Introduction
Postmenopausal women suffering from osteoporosis
have a higher risk of tooth and periodontal disease, which might be
related to alveolar bone loss (1,2). The
metabolic mechanism of alveolar bone is partly different from that
of other bones, and its biological metabolic cycle is very short
(3). Diosgenin (DIO), an aglycone of
the steroid saponin, is widely recognized as a phytoestrogen
(4,5), but the estrogen-like effect of DIO is
milder than real estrogen. A study indicated that DIO, at doses of
20–200 mg/kg, did not affect the uterine wet weight, epithelium
height, volume densities of endometrium, endometrial epithelia,
number of endometrial glands, or histological appearance of vaginal
epithelia in rats (6). In addition,
DIO shows adverse effects such as stimulatory action on growth of
mammary gland in ovariectomized (OVX) mouse (7). As for bone metabolism, previous studies
demonstrated that the application of DIO decreases bone loss in the
peripheral skeleton in animal models induced by ovariectomy
(8,9), but the effects of DIO on alveolar bone
still remain unclear. We therefore conducted a study to assess the
influence of DIO on alveolar bone in rats that had undergone
ovariectomy.
Long noncoding RNAs (lncRNAs), defined as RNAs
>200 nucleotides and lacking an open reading frame (ORF), were
identified from cDNA in 1991 (10,11).
Like microRNAs (miRNAs), lncRNAs are also considered to play
important roles in normal cellular physiological processes, with
tissue- and cell-specific expression patterns (12,13). The
limited studies on lncRNAs showed that they might play a vital role
in the occurrence and progression of skeletal diseases (14,15)
including osteoporosis (16,17). In studies on animal models, some
specific lncRNAs could be potential biomarkers for the diagnosis of
osteoporosis in the jaw of OVX mice have been reported (18).
In view of the importance of lncRNAs in bone
remodeling, we questioned if lncRNAs play a substantial role in the
action of DIO on the loss of alveolar bone. Therefore, we conducted
this study to assess the modulatory action of DIO on lncRNA and
mRNA expression profiles in alveolar bone in an OVX rat model.
Materials and methods
Animal grouping and treatments
There have been studies that used female rats aged 6
months that have received an ovariectomy as a model of osteoporosis
in postmenopausal women (19,20).
Forty-eight female Wistar rats aged 6 months, with an average
weight of 300±20.0 g, were obtained from the Experimental Animal
Center of the Academy of Military Medical Sciences. All the
experiments on animals gained approval of the Institutional Ethics
Committee of the Institute of Basic Theory, China Academy of
Chinese Medical Sciences. The rats were sham-operated (SHAM,
n=12) or bilaterally OVX (n=36) (21). We randomly divided the OVX rats into
the following three groups: OVX group (OVX, n=12), DIO group
(DIO, n=12), and estradiol valerate (EV) group (EV,
n=12). We treated the rats in the EV group with EV daily via
oral gavage at a dosage of 0.1 mg/kg body weight/day. In addition,
we treated the rats in the DIO group with DIO via oral gavage at a
dosage of 100 mg/kg body weight/day. At the same time, we treated
the rats in the SHAM and OVX groups with an equivalent volume of
distilled water via oral gavage. During the experiments, we fed all
the rats with standard chow. We started all of the treatments 1
week following OVX surgery, and the course continued for 12 weeks.
During the 12-week treatment period, no animal died.
Preparation of specimens
The average body weight of all rats was 365.84±26.85
g in the end of treatment. We anesthetized the animals with
xylazine at 12 mg/kg and ketamine at 80 mg/kg via intraperitoneal
injection and then euthanized them by exsanguination the day after
the last treatment. Under anesthesia, the abdominal aorta of rat
was punctured to collect 8–10 ml of blood specimens into
heparinized tubes. After exsanguinations, we performed cardiac
palpation on precordium of rats for five min to confirm cardiac
arrest. If the rats showed cardiac arrest, respiratory arrest and
mydriasis simultaneously, we confirmed the death of rat.
Subsequently, the blood specimens were separated by centrifugation
at 3,000 × g at a temperature of 4°C for 10 min and then aliquoted
and preserved at −80°C before use. We dissected the right mandibles
and then stored them at −20°C for determination of microstructure
and bone mineral density (BMD) with the help of micro-computerized
tomography (micro-CT). Afterwards, we used them for histological
examination. We dissected the left mandibles and reserved the bone
tissue between the incisor and molars and afterwards stored it at
−80°C for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) tests and microarray assay.
Biomarkers of bone turnover
Enzyme-linked immunosorbent assays (ELISAs; Beijing
Sunbio Biotech Co., Ltd., Beijing, China) were conducted to assess
plasma concentrations of bone resorption and bone formation
biomarkers, such as tartrate-resistant acid phosphatase (TRAP) and
alkaline phosphatase (ALP), in controlled, standardized and
duplicated experiments. An ELISA reader (BioTek Instruments, Inc.,
Winooski, VT, USA) was used to read absorption at 450 nm.
micro-CT assessments
We scanned the right mandible of each rat with a
high-resolution micro-CT system (Skyscan 1172 micro-CT system;
Bruker Corporation, Ettlingen, Germany) without sample preparation.
We applied the micro-CT system in accordance with a method
described previously (22). We
scanned each of the samples at a high resolution (6.8 µm). We
applied a 0.5 mm thick aluminum filter to remove noise from the
generated gray scale images. A global threshold (lower grey
threshold: 52, upper grey threshold: 255) was used for measurement
of the trabecular bone.
We captured the images of the mandible at a current
of 100 µA and a voltage of 100 keV to reconstruct a cubic region
starting 1.5 mm below the bottom of the first molar crown, which
served as the ‘volume of interest’ (VOI). We applied the standard
Skyscan software package for trabecular bone morphological
measurements within the VOI. In addition, we applied 3-D analyses
for assessment of the trabecular bone volume fraction (BV/TV), the
BMD, the trabecular separation (Tb.Sp), the trabecular thickness
(Tb.Th), the trabecular number (Tb.N), the degree of anisotropy
(DA) and the structural model index (SMI) for the same VOI
(23).
Histological examination
We fixed the right mandibles in formalin at a
concentration of 10%, decalcified mandible in EDTA at a
concentration of 14%, dehydrated them, and then embedded them in
paraffin. The cutting of sections was performed with a standard
microtome, and then the cut sections were affixed to glass slides
and stained sections using hematoxylin and eosin.
Analysis of lncRNA microarray
data
We prepared alveolar bone from six rats from the DIO
and OVX groups. The Rat LncRNA Array (Arraystar Inc., Rockville,
MD, USA) was used for profiling the lncRNAs and protein-coding
mRNAs in one experiment. In the microarray, there were
approximately 9300 lncRNAs from the UCSC all_mRNA records, NCBI
RefSeq and orthologs of rat lncRNAs databases and 15200
protein-coding mRNAs from the NCBI RefSeq database that were
evaluated at the same time.
We performed the lncRNA microarray assay at KangChen
Bio-tech (Shanghai, China). In brief, we isolated total RNA with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in accordance with the instructions. We amplified
one milligram of the total RNA from each group and transcribed
itinto fluorescent complementary RNA (cRNA). Then, we hybridized
the labeled cRNAs onto a 4×44 K Rat LncRNA Array. After washing the
slides, we scanned the arrays using an Agilent G2505B Microarray
Scanner. The analysis of the acquired array images was performed
using Agilent Feature Extraction software (v.10.7.3.1; Agilent
Technologies, Inc., Santa Clara, CA, USA). Finally, we performed
quantile normalization using Expander v5.1 software and processed
the data subsequently with the GeneSpring GX v.11.5.1 software
package (Agilent Technologies, Inc.). Threshold values of a fold
change ≥2 and P-value <0.05 were used for screening of
differentially expressed lncRNAs and mRNAs.
Ingenuity pathway analysis (IPA)
We imported the differentially expressed mRNAs from
array analyses into the Ingenuity Pathway Analysis Tool. The IPA
Tool identifies global functions, canonical pathways and biological
networks of a particular dataset on the basis of the Ingenuity
Pathways Knowledge Base (IPKB). Among the canonical pathways of
IPKB, the pathway ‘Role of Osteoblasts, Osteoclasts and
Chondrocytes in Rheumatoid Arthritis (ROOCRA)’ is unique and covers
almost all important molecules and signaling associated with
functions of osteoblasts and osteoclasts, such as differentiation,
mineralization, degradation, development, and apoptosis.
Considering the importance of the ROOCRA pathway in bone
metabolism, we further searched the differentially expressed mRNAs
allocated into the ROOCRA pathway, and these mRNAs were considered
key mRNAs.
