Introduction
Paraquat (N,N′-dimethyl-4,4′-bipyridinium
dichloride; PQ) is one of the most commonly used herbicides
worldwide (1,2). However, long-term accumulation or acute
intoxication of PQ may cause organ injury, resulting in a mortality
rate of >60–70% (3,4). Following oral administration, PQ is
absorbed through the gastrointestinal tract within 1–2 h and
accumulates in various organs, including the lung, liver, kidney
and central nervous system (5). PQ
primarily amasses within alveolar epithelial and bronchiolar Clara
cells of the lung, and results in acute lung injury and acute
respiratory distress syndrome, which is the leading cause of
mortality in patients with PQ poisoning (6). Although the exact pathogenic mechanism
of PQ remains largely unknown, PQ-induced pulmonary-specific
accumulation, excessive oxidative stress, inflammatory injury and
an imbalance in the deposition of the extracellular matrix, are
major contributors to lung injury following PQ intoxication
(7–9). However, pulmonary-targeted
accumulation, which results in acute lung injury, remains a
challenge for detoxification treatment.
P-glycoprotein (P-gp), a member of the ATP-binding
cassette (ABC) transporter family, is vital for many cellular
processes that require the transport of chemicals across the cell
membrane (10,11). P-gp hyperactivity causes drugs to be
pumped out of cells, resulting in chemotherapeutic agent and
antimicrobial drug resistance (12,13).
Previous studies have demonstrated that P-gp may be closely
associated with the removal of PQ from cells (14,15). In
corroboration with this association, P-gp levels are significantly
increased in rat alveolar type II cells following PQ exposure
(16) and a high level of P-gp has
been revealed to alleviate PQ toxicity in human epithelial colon
cancer cells and to significantly reduce rat lung tissue PQ
concentration (17,18). However, these protective effects were
reversed following treatment with a specific inhibitor of P-gp,
cyclosporine A (CsA) (18).
Therefore, P-gp appears to serve an important role in PQ transport
across cells and upregulating the activity of P-gp may be an
effective measure to attenuate the intracellular accumulation of PQ
and thus, its toxicity.
Nuclear factor erythroid-2 related factor 2 (Nrf2)
is a transcription factor that belongs to the cap ‘n’ collar family
of basic leucine zipper proteins, which is tightly regulated by
Kelch-like ECH-associated protein 1 (Keap1) (19). The Keap1-Nrf2 pathway, including its
targeted cytoprotective protein expression, is the fundamental
mechanism for cellular defence against oxidative and electrophilic
stress (20,21). Various natural compounds, including
cycloartenyl ferulate and resveratrol, inhibit PQ-induced oxidative
stress and apoptosis by activating the Nrf2 pathway (22,23). A
previous study demonstrated that Nrf2 overexpression attenuated PQ
toxicity in A549 cells and mice by activating heme oxygenase-1 and
NAD(P)H: Quinone oxidoreductase 1 (24). The primary focus of research on Nrf2
targets has been on detoxifying/antioxidant enzymes; however,
several ABC transporters are Nrf2 targets (25). Furthermore, previous studies have
revealed that the activation of Nrf2 is necessary to increase P-gp
activity (26–28). Therefore, the current study
hypothesized that Nrf2 may prevent organ injury in PQ poisoning by
increasing P-gp activation and reducing intracellular PQ
concentration.
The present study revealed that the Nrf2 gene was
overexpressed in the A549 cell line. Subsequent to this result, the
role of P-gp activation in PQ-challenged A549 cells was determined.
It was hypothesized that Nrf2 and P-gp activity may be potential
targets for the prevention of organ injury in PQ poisoning.
Materials and methods
Materials and reagents
PQ and the adenoviral (AD) system, including
plasmids containing the predesigned human Nrf2 gene and fetal
bovine serum (FBS), were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). RPMI-1640 medium was obtained from Gibco;
Thermo Fisher Scientific, Inc. (Waltham MA, USA). A549 cells were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). The Cell Counting Kit-8 (CCK-8) was
obtained from Dojindo Molecular Technologies, Inc. (Kumamoto,
Japan). Lactate dehydrogenase (LDH), superoxide dismutase (SOD),
malondialdehyde (MDA), tumor necrosis factor-α (TNF-α) and
interleukin-6 (IL-6) detection kits were supplied by Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Antibodies
against Nrf2 and P-gp were obtained from Abcam (Cambridge, UK). GFP
was purchased from Thermo Fisher Scientific, Inc. CsA (cat. no.
59865-13-3) was purchased from Sigma-Aldrich; Merck, KGaA.
Transfection and transfection
efficiency analysis
A549 cells were grown in a monolayer culture in
RPMI-1640 medium supplemented with 10% FBS at 37°C in a humidified
atmosphere with 5% CO2. Following this incubation, cells
(4×104 cells/well) were inoculated onto a 96-well
culture plate and left to grow in the logarithmic stage for 24 h at
37°C. A549 cells were transfected with adenoviral vectors
containing either AD-Nrf2 or AD-(GFP) (cat. no. PEP033; Thermo
Fisher Scientific, Inc.), at a multiplicity of infection of 50.
Control cells were cultured in RPMI-1640 medium only. The cells
were cultured in RPMI-1640 medium supplemented with 10% FBS and
maintained at 37°C in a 5% CO2-humidified incubator for
24 h. A fluorescence microscope (magnification, ×100; Olympus
CKX41SF; Olympus Corporation, Tokyo, Japan) was used to determine
the percentage of GFP synthesizing cells. The ratio of cells that
emitted green fluorescence in the same field of view was regarded
as the transfection efficiency. Nrf2 overexpression was also
confirmed via western blotting.
Cell culture and treatments
The current study included five groups: A control
group, a PQ group, a PQ + AD-Nrf2 group, a PQ + AD-Nrf2 + CsA group
and a control + CsA group. A549 cells were grown in a monolayer
culture in RPMI-1640 medium supplemented with 10% FBS and
maintained at 37°C in a 5% CO2-humidified incubator.
A549 cells (4×104 cells/well) were seeded onto a 96-well
culture plate. Following logarithmic stage growth for 24 h at 37°C,
A549 cells were transfected with AD-Nrf2 and preincubated with CsA
at a concentration of 12 µg/ml or vehicle control (0.1% ethanol).
