Introduction
At present, the aging phenomenon of social
population is increasingly obvious. The incidence of diabetes
mellitus (DM) is higher each year all over the world as a result of
many factors such as genetic factors, environment and social life
style (1). The latest survey data
show that in China there are >30 million DM patients aged >45
years (2), and most of the patients
with chronic renal insufficiency are DM patients whose common
clinical manifestation are proteinuria and gradual decline of renal
function (3). With a rather complex
pathogenesis, type 2 DM is the main type among DM patients. The
inflammatory response plays important roles in the occurrence and
development of diabetic nephropathy. C-reactive protein (CRP),
tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) play main
roles in promoting the inflammatory response among the systemic
inflammatory responses, regulating the patient's immune and
inflammatory response system (4,5).
Numerous studies have concluded that the long-term blood
hypercoagulability is one of the major causes of impaired renal
function in DM patients (6). In the
presence of proteinuria in patients with diabetic nephropathy, the
activity of coagulation factors in plasma is stronger than that in
the normal organism. In particular, endogenous coagulation factors
affect coagulation function in patients with one another (7). On the other hand, the number of
glomerular and tubular interstitial capillaries of patients with
diabetic nephropathy is smaller than that in the normal organism.
The decrease in the number of capillaries is mainly due to
apoptosis and clearance of vascular endothelial cells (8). In the adult organism, the normal
function of the vascular endothelial cells is maintained through
continuous proliferation of vascular endothelial cells to form new
vascular histiocytic germs (9).
Vascular endothelial cell is also one of the most important human
endocrine tissues, which can synthesize and release a large amount
of vasoactive substances, affecting vital organs of the body such
as kidney and liver (7). Therefore,
investigating the relationships of vascular endothelial function
and coagulation factors with renal function and levels of serum
inflammatory factors in patients with diabetic nephropathy may
provide a new clinical reference value for the diagnosis and
treatment of patients with diabetic nephropathy.
Patients and methods
General data
A total of 86 patients diagnosed with DM and
admitted to the 89th Hospital of the People's Liberation Army
(Weifang, China) from March 2014 to May 2017 were selected. Among
them, 38 patients with nephropathy were divided into the
observation group and 48 patients without nephropathy into the
control group. There were 45 males and 41 females aged 45–78 years
with an average age of 52.73±8.68 years. The patients enrolled met
the diagnostic criteria of World Health Organization (WHO) type 2
DM of 1997. The diagnostic criteria of diabetic nephropathy were
based on renal function, 24-h urinary protein and urinary
protein-creatinine ratio. Exclusion criteria: i) patients with
severe systemic infection; ⅱ) patients who used estrogen,
anti-coagulant or thrombolytic drugs; ⅲ) patients with malignant
tumor; ⅳ) patients with autoimmune diseases; v) patients with
severe liver and kidney dysfunction; ⅵ) patients with mental
illness; and ⅶ) patients with missing clinical data or who refused
to sign the informed consent.
The study was approved by the Ethics Committee of
the 89th Hospital of the People's Liberation Army. Patients who
participated in this study or their guardians, signed informed
consent and had complete clinical data.
Methods
All clinical data of patients including age, sex,
height, weight and duration of DM were retrospectively analyzed.
Fasting peripheral blood in all the patients after fasting and
water deprivation overnight for 10 h were collected. The upper
serum was taken to determine the relevant biochemical indicators.
The fasting plasma glucose (FPG) was determined by glucose oxidase
method. The glycated hemoglobin (HbA1c) was detected via
glycohemoglobin analyzer. The fasting insulin (FINS) level was
estimated via radioimmunity method with the kit provided by Beijing
Kepu Science and Technology Co., Ltd. (Beijing, China). The levels
of total cholesterol (TC), triglyceride (TG), low-density
lipoprotein cholesterol (LDL-C), high-density lipoprotein
cholesterol (HDL-C) and renal function indicators such as
cystatin-C (Cys-C), serum creatinine (SCr), urea, β2-microglobulin
were detected by the automatic biochemistry analyzer (Beckman-700,
Fullerton, CA, USA). The levels of CRP, TNF-α and IL-6 were
determined through immune turbidimetry. Immunomagnetic bead assay
was used to detect the fibrinogen (FIB), prothrombin time (PT) and
activated partial thromboplastin time (APTT). Homocysteine (Hcy)
and nitric oxide (NO) were detected through enzyme-linked
immunosorbent assay (ELISA). Brachial artery blood flow and
endothelium-dependent vasodilation through flow-mediated dilation
(FMD) were measured via two-dimensional ultrasound.