Build of mRNA-lncRNA coexpression
network
We selected all of the differentially expressed
lncRNAs and some of the key mRNAs to build the coexpression network
according to the method described previously (24,25). The
building of the network involved the following procedures: i)
preprocessing data- we used the median value of one mRNA with
different transcripts to represent the gene expression, without
exceptional treatment for lncRNA expression values; ii) screening
data-we removed the subsets of data in accordance with the lists of
the different kinds of lncRNA and selected mRNA; iii) then, we
calculated the Pearson's correlation coefficient and applied the
P-value for calculations between lncRNAs and key mRNAs; and iv)
screening on the basis of Pearson's correlation coefficients-we
considered values over 0.98 meaningful and used them to draw the
mRNA-lncRNA coexpression network using Cytoscape (v.2.8.3;
http://www.cytoscape.org) (26).
Analysis of mRNA-lncRNA coexpression
network
Closeness centrality is concerned with the shortest
distance of a node to all the other nodes in a network. Closeness
centrality of a certain node is the combined shortest distance from
all other nodes. Closeness centrality represents the potent
influence of a node on the network (27). The closeness scores of nodes in the
coexpression network were calculated using the CytoHubba plug-in
(28) in Cytoscape. We selected six
lncRNAs with the highest closeness scores and considered them
pivotal lncRNAs. Furthermore, six modules showing interaction
between pivotal lncRNAs and key mRNAs were built.
Validation of differentially expressed
mRNAs and lncRNAs using RT-qPCR
We performed RT-qPCR analyses for lncRNAs and mRNAs
with SYBR RT-qPCR kits (Takara Biotechnology Co., Ltd., Dalian,
China) and an ABI 7500 system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) in accordance with the instructions. We used an
internal control, Gapdh, to normalize the comparative
expression level of lncRNAs or mRNAs with the 2−ΔΔCt
cycle threshold technique.
Statistical analysis
We expressed the data as the mean ± standard
deviation. In addition, we used the Student-Newman-Keuls post hoc
tests and one-way ANOVA for determination of significant
differences between results using SPSS v.19.0 statistical software
package (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Influence of DIO on bone turnover
biomarkers
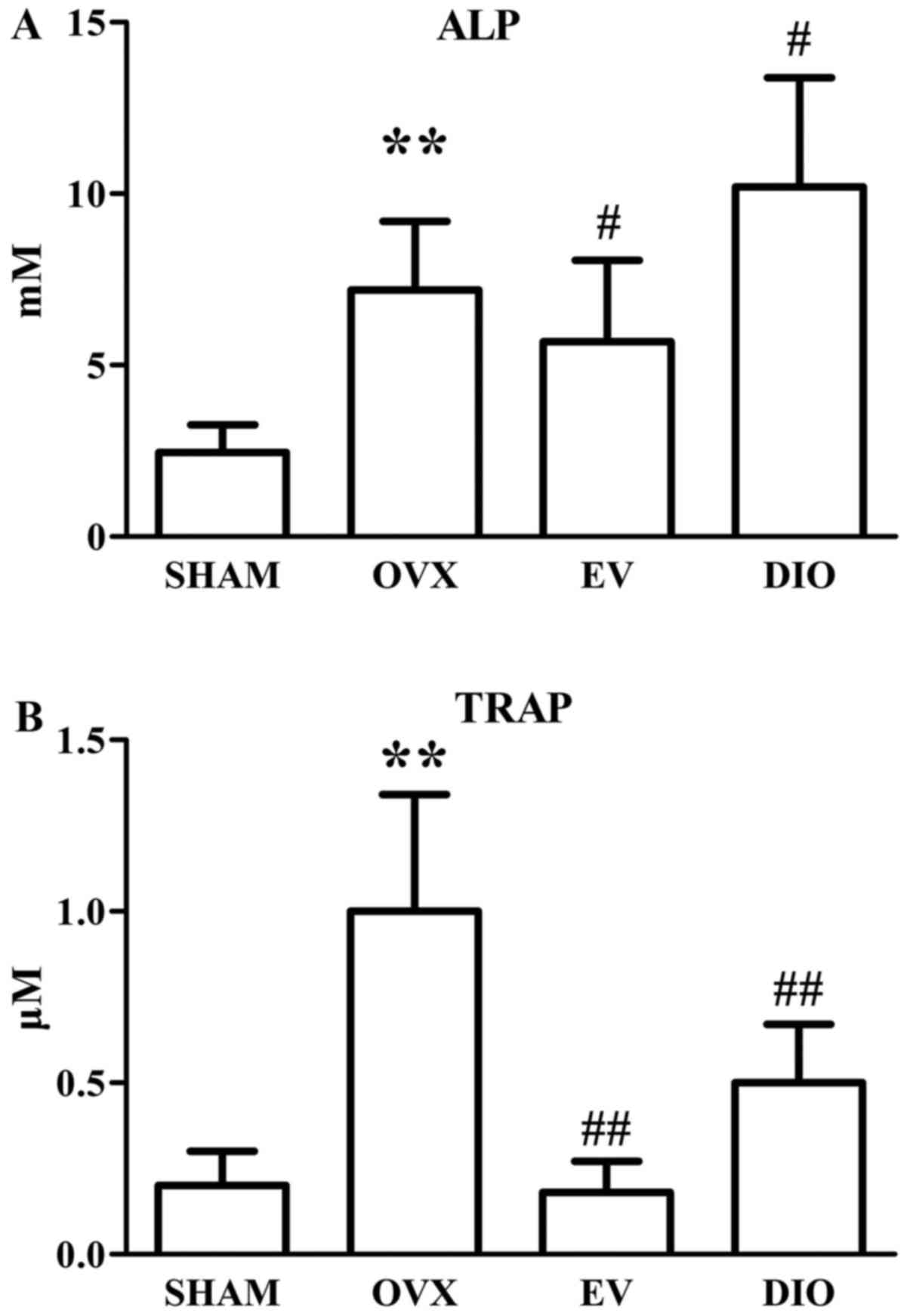
The plasma concentrations of ALP and TRAP in
different rats after a 12-week treatment are shown in Fig. 1A, B. After 12-week treatment, the ALP
and TRAP levels in OVX rats (7.20±2.01 mM, 1.01±0.34 µM,
respectively) were significantly higher than those in SHAM rats
(2.45±0.80 mM, 0.22±0.11 µM, respectively, P<0.01). Moreover,
the plasma ALP and TRAP levels in EV group rats (5.68±2.38 mM,
0.18±0.09 µM, respectively) were significantly lower than those in
OVX group rats (P<0.05 and P<0.01). Nevertheless, DIO
increased the level of ALP (10.20±3.18 mM) and decreased the level
of TRAP (0.50±0.17 µM) in rats compared to those in OVX group rats
(P<0.05 and P<0.01).
Influence of DIO on trabecular bone
microstructure and BMD
The evaluation of trabecular bone microstructure of
the four groups was conducted with micro-CT. The results suggested
that ovariectomy obviously reduced the BMD, Tb.N, Tb.Th and BV/TV
(BMD: 0.296±0.019 g/cm3, Tb.N: 1.530±0.510
mm−1, Tb.Th: 29.636±2.830 µm, BV/TV: 8.308±1.5840%,
P<0.01) and increased the SMI, Tb.Sp, and DA (SMI: 2.267±0.189,
Tb.Sp: 95.443±7.045 µm, DA: 1.943±0.101, respectively, P<0.01)
in comparison with the SHAM group (BMD: 0.786±0.099
g/cm3, BV/TV: 29.952±5.371%, Tb.Th: 84.001±6.540 µm,
Tb.Sp: 40.646±10.107 µm, Tb.N: 9.480±2.280 mm−1, SMI:
1.137±0.108, DA: 1.290±0.180) based on the morphometric parameters
of alveolar bone. The OVX-induced changes were significantly
inhibited after treatment with EV (BMD: 0.589±0.025
g/cm3, BV/TV: 19.321±1.567%, Tb.Th: 70.454±5.180 µm,
Tb.Sp: 59.053±10.360 µm, Tb.N: 6.810±1.610 mm−1, SMI:
1.498±0.120, DA: 1.555±0.167) or DIO (BMD: 0.477±0.071
g/cm3, BV/TV: 12.964±2.072%, Tb.Th: 39.893±7.550 µm,
Tb.Sp: 68.063±11.601 µm, Tb.N: 3.990±0.840 mm−1, SMI:
1.741±0.157, DA: 1.700±0.145) (Fig.
