Introduction
Bisphosphonates (BPs) accelerate osteoclast
apoptosis. The mechanism comprises strong inhibition of bone
resorption (1,2). BPs are effective for the treatment of
osteoporosis, Paget's disease, multiple myeloma, hypercalcemia of
malignancy and bone metastases from breast cancer and prostate
cancer (3–5). However, BP-related osteonecrosis of the
jaws (BRONJ) is a serious problem in patients treated with BPs
(6,7). The risk factors for BRONJ comprise
drug-associated, local and demographic/systemic factors.
Drug-associated risk factors include the potency of the specific
BP. Zoledronic acid (ZOL) is the most potent BP (8). It also carries the highest incidence of
BRONJ (9). The drugs that have
primarily been used in evaluated studies (10,11) on
BRONJ arising in rodents under BP therapy following tooth
extraction were ZOL and alendronate (12). Local risk factors include
dentoalveolar surgery, tooth extraction, periapical surgery and
periodontal surgery including osseous injury. Systemic risk factors
include corticosteroid therapy, diabetes and chemotherapeutic drugs
(13). A number of animal models of
BRONJ associated with risk factors such as corticosteroid therapy
(14), vitamin D deficiency
(15) and diabetes (16) combined with tooth extraction have
also been established.
Aging is an additional significant risk factor for
the development of BRONJ (17–19).
Bone aging is associated with oxidative stress (OS) as demonstrated
in both human studies and animal models (20–23). OS
occurs as result of overproduction of reactive oxygen species (ROS)
that is not balanced by an adequate level of antioxidants (24). BP treatment can produce OS (25), and continued local inflammation,
either with or without an associated infective process (26–29), can
produce ROS. Khandelwal et al (30) previously reported that treatment with
ZOL increased OS in the human breast cancer cell line MCF-7, and
this increase in OS was reversed by antioxidants. The authors
reported that ZOL can induce a dose-dependent but irreversible
autophagy by its effect on the mevalonate pathway and OS (30). It has also been demonstrated that OS
is associated with osteonecrosis (31–33).
Ichiseki et al (34) have
demonstrated that a single intraperitoneal injection of the
pro-oxidant DL-buthionine-(S,R)-sulfoximine (BSO) (500 mg/kg), an
oxidative stressor, in rats was by itself sufficient to induce
osteonecrosis. Osteonecrosis in the femoral head was confirmed at 5
(2 of 20 rats, 10%), 7 (7 of 20 rats, 35%), and 14 days following
BSO injection (8 of 20 rats, 40%) (34). To the best of our knowledge, no
reports to date have described animal models of BRONJ associated
with OS. To elucidate the mechanisms of the onset of BRONJ, the
present study focused on OS and investigated the effects of ZOL and
BSO in a short term on healing of surgically created palatal
defects.
Materials and methods
Animal handling
The present study was approved by the Ethics
Committee of the Hyogo College of Medicine (Hyogo, Japan; approval
number 16-078). Male 5-week-old C57BL/6J mice (n=40; body weight,
18–21 g) were obtained from Japan SLC, Inc. (Hamamatsu, Japan). The
animals were housed in a temperature-, humidity-, and
light-controlled room (23±3°C; 55±15%; 12-h light-dark cycle). Food
and water were available ad libitum.
Agents
ZOL [2-(imidazol-l-yl)-1-hydroxyethylidene-1,1-BP]
and BSO (DL-buthionine-(S,R)-sulfoximine) were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Experimental methods and design
Following 1 week of acclimatization, the 6-week-old
mice were randomly divided into four groups (n=5 each) and treated
with or without ZOL and with or without BSO (experimental groups:
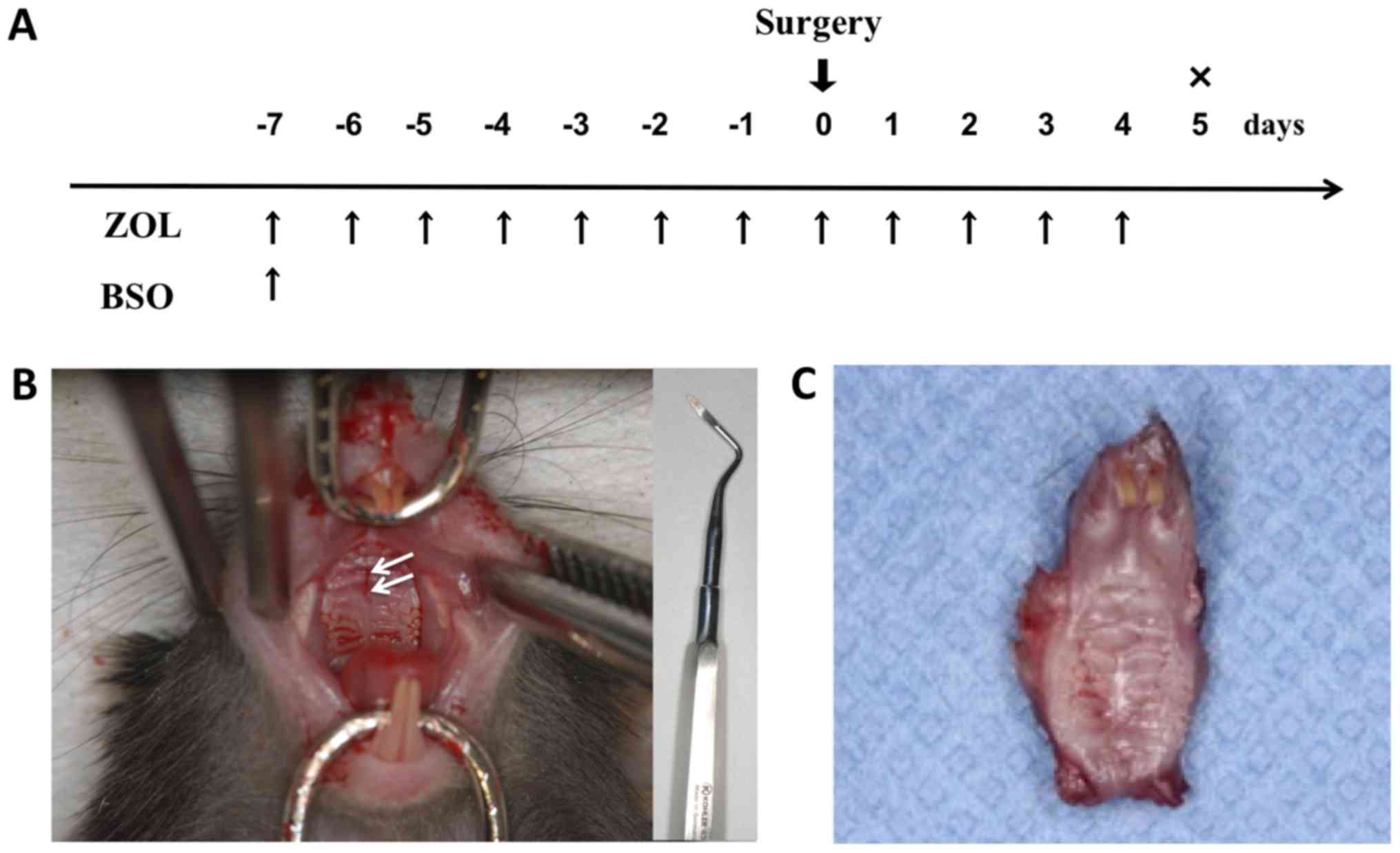
ZOL, BSO, and ZOL+BSO; control group: saline solution; Fig. 1A). A penetrating injury of the
midline palate was surgically created using a root elevator under
anesthesia with 2% isoflurane (Pfizer Japan, Inc., Tokyo, Japan)
(Fig. 1B). Dentoalveolar surgery is
a risk factor for the development of BRONJ in patients receiving
BPs (6). Therefore, tooth extraction
is commonly used to induce osteonecrosis in animal models. In the
present study, a penetrating injury of the midline palate was
surgically created using a root elevator as a less invasive surgery
than tooth extraction to minimize the suffering or distress of
eating with missing teeth. No problems were associated with the
presence of root fragments in the extraction socket. ZOL (250
µg/kg/day) and saline solution at the same dosage volume were