Statistical analysis
Statistical Product and Service Solutions (SPSS; IBM
Corp., Armonk, NY, USA) 19.0 software was used for data processing.
Data were collected and presented as (means ± SD), and Chi-square
test was used for the comparison of enumeration data. Pearsons
correlation analysis was used between the two factors. P<0.05
suggested that the diffidence was statistically significant.
Results
Comparison of general information
between the observation and control groups
The differences in age, sex, body mass index (BMI),
TC, TG, LDL-C and HDL-C were not statistically significant between
the observation and control groups. However, the DM duration in the
observation group was longer than that in the control group, and
levels of HbA1c, FPG and FINS were significantly higher than those
in the control group. All the differences were statistically
significant (p<0.05, Table
I).
 | Table I.Comparison of general information
between the observation and control groups. |
Table I.
Comparison of general information
between the observation and control groups.
| General
information | Observation group
(n=38) | Control group
(n=48) | P-value |
|---|
| Age (years) | 53.17±8.36 | 51.93±7.93 | 0.682 |
| Sex
(male/female) | 20/18 | 25/23 | 0.591 |
| BMI
(kg/m2) | 23.91±3.82 | 24.08±2.77 | 0.424 |
| DM duration
(years) | 10.79±2.53 | 6.36±3.17 | 0.013 |
| HbA1c (%) | 10.99±1.47 | 7.23±1.05 | 0.003 |
| FPG (mmol/l) | 11.39±1.76 | 8.27±0.95 | 0.001 |
| FINS (mIU/l) | 13.75±2.41 | 9.79±2.34 | 0.001 |
| TC (mmol/l) | 5.75±1.21 | 4.84±1.05 | 0.071 |
| TG (mmol/l) | 1.95±1.03 | 1.93±0.98 | 0.116 |
| LDL-C (mmol/l) | 3.11±0.97 | 2.97±0.86 | 0.493 |
| HDL-C (mmol/l) | 1.48±0.51 | 1.43±0.29 | 0.078 |
Comparison of vascular endothelial
functions between the observation and control groups
In the observation group, the peripheral serum Hcy
and brachial artery blood flow were higher than those in the
control group, and NO and FMD levels were lower than those in the
control group. The differences were statistically significant
(p<0.05, Table II).
 | Table II.Comparison of vascular endothelial
function between the observation and control groups. |
Table II.
Comparison of vascular endothelial
function between the observation and control groups.
| Related index | Observation group
(n=38) | Control group
(n=48) | P-value |
|---|
| Hcy (µmol/l) | 29.56±4.57 | 13.23±2.91 | 0.001 |
| NO (µmol/l) | 41.87±9.86 | 69.85±11.78 | 0.013 |
| Brachial artery blood
flow (ml/min) | 79.79±26.31 | 71.33±25.29 | 0.019 |
| FMD (%) | 3.49±0.26 | 4.73±0.41 | 0.001 |
Comparison of coagulation functions
between the observation and control groups
As for the comparison between the two groups of
patients, the difference in PT between the observation and control
groups was not significant. But the level of FIB in the observation
group was higher than that in the control group and APTT was
shorter than that in the control group. All the differences were
statistically significant (p<0.05, Table III).
 | Table III.Comparison of coagulation function
between the observation and control groups. |
Table III.