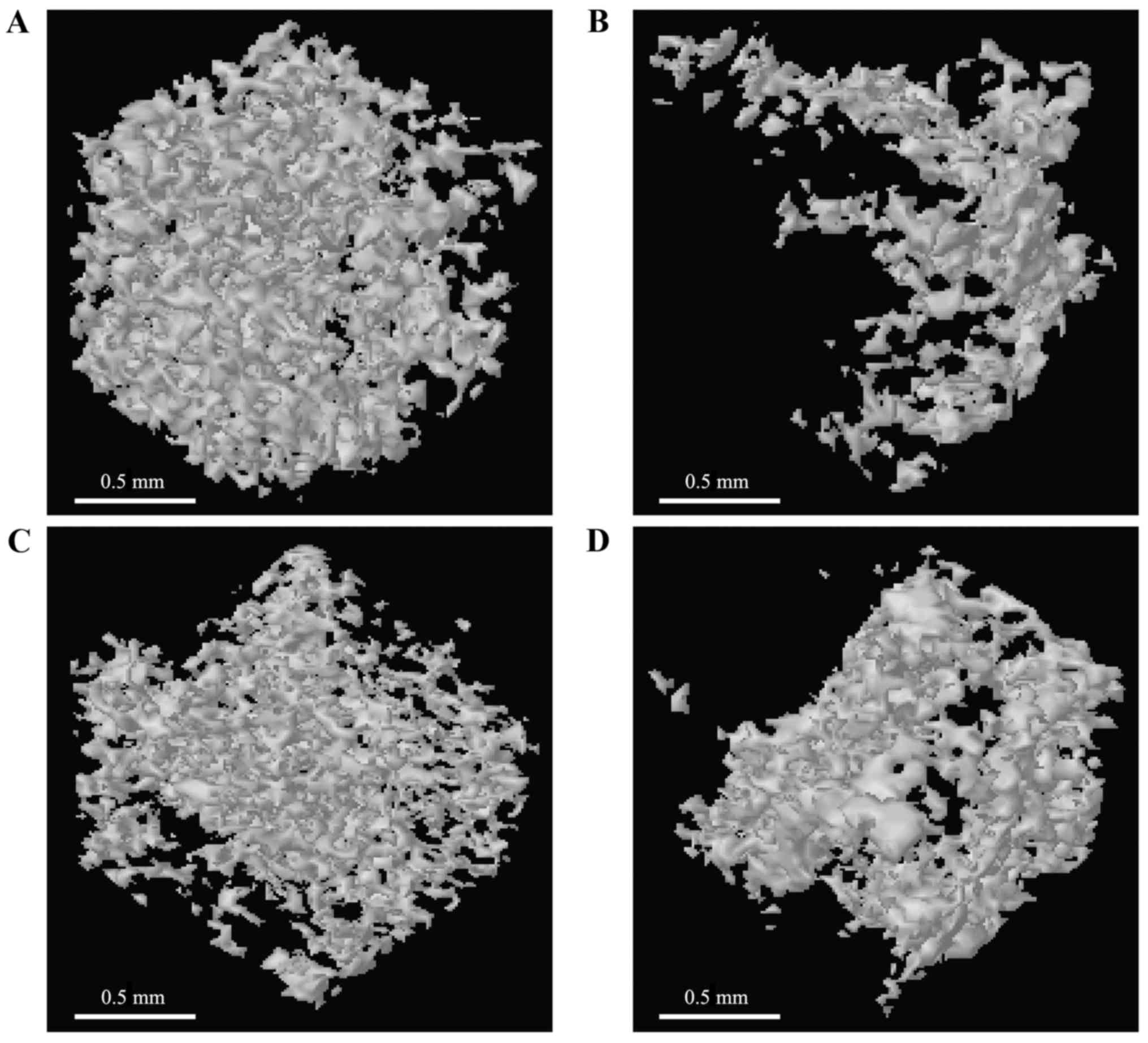
2A-G). Furthermore, impairment of the trabecula caused by
ovariectomy was relieved after treatment with EV or DIO (Fig. 3A-D).
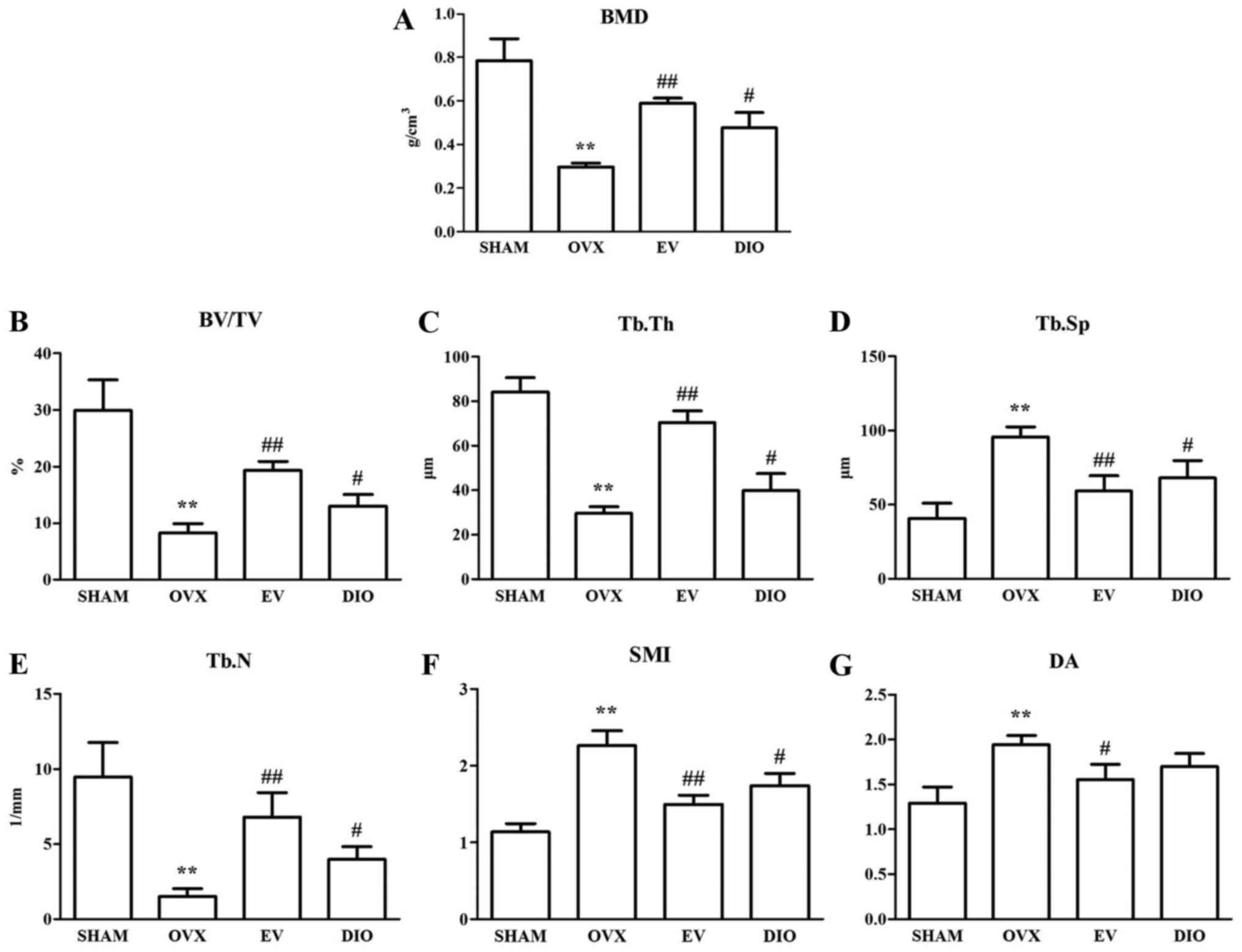 | Figure 2.Influence of DIO on BMD and
trabecular bone microarchitecture after 12-week treatment. (A) BMD,
(B) BV/TV, (C) Tb.Th, (D) Tb.Sp, (E) Tb.N, (F) SMI and (G) DA.