injected intraperitoneally from 7 days prior to the surgical
treatment to 4 days following the surgical treatment. The dosage
and duration of administration of ZOL was based on the protocols
described previously by Kobayashi et al (35). BSO (500 µg/kg/day) was administered 7
days prior to the surgical treatment as a single intraperitoneal
injection. The total maxillae were then harvested en bloc 5
days following the surgical treatment (Fig. 1C).
Bone histomorphometric analysis
To determine the bone histomorphometric parameters
of mouse femurs, the femurs from 4 mice in each group were
harvested at the same time as the total maxillae, stored in 70%
ethanol at 4°C, and analyzed using a micro-CT scanner (Scan
Xmate-L090; Comscan Techno Co., Ltd., Kanagawa, Japan). Scanning
was conducted at 75 kV and 105 mA with a spatial resolution of
~9.073 mm/pixel. For quantitative analysis, the bone volume
(BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N),
and trabecular separation (Tb.Sp) were determined using TRI/3D-BON
software version R9 (RATOC System Engineering Co., Ltd., Tokyo,
Japan).
Measurement of serum 8-OHdG
Blood samples (0.8 ml) were collected from the left
ventricle under anesthesia with 2% isoflurane for serum analysis 6
h following treatment with or without ZOL and with or without BSO
(n=5), and then they were euthanized by cervical dislocation. The
8-hydroxy-2′-deoxyguanosine (8-OHdG) level has been widely analyzed
as a marker of an individual's OS (36). The serum concentration of 8-OHdG was
measured using a highly sensitive ELISA kit (Highly Sensitive
8-OHdG Check ELISA kit; cat. no. KOG-HS10/E; Japan Institute for
the Control of Aging; Nikken SEIL Co., Ltd., Shizuoka, Japan)
according to the manufacturer's protocol. The absorbance at 405 nm
was determined using a microplate reader (Benchmark Plus™
Microplate Spectrophotometer; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Histopathology
The mice were euthanized by cervical dislocation
under anesthesia, induced by the inhalation of 5% isoflurane. The
maxillae were harvested from the control, ZOL, BSO and ZOL+BSO
groups. The tissue specimens were immediately placed in 10% neutral
buffered formalin at room temperature for 24 h and decalcified in
10% ethylenediaminetetraacetic acid at room temperature for 2
weeks. Paraffin sections (4-µm-thick) were cut using conventional
methods and stained with hematoxylin and eosin (H&E). Sections
were stained with hematoxylin for 5 min, washed with distilled
water, dipped in 0.1% ammonium solution several times and washed
again with 100% alcohol. The samples were stained with 1% eosin
solution for 20 sec at room temperature. The region of interest
(ROI) corresponded to the palatal bone including the surgically
perforated part and alveolar bone. The total numbers of osteocyte
lacunae and empty osteocytic lacunae were counted in four
non-overlapping defined ROIs at a magnification of ×200 under light
microscopy. Paraffin sections were cut again and stained with
Alcian blue for 30 min at room temperature to identify cartilage
and bone under light microscopy (magnification, ×200).
Tartrate-resistant acid phosphatase (TRAP) staining
was performed as described previously (16). Briefly, samples were placed in 0.2 M
acetate buffer [0.2 M sodium acetate and 50 mM L(+) tartaric acid
in double-distilled water; pH 5.0] for 20 min at room temperature.
The sections were then incubated with 0.5 mg/ml naphthol AS-MX
phosphate (Sigma-Aldrich; Merck KGaA) and 1.1 mg/ml Fast Red TR
Salt (Sigma-Aldrich; Merck KGaA) in 0.2 M acetate buffer for 1–4 h
at 37°C until the osteoclasts appeared bright red (37). The number of multinuclear
TRAP-positive cells was counted in four non-overlapping defined
ROIs at a magnification of ×200 under light microscopy.
For the canaliculi structure analysis, the bone
sections were incubated at room temperature for 30 min in silver
staining solution in the dark. The silver staining solution was
prepared by combining silver nitrate (2 volumes of 50% aqueous
solution; Wako Pure Chemical Industries, Ltd., Osaka, Japan) and
formic acid (1 volume of 1% solution containing 2% gelatin). The
sections were washed in distilled water and transferred to a 5%
aqueous sodium thiosulfate solution at room temperature for 5 min.
The number of canaliculi per osteocyte lacuna (N.Ot.Ca/Ot.Lc.) were
counted in 10 cells in 4 randomly selected non-overlapping defined
ROIs at ×400 magnification under light microscopy.
