Introduction
Despite the development of therapeutics in recent
years, gastric cancer is one of the most recurrent malignant types
of cancer and the second most common cause of cancer deaths
worldwide (1). Of all reported
cases, two-thirds of gastric cancer-related deaths have occurred in
developing countries with high-risk areas, including China, Japan
and Central and South America (2,3). Several
factors are known to serve important roles in promoting gastric
cancer, including familial genetics and environmental factors, such
as Helicobacter pylori infection, foods with high salt
content and smoking (4–7). Irrespective of traditional treatments
such as surgery, radiotherapy, and chemotherapy as well as novel
cancer-targeting therapies, the overall 5-year survival rate in
patients with gastric cancer is very low (8,9). Thus,
gastric cancer is a multi-factorial, complex disease, and the
detailed mechanisms regulating its development and progression
remain unclear. For these reasons, the identification of additional
biomarkers of gastric cancer and therapeutic targets is imperative
and necessary for improving the clinical outcome.
Tumor invasion and metastasis require proteolytic
degradation of the basement membrane and the extracellular matrix
(ECM). Fibronectin is an important ECM protein, which has been
implicated in many cancers and is known to be associated with
cancer proliferation and migration (10–13).
Fibronectin type III domain containing 1 (FNDC1), also known as
AGS8, contains a major component of the structural domain of
fibronectin (13). Recent studies
have demonstrated that FNDC1 is closely associated with the
development of many diseases (14–21);
however, the function of FNDC1 is still not clear. These studies
demonstrated that FNDC1 expression increased with skin tumor
progression and increasing tumor thickness (14). FNDC1 was demonstrated to be
hypermethylated in adenoid cystic carcinoma (15). In vitro, knockdown of FNDC1
could suppress the cellular proliferation and migration of prostate
cancer, while inducing apoptosis (16). van Ingen et al (17) performed a genome-wide association
study and identified that FNDC1 serves an important role as a
disease-contributing gene of acute otitis media in children. FNDC1
is also expressed in the kidney, may regulate G protein signaling
and has been implicated in the hypoxia-induced apoptosis of
cardiomyocytes (18–20). Recently, FNDC1 was reported to be
associated with vascular endothelial growth factor (VEGF)-mediated
cellular events, including tube formation, migration and
proliferation (21). To date,
however, the role of FNDC1 in the development and progression of
gastric cancer has not been evaluated.
In the present study, the expression pattern of
FNDC1 and correlations between its expression and the clinical
characteristics of gastric cancer were investigated for the first
time, to the best of our knowledge.
Patients and methods
Patients and tumor samples
Formalin-fixed tumor tissues from 98 patients (74
males, 24 females; age range, 25–83 years) including 25 paired
adjacent normal tissues (5 cm from tumor edges) were used for
immunohistochemical analysis. All patients who underwent surgical
resection for primary gastric cancer in the Department of General
Surgery in Nanfang Hospital (Guangzhou, China) from March 2010 to
December 2014 were enrolled in the present study. No patients had
received radiotherapy or chemotherapy prior to surgical resection.
Patients with any other types of cancer, or who missed follow-up
appointments were excluded from the present study. Each tumor was
assigned a histological type and a depth grading of infiltration
according to the World Health Organization classification (22). The differentiation grade and Tumor,
Node and Metastasis staging of gastric carcinoma were performed
according to the AJCC Cancer Staging Manual (23). Diagnosis was established by two
independent pathologists. The clinicopathological information of
the 98 patients with gastric cancer is presented in Table I. All the tissue specimens for the
present study were obtained from patients following provision of
written informed consent. The tissue samples obtained from the
tissue bank at Nanfang Hospital and this retrospective analysis
were approved by the Ethics Committee of Nanfang Hospital.
 | Table I.Association between
clinicopathological characteristics of gastric cancer and FNDC1
expression levels. |
Table I.
Association between
clinicopathological characteristics of gastric cancer and FNDC1
expression levels.