**P<0.01 vs. SHAM group; ##P<0.01 vs. OVX group;
#P<0.05 vs. OVX group. SHAM, sham-operation; OVX,
ovariectomy; EV, estradiol valerate; DIO, diosgenin. SHAM,
sham-operation; OVX, ovariectomy; EV, estradiol valerate; DIO,
diosgenin; BMD, bone mineral density; BV/TV, trabecular bone volume
fraction; Tb.Sp, trabecular separation; Tb.Th, trabecular
thickness; Tb.N, trabecular number; DA, degree of anisotropy; SMI,
structural model index. |
Influence of DIO on histomorphology of
alveolar bone
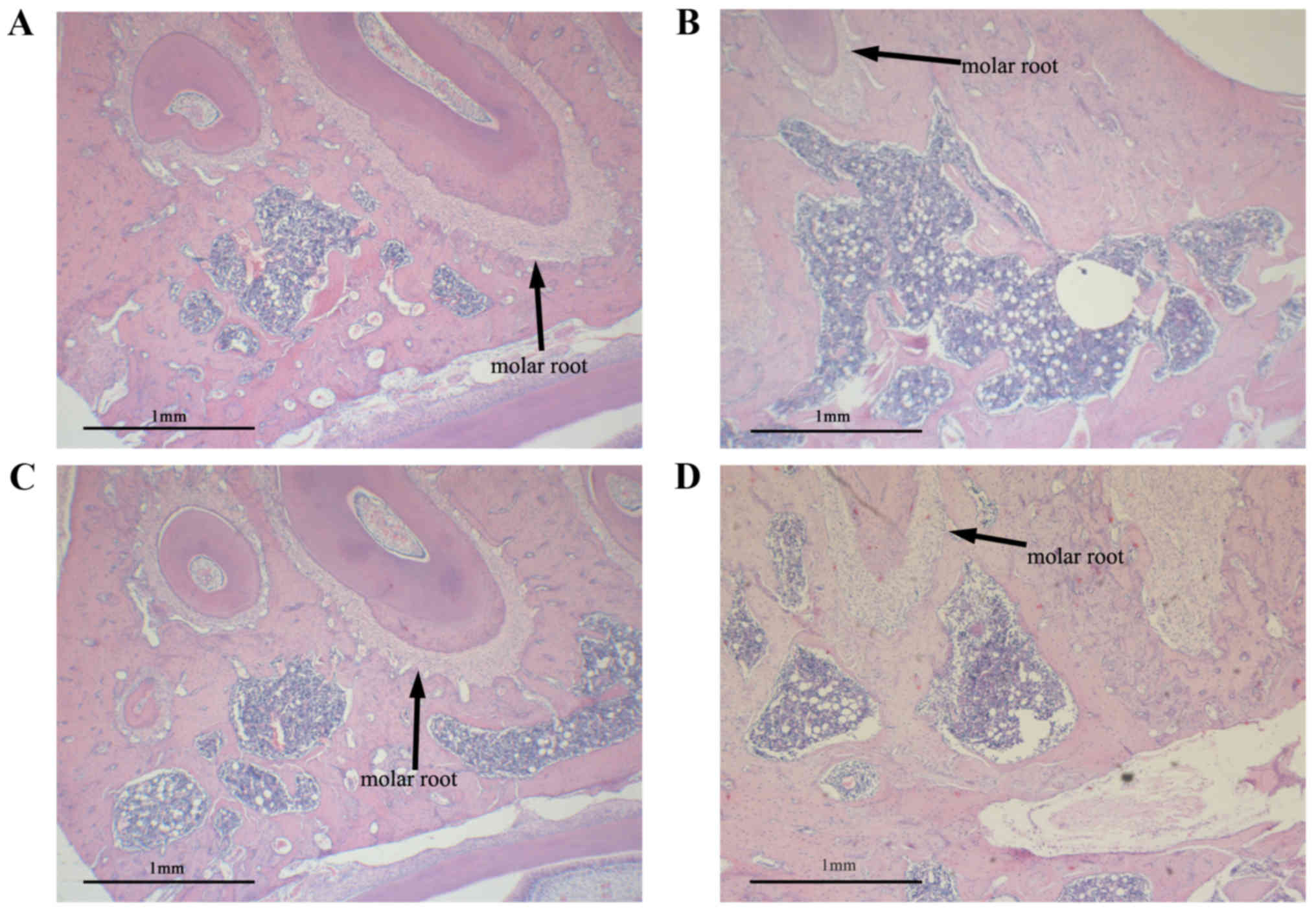
A histological examination was conducted to observe
the alveolar bone region of the first molar. The typical
histological sections of SHAM or OVX rats treated with vehicle, EV
or DIO are shown in Fig. 4. Sections
from rats in the SHAM group demonstrated thickened alveolar bone
and a small and scant medullary cavity (Fig. 4A) relative to those from the OVX
group. OVX significantly decreased the volume of alveolar bone and
increased the size of the medullary cavity (Fig. 4B). Alveolar bone volume was
significantly increased after 12 weeks of treatment with EV or DIO
(Fig. 4C and D), and the effect of
EV was greater than that of DIO. We mutually confirmed the results
of histological observations and micro-CT.
Influence of DIO on lncRNA and mRNA
expression profile
Microarray data analysis confirmed that there were
2409 differentially expressed lncRNAs and 3145 differentially
expressed mRNAs in alveolar bone from the DIO group in comparison
to those from the OVX group rats. Among the 2409 lncRNAs, 1311 were
upregulated and 1098 were down-regulated. Among the 3145 mRNAs,
1468 were shown upregulated and 1677 mRNAs were shown
down-regulated.
Influence of DIO on ROOCRA
pathway
A total of 24 differentially expressed mRNAs (key
mRNAs) in rat alveolar bone between the DIO group and the OVX group
were allocated into the ROOCRA pathway (Table I). Figs.
5 and 6 are schematic diagrams
illustrating the role of these key mRNAs in osteoblasts and
osteoclasts based on the ROOCRA pathway, respectively.
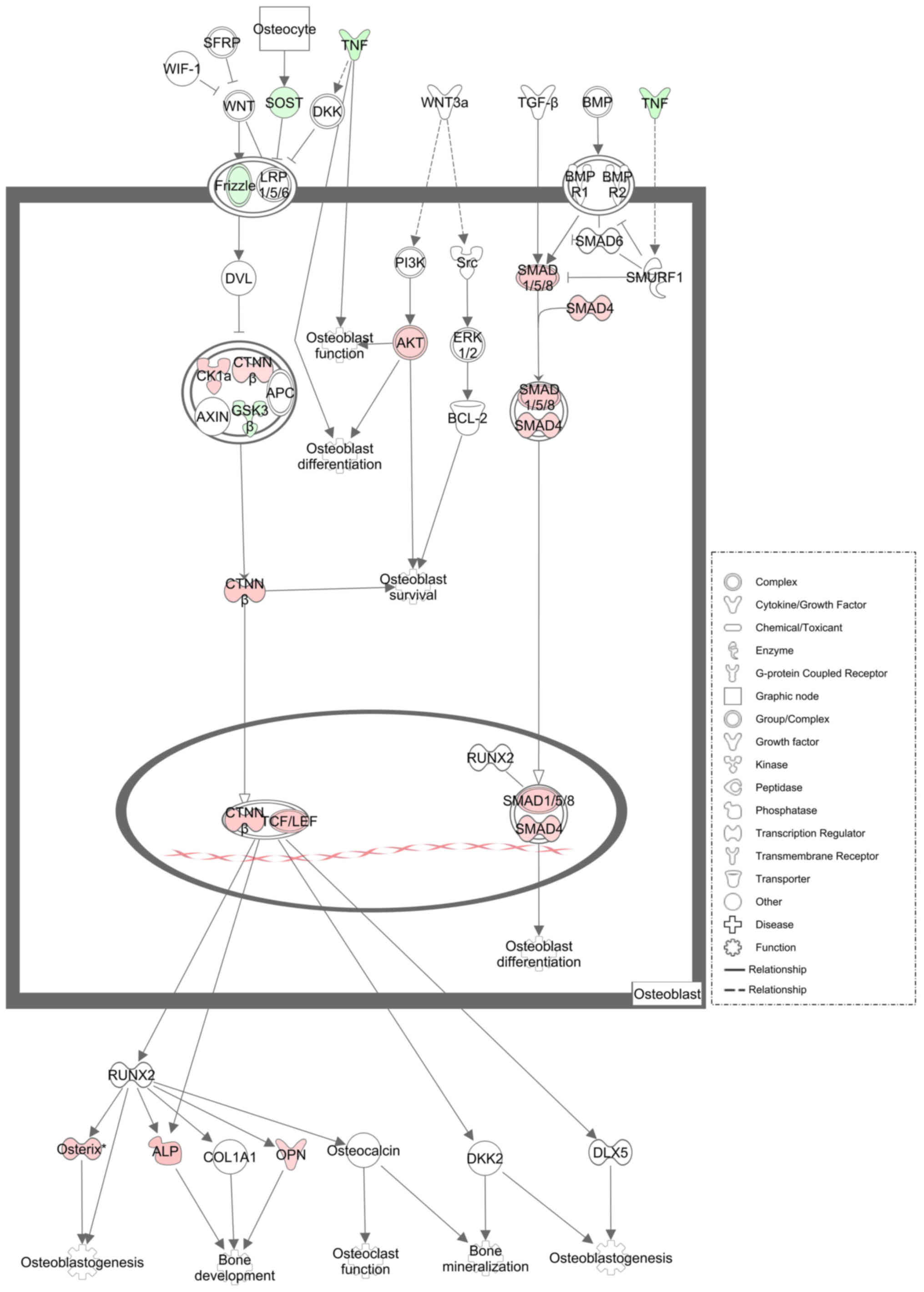 | Figure 5.Illustrative diagram elucidating the
differentially expressed mRNAs from rat alveolar bone between the
DIO group and OVX group in osteoblasts. The genes down-regulated
are shown as green, while the genes upregulated are shown as red.