Comparison of coagulation function
between the observation and control groups.
| Coagulation
function | Observation group
(n=38) | Control group
(n=48) | P-value |
|---|
| FIB (g/l) | 4.49±0.83 | 2.82±0.76 | 0.026 |
| PT (sec) | 11.93±1.31 | 12.08±1.45 | 0.573 |
| APTT (sec) | 30.88±2.79 | 35.06±3.07 | 0.046 |
Comparison of renal function levels
between the observation and control groups
The peripheral serum Cys-C, SCr, urea and
β2-microglobulin levels in observation group were significantly
higher than those in the control group and the differences were
statistically significant (p<0.05, Table IV).
 | Table IV.Comparison of renal function levels
between the observation and control groups. |
Table IV.
Comparison of renal function levels
between the observation and control groups.
| Related index | Observation group
(n=38) | Control group
(n=48) | P-value |
|---|
| Cys-C (µmol/l) | 1.47±0.35 | 0.96±0.17 | 0.001 |
| SCr (µmol/l) | 67±9 | 38±4 | 0.001 |
| Urea (mmol/l) | 5.7±0.8 | 2.9±0.5 | 0.001 |
| β2-microglobulin
(mg/l) | 4.3±0.3 | 2.7±0.2 | 0.001 |
Comparison of levels of peripheral
serum inflammatory factors between the observation and control
groups
Levels of peripheral serum CRP, TNF-α, IL-6 in the
observation group were significantly higher than those in the
control group and the differences were statistically significant
(p<0.05, Table V).
 | Table V.Comparison of levels of serum