Cells were subsequently incubated in RPMI-1640 medium supplemented
with 10% FBS and cultured at 37°C in a 5% CO2-humidified
incubator for 24 h and the experimental groups were administered 10
µl PQ (1×103 mol/l). Following further incubation for 24
h at 37°C, cells were harvested and used for subsequent
experimentation.
CCK-8 assay
Cell survival was assessed using a CCK-8 kit,
according to the manufacturer's protocol and viable cell density
was adjusted to 4×104 cells/ml. A549 cells
(4×104 cells/well) were seeded onto a 96-well culture
plate and cultured in RPMI-1640 medium supplemented with 10% FBS
and maintained at 37°C in a 5% CO2-humidified incubator
for 24 h. Growth medium was replaced with serum-free medium and
cells were cultured for a further 24 h. Subsequently, the
supernatant was discarded and 10 µl CCK-8 solution was added to
each well. Following incubation for 1 h at 37°C, absorbance was
measured at 450 nm using a Benchmark Plus Microplate
Spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
LDH release assay
The LDH release was examined using a LDH release
assay, according to the manufacturer's protocol. The release of LDH
was expressed as a percentage of the total LDH quantity in cells
treated with 2% Triton X-100.
MDA, SOD, TNF-α and IL-6
detection
The cells were centrifuged at 14,000 × g for 15 min
at 4°C and the supernatant was transferred to centrifuge tubes. The
levels of MDA (cat. no. A003-1), SOD (cat. no. A001-3), TNF-α (cat.
no. H052) and IL-6 (cat. no. H007) in cell supernatant was detected
using their respective ELISA kits, according to the manufacturer's
protocol. Samples were analyzed using a spectrophotometer. TNF-α,
IL-6 and SOD activities were expressed in pg/ml and MDA levels were
expressed as nmol/ml.
Western blot analysis
Total protein was extracted from A549 cells using
radioimmunoprecipitation assay (RIPA) buffer (Beyotime Institute of
Biotechnology, Haimen, China) and incubated on ice for 30 min. Cell
lysates were centrifuged for 20 min at 16,000 × g at 4°C. Total
protein was quantified using a bicinchoninic acid assay and 20 µg
protein/lane was separated via SDS-PAGE on a 10% gel. The separated
proteins were transferred onto nitrocellulose membranes (Thermo
Fisher Scientific, Inc.) and blocked with 5% non-fat milk for 1 h
at room temperature. The membranes were incubated with primary
antibodies against Nrf2 (1:1,000; cat. no. ab62352), P-gp (1:1,000;
cat. no. ab170904) or GAPDH (1:5,000; cat. no. ab8245; all Abcam,
Cambridge, UK) antibodies overnight at 4°C. Membranes were washed
with Tris-buffered saline Tween® 20. Following primary
incubation, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit (1:2,000; cat. no. 14708) or
mouse (1:2,000; cat. no. 14709; both Cell Signaling Technology,
Inc., Danvers, MA, USA) secondary antibodies for 2 h at room
temperature. Protein bands were visualized using the
chemiluminescence reagent (Thermo Fisher Scientific, Inc.).
Quantitative analysis was performed using Image J software (Version
1.8.0_172; National Institutes of Health, Bethesda, MD, USA). GAPDH
was utilized as the loading control.
High performance liquid chromatography
(HPLC) analysis
Transfected A549 cells exposed to PQ were harvested.
Following repeated freezing and thawing, cells were centrifuged at
16,000 × g for 5 min at 4°C. Cell supernatant (200 µl) was
subsequently collected and treated with an equivalent volume of
acetonitrile (200 µl; cat. no. A0793; TCI Development Co., Ltd.,
Shanghai, China). Following centrifugation at 12,000 × g for 15 min
at 4°C, 200 µl cell supernatant was filtered through an organic
solvent-compatible 0.45-µm syringe filter (cat. no. F512545; Sangon
Biotech Co., Ltd, Shanghai, China) and 20 µl was injected into an
Agilent 1100 series HPLC system (Agilent Technologies, Inc., Santa
Clara, CA, USA) equipped with a diode-array UV detector, on-line
degasser, autosampler, thermostat-columned compartment and
quaternary pump. Samples were eluted isocratically with an
isocratic elution composed of 4% mobile phase A (acetonitrile) and
96% mobile phase B (20 mM sodium dihydrogen phosphate, 0.4 mM
sodium heptanesulfonate and adjusted to pH 2.3) at a flow rate of
0.6 ml/min at 30°C. Separation was performed using an Agilent
Zorbax-SB-Aq column (internal diameter, 4.6 mm; length, 250 mm;
Agilent Technologies, Inc.) and results were detected at a
wavelength of 256 nm.
Statistical analysis
Experiments were performed at least three times and
data were presented as the mean ± standard deviation. Differences
between three or more groups were analyzed using one-way analysis
of variance followed by the least significant difference test.
P<0.05 was considered to indicate a statistically significant
result.
