Introduction
In 1997, Medzhitov et al (1) discovered the first human Toll protein,
which is toll like receptor 4 (TLR4), and confirmed the presence of
TLR4/ nuclear factor-κB (NF-κB) signaling in human. TLR4/NF-κB is
the main pathway mediating the signal transduction of bacterial
lipopolysacchride (LPS), and is also highly expressed in the heart
tissue (2,3). After binding to ligand, TLR4 can
activate MAPK and NF-κB, so as to regulate inflammation and
immune-related gene expression (4).
In an animal model of cardiac hypertrophy induced by aortic
ligation, it was found that the TLR4 gene-deficient mice had
significantly lower cardiac index (cardiac weight/body weight) and
smaller size of cardiomyocytes comparing with wild-type mice.
Moreover, blocking TLR4/NF-κB signaling pathway significantly
improved cardiac hypertrophy and cardiomyocyte apoptosis induced by
stress load, and reduced the sensitivity of myocardial cells to
inflammatory reactions, which in turn improved cardiac function
(5,6). Therefore, it is hypothesized that TLR4
and its signaling pathways may participate in the occurrence and
development of left ventricular remodeling by activating primary
immune mechanisms and inflammatory responses in impaired
myocardium. But this hypothesis remains to be confirmed. Myocardial
local RAS plays an important role in the occurrence and development
of ventricular remodeling. Angiotensin II (Ang II) is a major
effector of the renin angiotensinogen system (RAS), and as a
proinflammatory mediator, and Ang II mainly performs its function
through AT1a receptor (7). Studies have shown that TLR4 can
sensitively respond to Nak levels of LPS to induce cardiomyocyte
apoptosis, and RAS system blockers can completely inhibit
LPS-induced cardiomyocyte apoptosis both in vitro and in
vivo (8,9), suggesting TLR4-mediated signaling may
be related to renin-angiotensinogen system in the myocardium. LPS
from Escherichia coli can specifically agonize TLR4 without
activating other toll-like receptors (10). In this study, cultured neonatal rat
left ventricular myocytes (NRVMs) were stimulated with LPS, a
specific agonist of TLR4, to activate TLR4/NF-κB signaling pathway.
At the same time, NRVMs were treated with caffeic acid
phenethylester (CAPE), a specific inhibitor of NF-κB, to explore
the effects of TLR4/NF-κB signaling pathway on the expression of
angiotensinogen (ATG) and AT1a receptor, so as to
explore the mechanism of the role of TLR4/NF-κB in cardiovascular
diseases. This study was approved by the Ethics Committee of the
Second Affiliated Hospital of Dalian Medical University (Dalian,
China).
Materials and methods
Experimental subjects and main
reagents
Healthy newborn Sprague-Dawley rats (10 rats each
time, either male or female) aged 6–24 h were provided by the
Experimental Animal Center of Dalian Medical University. The
newborn rats were kept in cage, temperature 20–24°C, access to
breat milk, relative humidity 40–60%. LPS (055: B5), CAPE, type II
collagenase, trypsin and 5-bromodeoxyuridine (Brdu) were provided
by Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). TRIzol was from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Reverse
transcription kit was from Takara (Dalian, China). DMEM medium was
from Gibco; Thermo Fisher Scientific, Inc. Mouse striated
muscle-specific sarcomeric α-actin monoclonal antibody (cat. no.
MA1-26928), and SABC immunohistochemistry kit including
biotinylated secondary antibody, hydrogen peroxide, blocking serum,
ultra-sensitive ABC peroxidase mouse IgG staining kit (cat.
no.32052), all from Thermo Fisher Scientific, Inc. Rabbit
anti-mouse NF-κB p65 monoclonal antibody (cat. no. SC8008) was from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Primary cardiomyocyte culture and
grouping
Ventricles were collected from Sprague-Dawley rats,
and were cut into pieces. Then 0.1% trypsin and 0.1% type II
collagenase was added to make single cell suspension, and cardiac
fibroblasts and epithelial cells were removed by differential
adherence method. During the first 48 h of cell culture, 0.1 mmol/l
Brdu was added to inhibit the proliferation of cells except
cardiomyocytes. Cardiomyocytes showed radial or spiral-shaped
adherent growth, and obvious cardiomyocytes pulsation was observed
36 h later. Medium was changed at 48 h, and sub-fusion state was
reached at 96 h, then cells were cultured with serum-free DMEM.
After incubation for another 24 h, different interventions were
performed. Cells were divided into different groups: Experiment I:
i) Control group: No intervention factor was added into DMEM
medium; ii) LPS 10 ng/ml group: DMEM medium was added with LPS to a
final concentration of 10 ng/ml and iii) LPS 100 ng/ml group: DMEM
medium was added with LPS to final concentration of 1,000 ng/ml.
Intervention was performed for 24 h, and cells were collected. Then
expression of TLR4, ATG and AT1a receptor at mRNA level
was detected by RT-PCR. Experiment II: i) Control group: No
intervention factor was added into DMEM medium; ii) LPS 1,000 ng/ml
group: DMEM medium was added with LPS to a final concentration of
1,000 ng/ml; and iii) LPS 1,000 ng/ml + CAPE 20 µg/ml group: CAPE
was added to a final concentration of 20 µg/ml, and LPS was added
to a final concentration of 1 µg/ml 30 min later. Cells were
treated for 24 h, and expression of TLR4, ATG and AT1a
receptor at mRNA level was detected by RT-PCR. Each experiment was
repeated 3 times.
Identification of cardiomyocytes
Cardiomyocytes were seeded on glass slides and
observed under an inverted microscope (Olympus Corporation, Tokyo,
Japan). Cells were radial and swirling shape with transparent
cytoplasm and oval nuclei in the center. After incubation for 96 h,
slides were fixed with 4% paraformaldehyde for 20 min. After
washing with PBS 3 times, slides were immersed in 1% triton
solution for 5 min. SABC chemical staining was performed with
striated muscle-specific sarcomeric α-actin monoclonal antibody
(cat. no. ZM-0001) to identify cardiomyocytes.
Immunocytochemistry analysis of NF-κB
activation
Density of NRVMs was adjusted to 1×106
cells/ml, and cells were seeded on cell culture plates covered with
cover glasses. After treatment for 24 h, cover glasses were
removed, and plates were washed with PBS and fixed with 1%
paraformaldehyde (pH 7.0) at room temperature for 30 min and 0.1%
Triton X-100 (pH 7.0) at room temperature for 5 min. Rabbit
anti-mouse NF-κB p65 antibody (cat. no. SC8008; Santa Cruz
Biotechnology, Inc.) was added with a ratio of 1:100 and incubated
at 37°C for 60 min. Biotin-avidin reaction system was used to
detect the immune response. After DAB colorimetry and hematoxylin
counterstaining, slides were sealed. Immunocytochemistry results
were determined according to the following criteria: NF-κB exists
in the cytoplasm under normal circumstances, and enters nucleus
after activation. So, positive expression of NF-κB in nucleus
indicates the activation. Five visual fields were randomly selected
under the 200-fold optical microscope and the activation of NF-κB
was assessed by calculating the percentage of positive cells.