inflammatory factors between the observation and control
groups. |
Table V.
Comparison of levels of serum
inflammatory factors between the observation and control
groups.
| Inflammatory
factor | Observation group
(n=38) | Control group
(n=48) | P-value |
|---|
| CRP (mg/l) | 5.51±1.45 | 2.43±0.99 | 0.001 |
| TNF-α (ng/ml) | 10.98±1.34 | 6.07±1.12 | 0.001 |
| IL-6 (µg/l) | 9.57±1.65 | 6.82±1.39 | 0.001 |
Analysis of correlation of vascular
endothelial function and coagulation factors with renal function
and inflammatory factors
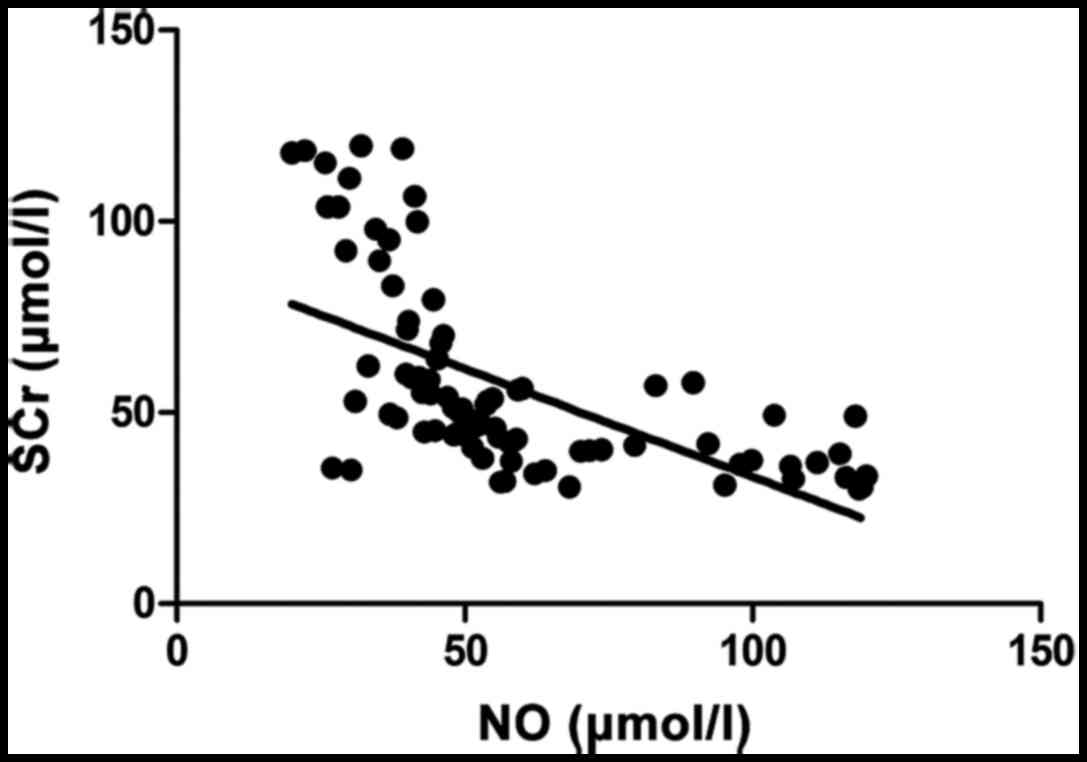
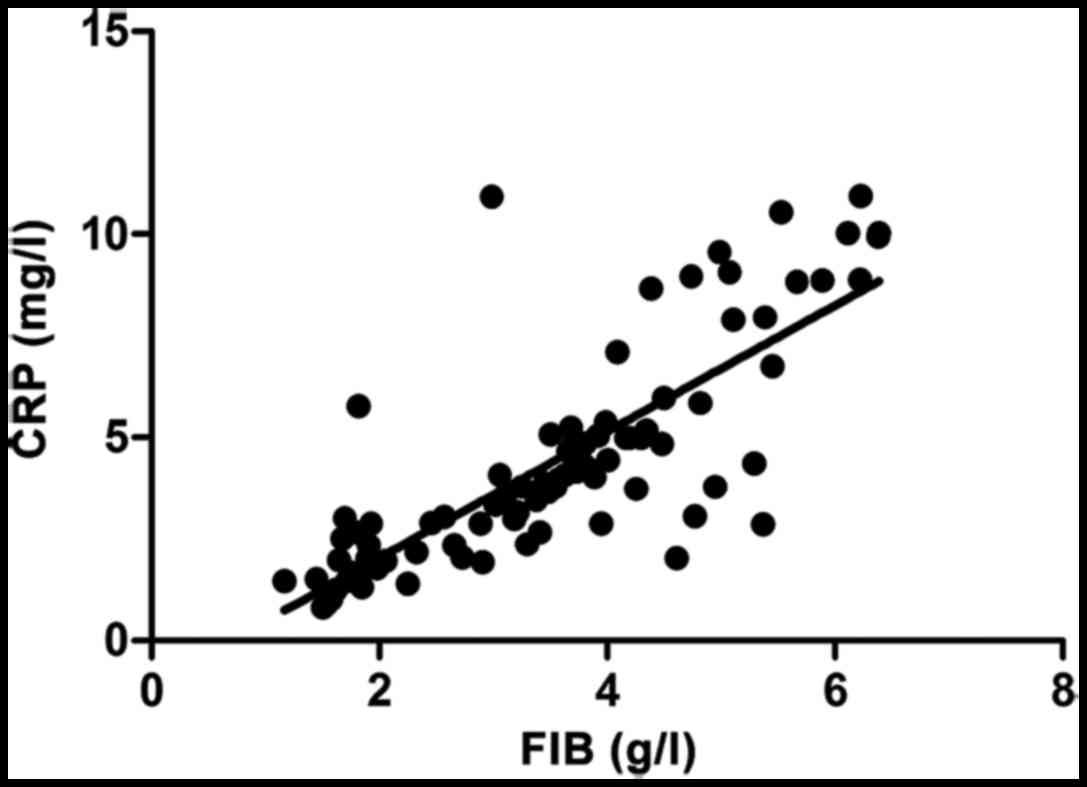
SCr (r=−0.683, p<0.001) and CRP (r=−0.696,
p<0.001) were negatively correlated with NO. SCr (r=0.713,
p<0.001) and CRP (r=0.747, p<0.001) were positively
correlated with FIB (Figs.
1–4).
Discussion
With the continuous changes in social lifestyle,
including living environment, dietary habits and other changes, the
incidence of DM is increasing. In particular, the incidence of DM
is approximately 10% in China, most of which is type 2 DM (10). The dysfunction of blood glucose
regulation results in the various target organ dysfunctions in the
whole body. Vascular disease is the most common disease found in
clinic. Diabetic nephropathy is one of the most common
complications, which is manifested as impaired renal function and
the presence of proteinuria (8).
