the WT
or MUT 3′-UTR of MUC4 and miR-150 mimics or controls. The
luciferase results indicated that overexpression of miR-150 in
TPC-1 cells co-transfected with MUC4 WT led to a significant
reduction of luciferase activity, while there was no such reduction
in the TPC-1 cells co-transfected with MUC4 MUT (Fig. 4B). These results suggested that MUC4
was the direct target of miR-150. The luciferase activity of
miR-150-transfected cells was markedly decreased compared with the
controls (Fig. 4B). The effects of
miR-150 upregulation on MUC4 mRNA and protein expression in TPC-1
cells were also analyzed. Compared with cells transfected with
negative control sequences, cells transfected with miR-150 mimics
exhibited significantly decreased mRNA and protein levels of MUC4
(Fig. 4C-E).
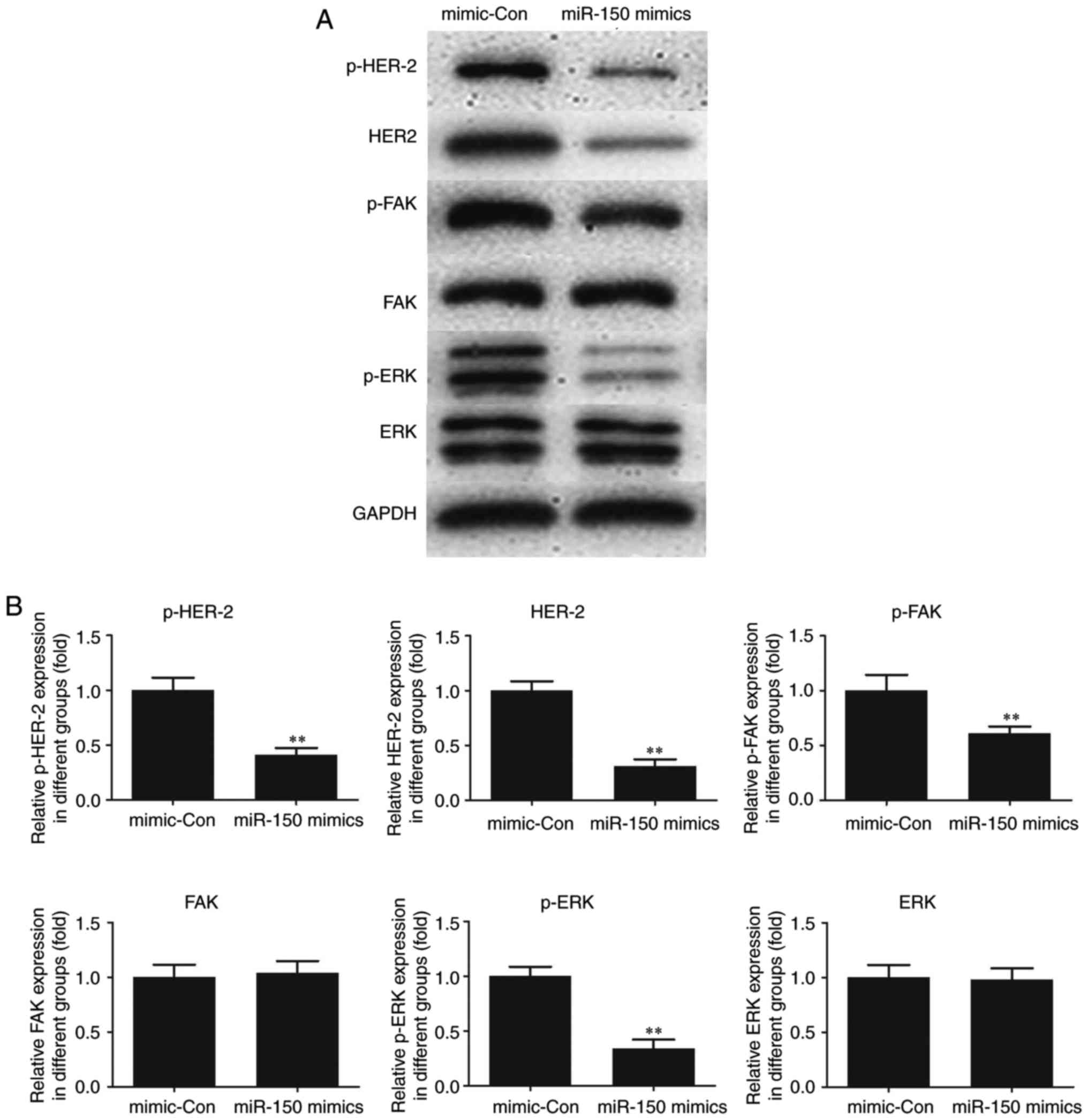
Effect of miR-150 on HER2 and FAK/ERK
downstream signaling
To elucidate the molecular mechanism of miR-150,
HER2, an interacting partner of MUC4 and downstream FAK/ERK
signaling were investigated in TPC-1 cells. As indicated in
Fig. 5, the expression of HER2 and
p-HER2 was significantly decreased following transfection of
miR-150 mimics in TPC-1 cells as compared with the control. A
significant decrease in p-FAK and p-ERK was also observed in
miR-150-transfected cells compared with the control, whereas no
significant changes in the expression levels of total FAK and ERK
were observed. The results suggested a critical role for MUC4 in
the expression of HER2 and p-HER2 and the phosphorylation of FAK
and ERK, key cancer survival molecules. In addition, the activation
of HER2 and FAK/ ERK signaling was indicated to be attenuated by
the upregulation of miR-150 in PTC.