Results
Transfection efficiency analysis
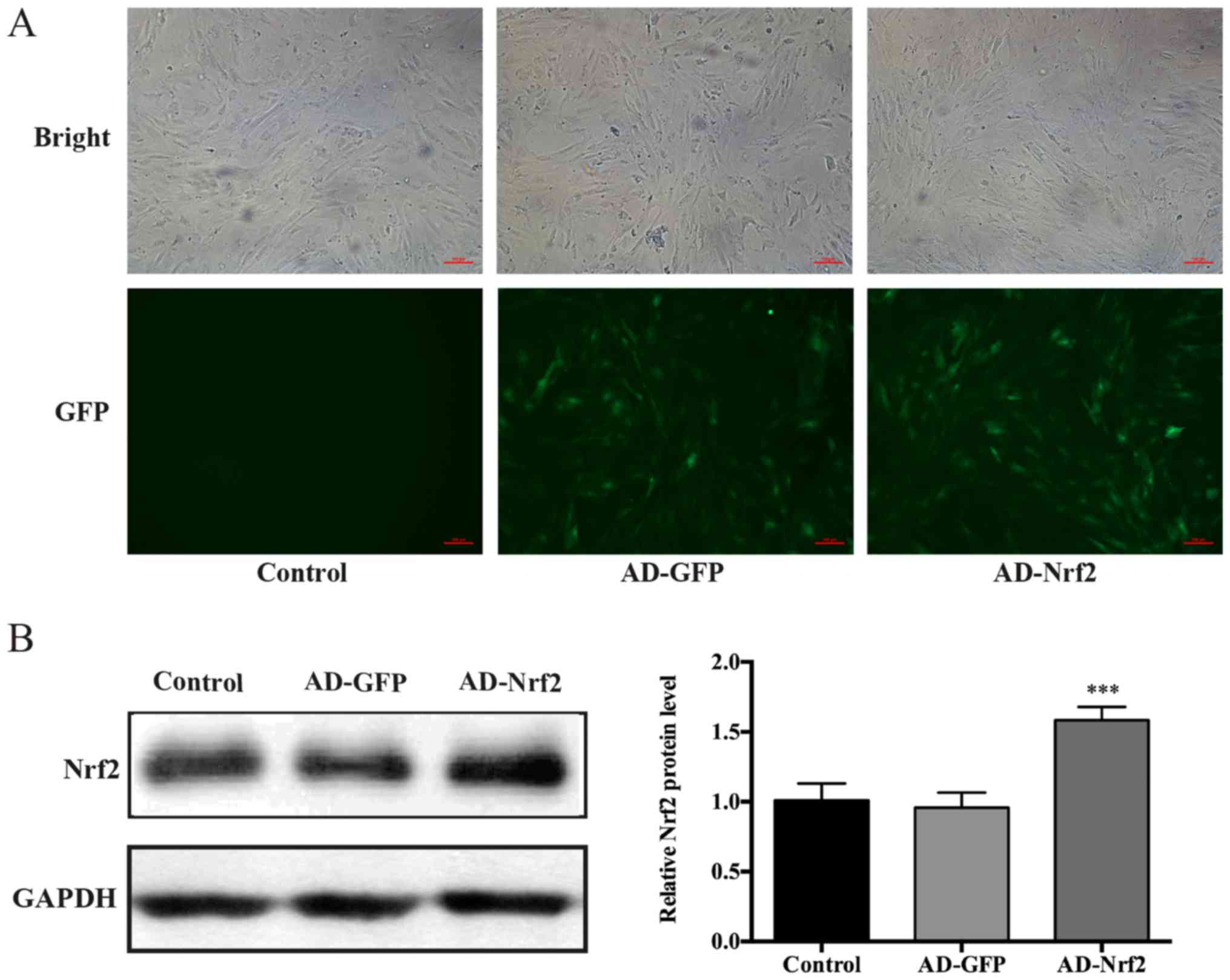
The results of transfection efficacy analysis
verified that the control group did not express green fluorescence
(Fig. 1A). From the green
fluorescence observed under a magnification of ×100, AD vector
transfection efficiency was determined to be >90% (data not
shown). The results of western blotting revealed that Nrf2
expression in cells transfected with AD-Nrf2 was significantly
higher compared with the control group (P<0.001; Fig. 1B), which demonstrated that the cell
model that was abundant for Nrf2 expression, and that the use of AD
vectors was successful.
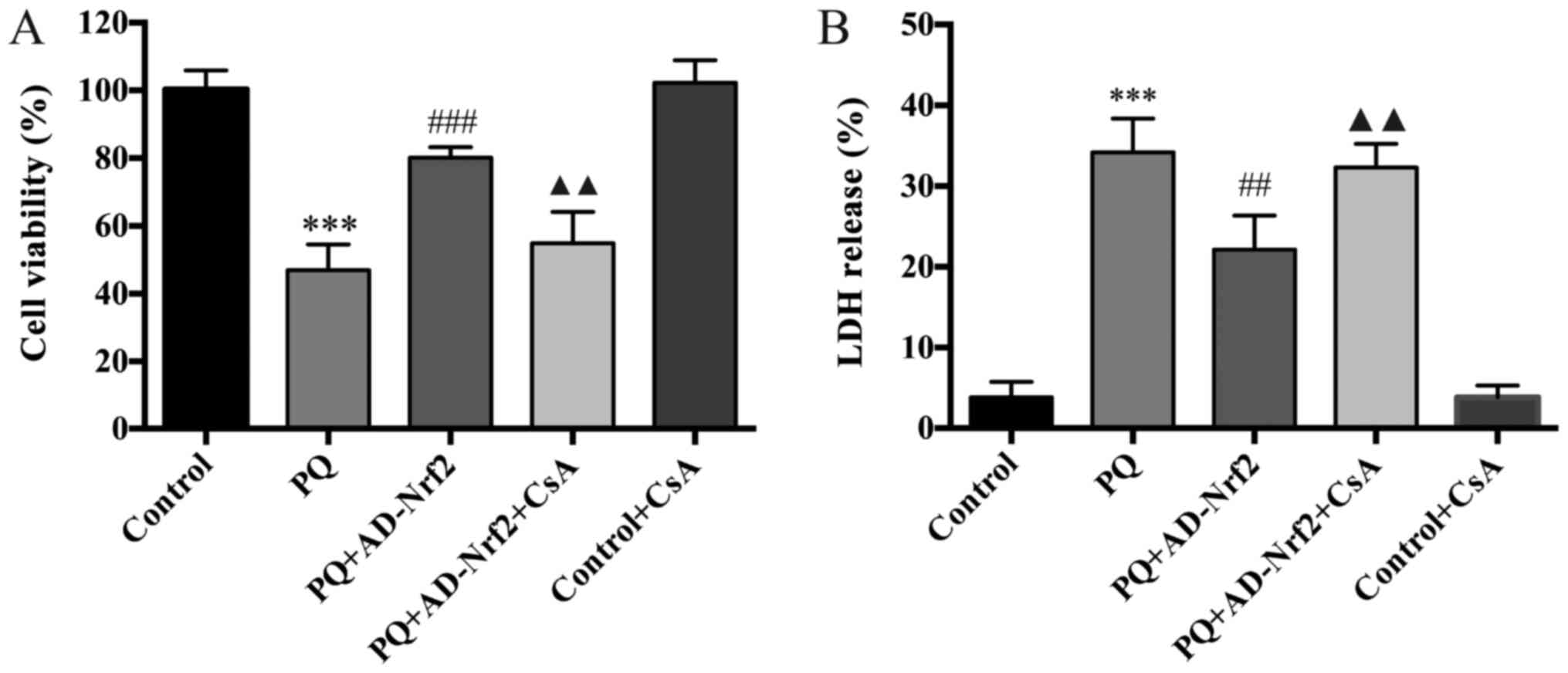
Cell viability
The results demonstrated that PQ exposure
significantly reduced A549 cell viability (P<0.001; Fig. 2A) and that PQ-induced cytotoxicity
was alleviated following Nrf2 treatment (P<0.001; Fig. 2A). However, treatment with CsA
significantly reversed the protective effects of Nrf2 on cell
viability following PQ exposure (P<0.01; Fig. 2A). No significant differences were
identified between the control and the control + CsA group.