Diabetic nephropathy is a common cause of clinical chronic renal
failure among many others, and the overreaction of inflammatory
system plays an important pathogenic role in diabetic nephropathy
(11). Type 2 DM is often
complicated with metabolic imbalance in adipocytes, which can
produce more inflammatory factors than normal levels and thus
resulting in an inflammatory response (12). The combination of diabetes with renal
injury leads to the severe ongoing inflammatory system response
(13). CRP, TNF-α and other factors
play an important role in the inflammatory system response process.
The inflammatory response causes the occurrence and aggravation of
atherosclerosis, which is also an important factor contributing to
the death of patients with diabetic nephropathy (14). The major role of IL-6 is to regulate
the immune system and to transmit important information in the
inflammatory system responses. TNF-α can enhance the stress
response of cells to the inflammatory reaction, lead to insulin
resistance and accelerate the renal injury in DM patients (15). It was found in this study that the
levels of peripheral inflammatory factors in DM patients
complicated with nephropathy were significantly higher than those
in patients with simple DM, and the differences were statistically
significant, which is consistent with the above conclusion
(p<0.05).
Numerous studies have shown that DM patients have
blood systems which are often manifested as hypercoagulability, and
they are more prone to the thrombosis compared with healthy people
(6). DM patients often have
different degrees of thrombosis which results in increased
mortality (16). Relevant data
reveal that the thrombus activation for DM patients is stronger
than that for normal body. A variety of plasma coagulation factors
are increased in patients with DM, further aggravating renal injury
(17). It was shown in this study
that the levels of renal function-related indicators in patients
with diabetic nephropathy were significantly higher than those in
diabetic patients without renal injury and APTT was shorter,
suggesting that patients with diabetic nephropathy have enhanced
endogenous coagulation function. The main pathologic and
physiological manifestations of DM patients are increased content
of extracellular matrix, increased quantity of mesangial cells and
glomerular sclerosis (18). Long
duration of DM, long-term poor blood glucose control, endocrine
metabolic disorders, the change of glomerular hemodynamics and
other reasons are the main factors leading to the occurrence and
aggravation of diabetic nephropathy (19). On the other hand, impaired vascular
endothelial function is also closely related to diabetic
nephropathy. Patients with long-term hyperglycemia are prone to
vascular endothelial dysfunction; disorder of the active substances
released from vascular synthesis, decreased level of vasodilator
active factor and increased content of vasoconstrictor factors, and
they are also easier to have vascular contracture and be exposed to
the increased risk of thrombosis (20). It was concluded in the study that the
blood of patients with diabetic nephropathy is hypercoagulable, and
the degree of vascular endothelial dysfunction is more severe than
that in patients only with diabetes. The difference was
statistically significant (p<0.05). Therefore, paying close
attention to the peripheral serum inflammatory factors, coagulation
factors and endothelial function in patients with diabetic
nephropathy will help to determine and treat the disease, improve
the prognosis, reduce mortality and provide new ideas for the
treatment of patients with diabetic nephropathy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS contributed to writing the manuscript as well as
collected and analyzed all clinical data of patients. CL analyzed
and interpreted the vascular endothelial functions of patients.
Both authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the 89th Hospital of the People's Liberation Army (Weifang, China).
Patients who participated in this study or their guardians, signed
informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mogensen CE and Schmitz O: The diabetic
kidney: From hyperfiltration and microalbuminuria to end-stage
renal failure. Med Clin North Am. 72:1465–1492. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Büyükkoçak S, Oztürk HS, Tamer MN, Kaçmaz
M, Çimen MYB and Durak I: Erythrocyte oxidant/antioxidant status of
diabetic patients. J Endocrinol Invest. 23:228–230. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonnet F and Cooper ME: Potential
influence of lipids in diabetic nephropathy: Insights from
experimental data and clinical studies. Diabetes Metab. 26:254–264.