Dynamic calcein labeling
At 9 and 2 days prior to euthanasia, 5 mice in each
group were administered an intraperitoneal injection of 10 mg/kg
calcein (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) for
double labeling. Non-fixed frozen sections (6-µm thickness) from
the maxilla were prepared with an adhesive film and a disposable
tungsten carbide blade [Cryofilm type 2C(9) and SL-T30 (UF),
respectively; SECTION LAB Co., Ltd., Hiroshima, Japan] according to
the method described by Kawamoto T and Kawamoto K (38), and calcein labeling was assessed. An
Olympus fluorescent microscope (Olympus Corporation, Tokyo, Japan)
was used, and the calcein double labels were analyzed with an
excitation wavelength of 485 nm and an emission wavelength of 510
nm at a magnification of ×200. The mineral apposition rate (MAR;
µm/day), defined as the distance between the midpoints of the
double label divided by the number of days between calcein
injections, was also measured (39).
Statistical analysis
All data are expressed as mean + or ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance followed by Bonferroni's multiple comparison
test (SPSS version 22.0 software; IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Body weight
There were no significant differences in body weight
among the control, ZOL, BSO and ZOL+BSO groups during the
experimental period (data not shown).
Macroscopic evaluation
In all groups, the surgical perforation exhibited
complete mucosal closure by the end of the study. No open wound or
bone exposure was noted in any group.
Bone histomorphometric analysis of the
distal femur
Bone histomorphometric analysis was used to
determine BV/TV, Tb.Th, Tb.N, and Tb.Sp (Table I). In the inter-group comparison,
significant differences in Tb.N (ZOL group, 4.45±0.65 1/mm; BSO
group, 3.07±0.42 1/mm; Table I) and
Tb.Sp (ZOL group, 197.40±37.41 µm; BSO group, 298.43±41.63 µm;
Table I) were observed between the
ZOL and BSO groups.
 | Table I.Bone histomorphometric analysis of
the distal femur. |
Table I.
Bone histomorphometric analysis of
the distal femur.
|
| Parameter |
|---|
|
|
|
|---|
| Group | BV/TV (%) | Tb.Th (µm) | Tb.N (1/mm) | Tb.Sp (µm) |
|---|
| Control | 11.33±2.56 | 31.21±3.36 | 3.61±0.53 | 250.30±46.68 |
| ZOL | 14.05±2.48 | 31.52±1.85 |
4.45±0.65a |
197.40±37.41a |
| BSO | 9.60±1.23 | 31.26±1.37 | 3.07±0.42 | 298.43±41.63 |
| ZOL+BSO | 12.10±2.23 | 31.13±1.64 | 3.88±0.56 | 230.47±39.76 |
Measurement of serum 8-OHdG
The 8-OHdG level was significantly increased by BSO
treatment (control group, 0.171±0.037 ng/ml; BSO group, 0.213±0.033
ng/ml; Table II).
 | Table II.Changes in serum 8-OHdG
concentration. |
Table II.
Changes in serum 8-OHdG
concentration.
| Group | 8-OHdG (ng/ml) |
|---|
| Control | 0.171±0.037 |
| ZOL | 0.169±0.028 |
| BSO |
0.213±0.033a |
| ZOL+BSO | 0.201±0.036 |
Histological evaluation
Sections of maxilla including the surgically
perforated part were stained with H&E and examined
histologically in all four groups. Complete epithelial coverage was
noted in all groups. Wound healing in the palate was assessed to
investigate the effect of ZOL and BSO on bone healing (Fig. 2A). The cartilage was less completely
formed around the surgically perforated part in the treatment
groups, especially in the BSO group, than in the control group
(Fig. 2B and C). Fibrous connective
tissue was present around the surgical perforation in the treatment
groups, especially in the ZOL+BSO group (Fig. 2B). Areas of necrotic bone with empty
osteocyte lacunae were observed in the palatal bone around the
surgical perforation in the ZOL+BSO group (Fig. 2D).
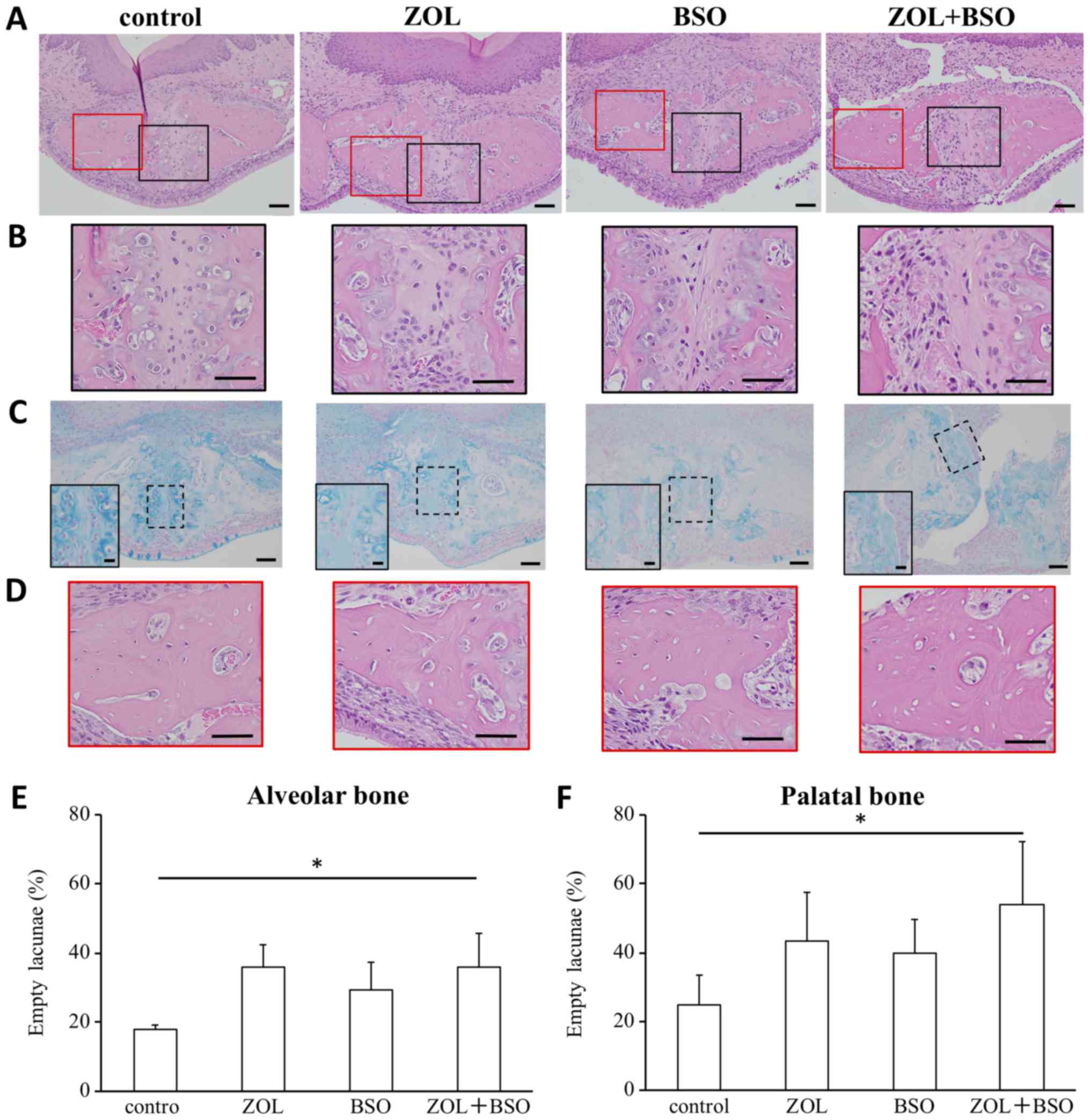 | Figure 2.Analysis of palatal bone including
the surgical perforation and alveolar bone. (A) Photomicrographs of
the surgical perforation. Hematoxylin and eosin stain; original
magnification, ×200. Scale bar, 50 µm. (B) Photomicrographs of
magnified black square area in (A). Scale bar, 50 µm. (C)
Photomicrographs of the surgical perforation. Alcian blue staining;
original magnification, ×200. Scale bar, 50 µm. Insets present the
higher magnification of the dotted square area. Scale bar, 10 µm.