|
|
| FNDC1
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Total | Low | High | χ2 | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
<60 | 53 | 13 | 40 | 0.528 | 0.467 |
|
≥60 | 45 | 14 | 31 |
|
|
| Sex |
|
|
|
|
|
|
Male | 74 | 23 | 51 | 1.886 | 0.170 |
|
Female | 24 | 4 | 20 |
|
|
| Tumor size
(diameter in cm) |
|
|
|
|
|
|
<5 | 51 | 15 | 36 | 0.184 | 0.668 |
| ≥5 | 47 | 12 | 35 |
|
|
| Clinical stage |
|
|
|
|
|
| I | 7 | 7 | 0 | 26.48 | <0.001 |
| II | 11 | 6 | 5 |
|
|
|
III | 62 | 11 | 51 |
|
|
| IV | 18 | 3 | 15 |
|
|
| T
classification |
|
|
|
|
|
|
T1+T2 | 11 | 8 | 3 | 12.669 | <0.001 |
|
T3+T4 | 87 | 19 | 68 |
|
|
| N
classification |
|
|
|
|
|
| N0 | 24 | 15 | 9 | 19.745 | <0.001 |
| N1 | 16 | 2 | 14 |
|
|
| N2 | 15 | 2 | 13 |
|
|
| N3 | 43 | 8 | 35 |
|
|
| Metastasis |
|
|
|
|
|
| No | 80 | 25 | 55 | 2.986 | 0.084 |
|
Yes | 18 | 2 | 16 |
|
|
| Pathologic
differentiation |
|
|
|
|
|
|
Well | 5 | 1 | 4 | 6.247 | 0.044 |
|
Moderate | 32 | 14 | 18 |
|
|
|
Poor | 61 | 12 | 49 |
|
|
Statistical analysis of FNDC1
expression in gastric cancer
To determine the expression pattern of FNDC1 in
gastric cancer, datasets from the Oncomine database (https://www.oncomine.org) were used. FNDC1 was queried
in the database, and the results were filtered by selecting gastric
cancer and cancer vs. normal analysis. The Cho, Derrico, and Wang
datasets obtained from the Oncomine database were embedded in the
NCBI GEO database (https://www.ncbi.nlm.nih.gov/geo/) at accession
numbers GSE13861, GSE13911 and GSE19826, respectively (24–26). The
Cancer Genome Atlas (TCGA) gastric database was analyzed by GEPIA
(http://gepia.cancer-pku.cn), a web-based
tool to deliver fast and customizable functionalities based on TCGA
and GTEx data (27). The following
settings were used for the analysis: ‘Expression on Box Plots’;
‘Gene=FNDC1’; ‘|log2FC| Cutoff=1’; ‘P-value Cutoff=0.01’;
‘Datasets=STAD’; ‘Log Scale=Yes’; ‘Jitter Size=0.4’; and ‘Match
TCGA normal and GTEx data’. The prognostic value of the FNDC1 gene
in gastric cancer was also analyzed using the Kaplan-Meier Plotter
(http://kmplot.com/analysis/). The
following settings were used for the analysis: ‘Overall survival’;
‘Post progression survival’; ‘auto select best cutoff’; ‘censore at
threshold’ (patients surviving over the selected threshold are
censored instead of excluded); ‘tumor stage all’; ‘tumor stage T
all’; ‘tumor stage N all’; ‘tumor stage M all’; ‘Lauren
classification all’; and ‘differentiation all’. The FNDC1 gene
probe set was 226930_at, and patients were split according to
median expression or expression at best cutoff for the probe. The
data were extracted from the Oncomine database, GEPIA website and
Kaplan-Meier Plotter between December 2017 and March 2018.
Immunohistochemistry analysis
Immunohistochemistry analysis was performed as
previously described. Formalin-fixed (fixed using 4% formalin at
room temperature for 24 h) and paraffin-embedded tissues were cut
into 4-µm-thick sections, followed by incubation at 65°C for 2 h.
Tissues were deparaffinized in xylene and then rehydrated in graded
alcohol and PBS. Following antigen retrieval by EDTA pre-incubated
with 5% normal bovine serum (Wuhan Boster Biological Technology
Ltd., Wuhan, China) at room temperature for 20 min, deparaffinized
sections were incubated overnight at 4°C with an optimal dilution
(1:100) of a primary polyclonal rabbit antibody against human FNDC1
(abs127634a; Absin Bioscience, Inc., Shanghai, China). Following
washing, the slides were incubated with horseradish peroxidase
conjugated-anti-rabbit IgG secondary antibodies (1:200; cat. no.
TA130023; OriGene Technologies, Inc., Beijing, China). Then,
reaction products were treated with diaminobenzidine (DAB; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA), counterstained
with hematoxylin at room temperature for 5 min, dehydrated, and
mounted. For negative controls, the primary antibodies were
omitted, but otherwise the methodology was the same. The Olympus
BX51 microscope (Olympus Corporation, Tokyo, Japan) was used to
capture images of the samples (magnification, ×200 and ×400). The
sections were reviewed and scored independently by two observers,
based on both the proportion of positively stained tumor cells and
the intensity of staining. The proportion of positive tumor cells
was scored as follows: 0 (no positive tumor cells), 1 (<10%
positive tumor cells), 2 (10–50% positive tumor cells), and 3
(>50% positive tumor cells). The intensity of staining was
graded according to the following criteria: 0 (no staining); 1
(weak staining=light yellow), 2 (moderate staining=yellow-brown),
and 3 (strong staining=brown). The staining index was calculated as
staining intensity score × proportion of positive tumor cells. An
optimal cut-off value was identified based on previous studies: A
score of ≥4 was defined as high FNDC1 expression and a score of ≤3
was defined as low FNDC1 expression (28–31).
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses. Data of FNDC1 expression level
obtained from the Oncomine database were analyzed using Student's
t-test. The correlation between FNDC1 expression and
clinicopathological parameters was measured by Pearson's
χ2 test. Overall survival curves were estimated by the
Kaplan-Meier method and log-rank test. Univariate and multivariate
Cox regression survival analysis was performed to determine the
independent prognostic markers. P<0.05 was considered
statistically significant.