2000.PubMed/NCBI
|
|
4
|
Ahn JH, Yu JH, Ko SH, Kwon HS, Kim DJ, Kim
JH, Kim CS, Song KH, Won JC, Lim S, et al: Taskforce Team of
Diabetes Fact Sheet of the Korean Diabetes Association: Prevalence
and determinants of diabetic nephropathy in Korea: Korea national
health and nutrition examination survey. Diabetes Metab J.
38:109–119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mima A: Inflammation and oxidative stress
in diabetic nephropathy: New insights on its inhibition as new
therapeutic targets. J Diabetes Res. 2013:2485632013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wada J and Makino H: Inflammation and the
pathogenesis of diabetic nephropathy. Clin Sci (Lond). 124:139–152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duran-Salgado MB and Rubio-Guerra AF:
Diabetic nephropathy and inflammation. World J Diabetes. 5:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jian W, Peng W, Xiao S, Li H, Jin J, Qin
L, Dong Y and Su Q: Role of serum vaspin in progression of type 2
diabetes: A 2-year cohort study. PLoS One. 9:e947632014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Šenolt L, Polanská M, Filková M, Cerezo
LA, Pavelka K, Gay S, Haluzík M and Vencovský J: Vaspin and
omentin: New adipokines differentially regulated at the site of
inflammation in rheumatoid arthritis. Ann Rheum Dis. 69:1410–1411.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Phalitakul S, Okada M, Hara Y and Yamawaki
H: Vaspin prevents TNF-α-induced intracellular adhesion molecule-1
via inhibiting reactive oxygen species-dependent NF-κB and PKCθ
activation in cultured rat vascular smooth muscle cells. Pharmacol
Res. 64:493–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karbek B, Bozkurt NC, Topaloglu O, Aslan
MS, Gungunes A, Cakal E and Delibasi T: Relationship of vaspin and
apelin levels with insulin resistance and atherosclerosis in
metabolic syndrome. Minerva Endocrinol. 39:99–105. 2014.PubMed/NCBI
|
|
12
|
Molitch ME, DeFronzo RA, Franz MJ, Keane
WF, Mogensen CE and Parving HH; American Diabetes Association, :
Diabetic nephropathy. Diabetes Care. 26 Suppl 1:S94–S98. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giannakopoulos B, Gao L, Qi M, Wong JW, Yu
DM, Vlachoyiannopoulos PG, Moutsopoulos HM, Atsumi T, Koike T, Hogg
P, et al: Factor XI is a substrate for oxidoreductases: Enhanced
activation of reduced FXI and its role in antiphospholipid syndrome
thrombosis. J Autoimmun. 39:121–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alzahrani SH and Ajjan RA: Coagulation and
fibrinolysis in diabetes. Diab Vasc Dis Res. 7:260–273. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Henry ML, Davidson LB, Wilson JE, McKenna
BK, Scott SA, McDonagh PF and Ritter LS: Whole blood aggregation
and coagulation in db/db and ob/ob mouse models of type 2 diabetes.
Blood Coagul Fibrinolysis. 19:124–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun X, Zhou X and Yan H: Hypercoagulation
aggravates renal dysfunction in patient with diabetic nephropathy.
Zhonghua Xue Ye Xue Za Zhi. 22:476–477. 2001.(In Chinese).
PubMed/NCBI
|
|
17
|
Patrassi GM, Martinelli S, Picchinenna A
and Girolami A: Contact phase of coagulation in diabetes mellitus
after aspirin administration. Folia Haematol Int Mag Klin Morphol
Blutforsch. 112:333–338. 1985.PubMed/NCBI
|
|
18
|
Patrassi GM, Vettor R, Padovan D and
Girolami A: Contact phase of blood coagulation in diabetes
mellitus. Eur J Clin Invest. 12:307–311. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen VM and Hogg PJ: Allosteric disulfide
bonds in thrombosis and thrombolysis. J Thromb Haemost.
4:2533–2541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Emsley J, McEwan PA and Gailani D:
Structure and function of factor XI. Blood. 115:2569–2577. 2010.
View Article : Google Scholar : PubMed/NCBI
|