LDH activity
The LDH activity of A549 cells was assessed to
determine PQ-induced cell injury. The LDH activity of PQ-exposed
cells was significantly increased when compared with the control
group (P<0.001; Fig. 2B).
However, treatment with Nrf2 reversed the PQ induced increase of
LDH activity (P<0.01; Fig. 2B).
Furthermore, LDH activity significantly increased in cells of the
PQ + AD-Nrf2 + CsA-treated group compared with those of the PQ +
AD-Nrf2 group (P<0.01; Fig. 2B).
No significant differences were identified between the control and
the control + CsA group.
SOD and MDA
Compared with the control group, a significant
reduction in SOD activity following PQ exposure was observed
(P<0.001; Fig. 3A). However,
treatment with AD-Nrf2 reversed this effect (P<0.01; Fig. 3A). The SOD activity of the PQ +
AD-Nrf2 + CsA-treated group was also significantly decreased
compared with those cells exposed to PQ + AD-Nrf2 treatment
(P<0.01; Fig. 3A).
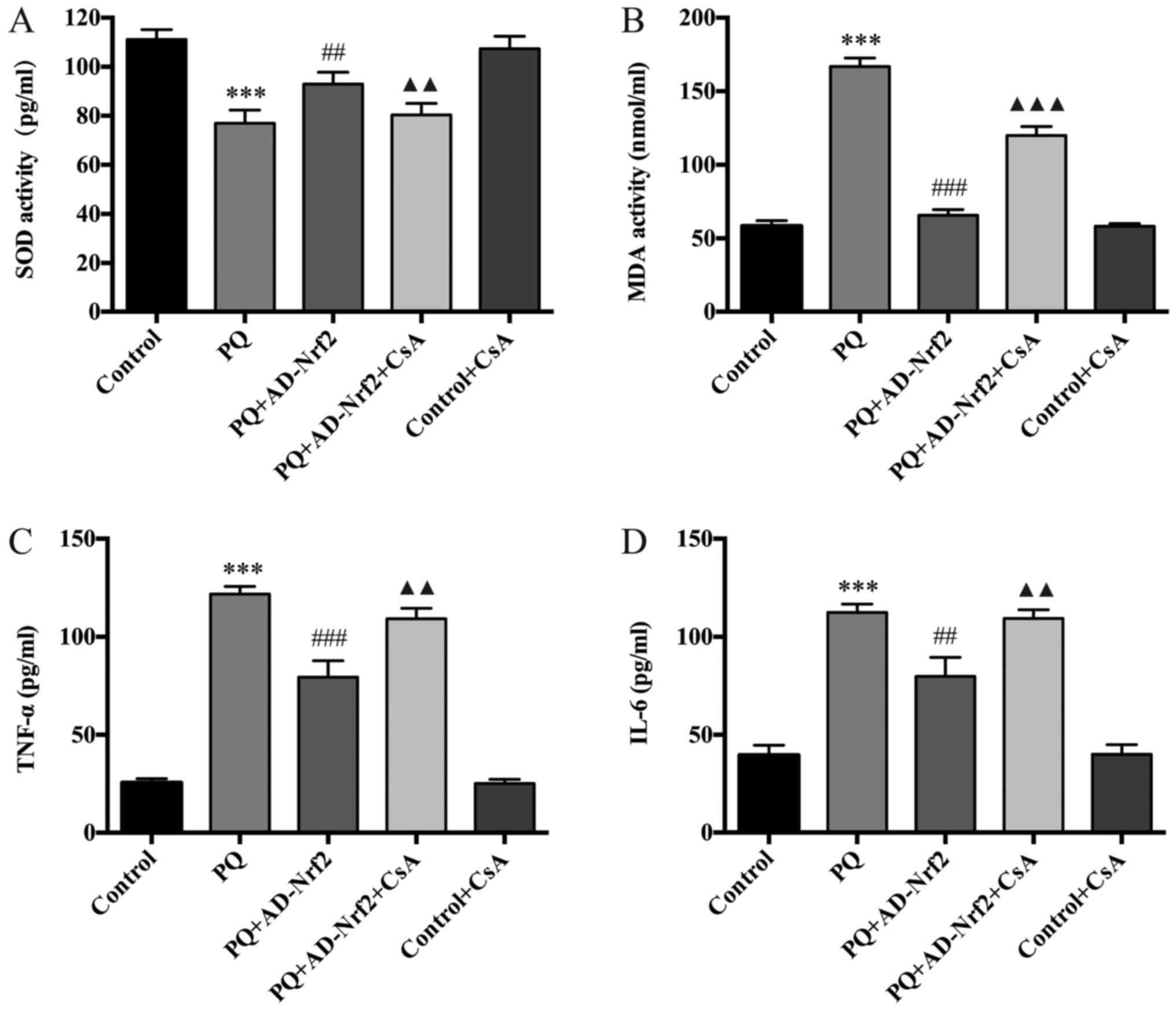 | Figure 3.Effect of Nrf2 and CsA on oxidative
stress and inflammation. (A) SOD activity, (B) MDA content, (C)
TNF-α protein expression and (D) IL-6 protein expression were
determined. Data are presented as the mean ± standard deviation
(n=4). ***P<0.001 vs. the control group;
###P<0.001 and ##P<0.01 vs. the PQ
group; ▲▲▲P<0.001 and ▲▲P<0.01 vs. the
PQ + AD-Nrf2 group. Nrf2, nuclear factor erythroid-2 related factor
2; CsA, cyclosporine A; SOD, superoxide dismutase; MDA,
malondialdehyde; TNF-α, tumor necrosis factor-α; IL-6,
interleukin-6; PQ, paraquat; AD, adenovirus. |
Cells of the PQ group exhibited a significant
increase in MDA concentration when compared with the control
(P<0.001; Fig. 3B). Whereas, the
MDA concentration of cells treated with PQ + AD-Nrf2 was
significantly reduced when compared with the PQ group (P<0.001;
Fig. 3B). However, treatment with
CsA reversed the protective effect of AD-Nrf2 (P<0.001; Fig. 3B). No significant differences were
identified in SOD activity or MDA concentration between the control
and the control + CsA group.