Results
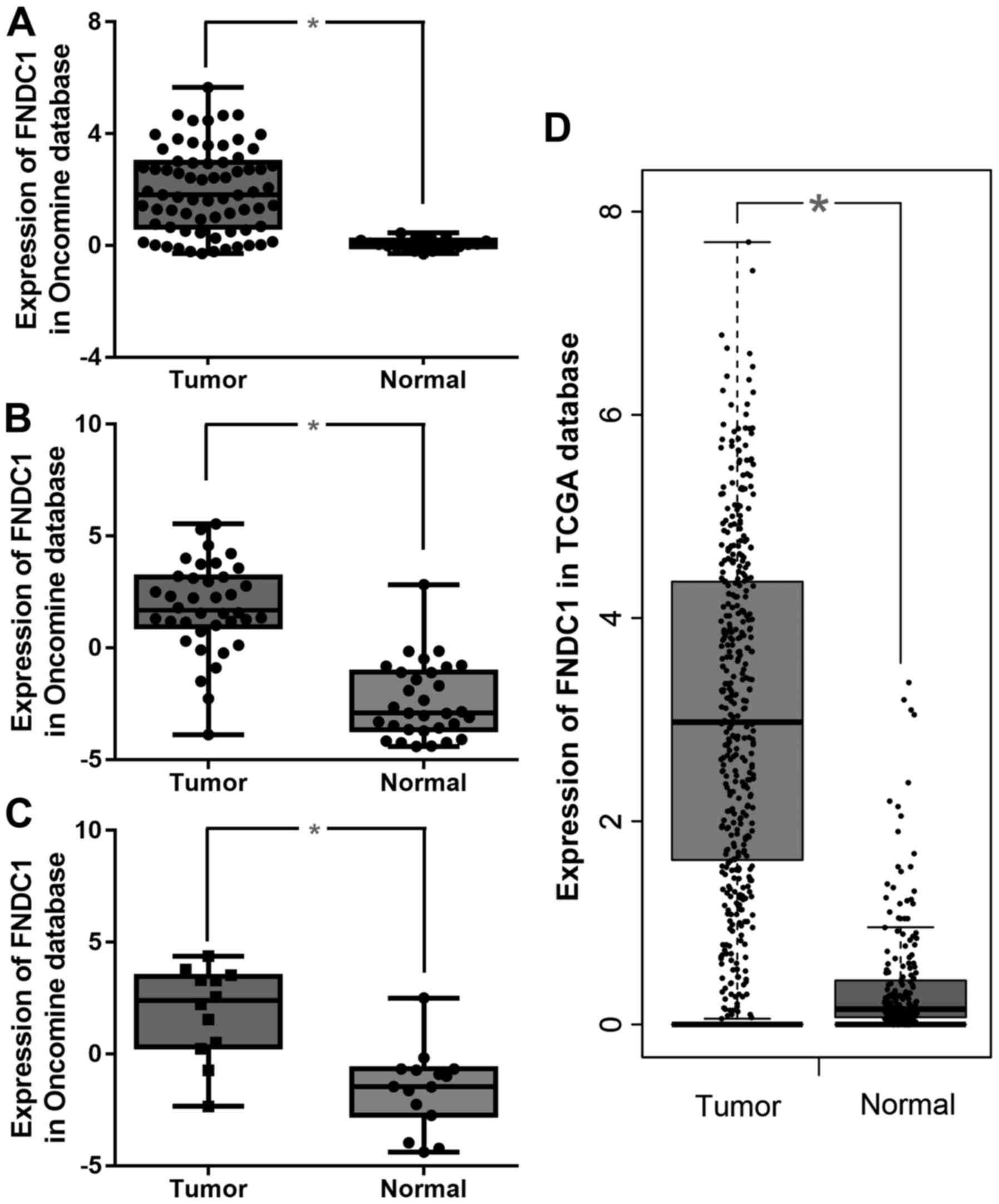
Analysis of FNDC1 gene expression
Data for FNDC1 gene expression were extracted from
the Oncomine database and TCGA gastric database for gastric cancer,
focusing on cancer vs. normal patient datasets. As presented in
Fig. 1, FNDC1 mRNA expression in
gastric cancer was demonstrated to be significantly upregulated in
tumor tissues compared with normal tissues in the Cho, Derrico, and
Wang datasets (Fig. 1A-C,
respectively) as well as in the TCGA database analyzed by GEPIA
(Fig. 1D).
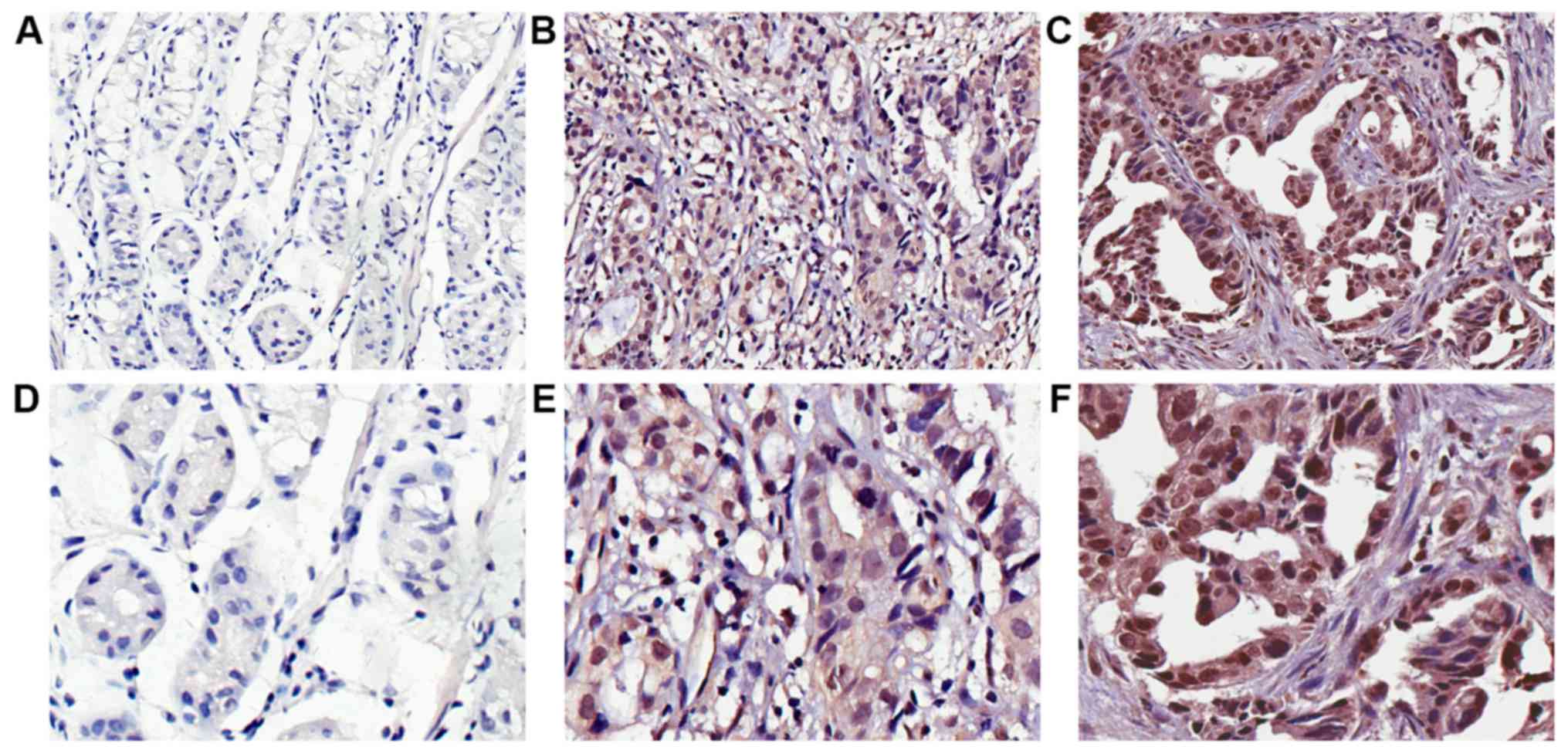
High expression of FNDC1 in human
gastric cancer tissues
The clinicopathological features of all 98 patients
with gastric cancer are summarized in Table I. The expressed FNDC1 was detected in
the nucleus and cytoplasm of the cancer cells, and FNDC1 was
predominately localized in the nucleus (Fig. 2). FNDC1 was expressed in 12% (3/25)
of paired adjacent normal tissues. Compared with these normal
tissues, a relatively high FNDC1 expression level was observed in
72.4% (71/98) of gastric cancer tissues and 27.6% (27/98) of cases
exhibited relatively low FNDC1 expression (Table I).
Upregulation of FNDC1 is associated
with advanced clinicopathological features of gastric cancer