(D) Photomicrographs of magnified red square area in (A). Scale
bar, 50 µm. The ratio of the number of empty osteocytic lacunae to
the total number of osteocyte lacunae was used to calculate the
percentage of dead osteocytes in the (E) alveolar and (F) palatal
bone. The numbers of total osteocyte lacunae and empty osteocytic
lacunae were counted in four non-overlapping defined regions of
interest at a magnification of ×200. *P<0.05. ZOL, zoledronic
acid; BSO, DL-buthionine-(S,R)-sulfoximine. |
The ratio of the number of empty osteocytic lacunae
to the total number of osteocyte lacunae was used to calculate the
percentage of dead osteocytes in the alveolar bone and palatal
bone. The number of empty osteocyte lacunae in the alveolar bone
(non-surgery area) and palatal bone (surgery area) was evaluated
(Fig. 2E and F). The number of empty
osteocyte lacunae in the alveolar bone was significantly increased
by treatment with ZOL+BSO (proportion of empty osteocyte lacunae
among total osteocyte lacunae: control group, 18.0±1.1%; ZOL group,
36.1±6.3%; BSO group, 29.4±7.9%; ZOL+BSO group, 35.9±9.8%). The
number of empty osteocyte lacunae in the palatal bone was
significantly increased by treatment with ZOL+BSO (proportion of
empty osteocyte lacunae among total osteocyte lacunae: control
group, 24.8±9.7%; ZOL group, 43.5±15.6%; BSO group, 39.7±11.1%;
ZOL+BSO group, 53.9±20.5%). More empty osteocyte lacunae were
present in the palatal bone around the surgical perforation than in
the alveolar bone in all groups.
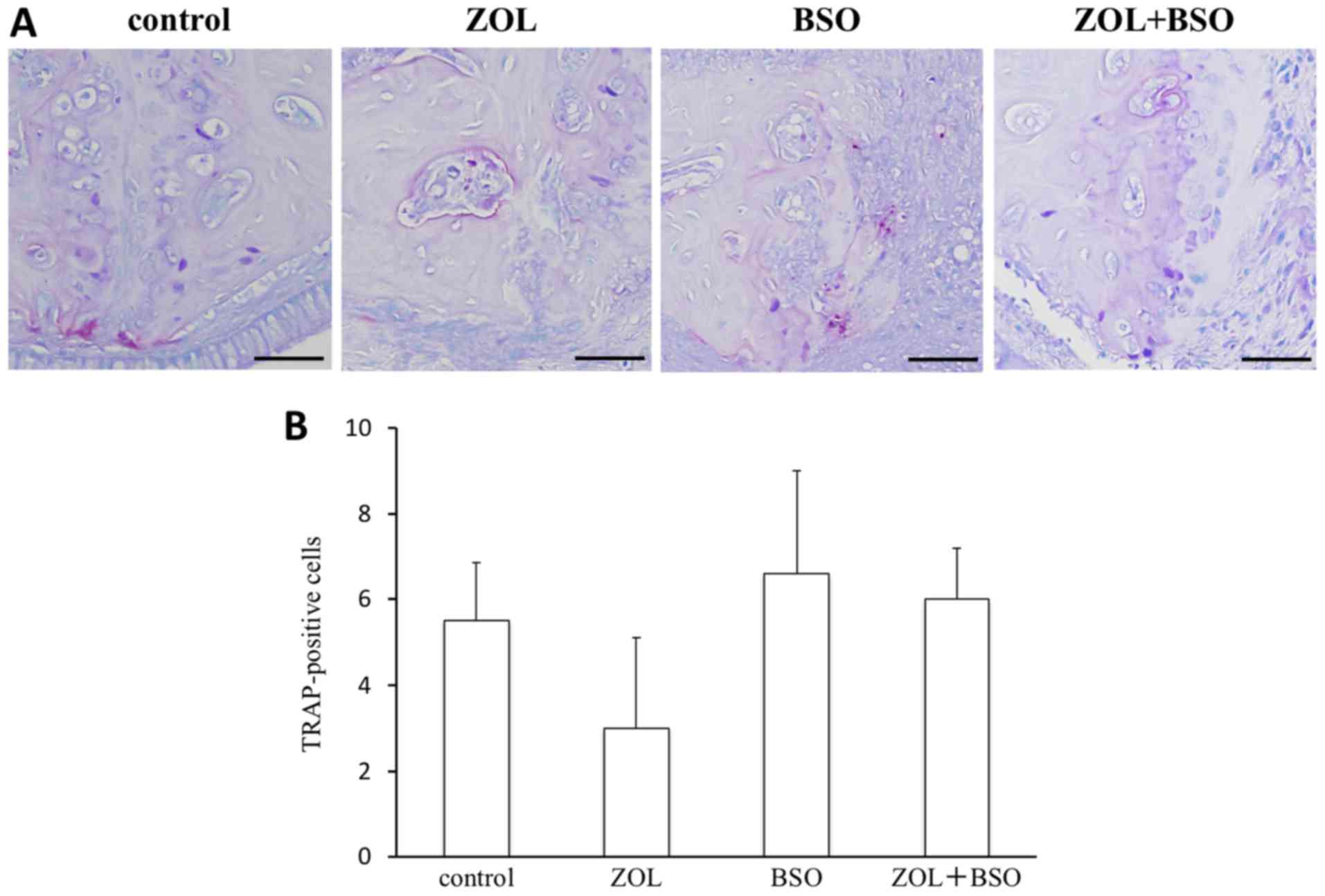
Osteoclast activity
TRAP-positive osteoclasts were present on the bone
surface of the palatal bone (Fig.