Detection of TLR4, AT1a
receptor and ATG mRNA expression in cardiomyocytes
Primers were synthesized by Takara. Total RNA was
extracted by using TRIzol reagent according to the instructions.
The quality of RNA samples was detected, and only the ones with
OD260/OD280 ratio between 1.6 and 1.8 were used in reverse
transcription. TLR4, AT1a receptor and ATG expression
were detected by two step method. Reverse transcription conditions:
30°C for 10 min, 50°C for 30 min and 99°C for 5 min. Primers used
in PCR reaction were: 5′-CGCTTTCAGCTTTGCCTTCATTAC-3′ (sense) and
5′-AGCTACTTCCTTGTGCCCTGTGAG-3′ (antisense) for TLR4, length of
amplified fragment was 555 bp; 5′-TTCAGGCCAAGACCTCC-3′ (sense) and
5′-CCAGCCGGGAGGTGCAGT-3′ (antisense) for ATG, length of amplified
fragment was 308 bp; 5′-GCACACTGGCAATGTAATGC-3′ (sense) and
5′-GTTGAACAGAACAAGTGACC-3′ (antisense) for AT1a, length
if amplified fragment was 385 bp; 5′-GTGGACGTTTATTGACTTCGG-3′
(sense) and 5′-TTCTTTGCTTTGCCTTTGC-3′ (antisense) for β-MHC, length
of amplified fragment was 399 bp; 5′-AACCCTAAGGCCAACCGTGAAAAG-3′
(sense) and 5′-TCATGAGGTAGTCTGTCAT-3′ (antisense) for endogenous
control β-actin, length of amplified fragment was 241 bp. TLR4 PCR
reaction conditions: 94°C for 2 min, then 30 cycles of 94°C for 30
sec, 60°C for 60 sec and 72°C for 1.5 min, and 72°C for 10 min. ATG
PCR reaction conditions: 94°C for 2 min, then 30 cycles of 94°C for
30 sec, 63°C for 60 sec and 72°C for 1.5 min, and 72°C for 10 min.
AT1a receptor PCR reaction conditions: 94°C for 2 min,
then 30 cycles of 94°C for 30 sec, 58°C for 45 sec and 72°C for 1.5
min, and 72°C for 10 min. PCR products were electrophoresed on a
1.5% agarose gel and results were photographed with a gel imager.
The ratio of the gray value of target gene to the gray value of
β-actin band was taken as the relative expression level of target
mRNA.
Statistical analysis
Data were processed by using SPSS 11.5 statistical
software (SPSS, Inc., Chicago, IL, USA). Each experiment was
performed 3 times, and the data are expressed as mean ± standard
deviation (mean ± SD) and analyzed by one-way ANOVA. Comparison
between the groups was made by analyzing data with Least
Significant Difference method. P<0.05 was considered to be
statistically significant.
Results
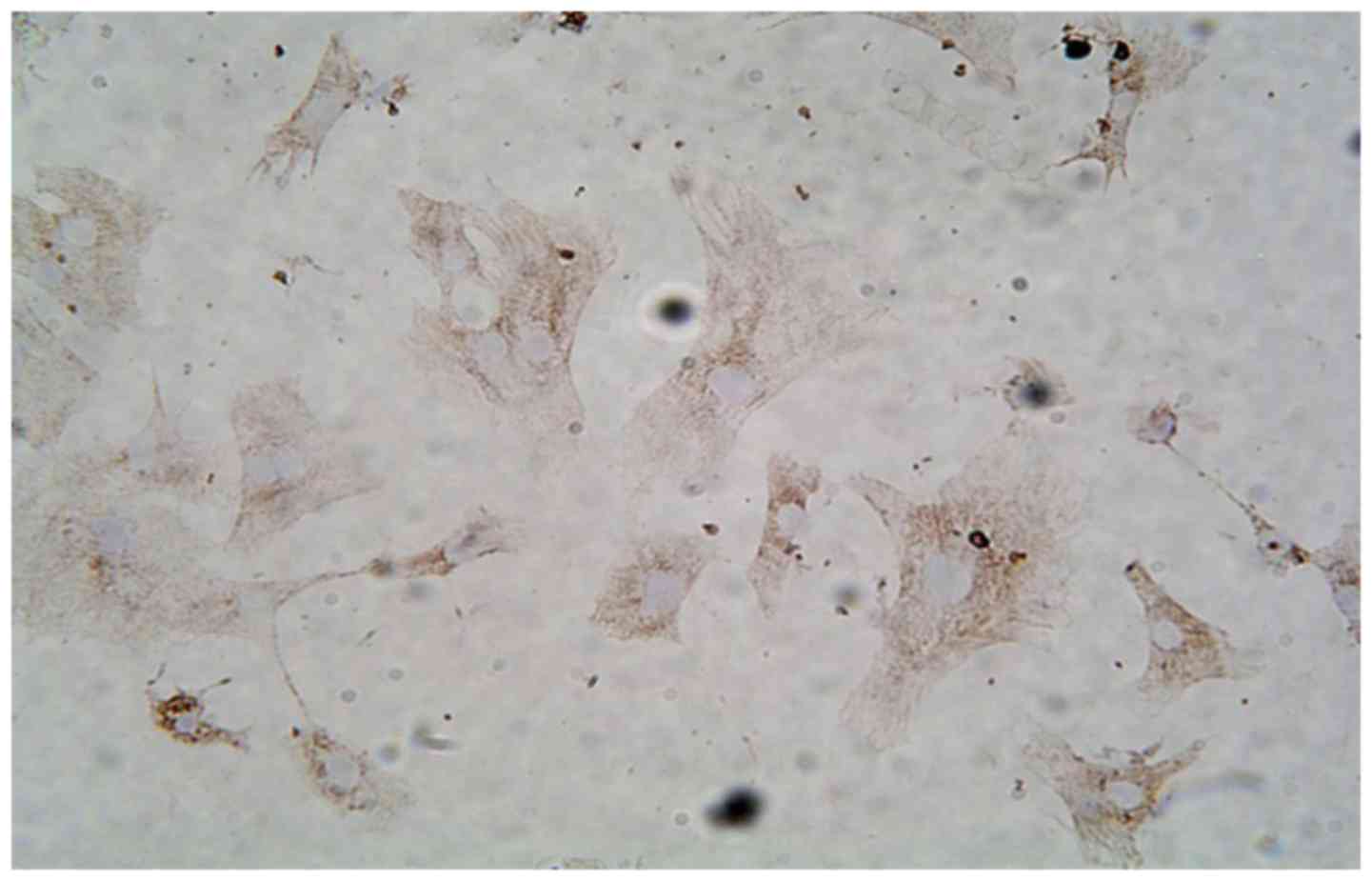
Culture and identification of
cardiomyocytes (NRVMs)
Fibroblasts were removed by using differential
adherence method. Cells were cultured for 24 h, and cells showed
long rod, polygon or fusiform shapes with concentric or radial
growth. Myocardial cell-specific synchronized beats were observed
at 48 h. Immunocytochemistry detection of striated muscle-specific
sarcomeric α-actin was used to identify cardiomyocytes, and the
purity of >95% (Fig. 1).