Discussion
miRNAs not only serve critical functions in
regulating numerous fundamental cellular processes, but may also
act as oncogenes or tumor suppressor genes in a variety of human
cancer types, including PTC (25).
Over the past few years, an increasing number of studies have been
focused on the role of miRNA expression in PTC (26,27). It
has been reported that miRNA dysregulation is associated with
pathological processes in PTC, as reflected by the extent of
extrathyroidal invasion, tumor size and the presence of lymph node
metastases (8). miRNA-146, −222 and
−221 are the most common miRNAs involved in these processes and
they are closely associated with tumor cell proliferation,
differentiation, migration and apoptosis (8). Clearly identifying the unique
expression profiles of cancer-specific miRNAs and their targets is
crucial for elucidating their role in tumorigenesis and miRNAs have
demonstrated potential in the development of cancer biomarkers and
novel therapeutic drugs (8). It has
been reported that miR-150 is up- or downregulated in a variety of
solid cancer types, including cervical, breast, lung, colorectal,
gastric, liver, pancreatic and esophageal cancer (19–21,28–30).
However, the role of miR-150 in human PTC has not been fully
elucidated.
In the present study, it was observed that miR-150
was decreased in PTC tissues and downregulated in human PTC cell
lines. Thus, PTC cells were transfected with miR-150 mimics to
upregulate the expression of miR-150. The transfection rate was
satisfactory, which was confirmed by RT-qPCR assay. The biological
effect of miR-150 on human PTC cells was then examined and the
results demonstrated that overexpression of miR-150 significantly
suppressed human PTC cell proliferation at 72 h following
transfection with the miR-150 mimic. In addition, the
overexpression of miR-150 significantly suppressed human PTC cell
metastasis. Further experiments were performed to determine the
potential underlying molecular mechanism of miR-150.
By means of bioinformatics analysis, a search for
target genes of miR-150 was performed and MUC4 was identified as a
potential target. MUC4 is a high molecular weight type I
transmembrane protein and alterations of MUC4 are often associated
with carcinomas (31). Upregulation
of MUC4 has been demonstrated in several cancer types, including
PTC (32). In a study of 98 patients
by Nam et al (33), the gene
expression of MUC4 increased by ~78-fold in PTC and the protein
staining scores of MUC4 also increased markedly in PTC compared
with normal thyroid tissue. The results indicated that high MUC4
expression was associated with small tumor size and the papillary
thyroid microcarcinoma subtype. MUC4 may serve a key function in
the early oncogenesis of PTC. However, the functional role of MUC4
in PTC and its dysregulation has not been clearly determined. In
the present study, the mRNA and protein levels of MUC4 were
identified to be significantly higher in PTC cells compared with
normal thyroid cells, whereas miR-150 mimics significantly
decreased the level of MUC4. Furthermore, the dual-luciferase
reporter system suggested that MUC4 was the direct target of
miR-150. Since there are numerous target genes of miR-150, rescue
experiments are required to further investigate miR-150 function as
a tumor suppressor via MUC4 in PTC. An in-depth study will be
conducted in the future.
The majority of cancer-associated cases of mortality
are attributed to metastasis (34).
Cancer cell metastasis involves a complicated series of phenotypic
and biochemical processes and events, including cell migration and
invasion (35). FAK serves a vital
function in tumor cell proliferation, survival and migration. FAK
deficiency results in reduced cell motility and enhanced focal
adhesion contact formation compared with control cells (36). FAK interactions with HER2 promote
tumorigenesis and micrometastatic cells express activated/p-FAK and
HER2, suggesting a role for HER2-FAK activation in malignant and
invasive growth (37). ERK is a
member of the mitogen-activated protein kinase family that is
involved in a broad range of cell functions and physiological
processes (38). ERK is a well-known
downstream signaling molecule of FAK and serves as a key regulatory
component in cell motility (39). In
the present study, decreased expression of HER2 and p-HER2 was
identified in miR-150-overexpressing PTC cells. A similar decrease
in p-FAK and p-ERK was also observed in miR-150-transfected cells,
indicating that MUC4 serves a key role in the activation of these
cancer-associated molecules and this effect may be partly modulated
through the alteration of miR-150 in PTC.