3A). The number of TRAP-positive osteoclasts was decreased by
ZOL treatment and increased by BSO treatment (number of
TRAP-positive cells: Control group, 5.5±1.4; ZOL group, 3.0±2.1;
BSO group, 6.6±2.4; ZOL+BSO group, 6.0±1.2). There were no
significant differences among the groups (Fig. 3B).
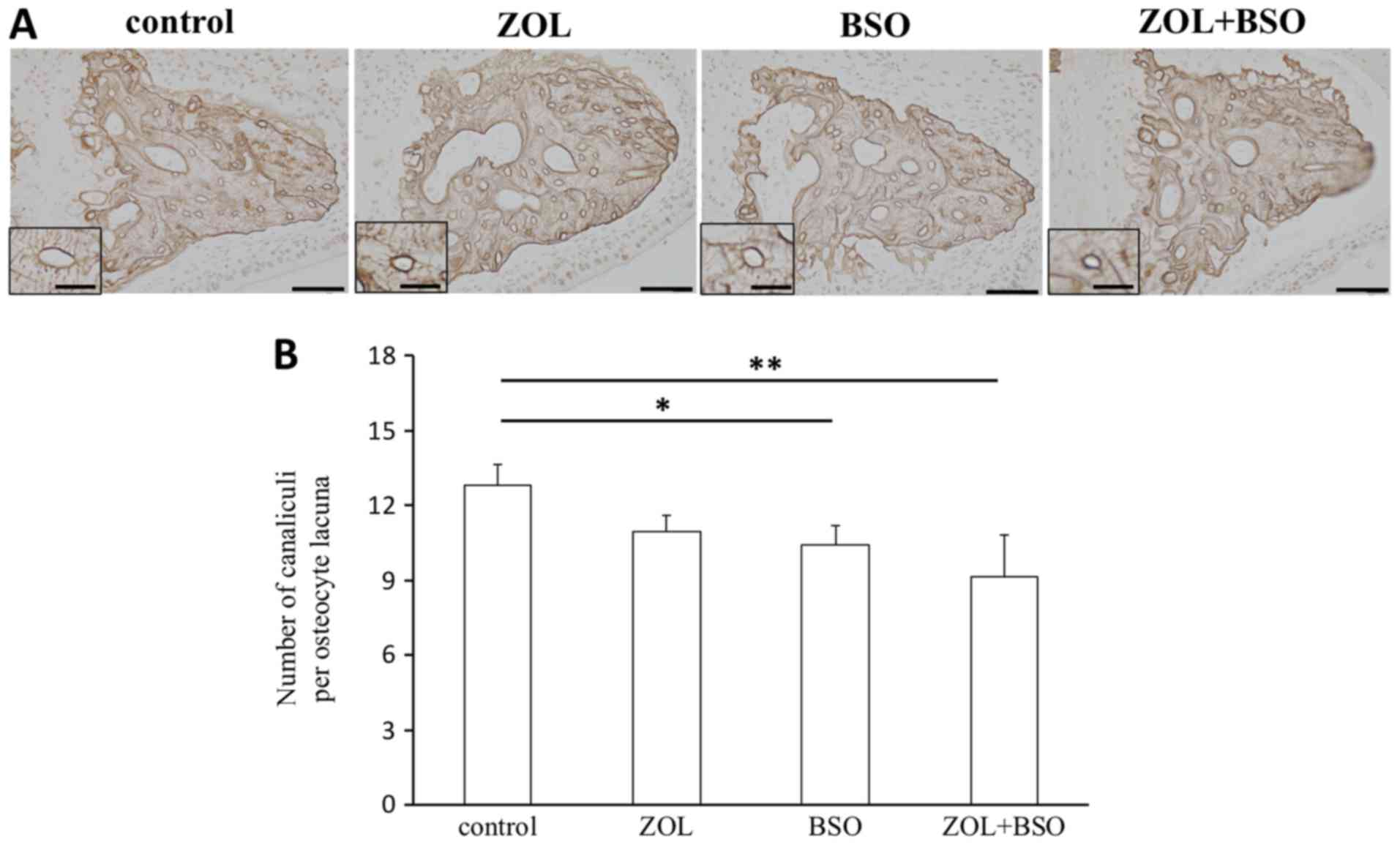
Osteocytic canalicular morphology
AgNOR staining was performed to investigate
morphological changes in the palatal bone (Fig. 4A). The N.Ot.Ca/Ot.Lc. was
significantly decreased by BSO treatment and ZOL+BSO treatment
compared with the control. N.Ot.Ca/Ot.Lc. was also markedly
decreased by ZOL treatment (Fig.
4B).