Results of cell viability assay
Cell viability was determined by trypan blue
staining and cell counting. The treatment factors used in this
study had no obvious effects on the growth of cardiomyocytes, and
the cell survival rate was over 95%.
LPS upregulates the expression of TLR4
mRNA in NRVMs
RT-PCR analysis showed that the expression of TLR4
mRNA in cardiomyocytes was relatively high. Compared with the
control group (TLR4 absorbance value was 0.301±0.112), TLR4 mRNA
expression was significantly increased after treatment with LPS for
24 h in a dose-dependent manner (LPS 10, 100 and 1,000 ng/ml, TLR4
absorbance. Values were 0.651±0.162, 0.801±0.217 and 1.052±0.227,
respectively, F=26.2, P<0.01). Compared with control group (TLR4
absorbance value was 0.301±0.112), treatment with 10, 100 and 1,000
ng/ml of LPS for 24 h increased the expression level of TLR4 by
1.17, 1.67 and 2.5 times, respectively (Fig. 2).
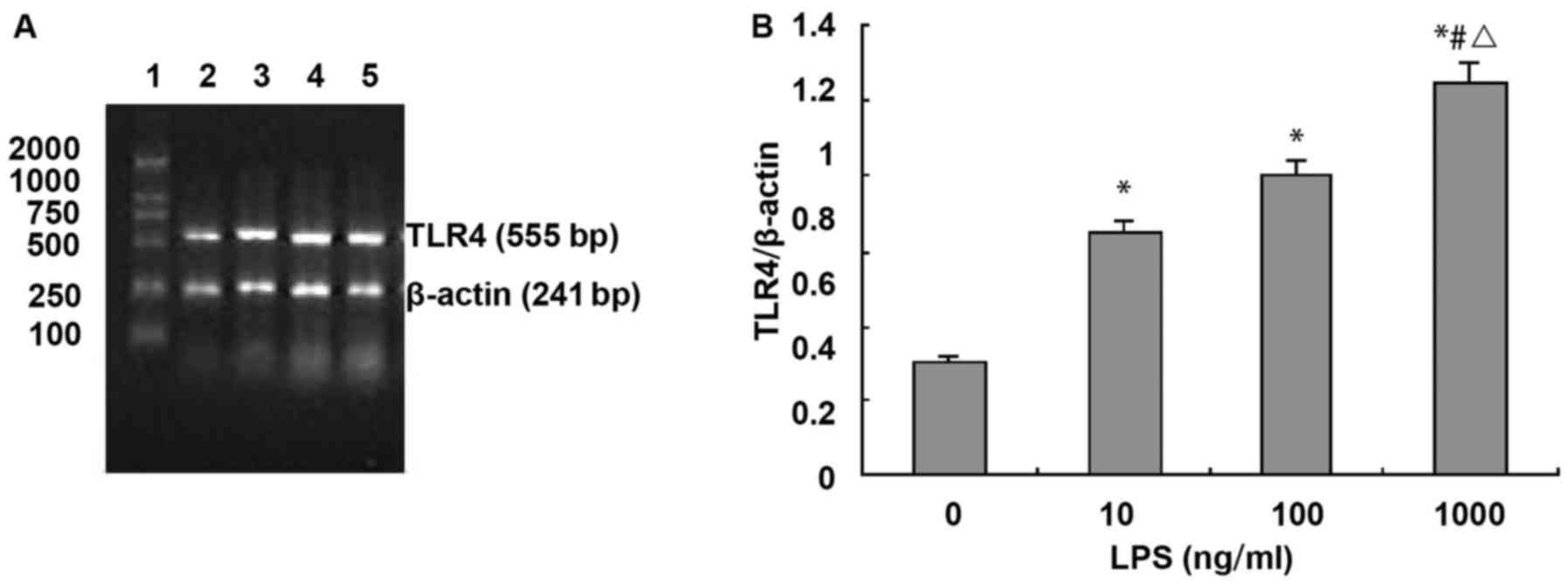 | Figure 2.LPS upregulates TLR4 mRNA expression
in NRVMs. (A) Agarose gel electrophoresis of TLR4 and β-actin PCR
amplification products: 1, DNA marker, 100, 250, 500, 750, 1,000
and 2,000 bp (from bottom to top); 2, normal control group; 3, LPS
10 ng/ml; 4, LPS 100 ng/ml; and 5, LPS 1,000 ng/ml. (B) Absorbance
value of TLR4 and β-actin PCR amplification bands (n=3). *P<0.01
compared with control group; #P<0.01 compared with
LPS 10 ng/ml; △P<0.05 compared with LPS 100
ng/ml. |
LPS upregulated the expression of ATG
mRNA in NRVMs
RT-PCR analysis showed that the expression level of
angiotensinogen mRNA was relatively low in cardiomyocytes. Compared
with control group (ATG absorbance values was 0.101±0.121),
expression of angiotensinogen mRNA was significantly increased in
NVMCs after LPS stimulation for 24 h in a dose-dependent manner
(LPS 10, 100 and 1,000 ng/ml, ATG absorbance values were
0.311±0.162, 0.511±0.179 and 0.651±0.209, respectively, F=28.9,
P<0.01). Compared with control group (ATG absorbance value was
0.101±0.121), treatment with 10, 100 and 1,000 ng/ml of LPS for 24
h increased the expression level of ATG 2, 4 and 5.5 times,
respectively (Fig. 3).
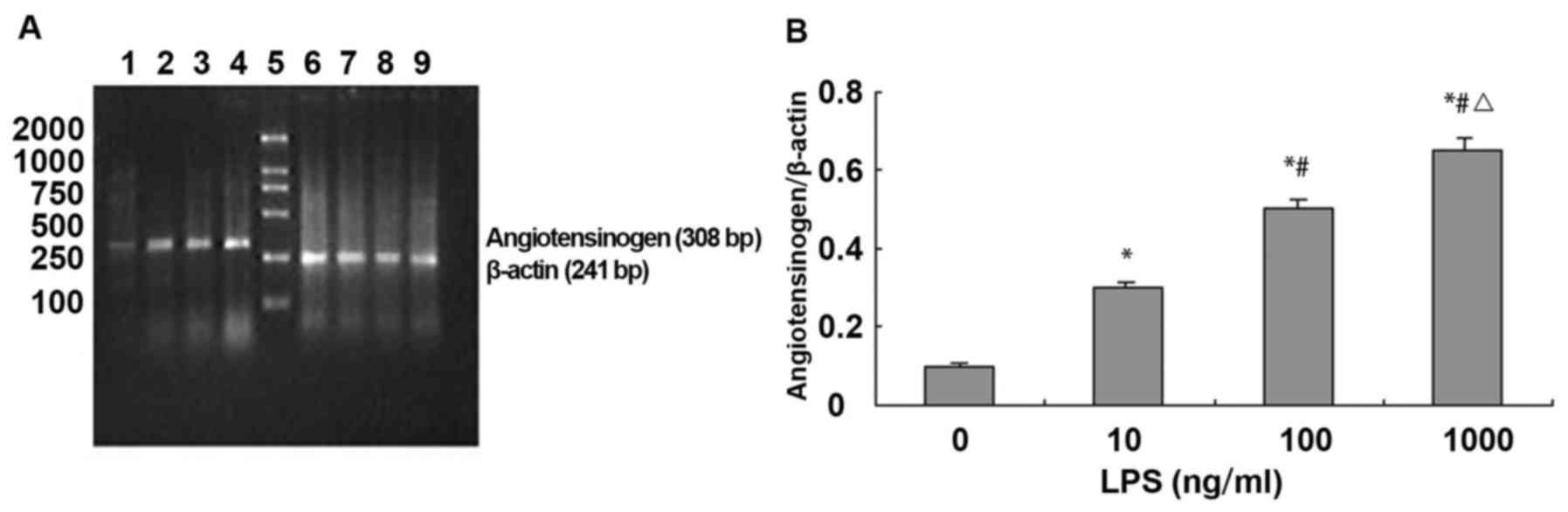 | Figure 3.LPS upregulates ATG mRNA expression in
NRVMs. (A) Agarose gel electrophoresis of ATG and β-actin PCR
amplification products: 1–4, agarose gel electrophoresis of ATG PCR
amplification products: 1, normal control group; 2, LPS 10 ng/ml;
3, LPS 100 ng/ml; 4, LPS 1,000 ng/ml; 5, DNA marker, 100, 250, 500,
750, 1,000 and 2,000 bp (from bottom to top); and 6–9, agarose gel
electrophoresis of β-actin PCR amplification products. (B)
Absorbance value of ATG and β-actin PCR amplification bands (n=3).