In summary, the findings of the present revealed
that miR-150 possesses antitumor properties and suppresses the
growth and malignant behavior of PTC cells, at least in part via
regulation of the downstream target MUC4. These findings may
provide a novel insight into diagnostic and therapeutic strategies
for PTC. However, the present study was limited by the fact that
only one tumor cell line was investigated, thus in-depth studies
will be conducted in the future in order to validate these
results.
Acknowledgements
The authors are would like to thank Professor
Caiping Huang of Head and Neck Surgery Department in Shanghai
Cancer Hospital for his kind surgery advice. In addition, the
authors appreciate the help provided by Dr Zhixin Xue of Changzhou
Wujin People's Hospital in collecting tissue samples.
References
|
1
|
Xiang D, Xie L, Xu Y, Li Z, Hong Y and
Wang P: Papillary thyroid microcarcinomas located at the middle
part of the middle third of the thyroid gland correlates with the
presence of neck metastasis. Surgery. 157:526–533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HY, Park WY, Lee KE, Park WS, Chung
YS, Cho SJ and Youn YK: Comparative analysis of gene expression
profiles of papillary thyroid microcarcinoma and papillary thyroid
carcinoma. J Cancer Res Ther. 6:452–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hughes DT, Haymart MR, Miller BS, Gauger
PG and Doherty GM: The most commonly occurring papillary thyroid
cancer in the United States is now a microcarcinoma in a patient
older than 45 years. Thyroid. 21:231–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chou CK, Yang KD, Chou FF, Huang CC, Lan
YW, Lee YF, Kang HY and Liu RT: Prognostic implications of miR-146b
expression and its functional role in papillary thyroid carcinoma.
J Clin Endocrinol Metab. 98:E196–E205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JC, Zhao JT, Clifton-Bligh RJ, Gill A,
Gundara JS, Ip JC, Glover A, Sywak MS, Delbridge LW, Robinson BG
and Sidhu SB: MicroRNA-222 and microRNA-146b are tissue and
circulating biomarkers of recurrent papillary thyroid cancer.
Cancer. 119:4358–4365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lang BH, Tang AH, Wong KP, Shek TW, Wan KY
and Lo CY: Significance of size of lymph node metastasis on
postsurgical stimulated thyroglobulin levels after prophylactic
unilateral central neck dissection in papillary thyroid carcinoma.
Ann Surg Oncol. 19:3472–3478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chruścik A and Lam AK: Clinical
pathological impacts of microRNAs in papillary thyroid carcinoma: A
crucial review. Exp Mol Pathol. 99:393–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang J, Cai W, Feng D, Teng H, Mao F,
Jiang Y, Hu S, Li X, Zhang Y, Liu B and Sun ZS: Genetic landscape
of papillary thyroid carcinoma in the Chinese population. J Pathol.
244:215–226. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hale BJ, Yang CX and Ross JW: Small RNA
regulation of reproductive function. Mol Reprod Dev. 81:148–159.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chi SW, Zang JB, Mele A and Darnell RB:
Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature.
460:479–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang XZ, Hang YK, Liu JB, Hou YQ, Wang N
and Wang MJ: Over-expression of microRNA-375 inhibits papillary
thyroid carcinoma cell proliferation and induces cell apoptosis by
targeting ERBB2. J Pharmacol Sci. 130:78–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borrelli N, Denaro M, Ugolini C, Poma AM,
Miccoli M, Vitti P, Miccoli P and Basolo F: miRNA expression
profiling of ‘noninvasive follicular thyroid neoplasms with
papillary-like nuclear features’ compared with adenomas and
infiltrative follicular variants of papillary thyroid carcinomas.
Mod Pathol. 30:39–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma X, Wei J, Zhang L, Deng D, Liu L, Mei
X, He X and Tian J: miR-486-5p inhibits cell growth of papillary
thyroid carcinoma by targeting fibrillin-1. Biomed Pharmacother.
80:220–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu L, Wang J, Li X, Ma J, Shi C, Zhu H,
Xi Q, Zhang J, Zhao X and Gu M: miR-204-5p suppresses cell
proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma.
Biochem Biophys Res Commun. 457:621–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vasilatou D, Papageorgiou S, Pappa V,
Papageorgiou E and Dervenoulas J: The role of microRNAs in normal
and malignant hematopoiesis. Eur J Haematol. 84:1–16. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He Y, Jiang X and Chen J: The role of
miR-150 in normal and malignant hematopoiesis. Oncogene.