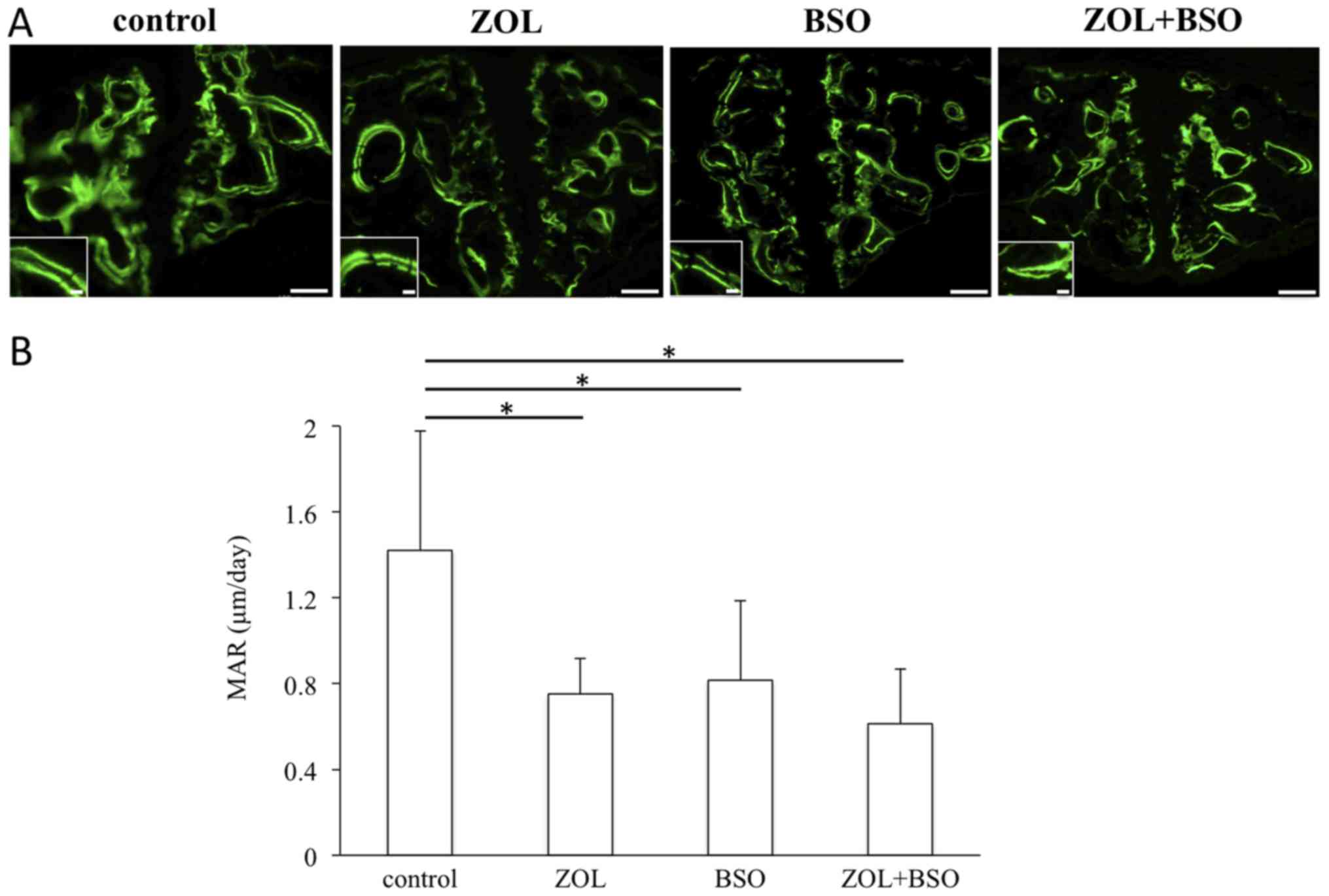
Bone dynamic parameters
Following calcein administration, the double
calcein-green labels were observed in the bones of the mice. Two
clear calcein-labeled lines were recognizable in the newly formed
bone around the palatal bone (Fig.
5A). The MAR was significantly lower in the treatment groups
than in the control group (Fig.
5B).
Discussion
In the present study, ZOL treatment tended to
increase the BV/TV and Tb.N of the femur and decrease the Tb.Sp in
mice. These findings may suggest that the experimental protocol
generated the expected anticatabolic effect of ZOL treatment in
bone. The bone remodeling rate is thought to be higher in the jaw
than femur throughout life (40).
Therefore, suppression of bone turnover by ZOL treatment may have
more profound effects on the jaw bones than long bones. The BSO
group exhibited significantly increased levels of 8-OHdG as a
marker of an individual's OS compared with the control group.
Bone tissue is continuously renewed by bone
remodeling, which comprises a dynamic interplay among bone cells
including osteoclasts, osteoblasts, and osteocytes (41). BPs induce osteoclast apoptosis, which
can be recognized by morphological changes in osteoclasts both
in vitro (42–44) and in vivo (42). The number of multinuclear
TRAP-positive cells was evaluated as osteoclasts. The number of
TRAP-positive cells was lower in the ZOL group than in the control
group. This result suggests that BP treatment may serve important
roles in the inhibition of bone turnover by osteoclasts in the bone
surgery area. Otherwise, only BSO treatment increased the number of
TRAP-positive osteoclasts. There was a difference in the number of
TRAP-positive osteoclasts between the BSO and ZOL groups. These
results suggest that BSO treatment is unrelated to osteoclast
apoptosis. OS occurs as a result of ROS overproduction. ROS have
opposite effects on osteoclast and osteoblast activity. ROS induce
the apoptosis of osteoblasts and osteocytes and activate the
differentiation of osteoclasts (24).
It was also observed that there were significantly
more empty osteocyte lacunae in the alveolar bone and palatal bone
in the ZOL+BSO group than in the control group. Empty osteocyte
lacunae were prone to increase by treatment with ZOL. This implies
that ZOL may have the potential to exacerbate bone damage. In all
groups, the number of empty osteocyte lacunae was higher around the
surgically created defect in the palatal than alveolar bone. When
bone becomes necrotic, bone repair is initiated by osteoclasts.
However, necrotic bone persists in the region because of osteoclast
suppression by ZOL. This is why dentoalveolar surgery is a risk
factor for the development of BRONJ in patients receiving BPs
(16). Empty osteocyte lacunae in
the alveolar bone and palatal bone were increased by single
treatment with ZOL or BSO, but not significantly. Empty osteocyte
lacunae were significantly increased by combined treatment with ZOL
and BSO. These results indicate that single treatment with ZOL or
BSO did not induce ONJ in the present study. This may have been
caused by shortages in the dosage and duration of administration of
these drugs. Long-term BP treatment seems to be an important risk
factor for BRONJ (45,46). However, combined treatment with ZOL
and BSO induced ONJ. The present results suggest that both of these
agents contribute to the onset of BRONJ in a short term. Future
experiments will aim to confirm the results of the current study by
determining whether inhibition of OS may prevent ZOL+BSO-induced
ONJ.