*P<0.01 compared with control group; #P<0.01
compared with LPS 10 ng/ml; △P<0.05 compared with LPS
100 ng/ml. |
LPS upregulates of AT1a
receptor mRNA expression in NRVMs
RT-PCR analysis showed that, compared with the
control group (TLR4 absorbance value was 0.501±0.141), expression
level of AT1a receptor mRNA in cardiomyocytes was
significantly increased in a dose-dependent manner (LPS 10, 100 and
1,000 ng/ml, AT1a receptor absorbance value was
0.701±0.182, 0.851±0.279 and 1.051±0.309, respectively, F=18.9,
P<0.01). Compared with control group (TLR4 absorbance value was
0.501±0.141), treatment with 10, 100 and 1,000 ng/ml of LPS for 24
h increased the expression level of AT1a receptor mRNA
by 0.4, 0.7 and 1.14 times, respectively (Fig. 4).
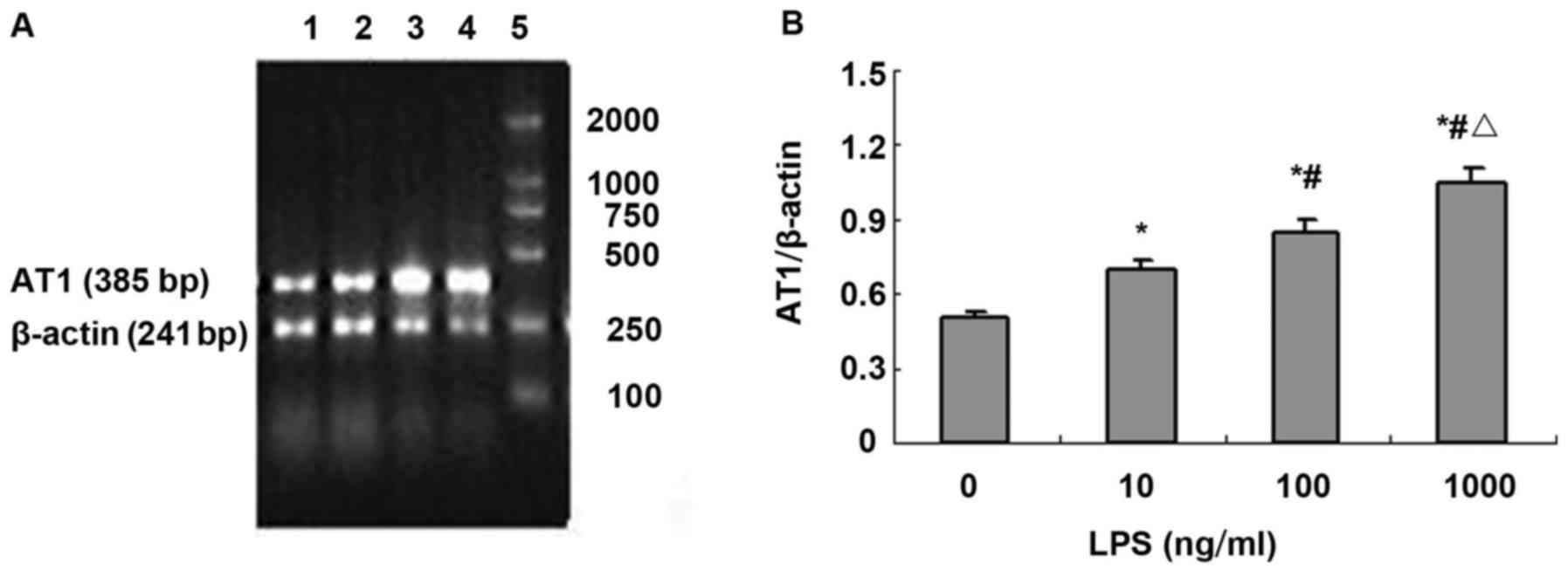 | Figure 4.LPS upregulates AT1a mRNA
expression in NRVMs. (A) Agarose gel electrophoresis of TLR4 and
β-actin PCR amplification products: 1, normal control group; 2, LPS
10 ng/ml; 3, LPS 100 ng/ml; 4, LPS 1,000 ng/ml; and 5, DNA marker,
100, 250, 500, 750, 1,000 and 2,000 bp (from bottom to top). (B)
Absorbance value of TLR4 and β-actin PCR amplification bands (n=3).