33:3887–3893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Ren X and Zhang X: Role of
microRNA-150 in solid tumors. Oncol Lett. 10:11–16. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Srivastava SK, Bhardwaj A, Singh S, Arora
S, Wang B, Grizzle WE and Singh AP: MicroRNA-150 directly targets
MUC4 and suppresses growth and malignant behavior of pancreatic
cancer cells. Carcinogenesis. 32:1832–1839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K,
Ren G, Su T, Pan Y, Feng B, et al: miR-150 promotes gastric cancer
proliferation by negatively regulating the pro-apoptotic gene EGR2.
Biochem Biophys Res Commun. 392:340–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Xi Y, Sun C, Zhang F, Jiang H, He
Q and Li D: CDK3 is a major target of miR-150 in cell proliferation
and anti-cancer effect. Exp Mol Pathol. 102:181–190. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou D, Li Z and Bai X: BRAFV600E and
RET/PTC promote proliferation and migration of papillary thyroid
carcinoma cells in vitro by regulating nuclear factor-κB. Med Sci
Monit. 23:5321–5329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Talmadge JE and Fidler IJ: AACR centennial
series: The biology of cancer metastasis: Historical perspective.
Cancer Res. 70:5649–5669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saiselet M, Gacquer D, Spinette A, Craciun
L, Decaussin-Petrucci M, Andry G, Detours V and Maenhaut C: New
global analysis of the microRNA transcriptome of primary tumors and
lymph node metastases of papillary thyroid cancer. BMC Genomics.
16:8282015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiu Z, Li H, Wang J and Sun C: miR-146a
and miR-146b in the diagnosis and prognosis of papillary thyroid
carcinoma. Oncol Rep. 38:2735–2740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kolanowska M, Wójcicka A, Kubiak A,
Świerniak M, Kotlarek M, Maciąg M, Gaj P, Koperski Ł, Górnicka B
and Jażdżewski K: Functional analysis of a novel,
thyroglobulin-embedded microRNA gene deregulated in papillary
thyroid carcinoma. Sci Rep. 7:99422017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Hu L, Tian C, Lu F, Wu J and Liu L:
microRNA-150 promotes cervical cancer cell growth and survival by
targeting FOXO4. BMC Mol Biol. 16:242015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang S, Chen Y, Wu W, Ouyang N, Chen J,
Li H, Liu X, Su F, Lin L and Yao Y: miR-150 promotes human breast
cancer growth and malignant behavior by targeting the pro-apoptotic
purinergic P2X7 receptor. PLoS One. 8:e807072013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao M, Hou D, Liang H, Gong F, Wang Y, Yan
X, Jiang X, Wang C, Zhang J, Zen K, et al: miR-150 promotes the
proliferation and migration of lung cancer cells by targeting SRC
kinase signalling inhibitor 1. Eur J Cancer. 50:1013–1024. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gautam SK, Kumar S, Cannon A, Hall B,
Bhatia R, Nasser MW, Mahapatra S, Batra SK and Jain M: MUC4 mucin-a
therapeutic target for pancreatic ductal adenocarcinoma. Expert
Opin Ther Targets. 21:657–669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh AP, Moniaux N, Chauhan SC, Meza JL
and Batra SK: Inhibition of MUC4 expression suppresses pancreatic
tumor cell growth and metastasis. Cancer Res. 64:622–630. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nam KH, Noh TW, Chung SH, Lee SH, Lee MK,
Hong SW, Chung WY, Lee EJ and Park CS: Expression of the membrane
mucins MUC4 and MUC15, potential markers of malignancy and
prognosis, in papillary thyroid carcinoma. Thyroid. 21:745–750.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Valderrama-Treviño I A, Barrera-Mera B,
Ceballos-Villalva C J and Montalvo-Javé E E: Hepatic metastasis
from colorectal cancer. Euroasian J Hepatogastroenterol. 7:166–175.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun J, Luo Q, Liu L, Yang X, Zhu S and
Song G: Salinomycin attenuates liver cancer stem cell motility by
enhancing cell stiffness and increasing F-actin formation via the
FAK-ERK1/2 signalling pathway. Toxicology. 384:1–10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao X and Guan JL: Focal adhesion kinase
and its signaling pathways in cell migration and angiogenesis. Adv
Drug Deliv Rev. 63:610–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J and Hochwald SN: The role of FAK
in tumor metabolism and therapy. Pharmacol Ther. 142:154–163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mendoza MC, Vilela M, Juarez JE, Blenis J
and Danuser G: ERK reinforces actin polymerization to power
persistent edge protrusion during motility. Sci Signal. 8:ra472015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Natarajan M, Hecker TP and Gladson CL: FAK
signaling in anaplastic astrocytoma and glioblastoma tumors. Cancer
J. 9:126–133. 2003. View Article : Google Scholar : PubMed/NCBI
|