Osteocytes are embedded in the bone matrix within a
network of lacunae and canaliculi. Osteocytes use their dendritic
processes to communicate with each other, bone surface cells such
as osteoblasts and osteoclasts, and vasculature cells (47). Osteocytes produce and secrete
sclerostin and receptor activator of nuclear factor-κB ligand to
communicate indirectly with bone-associated cells. The osteocyte
has a key role in regulating bone turnover. Busse et al
(48) and Dunstan et al
(49) previously revealed
age-associated reduction in osteocyte viability. Kobayashi et
al (50) recently demonstrated a
similar reduction in both canalicular density and number in aged
murine and oxidative-damaged osteocytes, supporting the hypothesis
that aging and/or redox imbalances in osteocytes commonly
exacerbate the impairment of osteocytic canalicular networks and
reduce survival in mammals. The present study reported a 16%
reduction in N.Ot.Ca/Ot.Lc. in the palatal bone with BSO treatment.
Otherwise, the N.Ot.Ca/Ot.Lc. was slightly decreased by ZOL
treatment, but not significantly. The MAR was also evaluated. The
MAR was lower in the treatment groups than in the control group and
was lowest in the ZOL+BSO group. These results suggest that BP and
BSO treatments serve an important role in the inhibition of bone
turnover following dentoalveolar surgery.
The presence of bacterial colonies around the
surgical perforation was not evaluated. Howie et al
(11) recently established a model
for osteonecrosis of the jaw with zoledronate treatment following
repeated major trauma, and there was no detectable bacterial
colonization at 1 week following extraction in either the control
or zoledronate-treated rats. The term ‘osteonecrosis’ in BRONJ is
associated with aseptic necrosis. According to the 2014 position
paper of the American Association of Oral and Maxillofacial
Surgeons (42), stage 1 BRONJ does
not comprise bacterial infection. Therefore, stage 1 BRONJ can be
defined as an osteonecrosis type of BRONJ.
In conclusion, investigation of this model has
demonstrated that osteonecrosis induced by BSO treatment was
similar to that induced by ZOL treatment. ZOL treatment may
primarily target inhibition of osteoclasts, and BSO treatment
affects osteocytes. OS in osteocytes exacerbate the impairment of
osteocytic canalicular networks. Both BPs and OS suppressed bone
turnover. As a result, BPs and OS may induce osteonecrosis
following invasive dentoalveolar surgery. OS has been demonstrated
as an additional risk factor for the development of BRONJ.
Acknowledgements
The authors would like to thank Professor Takashi
Daimon (Department of Medical Informatics, Hyogo College of
Medicine, Nishinomiya, Japan) for assisting with the statistical
analysis and Ms Shinobu Osawa for her technical assistance.
Funding
The present study was supported by JSPS KAKENHI
[grant nos. 15K11332, 18K09825 (to KT), 18K17124 (JT), and 26861761
(MY)], and by a Grant-in-Aid for Researchers [Hyogo College of
Medicine, 2016 (to KT)] and a Grant-in-Aid for Graduate Students
[Hyogo College of Medicine, 2017 (to JT)].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT, KT and HK conceived and designed the present
study. JT, KT, HH, MU, HM and MY performed the experiments. JT, KT,
HH, KY, KN and HK analyzed the data and performed statistical
analysis. JT, KT, KN and HK wrote, reviewed and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in compliance
with the study protocol, which was reviewed by the Animal Care and
Use Committee of Hyogo College of Medicine (Nishinomiya, Japan) in
accordance with the Act on Welfare and Management of Animals (Law
No. 105, Japan), the Standards Relating to the Care and Management
of Laboratory Animals and Relief of Pain (Japanese Ministry of
Environment, Notice No. 88, 2006), and the Fundamental Guidelines
for Proper Conduct of Animal Experiment and Related Activities in
Academic Research Institutions (Japanese Ministry of Education,
Culture, Sports, Science and Technology, Notice No. 71, 2006). Our
proposed study was approved by the committee under the
institutional approval no. 16-078.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Migliorati CA, Casiglia J, Epstein J,
Jacobsen PL, Siegel MA and Woo SB: Managing the care of patients
with bisphosphonate-associated osteonecrosis: An American Academy
of Oral Medicine position paper. J Am Dent Assoc. 136:1658–1668.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng A, Mavrokokki A, Carter G, Stein B,
Fazzalari NL, Willson DF and Goss AN: The dental implications of
bisphosphonates and bone disease. Aust Dent J. 50 (4 Suppl
2):S4–S13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bertoldo F, Santini D and Lo Cascio V:
Bisphosphonates and osteomylelitis of the jaw: A pathogenic puzzle.
Nat Clin Pract Oncol. 4:711–721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Licata AA: Discovery, clinical
development, and therapeutic uses of bisphosphonates. Ann
Pharmacother. 39:668–677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michaelson MD and Smith MR:
Bisphosphonates for treatment and prevention of bone metastases. J
Clin Oncol. 23:8219–8224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruggiero SL, Dodson TB, Assael LA,
Landesberg R, Marx RE and Mehrotra B: American Association of Oral
and Maxillofacial Surgeons: American Association of Oral and
Maxillofacial Surgeons position paper on bisphosphonate-related
osteonecrosis of the jaws-2009 update. J Oral Maxillofac Surg. 67
Suppl 5:2–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Urade M, Tanaka N, Furusawa K, Shimada J,
Shibata T, Kirita T, Yamamoto T, Ikebe T, Kitagawa Y and Fukuta J:
Nationwide survey for bisphosphonate-related osteonecrosis of the
jaws in Japan. J Oral Maxillofac Surg. 69:e364–e371. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doggrell SA: Clinical efficacy and safety
of zoledronic acid in prostate and breast cancer. Expert Rev
Anticancer Ther. 9:1211–1218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reid IR and Cornish J: Epidemiology and
pathogenesis of osteonecrosis of the jaw. Nat Rev Rheumatol.
8:90–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JH, Park YB, Li Z, Shim JS, Moon HS,
Jung HS and Chung MK: Effect of alendronate on healing of
extraction sockets and healing around implants. Oral Dis.
17:705–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Howie RN, Borke JL, Kurago Z, Daoudi A,
Cray J, Zakhary IE, Brown TL, Raley JN, Tran LT, Messer R, et al: A
model for osteonecrosis of the jaw with zoledronate treatment
following repeated major trauma. PLoS One. 17:e01325202015.