White represents genes not chosen but introduced into the network
by relation. DIO, diosgenin; OVX, ovariectomy; TNF, tumor necrosis
factor; SOST, sclerostin; CK1α, casein kinase 1 alpha; CTNNβ,
catenin beta; GSK3β, glycogen synthase kinase 3 beta; AKT,
serine/threonine kinase; SMAD1/5/8, SMAD family member 1/5/8;
SMAD4, SMAD family member 4; TCF/LEF, transcription factor protein;
ALP, alkaline phosphatase; OPN, osteopontin. |
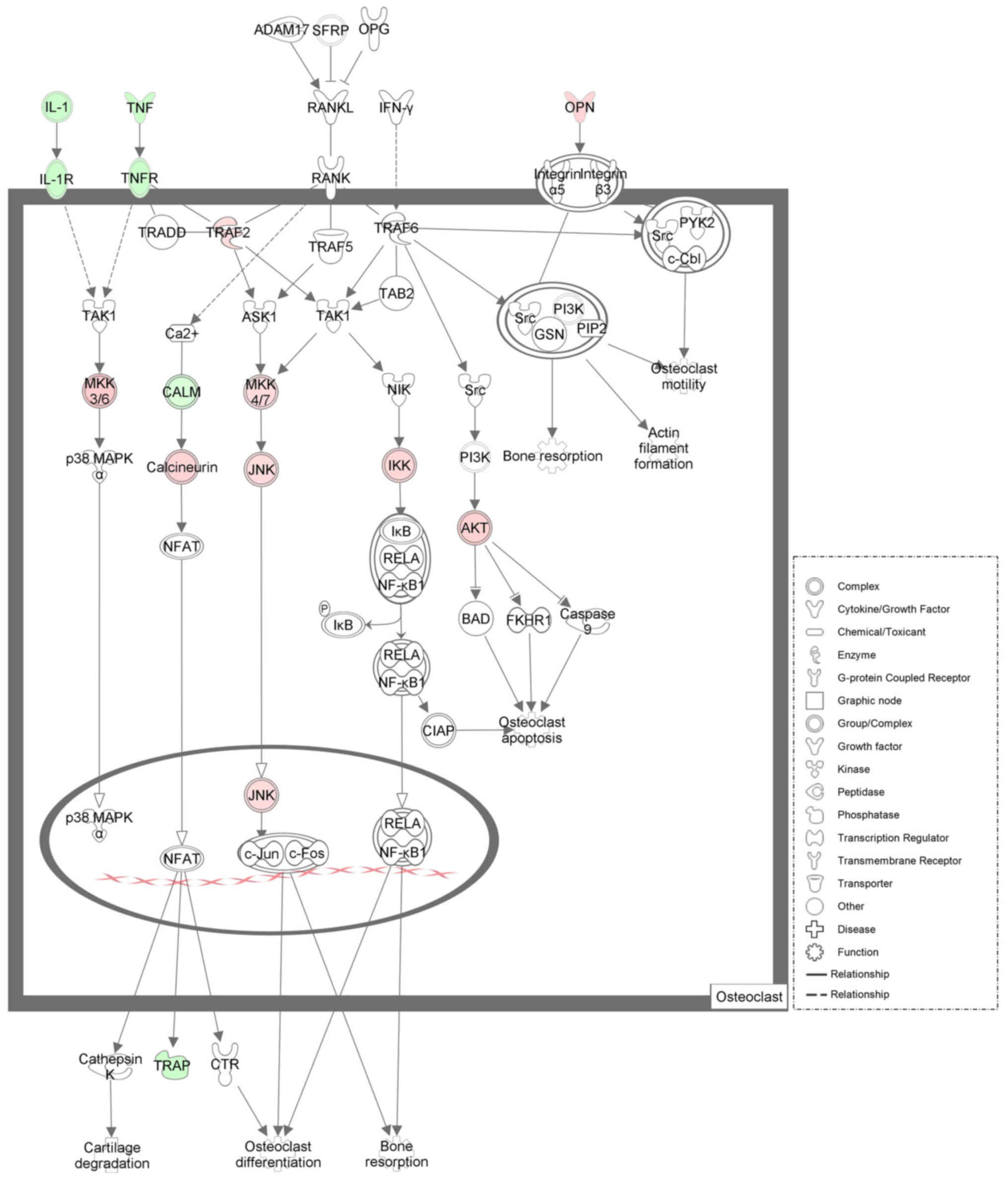 | Figure 6.Illustrative diagram elucidating the
differentially expressed mRNAs from rat alveolar bone between the
DIO group and OVX group in osteoclasts. The genes down-regulated
are shown as green, while the genes upregulated are shown as red.
White represents genes not chosen but introduced into the network
by relation. DIO, diosgenin; OVX, ovariectomy; IL-1, interleukin 1;
IL-1R, interleukin 1 receptor; TNF, tumor necrosis factor; TNFR,
tumor necrosis factor receptor; OPN, osteopontin; TRAF2, TNF
receptor associated factor 2; MKK3/6, mitogen-activated protein
kinase kinase 3/6; MKK4/7, mitogen-activated protein kinase kinase
4/7; CALM, calmodulin; JNK, c-Jun N-terminal kinase; IKK, I-kappaB
kinase; AKT, serine/threonine kinase; TRAP, tartrate-resistant acid
phosphatase. |
 | Table I.Key mRNAs associated with the
regulatory effect of diosgenin on osteoblasts and osteoclast. |
Table I.
Key mRNAs associated with the
regulatory effect of diosgenin on osteoblasts and osteoclast.
| Gene Symbol | Fold change | Gene symbol | Fold change |
|---|
| Tcf2 | 6.7297165 | Mapk9 | 2.0351413 |
| Alpl | 3.4451298 | Ctnnb1 | 2.0331123 |
| Map2k6 | 3.0244298 | Traf2 | 2.0252916 |
| Smad8 | 2.8872466 | Il1b | −14.1060169 |
| Sp7 | 2.8651835 | Tnf | −10.851363 |
| Akt2 | 2.6869522 | Calml3 | −4.2190773 |
| Csnk1a1 | 2.6036916 | Acp5 | −3.5965811 |
| Ppp3r1 | 2.5592904 | Il1r1 | −2.8160289 |
| Spp1 | 2.4752330 | Sost | −2.7645216 |
| Smad4 | 2.4189853 | Fzd9 | −2.3562909 |
| Chuk | 2.3533404 |
Tnfrsf1a | −2.2219197 |
| Map2k4 | 2.0987597 | Gsk3b | −2.0772502 |
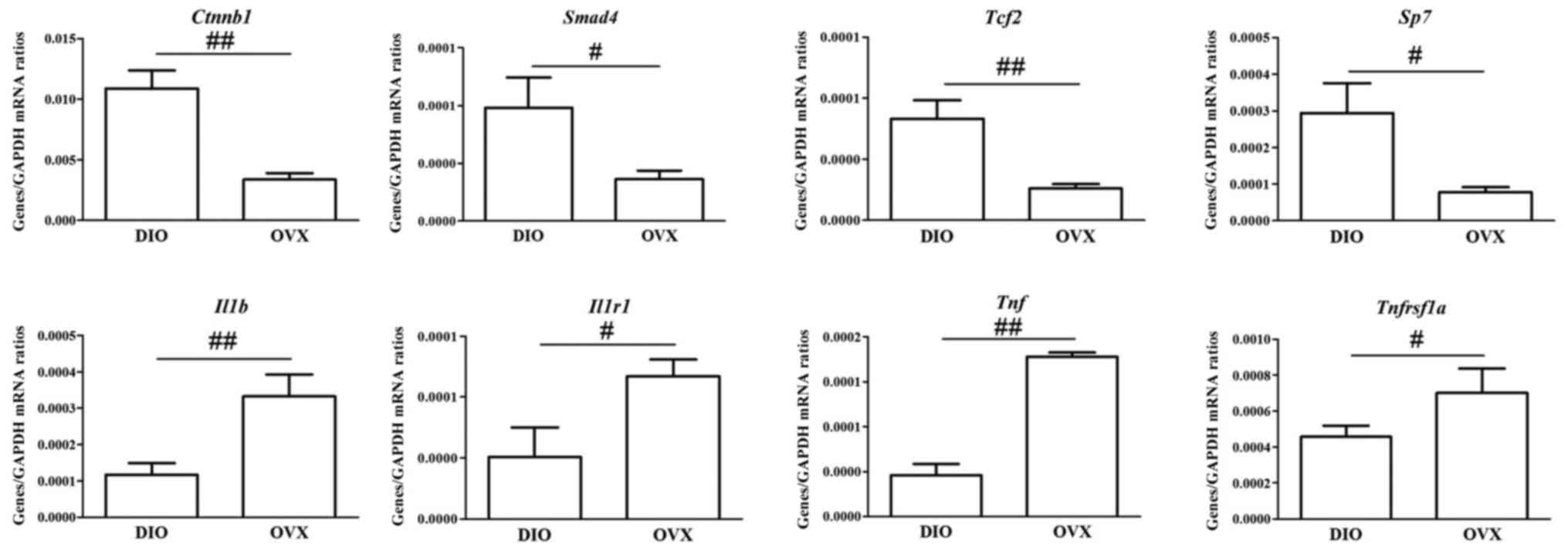
Key mRNA and lncRNA coexpression
network associated with regulatory effect of DIO
Considering the positive regulatory effect of DIO on
osteoblasts and the negative regulatory effect on osteoclasts shown
in the ROOCRA pathway, we selected eight of twenty-four key mRNAs
(Ctnnb1, Smad4, Tcf2, Sp7, Il1b, Il1r1, Tnf and
Tnfrsf1a) and all of the differentially expressed lncRNAs to
construct the coexpression network. A total of 1656 nodes and 5341
edges were obtained in the coexpression network (data not
shown).