TNF-α and IL-6
When compared with the control group, significant
increases in TNF-α and IL-6 were observed in the PQ group
(P<0.001; Fig. 3C and D). AD-Nrf2
treatment reversed these increases (TNF-α, P<0.001; IL-6,
P<0.01; Fig. 3C and D) and the
levels of TNF-α and IL-6 in the PQ + AD-Nrf2 + CsA-treated group
were significantly increased compared with cells exposed to PQ +
AD-Nrf2 (P<0.01; Fig. 3C and D).
No significant differences were identified in the levels of TNF-α
or IL-6 expression between the control and the control + CsA
group.
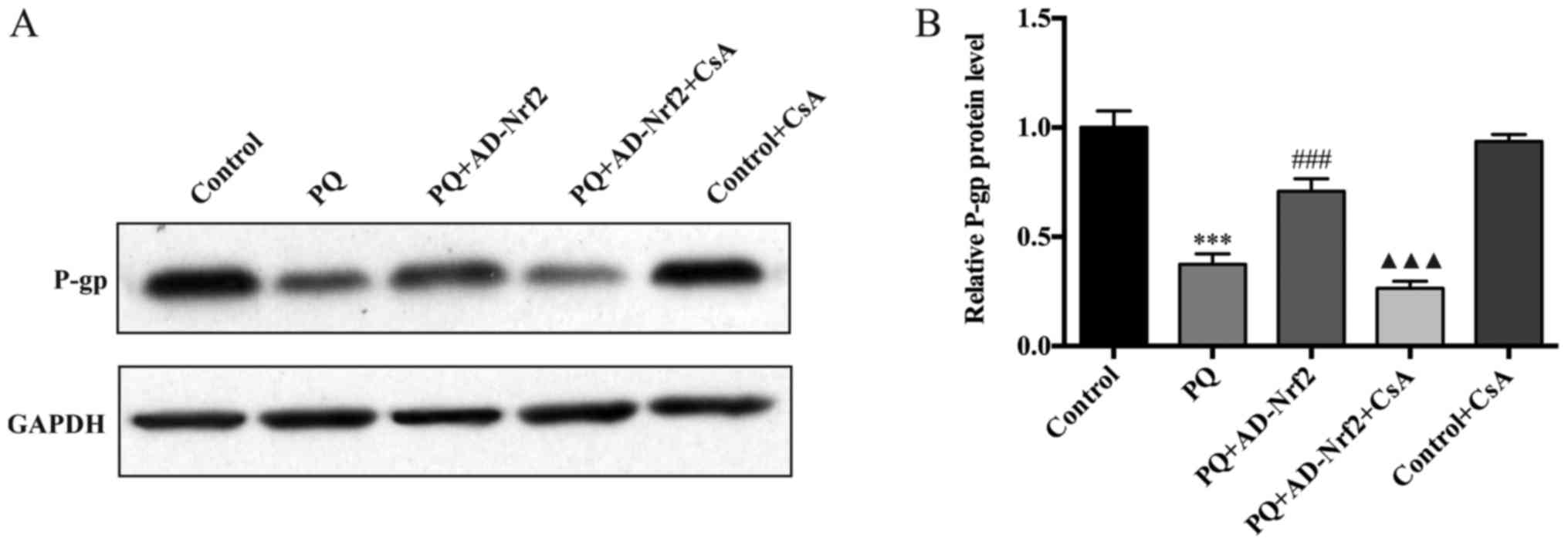
P-gp protein expression
Treatment with PQ induced a significant decrease of
P-gp protein levels when compared with the control group (Fig. 4A). However, P-gp protein expression
increased ~1.9-fold in the AD-Nrf2-treated group compared with the
PQ group (P<0.001; Fig. 4B).
Treatment with CsA was demonstrated to reverse the increase in P-gp
expression in the PQ + AD-Nrf2 + CsA group compared with the PQ +
AD-Nrf2 group (P<0.001; Fig. 4B).
No significant differences were identified in the level of P-gp
protein expression in the control group compared with the control +
CsA group.
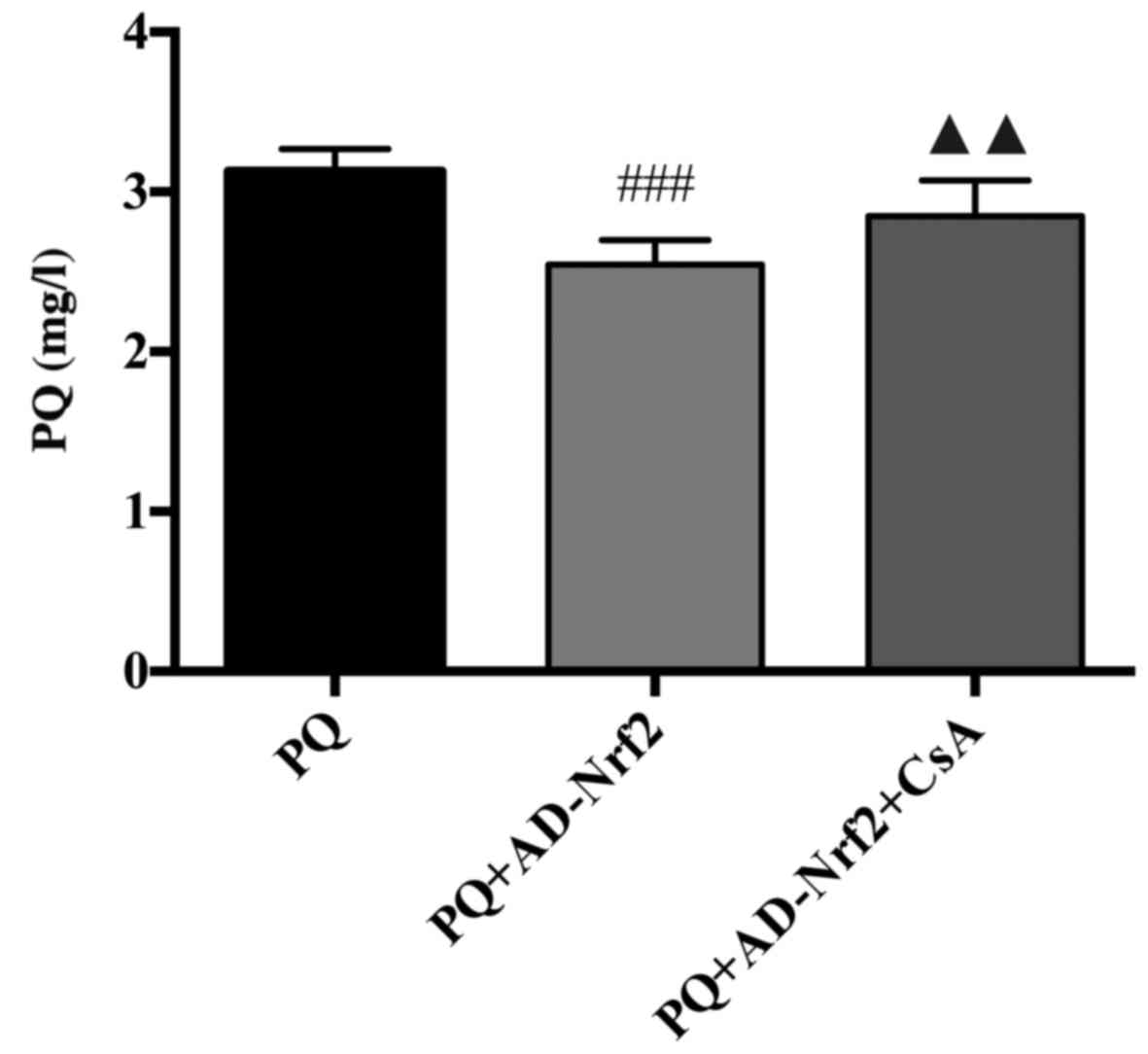
PQ concentration
The concentration of PQ in the PQ-treated group was
3.14±0.14 mg/l. Cells that received PQ + AD-Nrf2 treatment
exhibited a significant decrease in PQ concentration compared with
the PQ group (2.53±0.15; P<0.001; Fig. 5). Furthermore, cells of the PQ +
AD-Nrf2 + CsA group significantly reversed the effect of AD-Nrf2
treatment, resulting in a PQ concentration of 2.85±0.22 mg/l
(P<0.01; Fig. 5).
Discussion
PQ intoxication and the subsequent selective
accumulation of PQ molecules results in multi-organ failure and
severe pulmonary injury when in the lung (29). The current study successfully
constructed Nrf2-overexpressed A549 cells that exhibited resistance
to PQ toxicity. Nrf2 treatment was revealed to upregulate P-gp
activity and subsequently reduce the intracellular accumulation of
PQ and cell injury. However, these results were reversed following
treatment with the specific inhibitor of P-gp (CsA). To the best of
our knowledge, this is the first study to assess the role of Nrf2
treatment on P-gp expression and PQ concentration in PQ exposed
cells, which may serve as potential therapeutic targets for PQ
detoxification.
Nrf2 is a key transcription factor that has been
demonstrated to be an effective target for the prevention or
treatment of various human diseases, which include cardiovascular
diseases, neurodegenerative diseases, neuropsychiatric disorders
and cancer (30–34). Previous studies have revealed that
the upregulation of Nrf2 is critical for cytoprotection against
various types of cell injury, which include hydrogen
peroxide-induced oxidative stress and inflammation (35–37). In
another previous study, mifepristone-induced Nrf2 gene
overexpression in the lungs ameliorated PQ-induced injury by
activating the Nrf2-antioxidant response element (ARE) pathway
(38). In addition, silent
information regulator 2-related enzyme 1 was demonstrated to
trigger the Nrf2/ARE antioxidant pathway and protect against lung
injury induced by PQ poisoning (39). These studies have revealed the