*P<0.01 compared with control group; #P<0.01
compared with LPS 10 ng/ml; △P<0.05 compared with LPS
100 ng/ml. |
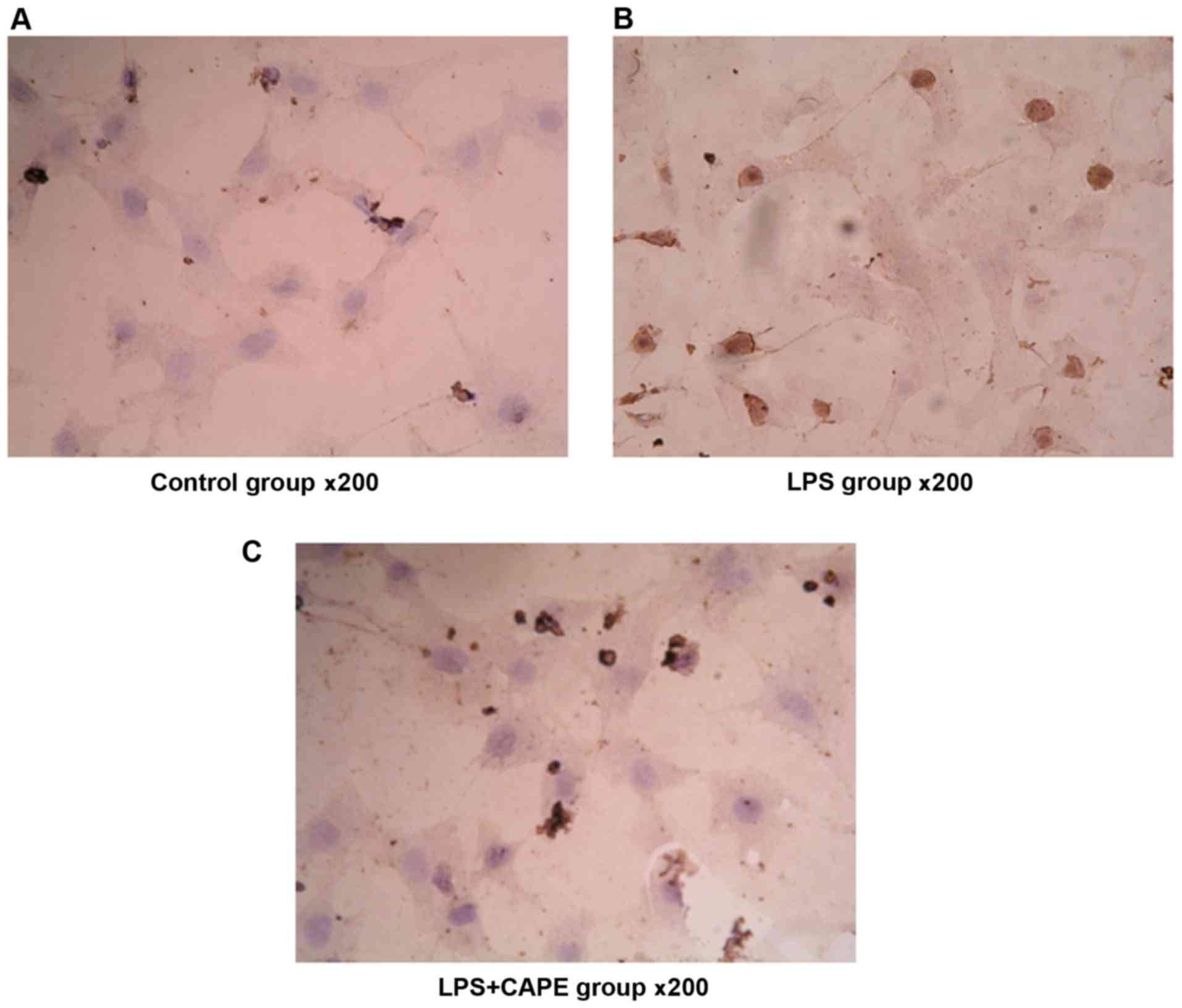
Effect of NF-κB inhibitor CAPE on
LPS-induced activation of NF-κB in NRVMs
Immunocytochemistry staining showed that expression
level of NF-κB p65 was low. After stimulation with LPS (1,000
ng/ml) for 24 h, more nuclei showed brownish yellow, indicating the
nuclear translocation of NF-κB p65 after LPS stimulation. There was
almost no nuclear staining after treatment with NF-κB inhibitor
CAPE (20 µg/ml), suggesting that CAPE inhibits the activation of
NF-κB by LPS (Fig. 5).
Effect of NF-κB inhibitor CAPE on
LPS-induced TLR4 mRNA expression in NRVMs
Compared with control group, expression level of
TLR4 mRNA was significantly increased after treatment with LPS
(1,000 ng/ml) for 24 h (absorbance value 0.301±0.112 vs.
1.052±0.227, P<0.01). CAPE (20 µg/ml) inhibited TLR4 mRNA
(absorbance value: 1.052±0.227 vs. 0.411±0.123, P<0.01)
(Fig. 6).
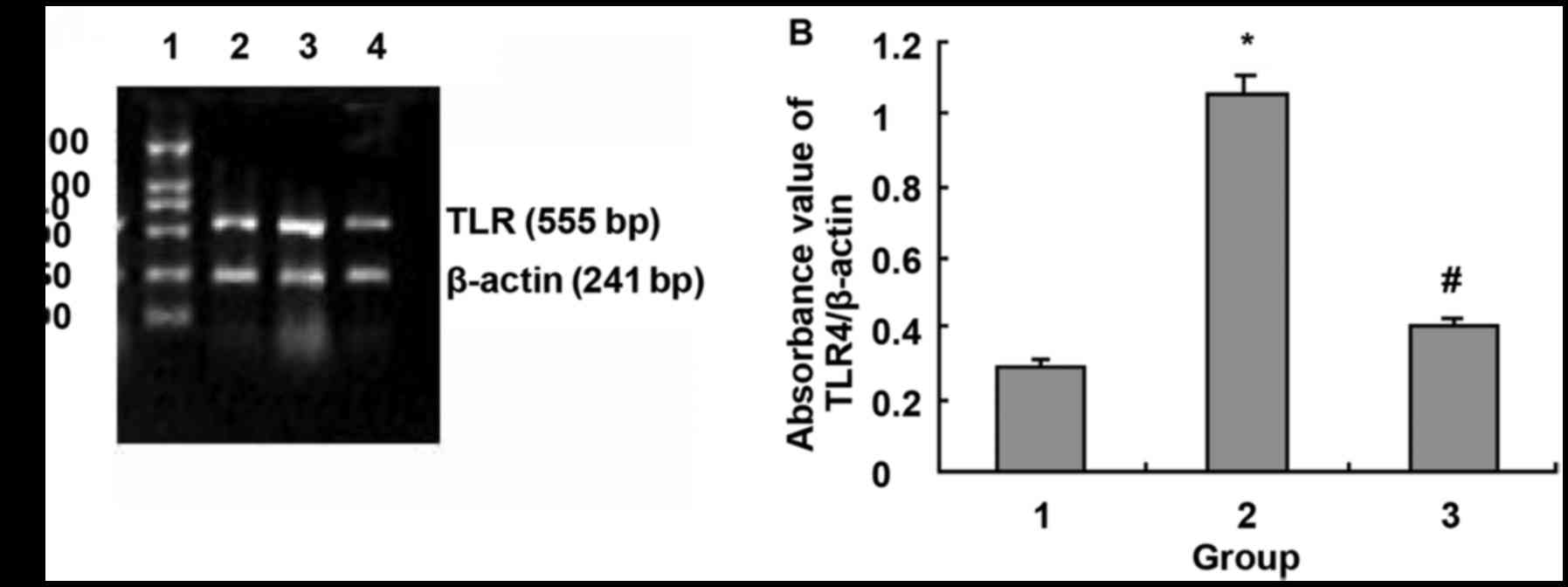 | Figure 6.Effects of NF-κB blocker CAPE on
LPS-induced TLR4 mRNA expression. (A) Agarose gel electrophoresis
of TLR4 and β-actin PCR amplification products: 1, DNA marker, 100,
250, 500, 750, 1,000 and 2,000 bp (from bottom to top); 2, normal
control group; 3, LPS 1,000 ng/ml; and 4, LPS 1,000 ng/ml + CAPE 20
µg/ml. (B) Absorbance value of TLR4 and β-actin PCR amplification
bands. 1, Normal control group; 2, LPS 1,000 ng/ml; and 3, LPS
1,000 ng/ml + CAPE 20 µg/ml. *P<0.01 compared with control
group; #P<0.01 compared with LPS 1,000 ng/ml. |
Effect of NF-κB inhibitor CAPE on
LPS-induced ATG mRNA expression in NRVMs
Compared with control group, LPS (1,000 ng/ml)
stimulation for 24 h significantly upregulated ATG mRNA expression
in NRVMs (absorbance value 0.101±0.112 vs. 0.652±0.227, P<0.01).