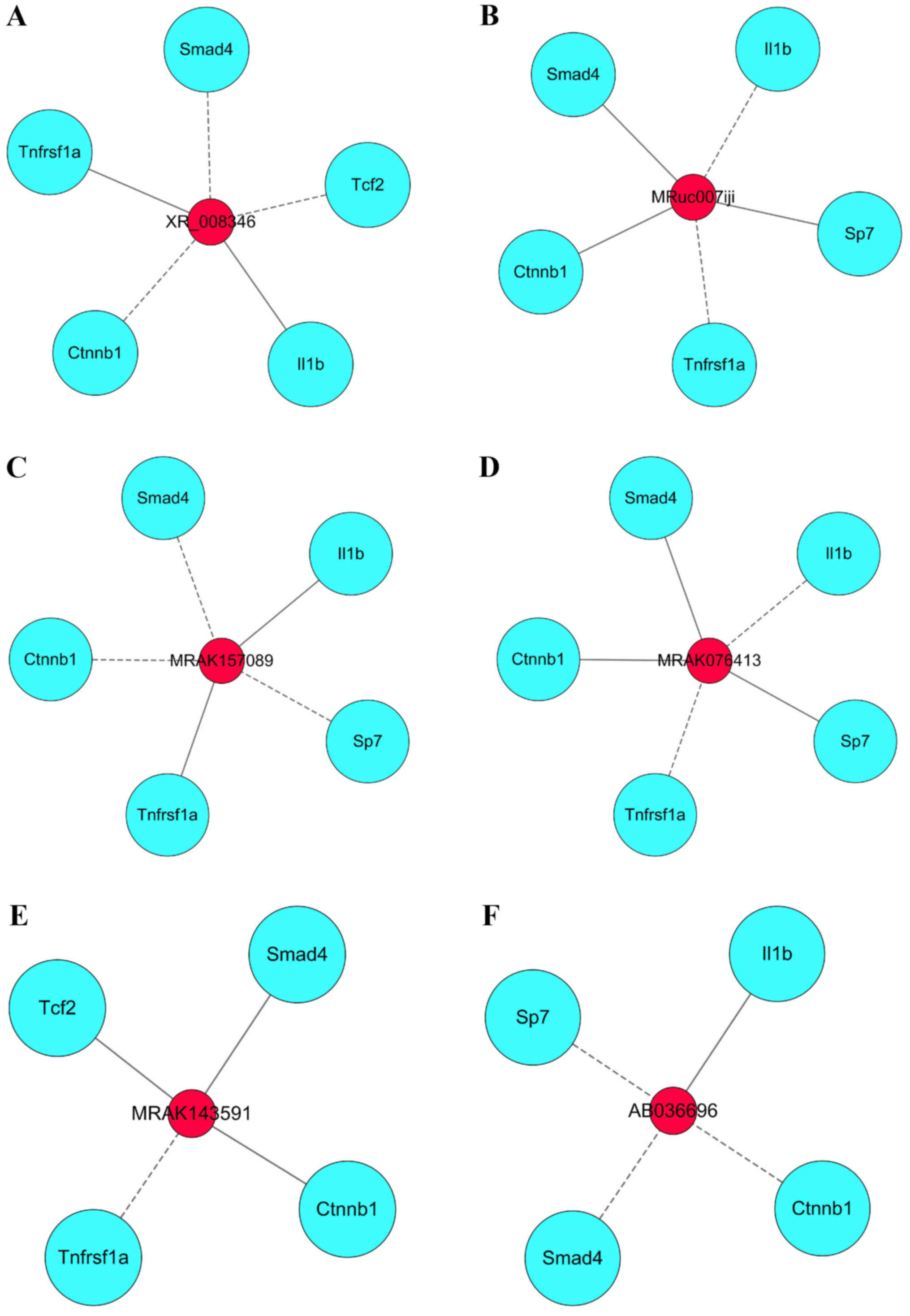
Pivotal lncRNAs in the coexpression
network associated with the regulatory effect of DIO
We regarded six lncRNAs with high closeness scores
as the pivotal lncRNAs that could have multiple regulatory effects
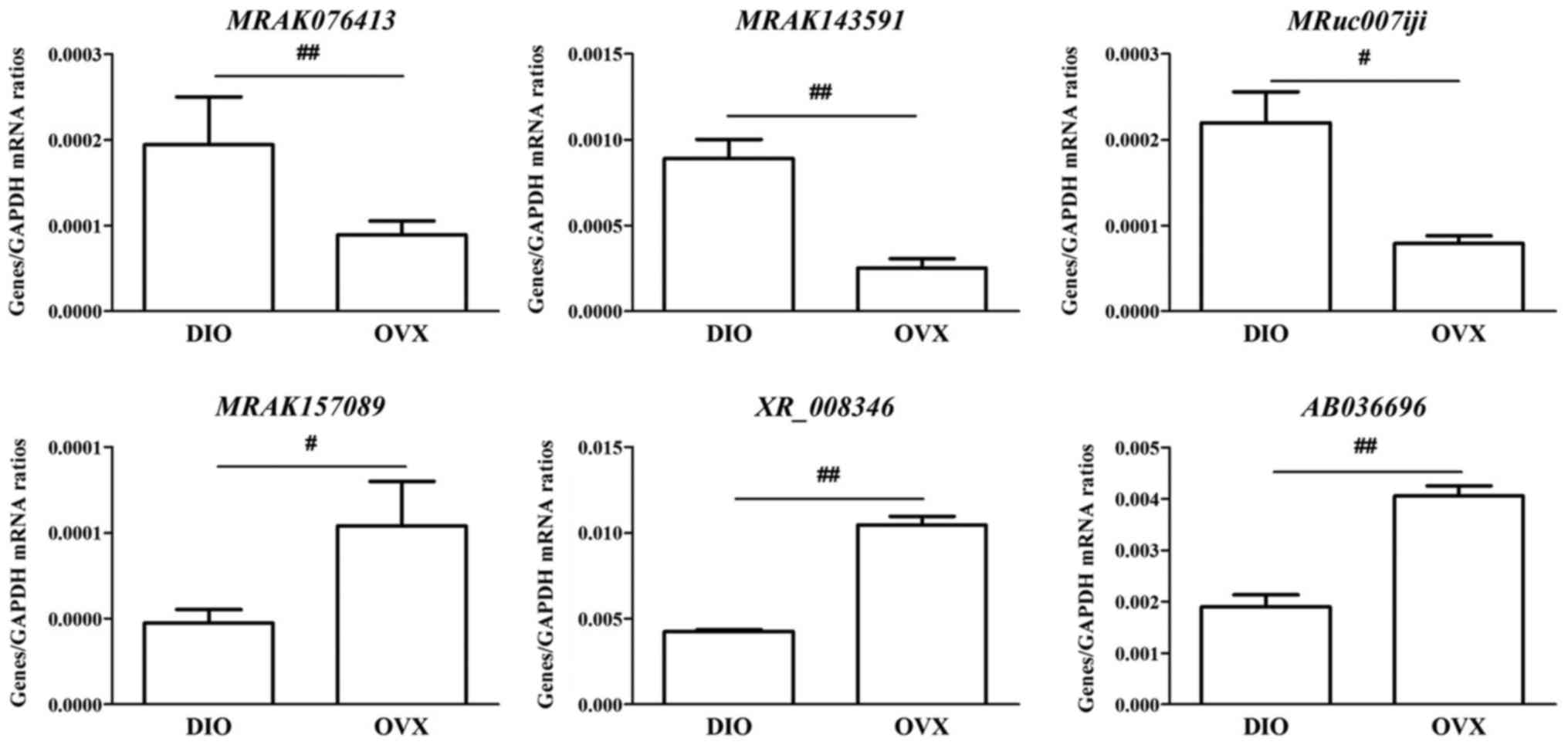
on mRNAs. The six pivotal lncRNAs are listed in Table II. We further established six
modules displaying the interaction between the six pivotal lncRNAs
and eight key mRNAs (Fig. 7A-F).
 | Table II.Pivotal lncRNAs in the co-expression
network associated with the regulatory effect of diosgenin. |
Table II.