To investigate the role of FNDC1 in gastric cancer,
its expression was examined using immunohistochemistry in 98
paraffin-embedded archived human gastric cancer tissues, including
7 cases at clinical stage I, 11 cases at clinical stage II, 62
cases at clinical stage III, and 18 cases at clinical stage IV
(Table I). As presented in Table I, significant associations were
observed between FNDC1 expression and clinical stage (P<0.001),
T classification (P<0.001), N classification (P<0.001), and
pathological differentiation (P=0.044). However, the expression of
FNDC1 was not associated with age (P=0.467), sex (P=0.17), tumor
size (P=0.668), or metastasis (P=0.084). These results indicated a
significant association between FNDC1 expression and the prognosis
of gastric cancer.
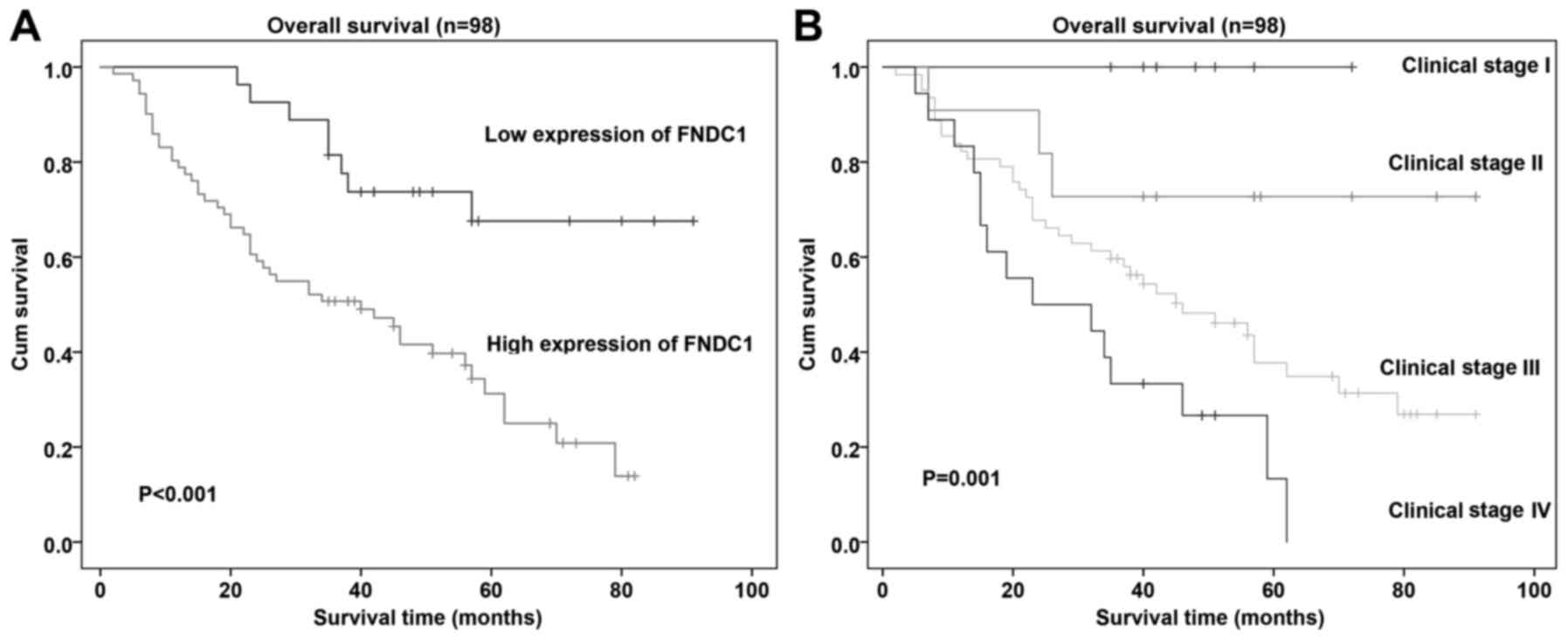
High FNDC1 expression in gastric
cancer tissues correlates with poor patient survival
Cox regression analysis was used to determine
whether FNDC1 expression could serve as a risk factor. As presented
in Table II, using univariate Cox
regression analyses, it was demonstrated that high FNDC1 expression
level was associated with a significantly increased risk of death