CAPE (20 µg/ml) inhibited ATG mRNA expression (absorbance value:
0.652±0.227 vs. 0.191±0.123, P<0.01) (Fig. 7).
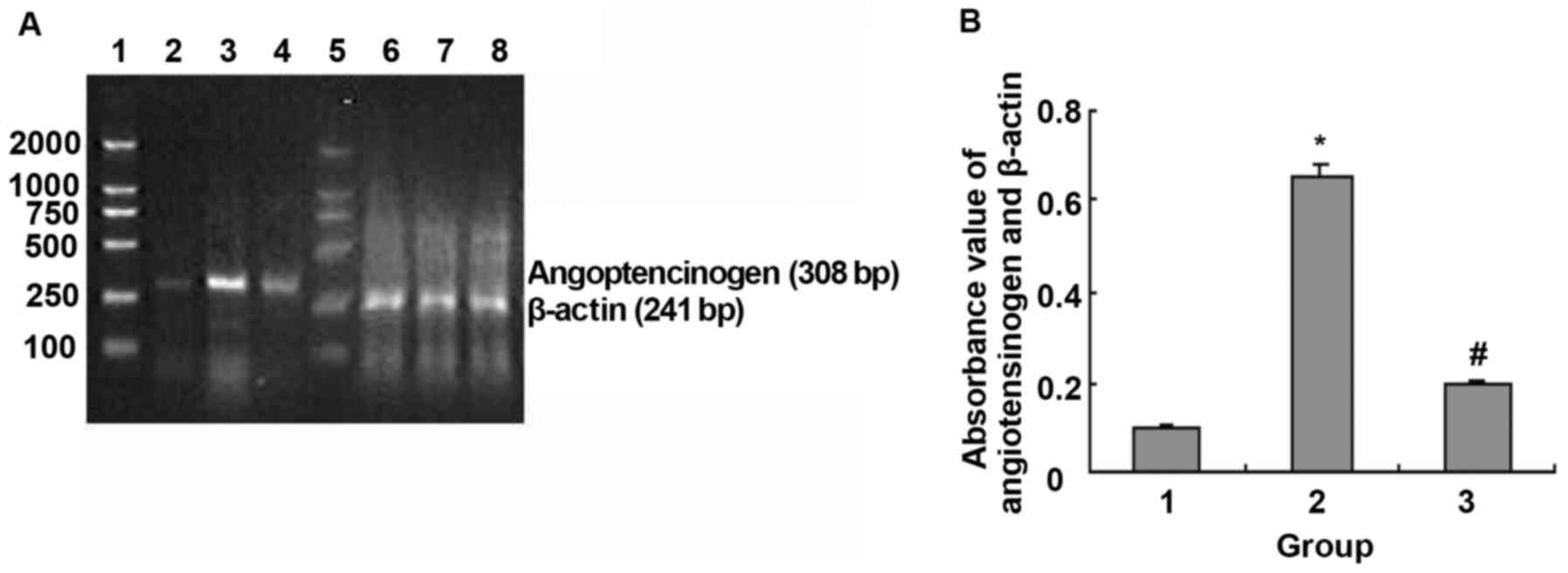 | Figure 7.NF-κB blocker CAPE modulated
LPS-induced angiotensinogen mRNA expression. (A) Agarose gel
electrophoresis of ATG and β-actin PCR amplification products: 1,
5, DNA marker, 100, 250, 500, 750, 1,000 and 2,000 bp (from bottom
to top); 2–4, ATG PCR amplification products: 2, normal control
group; 3, LPS 1,000 ng/ml and; 4, LPS 1,000 ng/ml + CAPE 20 µg/ml;
6–8, β-actin PCR amplification products: 6, normal control group;
7, LPS 1,000 ng/ml; and 8, LPS 1,000 ng/ml + CAPE 20 µg/ml. (B)
Absorbance value of angiotensinogen and β-actin PCR amplification
bands: 1, Normal control group; 2, LPS 1,000 ng/ml; and 3, LPS
1,000 ng/ml + CAPE 20 µg/ml. *P<0.01 compared with control
group; #P<0.01 compared with LPS 1,000 ng/ml. |
NF-κB inhibitor CAPE regulates
LPS-induced NRVMs AT1a receptor mRNA expression
Compared with control group, LPS (1,000 ng/ml)
stimulation for 24 h significantly upregulated AT1 mRNA
expression in NRVMs (absorbance value 0.501±0.112 vs. 1.05±0.227,
P<0.01). CAPE (20 µg/ml) inhibited AT1a mRNA
expression (absorbance value: 1.05±0.227 vs. 0.711±0.223,
P<0.01) (Fig. 8).