Pivotal lncRNAs in the co-expression
network associated with the regulatory effect of diosgenin.
| lncRNA name | Fold change | Closeness
score |
|---|
| XR_008346 | −2.1672872 | 788.4166667 |
| MRuc007iji | 2.4346808 | 788.1666667 |
| MRAK157089 | −2.4197937 | 788.1666667 |
| MRAK076413 | 2.5446928 | 788.1666667 |
| MRAK143591 | 2.5193525 | 785.5 |
| AB036696 | −2.2948153 | 780.25 |
Validation of pivotal lncRNAs and key
mRNAs by RT-qPCR
We evaluated the expression of eight key mRNAs and
six pivotal lncRNAs in the coexpression network or modules
associated with the regulatory effect of DIO. In general, the
RT-qPCR data were consistent with the results found in the
microarray analysis (Figs. 8 and
9).
Discussion
Phytoestrogens are a diverse group of plant-derived
compounds that can bind to estrogen receptors (ERs) and mimic some
actions of estrogen through the activation or inactivation of
certain genes (29). It is
acknowledged that osteoporosis in postmenopausal women is induced
by estrogen deficiency and many phytoestrogens can be used to
mitigate osteoporosis. DIO, as a phytoestrogen, had been proven to
be a potential anti-osteopenic agent (30,31), but
the mechanism of this effect is not fully understood. lncRNAs play
a significant role in developing and maintaining the phenotypes or
functions of cells (32), including
the cells within bone (33). We
conducted this study to explore the role of lncRNAs in the
anti-osteoporotic influence of DIO in alveolar bone of OVX
rats.
Animal models induced by OVX mimics high-turnover
bone loss in human such as postmenopausal women. High-turnover bone
loss is characterized by excessively high bone formation and bone
resorption processes (34). BMD was
significantly reduced after ovariectomy as a result of an elevation
in alveolar bone turnover among the OVX rats in comparison to the
Sham rats. However, the BMD of the alveolar bone was augmented
following treatment with EV or DIO in comparison to the OVX group.
As plasma biomarkers, levels of both TRAP and ALP were raised in
the OVX group rats in comparison to those in the SHAM group. The
treatment with EV for 12 weeks lowered the increases in the two
biomarkers significantly. It suggested that estrogen was able to
lower both bone formation and bone resorption in OVX rats
synchronously. Our findings were similar to those of other
researchers (35–37). However, DIO not only decreased the
level of TRAP but increased the level of ALP (Fig. 1A and B), indicating that DIO only
acts as an estrogen-like agent and is not identical to estrogen.
DIO may have specific targeting molecules or pathways.
The 3-D bone microstructure analysis using micro-CT
demonstrated significant changes in Tb.N, Tb.Th, Tb.Sp, BV/TV, and
SMI, which indicated that there was less loss of alveolar bone in
DIO- or EV-treated rats in comparison to the OVX group. The
anti-osteoporotic influence of EV on alveolar bone was more
powerful than DIO (Fig. 2). The
morphological findings of alveolar bone (Fig. 4) were consistent with the micro-CT
evaluation (Fig. 3).
We found that DIO had a strong anti-osteoporotic
influence on alveolar bone according to the results from
histological observation, micro-CT, the assays of BMD, and bone
turnover biomarkers.
We evaluated the lncRNA and mRNA profiles using a
microarray in order to confirm the anti-osteoporotic influence of
DIO on alveolar bone. As the functions of differentially expressed
lncRNAs were largely unclear, we first explored the roles of
differentially expressed mRNAs in the anti-osteopenic effects of
DIO. The ROOCRA pathway in the IPA database is a unique pathway and
covers almost all of the important molecules and signaling
associated with functions of osteoblasts and osteoclasts, such as
differentiation, mineralization, degradation, development, and
apoptosis. We found that 24 differentially expressed mRNAs were
associated with the ROOCRA pathway (Table I). As shown in Fig. 5, we found that DIO could promote the
bone formation process via increasing signaling of the Wnt and BMPs
pathways, two recognized signaling pathways regulating the
osteogenic differentiation of mesenchymal stem cells or
preosteoblasts (38,39). At the mRNA level, the expression of
some transcription factors or complexes in osteoblasts, such as
Smad4, Smad8, and beta-catenin/Tcf, and the expression of some
downstream molecules, such as Osterix, Alp and osteopontin (OPN),
were all increased. As shown in Fig.
6, we found that DIO could inhibit the bone resorption process
via decreasing two potent stimulators of osteoclastogenesis [tumor
necrosis factor-alpha (TNF-alpha) and interleukin-1 beta
(IL-1beta)] and their receptors (IL-1R and TNF-R1) (40,41).
Particularly, the results from the microarray assay showed the
expression of Il1b and Tnf (−14.106-and −10.851-fold
changes, respectively) were strongly inhibited by DIO. In addition,
the mRNA expression of TRAP in alveolar bone was shown to be
down-regulated after a 12-week DIO treatment. In brief, the results
from the microarray and pathway analysis suggested that the
anti-osteoporotic influence of DIO on alveolar bone was attributed
to enhanced bone formation via increasing the expression of some
transcription factors (e.g., Ctnnb1, Tcf2, Smad4, and
Smad8) and to a lowered bone resorption via decreasing the
expression of some proinflammatory cytokines or receptors (e.g.,
Tnf, Il1b, Il1r1, and Tnfrsf1a).
Based on results from the pathway analysis of
differentially expressed mRNAs, we focused on eight key mRNAs
(Ctnnb1, Tcf2, Smad4, Smad8, Tnf, Il1b, Il1r1, and
Tnfrsf1a) and further established a lncRNA-mRNA coexpression
network associated with the anti-bone loss effect of DIO to
identify pivotal lncRNAs that could regulate the eight key mRNAs
mentioned above.
A large coexpression network consisting of 1656
nodes and 5341 edges was constructed. However, almost all of the
lncRNAs in the coexpression network were not annotated. This was a
limitation of our study. Fortunately, some researchers hold that
hub analysis of the coexpression network could overcome this
limitation to some degree (42,43).
Closeness is one of the most important metrics for finding hubs in
a network (27,44). Closeness is the length of the
shortest path from one node to another. To evaluate how close any
one lncRNA is to eight key mRNAs in this network, we used a
closeness score to find the lncRNAs that are most likely to be
regulators of the eight key mRNAs. Six pivotal lncRNAs with the
highest closeness scores (XR_008346, MRuc007iji, MRAK157089,
MRAK076413, MRAK143591, and AB036696) were found. Then, we built
six modules that hint at which of these pivotal lncRNAs might have
positive or negative regulatory effects on key mRNAs, but we did
not find any reports on the relationships between pivotal lncRNAs
and key mRNAs. In the future, to reveal the underlying mechanisms
of these lncRNAs, further research is necessary.
In conclusion, DIO inhibits ovariectomy-induced loss
of alveolar bone in rats via promoting bone formation and
inhibiting bone resorption. The mechanism of this anti-osteoporotic
influence of DIO probably lies in the global modulation of the mRNA
and lncRNA expression profiles. Of note, six pivotal lncRNAs
(XR_008346, MRuc007iji, MRAK157089, MRAK076413, MRAK143591 and
AB036696), which may regulate the expression of eight key mRNAs
(Ctnnb1, Tcf2, Smad4, Smad8, Tnf, Il1b, Il1r1 and
Tnfrsf1a), play crucial roles in this process. Our study
indicates that DIO can potentially be used as a drug or health
supplements for postmenopausal females with alveolar bone loss.
Acknowledgements
Not applicable.
Funding
The present study was supported by grant from the
National Natural Science Foundation of China (grant no. 81473450),
the Beijing Foundation for Science and Technology Development of
Traditional Chinese Medicine (grant no. JJ2015-54) and the
Fundamental Research Funds for the Central Public Welfare Research
Institutes (grant no. YZ-1780).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ, GGX and DJ were involved in the study conception
and design. ZZ, YC and LX were involved in analysis and
interpretation of data. ZZ, YC, GGX and ZW collected data. ZZ, GGX
and DJ wrote the article. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal use protocol has been reviewed and
approved by Institutional Ethics Committee of the Institute of
Basic Theory, China Academy of Chinese Medical Sciences (Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taguchi A, Tanimoto K, Suei Y and Wada T:
Tooth loss and mandibular osteopenia. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 79:127–132. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krall EA, Garcia RI and Dawson-Hughes B:
Increased risk of tooth loss is related to bone loss at the whole
body, hip and spine. Calcif Tissue Int. 59:433–437. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ikeo T, Goda S and Domae E: Metabolism of
alveolar bone. Clin Calcium. 16:117–121. 2006.(In Japanese).