in patients with gastric cancer (P=0.001) compared with that in
patients with low FNDC1 expression level. Using multivariate Cox
regression analysis, it was also determined that FNDC1 could be an
important factor for predicting poor survival when FNDC1 expression
(P=0.032) and clinical stage were included (P=0.031; Table II). Patients with high FNDC1
expression levels had shorter overall survival (OS) times compared
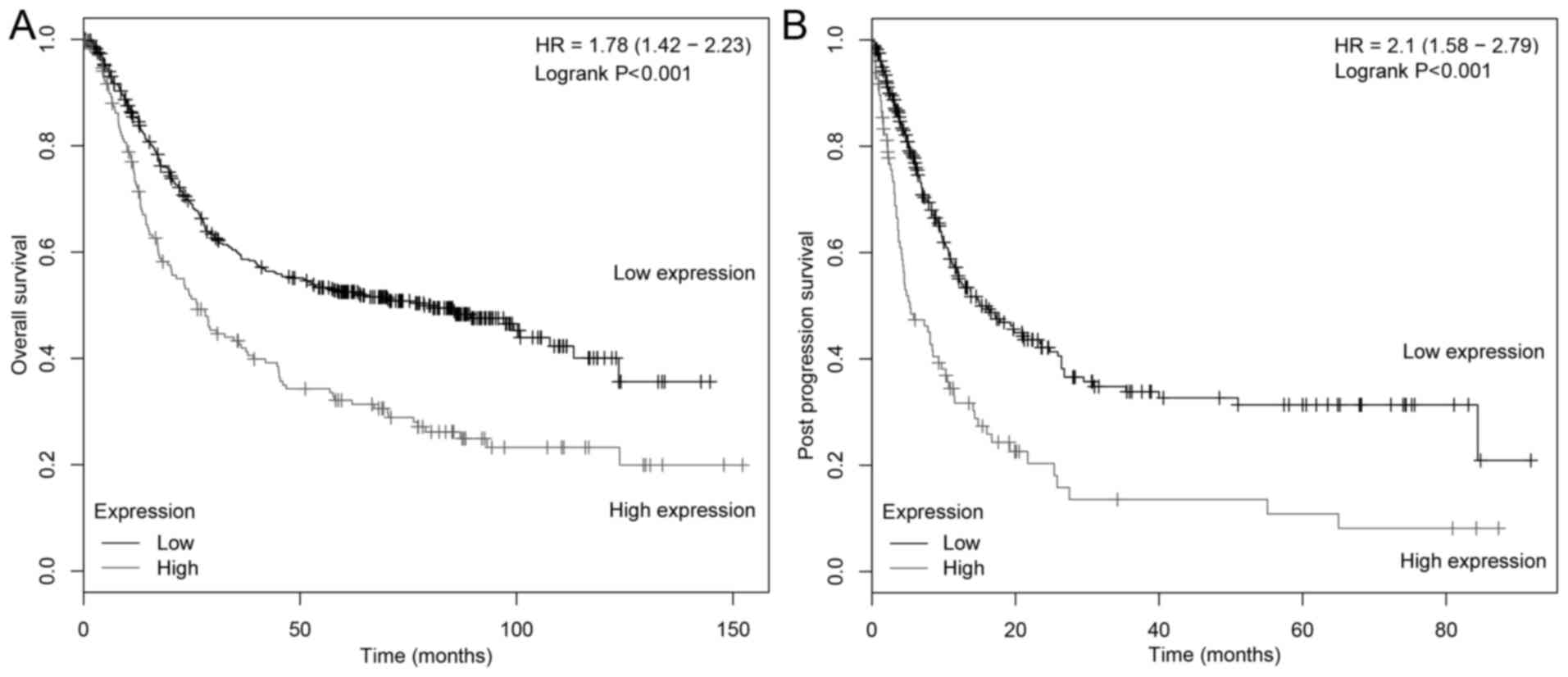
with patients with low FNDC1 expression levels (Fig. 3). To further understand the
association between survival and FNDC1 expression in gastric
cancer, the correlation between FNDC1 expression level and the
survival of patients with gastric cancer was evaluated using the
Kaplan-Meier Plotter (Fig. 4) and it
was demonstrated that low FNDC1 expression level is a favorable
prognostic factor for OS and post-progression survival (PPS;
P<0.001, n=631; P<0.001, n=384, respectively) in patients
with gastric cancer. Collectively, these results indicated that
overexpression of FNDC1 in patients with primary gastric cancer is
correlated with poor survival.
 | Table II.Univariate and multivariate analyses
of various prognosis parameters in 98 patients with gastric cancer
using Cox regression model. |
Table II.