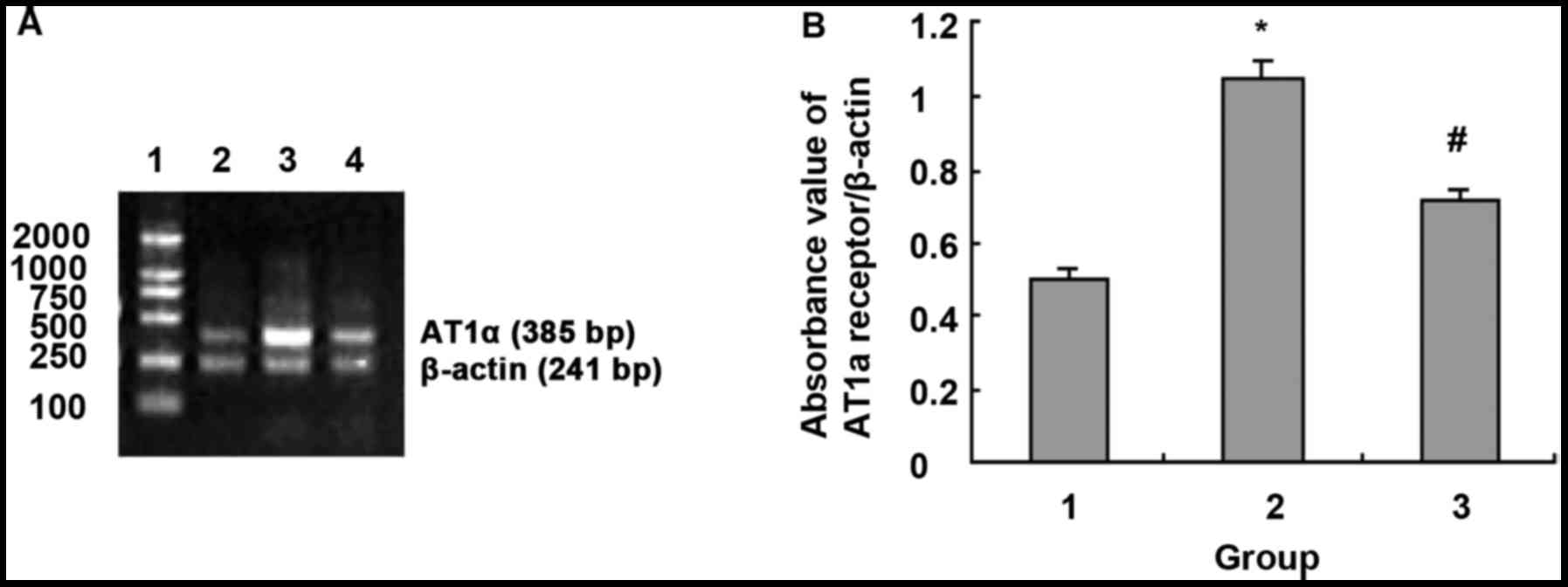 | Figure 8.NF-κB blocker CAPE regulated
LPS-induced AT1a receptor mRNA expression. (A) Agarose
gel electrophoresis of TLR4 and β-actin PCR amplification products:
1, DNA marker, 100, 250, 500, 750, 1,000 and 2,000 bp (from bottom
to top); 2, normal control group; 3, LPS 1,000 ng/ml; and 4, LPS
1,000 ng/ml + CAPE 20 µg/ml. (B) Absorbance value of
AT1a receptor and β-actin PCR amplification bands. 1,
Normal control group; 2, LPS 1,000 ng/ml; 3, LPS 1,000 ng/ml + CAPE
20 µg/ml. *P<0.01 compared with control group;
#P<0.01 compared with LPS 1,000 ng/ml. |
Discussion
Recently cloned human TLR4 is a transmembrane
receptor that mediates inflammation and the innate immune response.
Studies have shown that TLR4 is highly expressed in cardiomyocytes,
and TLR4-mediated signaling plays an important role in the
pathogenesis of cardiovascular diseases such as heart failure,
atherosclerosis, myocarditis and myocardial remodeling (3–5). TLR4
was found to be a portal protein that mediates LPS signaling
(10). LPS is the main component
cell wall outer membrane of gram-negative bacteria, and is the most
common pathogen-associated molecular pattern (PMAPs). In this
study, the purified E. coli LPS was used as a TLR4 specific
agonist to study the effect of TLR4 activation on the renin-ATG in
neonatal rat cardiomyocytes. This study found that LPS upregulated
TLR4 mRNA expression by agonizing TLR4 in a dose-dependent manner.
TLR4 as a transmembrane signal transduction receptor, its
expression level directly affects its signal transduction.
Upregulation of TLR4 may increase the sensitivity of myocardial
cells to injury stimuli. We also found that LPS activated TLR4 and
upregulated expression of ATG and AT1a receptor mRNA in
a dose-dependent manner. Therefore, we believe that TLR4 activation
mediated the expression of ATG and AT1a receptor. TLR4
signaling pathway may participate in the occurrence and development
of left ventricular remodeling and other cardiovascular diseases by
activating the renin-ATG system.
Renin-ATG system in circulating blood rapidly
regulates blood pressure, salt metabolism and homeostasis via Ang
II. With the development and application of molecular biology
techniques, it has been found that there is an independent RAS in
the heart, and activation of local myocardial RAS regulates
cardiomyocyte growth, proliferation, differentiation and apoptosis
(7). ATG is the most basic substance
of RAS. ATG produces Ang I and angiotensin-converting enzyme under
the action of renin, angiotensin and cathepsin, which in turn
degrade Ang I to Ang II, and Ang II can also be produced by a
renin-independent ACE-independent pathway. Ang II performs it
functions by highly specifically binding to its receptor on cell
membrane surface. Almost all known effects of Ang II on
inflammatory responses regulation, cell growth (such as tissue
fibrosis, hypertrophy, and tissue remodeling) are mediated by
AT1a receptor (7,11). This study showed that TLR4 activation
significantly enhanced the expression of angiotensinogen and
AT1 receptor, and the 1.17 times increase in expression
level of TLR4 was followed by the 2 times increase in expression
level of ATG, and the 1.67 times increase in expression level of
TLR4 was followed by the 4 times increase in expression level of
ATG. At the same time, with the increase in AT1a
receptor expression, effect of RAS is further strengthened. RAS
constricts vascular smooth muscle, arrests sodium retention,
inhibits renin secretion, promotes endothelin secretion, increases
vasopressin release, increases blood pressure, activates the
sympathetic nervous system, stimulates cardiac hypertrophy,
stimulates vascular and cardiac fibrosis, and increases myocardial
contractility, induces arrhythmias, activates plasminogen activator
inhibitor 1, stimulates peroxide formation, and participates in
almost every aspect of the cardiovascular system via
AT1a receptor. Therefore, TLR4 signaling pathway not
only plays an important role in left ventricular remodeling, but
also has important functions in the occurrence and development of
other cardiovascular diseases.
More importantly, most patients with cardiovascular
disease show no definite infection factors. In chronic and
sub-acute states, such as scaling, smoking, periodontal disease or
chronic infection and exercise, LPS level in vivo is
maintained at pg/ml to ng/ml level (8). Studies have shown that cardiomyocytes
still can sensitively respond to Nak levels of LPS through TLR4;
although this response is not enough to cause blood pressure
depression, production of TNF-α and interleukin-1β (IL-1β) and
other cardiac myocyte-reactive factors such as endotoxemia, it can
induce cardiomyocyte apoptosis (8).
Consistent with previous studies, in our study, LPS at the level of
10 ng/ml still increased the expression of TLR4, ATG and AT1.
Moreover, macromolecular degradation products in the body,
intracellular components released after cell rupture, and gene
products activated by inflammation can activate TLR4 and promote
the signal cascade (12). Chronic
TLR4 signaling and RAS activation may be a major contributor to
progressive dysfunction in target organs. NF-κB is a group of
multidirectional nuclear transcription regulators. In general,
NF-κB has two subunits, p50 and p65, which form heterodimers and
exist in the cytoplasm in an inactive form. Inhibitors κB (IκBs)
control the activation of NF-κB. They bind to NF-κB dimers to mask
the nuclear localization region of NF-κB. Phosphorylation and
activation of IκB kinase (IKK) can lead to the dissociation IκBs
from NF-κB, which in turn activate NF-κB. Then, NF-κB will
translocate from cytoplasm to the nucleus and binds to target genes
and regulates a series of target genes involved in cardiovascular
pathophysiology, including cytokines, angiotensinogen, and
AT1 receptors (13). In
this study, nuclear translocation of NF-κB p65 was significantly
increased in cardiomyocytes after treatment with LPS for 24 h. CAPE
inhibited the activation of NF-κB p65, and also inhibited the
upregulation of expression of ATG and AT1 receptor
induced by LPS. Since NF-κB plays an important regulatory role on
the expression of angiotensinogen and AT1 receptor genes
(12), TLR4/NF-κB pathway is thought
to mediate upregulation of the expression of ATG and
AT1a receptor induced by LPS in NRVMs, and NF-κB plays a
key regulatory role in TLR4-mediated effects described above.