PubMed/NCBI
|
|
4
|
Au AL, Kwok CC, Lee AT, Kwan YW, Lee MM,
Zhang RZ, Ngai SM, Lee SM, He GW and Fung KP: Activation of
iberiotoxin-sensitive, Ca2+-activated K+ channels of porcine
isolated left anterior descending coronary artery by diosgenin. Eur
J Pharmacol. 502:123–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jesus M, Martins AP, Gallardo E and
Silvestre S: Diosgenin: Recent highlights on pharmacology and
analytical methodology. J Anal Methods Chem. 2016:41562932016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Medigović I, Ristić N, Živanović J,
Šošić-Jurjević B, Filipović B, Milošević V and Nestorović N:
Diosgenin does not express estrogenic activity: A uterotrophic
assay. Can J Physiol Pharmacol. 92:292–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aradhana, Rao AR and Kale RK: Diosgenin-a
growth stimulator of mammary gland of ovariectomized mouse. Indian
J Exp Biol. 30:367–370. 1992.PubMed/NCBI
|
|
8
|
Zhang Z, Song C, Fu X, Liu M, Li Y, Pan J,
Liu H, Wang S, Xiang L, Xiao GG and Ju D: High-dose diosgenin
reduces bone loss in ovariectomized rats via attenuation of the
RANKL/OPG ratio. Int J Mol Sci. 15:17130–17147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Folwarczna J, Zych M, Nowinska B, Pytlik
M, Bialik M, Jagusiak A, Lipecka-Karcz M and Matysiak M: Effect of
diosgenin, a steroidal sapogenin, on the rat skeletal system. Acta
Biochim Pol. 63:287–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown CJ, Ballabio A, Rupert JL,
Lafreniere RG, Grompe M, Tonlorenzi R and Willard HF: A gene from
the region of the human X inactivation centre is expressed
exclusively from the inactive X chromosome. Nature. 349:38–44.
1991. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartolomei MS, Zemel S and Tilghman SM:
Parental imprinting of the mouse H19 gene. Nature. 351:153–155.
1991. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwok ZH and Tay Y: Long noncoding RNAs:
Lincs between human health and disease. Biochem Soc Trans.
45:805–812. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deniz E and Erman B: Long noncoding RNA
(lincRNA), a new paradigm in gene expression control. Funct Integr
Genomics. 17:135–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei B, Wei W, Zhao B, Guo X and Liu S:
Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate
osteogenic differentiation and proliferation in non-traumatic
osteonecrosis of femoral head. PLoS One. 12:e01690972017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xing D, Liang JQ, Li Y, Lu J, Jia HB, Xu
LY and Ma XL: Identification of long noncoding RNA associated with
osteoarthritis in humans. Orthop Surg. 6:288–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Li Y and Zhang Y, Ma L, Lin L,
Meng J, Jiang L, Wang L, Zhou P and Zhang Y: LncRNA MEG3 inhibited
osteogenic differentiation of bone marrow mesenchymal stem cells
from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed
Pharmacother. 89:1178–1186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tong X, Gu PC, Xu SZ and Lin XJ: Long
non-coding RNA-DANCR in human circulating monocytes: A potential
biomarker associated with postmenopausal osteoporosis. Biosci
Biotechnol Biochem. 79:732–737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao L, Fu J, Tian Y and Wu J: Systematic
analysis of lncRNAs, miRNAs and mRNAs for the identification of
biomarkers for osteoporosis in the mandible of ovariectomized mice.
Int J Mol Med. 40:689–702. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li CM, Dong XL, Fan XD, Wu JH, Wang QH,
Tian XL, Guo DJ, Wong MS, Qiu TQ and Chan SW: Aqueous extract of
danshen (Salvia miltiorrhiza Bunge) protects ovariectomized rats
fed with high-fat diet from endothelial dysfunction. Menopause.
20:100–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maïmoun L, Brennan-Speranza TC, Rizzoli R
and Ammann P: Effects of ovariectomy on the changes in
microarchitecture and material level properties in response to hind
leg disuse in female rats. Bone. 51:586–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lane NE, Yao W, Kinney JH, Modin G,
Balooch M and Wronski TJ: Both hPTH(1–34) and bFGF increase
trabecular bone mass in osteopenic rats but they have different
effects on trabecular bone architecture. J Bone Miner Res.
18:2105–2115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Pham SM and Crabbe DL:
High-resolution Micro-CT evaluation of mid- to long-term effects of
estrogen deficiency on rat trabecular bone. Acad Radiol.
10:1153–1158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Müller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu G, Yao W, Wang J, Ma X, Xiao W, Li H,
Xia D, Yang Y, Deng K, Xiao H, et al: LncRNAs expression signatures
of renal clear cell carcinoma revealed by microarray. PLoS One.
7:e423772012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao Q, Liu C, Yuan X, Kang S, Miao R,
Xiao H, Zhao G, Luo H, Bu D, Zhao H, et al: Large-scale prediction
of long non-coding RNA functions in a coding-non-coding gene
co-expression network. Nucleic Acids Res. 39:3864–3878. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu J, Li C and Ji W: Identification of
genes in ulcerative colitis associated colorectal cancer based on
centrality analysis of co-expression network. Neoplasma.
62:756–764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: CytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol. 8 Suppl 4:S112014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Turner JV, Agatonovic-Kustrin S and Glass
BD: Molecular aspects of phytoestrogen selective binding at
estrogen receptors. J Pharm Sci. 96:1879–1885. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hung YT, Tikhonova MA, Ding SJ, Kao PF,
Lan HH, Liao JM, Chen JH, Amstislavskaya TG and Ho YJ: Effects of
chronic treatment with diosgenin on bone loss in a
d-galactose-induced aging rat model. Chin J Physiol. 57:121–127.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao S, Niu F, Xu CY, Liu Y, Ye L, Bi GB,
Chen L, Tian G and Nie TH: Diosgenin prevents bone loss on retinoic
acid-induced osteoporosis in rats. Ir J Med Sci. 185:581–587. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun M and Kraus WL: From discovery to
function: the expanding roles of long noncoding RNAs in physiology
and disease. Endocr Rev. 36:25–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huynh NP, Anderson BA, Guilak F and
McAlinden A: Emerging roles for long noncoding RNAs in skeletal
biology and disease. Connect Tissue Res. 58:116–141. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeh JK, Chen MM and Aloia JF:
Ovariectomy-induced high turnover in cortical bone is dependent on
pituitary hormone in rats. Bone. 18:443–450. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dabbaghmanesh MH, Noorafshan A, Talezadeh
P, Tanideh N, Koohpeyma F, Iraji A, Bakhshayeshkaram M and
Montazeri-Najafabady N: Stereological investigation of the effect
of Elaeagnus angustifolia fruit hydroalcoholic extract on
osteoporosis in ovariectomized rats. Avicenna J Phytomed.
7:261–274. 2017.PubMed/NCBI
|
|
36
|
Li S, Zhang W, Duan F, Liu W, Sun X and
Pan X: The preventive and therapeutic roles of phytoestrogen
α-Zearalanol on osteoporetic rats due to ovariectomization. Iran J
Basic Med Sci. 19:1216–1221. 2016.PubMed/NCBI
|
|
37
|
You MK, Kim DW, Jeong KS, Bang MA, Kim HS,
Rhuy J and Kim HA: St. John's Wort (Hypericum perforatum)
stimulates human osteoblastic MG-63 cell proliferation and
attenuates trabecular bone loss induced by ovariectomy. Nutr Res
Pract. 9:459–465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marcellini S, Henriquez JP and Bertin A:
Control of osteogenesis by the canonical Wnt and BMP pathways in
vivo: Cooperation and antagonism between the canonical Wnt and BMP
pathways as cells differentiate from osteochondroprogenitors to
osteoblasts and osteocytes. Bioessays. 34:953–962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin GL and Hankenson KD: Integration of
BMP, Wnt, and notch signaling pathways in osteoblast
differentiation. J Cell Biochem. 112:3491–3501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kudo O, Fujikawa Y, Itonaga I, Sabokbar A,
Torisu T and Athanasou NA: Proinflammatory cytokine
(TNFalpha/IL-1alpha) induction of human osteoclast formation. J
Pathol. 198:220–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dou C, Cao Z, Yang B, Ding N, Hou T, Luo
F, Kang F, Li J, Yang X, Jiang H, et al: Changing expression
profiles of lncRNAs, mRNAs, circRNAs and miRNAs during
osteoclastogenesis. Sci Rep. 6:214992016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu L, Xu Q, Zhang H, Li M, Zhu C, Jiang M,
Sang X, Zhao Y, Sun Q and Zhao H: A new avenue for obtaining
insight into the functional characteristics of long noncoding RNAs
associated with estrogen receptor signaling. Sci Rep. 6:317162016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghosh R and Lerman K: Parameterized
centrality metric for network analysis. Phys Rev E Stat Nonlin Soft
Matter Phys. 83:0661182011. View Article : Google Scholar : PubMed/NCBI
|