Univariate and multivariate analyses
of various prognosis parameters in 98 patients with gastric cancer
using Cox regression model.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | Cases (n) | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| Age (years) |
|
|
|
|
|
|
<60 | 53 | 0.413 | 1.246
(0.736–2.110) |
|
|
|
≥60 | 45 |
|
|
|
|
| Sex |
|
|
|
|
|
|
Male | 74 | 0.118 | 1.593
(0.889–2.854) |
|
|
|
Female | 24 |
|
|
|
|
| Tumor size
(diameter in cm) |
|
|
|
|
|
|
<5 | 51 | 0.028 | 1.811
(1.065–3.078) | 0.056 | 1.684
(0.986–2.877) |
| ≥5 | 47 |
|
|
|
|
| Clinical stage |
|
|
|
|
|
| I | 7 | <0.001 | 2.343
(1.524–3.603) | 0.031 | 2.907
(1.105–7.653) |
| II | 11 |
|
|
|
|
|
III | 62 |
|
|
|
|
| IV | 18 |
|
|
|
|
| T
classification |
|
|
|
|
|
|
T1+T2 | 11 | 0.122 | 2.507
(0.783–8.024) |
|
|
|
T3+T4 | 87 |
|
|
|
|
| N
classification |
|
|
|
|
|
| N0 | 24 | 0.026 | 1.299
(1.031–1.635) | 0.861 | 0.977
(0.752–1.269) |
| N1 | 16 |
|
|
|
|
| N2 | 15 |
|
|
|
|
| N3 | 43 |
|
|
|
|
| Metastasis |
|
|
|
|
|
| No | 80 | 0.016 | 2.124
(1.150–3.924) | 0.370 | 0.592
(0.188–1.862) |
|
Yes | 18 |
|
|
|
|
| Pathologic
differentiation |
|
|
|
|
|
|
Well | 5 | 0.041 | 1.732
(1.022–2.936) | 0.262 | 1.355
(0.797–2.306) |
|
Moderate | 32 |
|
|
|
|
|
Poor | 61 |
|
|
|
|
| FNDC1
expression |
| 0.001 | 3.543
(1.667–7.533) | 0.032 | 2.326
(1.073–5.038) |
|
Low | 27 |
|
|
|
|
|
High | 71 |
|
|
|
|
Discussion
Gastric cancer is a common malignant neoplasm that
poses a serious threat to human life and health. Hence, it is
considerably important to investigate the pathogenesis of gastric
cancer and identify highly sensitive and specific molecular
biomarkers for gastric cancer. Fibronectin is an important ECM
protein that has been reported to promote invasiveness of gastric
cancer cells, and the serum fibronectin levels of patients with
gastric cancer were significantly higher than those in the healthy
controls (32,33). FNDC1 contains a major component of
the structural domain of fibronectin (10–13). The
present study indicated that FNDC1 expression is higher in gastric
cancer tissues than in adjacent normal tissues. FNDC1 may serve a
role in tumor invasion in gastric cancer, as there were significant
associations between FNDC1 expression and the depth of tumor
invasion, lymph node metastasis, clinical stage and poor
differentiation. Consistent with the present results, Oncomine and
TCGA database analyses demonstrated that FNDC1 was overexpressed in
gastric cancer tissues. Furthermore, it was observed that high
FNDC1 protein expression was an independent prognostic factor for
gastric cancer and was significantly correlated with poor survival.
Bioinformatic databases were also used to confirm the association
between FNDC1 expression and prognosis in gastric cancer patients.
Results obtained using the Kaplan-Meier Plotter verified the
finding that high FNDC1 expression was associated with poor OS and
PPS in patients with gastric cancer.
Only limited data have been reported regarding the
function of FNDC1. Chronic inflammation is a well-documented risk
factor in cancer development (34–36).
FNDC1 was initially identified as a desmoplastic response-related
gene and seemed to have a role in inflammation (14). FNDC1 expression was correlated with
skin tumor progression and could be induced by treatment with
transforming growth factor-β, interleukin-1, and tumor necrosis
factor-α in vitro (14).
Notably, 12% of normal adjacent tissues in the present study
exhibited positive FNDC1 staining. These tissues were demonstrated
to exhibit atrophic gastritis and low-grade dysplasia. This
suggested that the positive staining of these tissues may be
associated with the malignant progression of gastric tissue. A
previous study on acute otitis media in children identified that
associated variants were significantly correlated with the
expression levels and methylation status of FNDC1, and mouse
homolog Fndc1 was upregulated under proinflammatory conditions,
such as in lipopolysaccharide treatment (17). FNDC1 has been demonstrated to have an
important role in the regulation of proliferation, apoptosis, and
migration in prostate cancer (16).