It has been reported that the antioxidant PDTC
downregulates the increased expression of TLR4 induced by LPS and
IL-1β in both cardiomyocytes and microvascular endothelial cells,
and PDTC also downregulates TLR4 expression in normal
cardiomyocytes (2). An et al
(14) showed that TLR4 expression is
dependent on NF-κB activation. Previous studies have shown that
changes in TLR4 expression on the cell surface directly alter the
response of cells to LPS. Harju et al (15) injected LPS into the amniotic membrane
of mice at different stages of pregnancy. Expression of TLR4 in
fetal membranes increased with pregnancy, and the expression level
of TLR4 controlled the expression of cytokines, suggesting that the
expression of the receptor is related to the intensity of its
effect. In this study, LPS upregulated TLR4 expression, and CAPE
significantly inhibited LPS-induced upregulation of TLR4
expression, suggesting that activity of NF-κB has an important
regulatory role on TLR4 expression. In addition, the binding of
TLR4 to ligand can also activate NF-κB, whereas NF-κB regulates
TLR4 expression. There may be a positive feedback regulation
mechanism between them. Upregulation of TLR4 expression after NF-κB
activation enhances LPS-mediated TLR4/NF-κB signaling, which
aggravates LPS-induced cardiomyocyte injury. However, how NF-κB
regulates TLR4 expression is unclear. It is possible that the
nuclear translocation of NF-κB may promote the transcription of
TLR4 gene, or cytokines downstream NF-κB stimulate the expression
of TLR4. More studies are needed to answer these questions.
In conclusion, activation of TLR4/NF-κB by LPS
positively regulates TLR4 expression. Upregulation of TLR4 may
aggravate the damage to NRVMs caused by LPS. TLR4/NF-κB signaling
upregulates the expression of ATG and AT1 receptors in
NRVMs, suggesting that TLR4/NF-κB-mediated activation of the local
renin-ATG system is one of the mechanisms involved in the
pathogenesis of cardiovascular diseases. NF-κB plays a key
regulatory role in LPS-mediated effects mentioned above.
Intervention with TLR4/NF-κB signaling may be a new target for the
prevention and treatment of left ventricular remodeling or other
cardiovascular diseases.
Acknowledgements
Not applicable.
Funding
This project was supported by the National Natural
Science Foundation of China (no. 30371568).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
HJ was responsible for PCR and the writing of the
paper. PQ provided ideas of selecting topics and revised the paper.
HYW was responsible for conducting experiments and statistical
methods. JWW was a major contributor in the collection of data,
modification of pictures and statistical analysis. DYL was
responsible for reviewing literature and summing up the data. NN
was responsible for cell culture. GHL was responsible for assisting
HJ to perform the experi-ment and collection and assembly of data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Second Affiliated Hospital of Dalian Medical University
(Dalian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila Toll
protein signals activation of adaptive immunity. Nature.
388:394–397. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frantz S, Kobzik L, Kim YD, Fukazawa R,
Medzhitov R, Lee RT and Kelly RA: Toll4 (TLR4) expression in
cardiac myocytes in normal and failing myocardium. J Clin Invest.
104:271–280. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu L, Wang Y, Cao ZY, Wang MM, Liu XM,
Gao T, Hu QK, Yuan WJ and Lin L: Upregulated TLR4 in cardiomyocytes
exacerbates heart failure after long-term myocardial infarction. J
Cell Mol Med. 19:2728–2740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W,
Deng C, Fan C, Di S, Sun Y, et al: The emerging role of Toll-like
receptor 4 in myocardial inflammation. Cell Death Dis. 7:e22342016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ehrentraut H, Ehrentraut Felix S, Boehm O,
El Aissati S, Foltz F, Goelz L, Goertz D, Kebir S, Weisheit C, Wolf
M, et al: Tlr4 deficiency protects against cardiac pressure
overload induced hyperinflammation. PLoS One. 10:e01429212015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ha T, Li Y, Hua F, Ma J, Gao X, Kelley J,
Zhao A, Haddad GE, Williams DL and Browder William I: Reduced
cardiac hypertrophy in toll-like receptor 4-deficient mice
following pressure overload. Cardiovasc Res. 68:224–234. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrario CM: Cardiac remodelling and RAS
inhibition. Ther Adv Cardiovasc Dis. 10:162–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li HL, Suzuki J, Bayna E, Zhang FM, Dalle
Molle E, Clark A, Engler RL and Lew WY: Lipopolysaccharide induces
apoptosis in adult rat ventricular myocytes via cardiac AT(1)
receptors. Am J Physiol Heart Circ Physiol. 283:H461–H467. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abareshi A, Norouzi F, Asgharzadeh F,
Beheshti F, Hosseini M, Farzadnia M and Khazaei M: Effect of
angiotensin-converting enzyme inhibitor on cardiac fibrosis and
oxidative stress status in lipopolysaccharide-induced inflammation
model in rats. Int J Prev Med. 8:692017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tapping RI, Akashi S, Miyake K, Godowski
PJ and Tobias PS: Toll-like receptor 4, but not toll-like receptor
2, is a signaling receptor for Escherichia and
Salmonella lipopolysaccharides. J Immunol. 165:5780–5787.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cervenka L, Vanecková I, Husková Z,
Vanourková Z, Erbanová M, Thumová M, Skaroupková P, Opocenský M,
Malý J, Chábová VC, et al: Pivotal role of angiotensin II receptor
subtype 1A in the development of two-kidney, one-clip hypertension:
Study in angiotensin II receptor subtype 1A knockout mice. J
Hypertens. 26:1379–1389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boza P, Ayala P, Vivar R, Humeres C,
Cáceres FT, Muñoz C, García L, Hermoso MA and Díaz-Araya G:
Expression and function of toll-like receptor 4 and inflammasomes
in cardiac fibroblasts and myofibroblasts: IL-1β synthesis,
secretion, and degradation. Mol Immunol. 74:96–105. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones WK, Brown M, Ren X, He S and
McGuinness M: NF-kappaB as an integrator of diverse signaling
pathways: The heart of myocardial signaling? Cardiovasc Toxicol.
3:229–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
An H, Yu Y, Zhang M, Xu H, Qi R, Yan X,
Liu S, Wang W, Guo Z, Guo J, et al: Involvement of ERK, p38 and
NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9
gene expression induced by lipopolysaccharide in mouse dendritic
cells. Immunology. 106:38–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harju K, Ojaniemi M, Rounioja S, Glumoff
V, Paananen R, Vuolteenaho R and Hallman M: Expression of toll-like
receptor 4 and endotoxin responsiveness in mice during perinatal
period. Pediatr Res. 57:644–648. 2005. View Article : Google Scholar : PubMed/NCBI
|