important role of Nrf2 in the prevention of PQ toxicity. Consistent
with these results, the Nrf2 gene-transfected A549 cells of the
current study presented resistance to PQ toxicity, as evidenced by
an elevated cell viability, a decreased LDH activity, an improved
oxidative stress response and an attenuation of inflammation, with
decreased TNF-α and IL-6 levels following PQ challenge.
In the present study, it was demonstrated that the
overexpressed Nrf2 gene also decreased intracellular PQ
concentrations, which indicated that certain membrane transporters
may be involved in Nrf2-induced cytoprotection against PQ toxicity.
Not including phase-II detoxification enzymes, the activities of
Nrf2-regulated phase-III drug transporters represent a cellular
mechanism that protects against xenobiotics (40). Maher et al (41) revealed that the activation of the
Nrf2 pathway may stimulate the coordinated induction of hepatic
multi-drug resistance-associated proteins, which are ATP-dependent
efflux transporters that serve an important role in cellular
defense against various xenobiotics. The activities of other
membrane transporters, including organic cation transporters, are
also induced by Nrf2 (42). Since
P-gp has been extensively studied and demonstrated to be closely
associated with PQ excretion (17,43,44), the
present study further assessed whether P-gp served a role in the
Nrf2-induced inhibition of PQ accumulation and cytoprotection in
PQ-challenged A549 cells.
In the current study, P-gp protein levels
significantly increased following Nrf2 overexpression in A549
cells. Upregulated P-gp was also demonstrated to reduce
intracellular PQ concentrations, which was ameliorated following
treatment with the specific inhibitor of P-gp (CsA). Furthermore,
Nrf2-induced cytoprotection against oxidative stress and
inflammation was inhibited following CsA treatment. These results
indicate that P-gp serves a vital role in PQ transport and in the
protective effects of Nrf2 against PQ toxicity. The current study
corroborated with several previous studies (38,39). It
has been demonstrated that an increased P-gp protein level exerts
protective effects against PQ-induced toxicity in vitro and
in vivo, by inhibiting the accumulation of intracellular PQ,
indicating that PQ may be a P-gp substrate (18). Several other studies have revealed
that a decrease of P-gp activity at the blood-brain barrier leads
to the increased accumulation of neurotoxicants in the brain
(45–47).
The present study had several limitations.
Experiments of the current study were performed in vitro,
meaning that the influences of Nrf2 and P-gp in vivo are
unknown. In addition, Nrf2 overexpression in PQ poisoning may also
activate other various key efflux transporters excluding P-gp,
which include multidrug resistance-associated proteins and organic
anion transporting polypeptide 2. Thus, additional studies are
required to assess Nrf2 and the role of other transporters in
vitro and in vivo.
In summary, the current study demonstrated that the
Nrf2-mediated increase of P-gp is an important theoretical pathway
against PQ toxicity, which reduces intracellular PQ concentrations.
Therefore, the present study provides a novel therapeutic target
for PQ accumulation and toxicity within the lung.