Previous studies have also reported that FNDC1 was expressed in
kidney and heart tissue and served a role in hypoxia-induced
apoptosis of cardiomyocytes by interacting with Gβγ38 (18). FNDC1-Gβγ signal input also affects
the function of CX43 and cell permeability, thus increasing the
sensitivity of cells to hypoxic stress (19). Angiogenesis serves a critical role in
malignant tumor growth and metastasis and is regulated by
proangiogenic and antiangiogenic factors (37). VEGF is associated with physiological
and pathological angiogenesis, which enhances the permeability of
blood vessels, reduces endothelial cell apoptosis, activates
stromal proteolysis, and promotes the proliferation and migration
of endothelial cells (38,39). Recently, Hayashi et al
(21) demonstrated that knockdown of
FNDC1 in endothelial cells inhibited VEGF-mediated cellular events,
including cell growth, migration and proliferation. These results
may be analogous to the results of the present study of gastric
cancer. FNDC1 seems to have an important role in cellular
proliferation and angiogenesis, which are necessary for tumor
growth and metastasis.
To the best of our knowledge, this is the first
study to investigate the association between FNDC1 expression
levels and the clinicopathological features of patients with
gastric cancer. Expression of FNDC1 was associated with unfavorable
clinical characteristics and poor prognosis of patients with
gastric cancer, suggesting that FNDC1 may have a positive
regulatory role in gastric cancer by acting as a tumor-promotor
gene. However, the present study is a retrospective observational
study, and the results may not be representative of other gastric
cancer populations. Further studies are required to determine the
potential function of FNDC1 expression in tumor invasion and
metastasis to verify the molecular basis of FNDC1 expression in
gastric cancer. In addition, research on the changes in fibronectin
levels following treatment of patients with gastric cancer should
be performed in future studies.
In conclusion, the present results demonstrated that
high FNDC1 expression level in human gastric cancer is
significantly correlated with the progression as well as poor
prognosis of the tumor. The present findings support the
possibility that FNDC1 expression levels may be an important
prognostic indicator and potential therapeutic target in gastric
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangdong Province (grant no.
2017A030313472).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MZ and YiZ contributed equally to this study. MZ
assisted in the design of the study, performed experiments,
analyzed data and drafted the manuscript. YiZ contributed to the
study design, interpreted data, and helped with the manuscript
revision. FY and YP contributed to data analysis and helped draft
and revise the manuscript. JW and JY provided technical support and
assisted with the manuscript revision. WZ contributed to the study
design and helped draft the manuscript. YaZ contributed to the
study design, helped revise the manuscript and provided funding.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The collection of tissue samples used for this study
was approved by the Ethics Committee of Nanfang Hospital
(Guangzhou, China) and all patients provided informed written
consent.
Patient consent for publication
All participants gave informed written consent prior
to taking part in this study. All samples were anonymized.
Identifying information, including names, initials, date of birth
or hospital numbers, images or statements were not included in the
manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah MA and Ajani JA: Gastric cancer-an
enigmatic and heterogeneous disease. JAMA. 303:1753–1754. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki H, Iwasaki E and Hibi T:
Helicobacter pylori and gastric cancer. Gastric Cancer. 12:79–87.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kato S, Tsukamoto T, Mizoshita T, Tanaka
H, Kumagai T, Ota H, Katsuyama T, Asaka M and Tatematsu M: High
salt diets dose-dependently promote gastric chemical carcinogenesis
in Helicobacter pylori-infected Mongolian gerbils associated with a
shift in mucin production from glandular to surface mucous cells.
Int J Cancer. 119:1558–1566. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Milne AN, Carneiro F, O'Morain C and
Offerhaus GJ: Nature meets nurture: Molecular genetics of gastric
cancer. Hum Genet. 126:615–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gomceli I, Demiriz B and Tez M: Gastric
carcinogenesis. World J Gastroenterol. 18:5164–5170.
2012.PubMed/NCBI
|
|
8
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wagner AD and Moehler M: Development of
targeted therapies in advanced gastric cancer: Promising
exploratory steps in a new era. Curr Opin Oncol. 21:381–385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao Y, Liu X, Lu W, Chen Y, Wu X, Li M,
Wang XA, Zhang F, Jiang L, Zhang Y, et al: Fibronectin promotes
cell proliferation and invasion through mTOR signaling pathway
activation in gallbladder cancer. Cancer Lett. 360:141–150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fernandez-Garcia B, Eiró N, Marín L,
González-Reyes S, González LO, Lamelas ML and Vizoso FJ: Expression
and prognostic significance of fibronectin and matrix
metalloproteases in breast cancer metastasis. Histopathology.
64:512–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
von Au A, Vasel M, Kraft S, Sens C, Hackl
N, Marx A, Stroebel P, Hennenlotter J, Todenhöfer T, Stenzl A, et
al: Circulating fibronectin controls tumor growth. Neoplasia.
15:925–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao M, Craig D, Lequin O, Campbell ID,
Vogel V and Schulten K: Structure and functional significance of
mechanically unfolded fibronectin type III1 intermediates. Proc
Natl Acad Sci USA. 100:14784–14789. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anderegg U, Breitschwerdt K, Köhler MJ,
Sticherling M, Haustein UF, Simon JC and Saalbach A: MEL4B3, a
novel mRNA is induced in skin tumors and regulated by TGF-beta and
pro-inflammatory cytokines. Exp Dermatol. 14:709–718. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bell A, Bell D, Weber RS and El-Naggar AK:
CpG island methylation profiling in human salivary gland adenoid
cystic carcinoma. Cancer. 117:2898–2909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Das DK, Naidoo M, Ilboudo A, Park JY, Ali
T, Krampis K, Robinson BD, Osborne JR and Ogunwobi OO: miR-1207-3p
regulates the androgen receptor in prostate cancer via
FNDC1/fibronectin. Exp Cell Res. 348:190–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Ingen G, Li J, Goedegebure A, Pandey
R, Li YR, March ME, Jaddoe VW, Bakay M, Mentch FD, Thomas K, et al:
Genome-wide association study for acute otitis media in children
identifies FNDC1 as disease contributing gene. Nat Commun.
7:127922016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato M, Cismowski MJ, Toyota E, Smrcka AV,
Lucchesi PA, Chilian WM and Lanier SM: Identification of a
receptor-independent activator of G protein signaling (AGS8) in
ischemic heart and its interaction with Gbetagamma. Proc Natl Acad
Sci USA. 103:797–802. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato M, Jiao Q, Honda T, Kurotani R,
Toyota E, Okumura S, Takeya T, Minamisawa S, Lanier SM and Ishikawa
Y: Activator of G protein signaling 8 (AGS8) is required for
hypoxia-induced apoptosis of cardiomyocytes: Role of G betagamma
and connexin 43 (CX43). J Biol Chem. 284:31431–31440. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu CT, Garnaas MK, Tin A, Kottgen A,
Franceschini N, Peralta CA, de Boer IH, Lu X, Atkinson E, Ding J,
et al: Genetic association for renal traits among participants of
African ancestry reveals new loci for renal function. PLoS Genet.
7:e10022642011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayashi H, Al Mamun A, Sakima M and Sato
M: Activator of G-protein signaling 8 is involved in VEGF-mediated
signal processing during angiogenesis. J Cell Sci. 129:1210–1222.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO classification of tumours of the digestive system.
4th. International Agency for Research on Cancer; Lyon: 2010
|
|
23
|
Amin MB, Edge S, Greene FL, Schilsky RL,
Gaspar LE, Washington MK, Sullivan DC, Brookland RK, Brierley JD,
Balch CM, et al: AJCC cancer staging manual. 8th ed. Springer; New
York: 2017, View Article : Google Scholar
|
|
24
|
Cho JY, Lim JY, Cheong JH, Park YY, Yoon
SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, et al: Gene expression
signature-based prognostic risk score in gastric cancer. Clin
Cancer Res. 17:1850–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Errico M, de Rinaldis E, Blasi MF, Viti
V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D,
et al: Genome-wide expression profile of sporadic gastric cancers
with microsatellite instability. Eur J Cancer. 45:461–469. 2009.
View Article : Google Scholar
|
|
26
|
Wang Q, Wen YG, Li DP, Xia J, Zhou CZ, Yan
DW, Tang HM and Peng ZH: Upregulated INHBA expression is associated
with poor survival in gastric cancer. Med Oncol. 29:77–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45(W1):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wan J, Liu H, Feng Q, Liu J and Ming L:
HOXB9 promotes endometrial cancer progression by targeting E2F3.
Cell Death Dis. 9:5092018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Fu D, Chen Y, Su J, Wang Y, Li X,
Zhai W, Niu Y, Yue D and Geng H: G3BP1 promotes tumor progression
and metastasis through IL-6/G3BP1/STAT3 signaling axis in renal
cell carcinomas. Cell Death Dis. 9:5012018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Z, Yang F, Zhang Y, Wang X, Shi J,
Wei H, Sun F and Yu Y: Girdin protein: A potential metastasis
predictor associated with prognosis in lung cancer. Exp Ther Med.
15:2837–2843. 2018.PubMed/NCBI
|
|
31
|
Liu Y, Yao X, Zhang Q, Qian L, Feng J,
Bian T, Zhang J and Tian Y: Expression of Kruppel-like factor 8 and
Ki67 in lung adenocarcinoma and prognosis. Exp Ther Med.
14:1351–1356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li D, Ding J, Wang X, Wang C and Wu T:
Fibronectin promotes tyrosine phosphorylation of paxillin and cell
invasiveness in the gastric cancer cell line AGS. Tumori.
95:769–779. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tas F, Bilgin E, Karabulut S, Tastekin D
and Duranyildiz D: Levels of serum fibronectin as a biomarker in
gastric cancer patients: Correlation with clinical diagnosis and
outcome. Mol Clin Oncol. 4:655–659. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Byrne KJ and Dalgleish AG: Chronic
immune activation and inflammation as the cause of malignancy. Br J
Cancer. 85:473–483. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aggarwal BB, Shishodia S, Sandur SK,
Pandey MK and Sethi G: Inflammation and cancer: How hot is the
link? Biochem Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Poon RT, Fan ST and Wong J: Clinical
implications of circulating angiogenic factors in cancer patients.
J Clin Oncol. 19:1207–1225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tischer E, Gospodarowicz D, Mitchell R,
Silva M, Schilling J, Lau K, Crisp T, Fiddes JC and Abraham JA:
Vascular endothelial growth factor: A new member of the
platelet-derived growth factor gene family. Biochem Biophys Res
Commun. 165:1198–1206. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kowanetz M and Ferrara N: Vascular
endothelial growth factor signaling pathways: Therapeutic
perspective. Clin Cancer Res. 12:5018–5022. 2006. View Article : Google Scholar : PubMed/NCBI
|