Acknowledgements
Not applicable.
Funding
The current study was supported by grants from the
Natural Science Foundation of Zhejiang Province (grant no.
LQ14H150002), the Core Project of Medicine and Health Care Platform
in Zhejiang Province (grant no. 2016RCA021), the Traditional
Chinese Medical Project of Zhejiang Province (grant no. 2015ZZ015)
and the Key Construction Academic Subject (Medical Innovation) of
Zhejiang Province (grant no. 11-CX26).
Availability of data and materials
All data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GLH and ZQL designed the study. BW prepared the
manuscript, participated in experimental design and guided students
to complete experiments and statistical anlaysis. HXL, JL, YJG,
YHT, ZJC and LFH collected the data. GJZ analyzed the data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yao R, Cao Y, He YR, Lau WB, Zeng Z and
Liang ZA: Adiponectin attenuates lung fibroblasts activation and
pulmonary fibrosis induced by paraquat. PLoS One. 10:e01251692015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cherukuri H, Pramoda K, Rohini D, Thunga
G, Vijaynarayana K, Sreedharan N, Varma M and Pandit V:
Demographics, clinical characteristics and management of herbicide
poisoning in tertiary care hospital. Toxicol Int. 21:209–213. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seok SJ, Gil HW, Jeong DS, Yang JO, Lee EY
and Hong SY: Paraquat intoxication in subjects who attempt suicide:
Why they chose paraquat. Korean J Intern Med. 24:247–251. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Senarathna L, Eddleston M, Wilks MF,
Woollen BH, Tomenson JA, Roberts DM and Buckley NA: Prediction of
outcome after paraquat poisoning by measurement of the plasma
paraquat concentration. QJM. 102:251–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gil HW, Hong JR, Jang SH and Hong SY:
Diagnostic and therapeutic approach for acute paraquat
intoxication. J Korean Med Sci. 29:1441–1449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koepsell H: Polyspecific organic cation
transporters: Their functions and interactions with drugs. Trends
Pharmacol Sci. 25:375–381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He X, Wang L, Szklarz G, Bi Y and Ma Q:
Resveratrol inhibits paraquat-induced oxidative stress and
fibrogenic response by activating the nuclear factor erythroid
2-related factor 2 pathway. J Pharmacol Exp Ther. 342:81–90. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
She X, Hong G, Tan J, Zhao G, Li M and Lu
Z: The protective effect of ulinastatin on paraquat-induced injury
in HK-2 cells and the underlying mechanisms. Zhonghua Lao Dong Wei
Sheng Zhi Ye Bing Za Zhi. 33:501–506. 2015.(In Chinese). PubMed/NCBI
|
|
9
|
Yao R, Zhou Y, He Y, Jiang Y, Liu P, Ye L,
Zheng Z, Lau WB, Cao Y and Zeng Z: Adiponectin protects against
paraquat-induced lung injury by attenuating oxidative/nitrative
stress. Exp Ther Med. 9:131–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kimura M, Yamamoto T, Zhang J, Itoh K, Kyo
M, Kamiya T, Aburatani H, Katsuoka F, Kurokawa H, Tanaka T, et al:
Molecular basis distinguishing the DNA binding profile of Nrf2-Maf
heterodimer from that of Maf homodimer. J Biol Chem.
282:33681–33690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aleksunes LM and Manautou JE: Emerging
role of Nrf2 in protecting against hepatic and gastrointestinal
disease. Toxicol Pathol. 35:459–473. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou SF: Structure, function and
regulation of P-glycoprotein and its clinical relevance in drug
disposition. Xenobiotica. 38:802–832. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bosquillon C: Drug transporters in the
lung-do they play a role in the biopharmaceutics of inhaled drugs?
J Pharm Sci. 99:2240–2255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lacher SE, Gremaud JN, Skagen K, Steed E,
Dalton R, Sugden KD, Cardozo-Pelaez F, Sherwin CM and Woodahl EL:
Absence of P-glycoprotein transport in the pharmacokinetics and
toxicity of the herbicide paraquat. J Pharmacol Exp Ther.
348:336–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang H, Lu-Bo Y and Haddad GG: A
Drosophila ABC transporter regulates lifespan. PLoS Genet.
10:e10048442014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lehmann T, Kohler C, Weidauer E, Taege C
and Foth H: Expression of MRP1 and related transporters in human
lung cells in culture. Toxicology. 167:59–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Silva R, Palmeira A, Carmo H, Barbosa DJ,
Gameiro M, Gomes A, Paiva AM, Sousa E, Pinto M, Bastos Mde L and
Remião F: P-glycoprotein induction in Caco-2 cells by newly
synthetized thioxanthones prevents paraquat cytotoxicity. Arch
Toxicol. 89:1783–1800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dinis-Oliveira RJ, Remiao F, Duarte JA,
Ferreira R, Sanchez Navarro A, Bastos ML and Carvalho F:
P-glycoprotein induction: An antidotal pathway for paraquat-induced
lung toxicity. Free Radic Biol Med. 41:1213–1224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Itoh K, Tong KI and Yamamoto M: Molecular
mechanism activating Nrf2-Keap1 pathway in regulation of adaptive
response to electrophiles. Free Radic Biol Med. 36:1208–1213. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kobayashi A, Kang MI, Okawa H, Ohtsuji M,
Zenke Y, Chiba T, Igarashi K and Yamamoto M: Oxidative stress
sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to
regulate proteasomal degradation of Nrf2. Mol Cell Biol.
24:7130–7139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong GL, Liu JM, Zhao GJ, Wang L, Liang G,
Wu B, Li MF, Qiu QM and Lu ZQ: The reversal of paraquat-induced
mitochondria-mediated apoptosis by cycloartenyl ferulate, the
important role of Nrf2 pathway. Exp Cell Res. 319:2845–2855. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li S, Zhao G, Chen L, Ding Y, Lian J, Hong
G and Lu Z: Resveratrol protects mice from paraquat-induced lung
injury: The important role of SIRT1 and NRF2 antioxidant pathways.
Mol Med Rep. 13:1833–1838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu H, Chang Z, Han W, Wang L and Hong G:
Curcumin reduces paraquat-induced oxidative injury in A549 cells by
activation of the Nrf2-ARE pathway. Zhonghua Lao Dong Wei Sheng Zhi
Ye Bing Za Zhi. 32:44–49. 2014.(In Chinese). PubMed/NCBI
|
|
25
|
Adachi T, Nakagawa H, Chung I, Hagiya Y,
Hoshijima K, Noguchi N, Kuo MT and Ishikawa T: Nrf2-dependent and
-independent induction of ABC transporters ABCC1, ABCC2, and ABCG2
in HepG2 cells under oxidative stress. J Exp Ther Oncol. 6:335–348.
2007.PubMed/NCBI
|
|
26
|
Cen J, Zhang L, Liu F, Zhang F and Ji B:
Long-term alteration of reactive oxygen species led to multidrug
resistance in MCF-7 cells. Oxid Med Cell Longev. 2016:70534512016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J, Zhu Y, Li F, Zhang G, Shi J, Ou R,
Tong Y, Liu Y, Liu L, Lu L and Liu Z: Spica prunellae and its
marker compound rosmarinic acid induced the expression of efflux
transporters through activation of Nrf2-mediated signaling pathway
in HepG2 cells. J Ethnopharmacol. 193:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghanem CI, Rudraiah S, Bataille AM, Vigo
MB, Goedken MJ and Manautou JE: Role of nuclear factor-erythroid
2-related factor 2 (Nrf2) in the transcriptional regulation of
brain ABC transporters during acute acetaminophen (APAP)
intoxication in mice. Biochem Pharmacol. 94:203–211. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gawarammana IB and Buckley NA: Medical
management of paraquat ingestion. Br J Clin Pharmacol. 72:745–757.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai Y, Wang X, Zhao S, Ma C, Cui J and
Zheng Y: Sulforaphane protects against cardiovascular disease via
Nrf2 activation. Oxid Med Cell Longev. 2015:4075802015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Oliveira MR, Nabavi SF, Habtemariam S,
Erdogan Orhan I, Daglia M and Nabavi SM: The effects of baicalein
and baicalin on mitochondrial function and dynamics: A review.
Pharmacol Res. 100:296–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Oliveira MR: Phloretin-induced
cytoprotective effects on mammalian cells: A mechanistic view and
future directions. Biofactors. 42:13–40. 2016.PubMed/NCBI
|
|
33
|
de Oliveira MR, Nabavi SF, Manayi A,
Daglia M, Hajheydari Z and Nabavi SM: Resveratrol and the
mitochondria: From triggering the intrinsic apoptotic pathway to
inducing mitochondrial biogenesis, a mechanistic view. Biochim
Biophys Acta. 1860:727–745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oliveira MR, Nabavi SF, Daglia M,
Rastrelli L and Nabavi SM: Epigallocatechin gallate and
mitochondria-A story of life and death. Pharmacol Res. 104:70–85.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hichor M, Sampathkumar NK, Montanaro J,
Borderie D, Petit PX, Gorgievski V, Tzavara ET, Eid AA, Charbonnier
F, Grenier J and Massaad C: Paraquat induces peripheral myelin
disruption and locomotor defects: Crosstalk with LXR and wnt
pathways. Antioxid Redox Signal. 27:168–183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Oliveira MR, de Souza ICC and Furstenau
CR: Carnosic acid induces anti-inflammatory effects in
paraquat-treated SH-SY5Y cells through a mechanism involving a
crosstalk between the Nrf2/HO-1 axis and NF-kB. Mol Neurobiol.
55:890–897. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shinjo T, Tanaka T, Okuda H, Kawaguchi AT,
Oh-Hashi K, Terada Y, Isonishi A, Morita-Takemura S, Tatsumi K,
Kawaguchi M and Wanaka A: Propofol induces nuclear localization of
Nrf2 under conditions of oxidative stress in cardiac H9c2 cells.
PLoS One. 13:e01961912018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hong GL, Cai QQ, Tan JP, Jiang XZ, Zhao
GJ, Wu B, Li MF, Qiu QM and Lu ZQ: Mifepristone-inducible
recombinant adenovirus attenuates paraquat-induced lung injury in
rats. Hum Exp Toxicol. 34:32–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding YW, Zhao GJ, Li XL, Hong GL, Li MF,
Qiu QM, Wu B and Lu ZQ: SIRT1 exerts protective effects against
paraquat-induced injury in mouse type II alveolar epithelial cells
by deacetylating NRF2 in vitro. Int J Mol Med. 37:1049–1058. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu C, Li CY and Kong AN: Induction of
phase III and III drug metabolism/transport by xenobiotics. Arch
Pharm Res. 28:249–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maher JM, Dieter MZ, Aleksunes LM, Slitt
AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, et
al: Oxidative and electrophilic stress induces multidrug
resistance-associated protein transporters via the nuclear
factor-E2-related factor-2 transcriptional pathway. Hepatology.
46:1597–1610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jeddi F, Soozangar N, Sadeghi MR, Somi MH,
Shirmohamadi M, Eftekhar-Sadat AT and Samadi N: Nrf2 overexpression
is associated with P-glycoprotein upregulation in gastric cancer.
Biomed Pharmacother. 97:286–292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zerin T, Kim YS, Hong SY and Song HY:
Protective effect of methylprednisolone on paraquat-induced A549
cell cytotoxicity via induction of efflux transporter,
P-glycoprotein expression. Toxicol Lett. 208:101–107. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
She XR, Tian X, Fan XK, Hong GL, Zhao GJ,
Li MF and Lu ZQ: The effects of P-glycoprotein expression induced
by ulinastatin on HK-2 cells damage induced by paraquat. Zhonghua
Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 34:805–809. 2016.(In
Chinese). PubMed/NCBI
|
|
45
|
Bartels AL, Kortekaas R, Bart J, Willemsen
AT, de Klerk OL, de Vries JJ, van Oostrom JC and Leenders KL:
Blood-brain barrier P-glycoprotein function decreases in specific
brain regions with aging: A possible role in progressive
neurodegeneration. Neurobiol Aging. 30:1818–1824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee G and Bendayan R: Functional
expression and localization of P-glycoprotein in the central
nervous system: Relevance to the pathogenesis and treatment of
neurological disorders. Pharm Res. 21:1313–1330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Furuno T, Landi MT, Ceroni M, Caporaso N,
Bernucci I, Nappi G, Martignoni E, Schaeffeler E, Eichelbaum M,
Schwab M and Zanger UM: Expression polymorphism of the blood-brain
barrier component P-glycoprotein (MDR1) in relation to Parkinson's
disease. Pharmacogenetics. 12:529–534. 2002. View Article : Google Scholar : PubMed/NCBI
|