View Article : Google Scholar
|
|
12
|
Poubel VLDN, Silva CAB, Mezzomo LAM, De
Luca Canto G and Rivero ERC: The risk of osteonecrosis on alveolar
healing after tooth extraction and systemic administration of
antiresorptive drugs in rodents: A systematic review. J
Craniomaxillofac Surg. 46:245–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Advisory T ask Force on
Bisphosphonate-Related Ostenonecrosis of the Jaws, American
Association of Oral and Maxillofacial Surgeons: American
Association of Oral and Maxillofacial Surgeons position paper on
bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac
Surg. 65:369–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sonis ST, Watkins BA, Lyng GD, Lerman MA
and Anderson KC: Bony changes in the jaws of rats treated with
zoledronic acid and dexamethasone before dental extractions mimic
bisphosphonate-related osteonecrosis in cancer patients. Oral
Oncol. 45:164–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hokugo A, Christensen R, Chung EM, Sung
EC, Felsenfeld AL, Sayre JW, Garrett N, Adams JS and Nishimura I:
Increased prevalence of bisphosphonate-related osteonecrosis of the
jaw with vitamin D deficiency in rats. J Bone Miner Res.
25:1337–1349. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takaoka K, Yamamura M, Nishioka T, Abe T,
Tamaoka J, Segawa E, Shinohara M, Ueda H, Kishimoto H and Urade M:
Establishment of an animal model of bisphosphonate-related
osteonecrosis of the jaws in spontaneously diabetic torii rats.
PLoS One. 14:e01443552015. View Article : Google Scholar
|
|
17
|
Bamias A, Kastritis E, Bamia C,
Moulopoulos LA, Moulopoulos I, Bozasb G, Koutsoukou V, Gika D,
Anaqnostopoulos A, Papadimitriou C, et al: Osteonecrosis of the jaw
in cancer after treatment with bisphosphonates: Incidence and risk
factors. J Clin Oncol. 23:8580–8587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marx RE, Sawatari Y, Fortin M and Broumand
V: Bisphosphonate-induced exposed bone
(osteonecrosis/osteopetrosis) of the jaws: Risk factors,
recognition, prevention, and treatment. J Oral Maxillofac Surg.
63:1567–1575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jadu F, Lee L, Pharoah M, Reece D and Wang
L: A retrospective study assessing the incidence, risk factors and
comorbidities of pamidronate-related necrosis of the jaws in
multiple myeloma patients. Ann Oncol. 18:2015–2019. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Almeida M, Han L, Martin-Millan M, Plotkin
LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T,
Parfitt AM, et al: Skeletal involution by age-associated oxidative
stress and its acceleration by loss of sex steroids. J Biol Chem.
282:27285–27297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Almeida M, Ambrogini E, Han L, Manolagas
SC and Jilka RL: Increased lipid oxidation causes oxidative stress,
increased peroxisome proliferator-activated receptor-gamma
expression, and diminished pro-osteogenic Wnt signaling in the
skeleton. J Biol Chem. 284:27438–27448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nojiri H, Saita Y, Morikawa D, Kobayashi
K, Tsuda C, Miyazaki T, Saito M, Marumo K, Yonezawa I, Kaneko K, et
al: Cytoplasmic superoxide causes bone fragility owing to
low-turnover osteoporosis and impaired collagen cross-linking. J
Bone Miner Res. 26:2682–2694. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Almeida M and O'Brien CA: Basic biology of
skeletal aging: Role of stress response pathways. J Gerontol Biol
Sci Med Sci. 68:1197–1208. 2013. View Article : Google Scholar
|
|
24
|
Domazetovic V, Marcucci G, Iantomasi T,
Brandi ML and Vincenzini MT: Oxidative stress in bone remodeling:
Role of antioxidants. Clin Cases Miner Bone Metab. 14:209–216.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koçer G, Naziroğlu M, Çelik Ö, Önal L,
Özçelik D, Koçer M and Sönmez TT: Basic fibroblast growth factor
attenuates bisphosphonate-induced oxidative injury but decreases
zinc and copper levels in oral epithelium of rat. Biol Trace Elem
Res. 153:251–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Awodele O, Olayemi SO, Nwite JA and
Adeyemo TA: Investigation of the levels of oxidative stress
parameters in HIV and HIV-TB co-infected patients. J Infect Dev
Ctries. 6:79–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lebreton F, van Schaik W, Sanguinetti M,
Posteraro B, Torelli R, Lee Bras F, Vemeuil N, Zhang X, Giard JC,
Dhalluin A, et al: AsrR is an oxidative stress sensing regulator
modulating Enterococcus faecium opportunistic traits, antimicrobial
resistance, and pathogenicity. PLoS Pathog. 8:e10028342012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McDevitt CA, Ogunniyi AD, Valkov E,
Lawrence MC, Kobe B, McEwan AG and Paton JC: A molecular mechanism
for bacterial susceptibility to zinc. PLoS Pathog. 7:e10023572011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moye-Rowley WS: Transcription factors
regulating the response to oxidative stress in yeast. Antioxid
Redox Signal. 4:123–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khandelwal VK, Mitrofan LM, Hyttinen JM,
Chaudhari KR, Buccione R, Kaarniranta K, Ravingerová T and
Mönkkönen J: Oxidative stress plays an important role in zoledronic
acid-induced autophagy. Physiol Res. 63 Suppl 4:S601–S612.
2014.PubMed/NCBI
|
|
31
|
Kuribayashi M, Fujioka M, Takahashi KA,
Arai Y, Ishida M, Goto T and Kubo T: Vitamin E prevents
steroid-induced osteonecrosis in rabbits. Acta Orthop. 81:154–160.
2010. View Article : Google Scholar
|
|
32
|
Ichiseki T, Matsumoto T, Nishino M,
Kaneuji A and Katsuda S: Oxidative stress and vascular permeability
in steroid-induced osteonecrosis model. J Orthop Sci. 9:509–515.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ichiseki T, Kaneuji A, Katsuda S, Ueda Y,
Sugimori T and Matsumoto T: DNA oxidation injury in bone early
after steroid administration is involved in the pathogenesis of
steroid-induced osteonecrosis. Rheumatology (Oxford). 44:456–460.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ichiseki T, Kaneuji A, Ueda Y, Nakagawa S,
Mikami T, Fukui K and Matsumoto T: Osteonecrosis development in a
novel rat model characterized by a single application of oxidative
stress. Arthritis Rheum. 63:2138–2141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kobayashi Y, Hiraga T, Ueda A, Wang L,
Matsumoto-Nakano M, Hata K, Yatani H and Yoneda T: Zoledronic acid
delays wound healing of the tooth extraction socket, inhibits oral
epithelial cell migration, and promotes proliferation and adhesion
to hydroxyapatite of oral bacteria, without causing osteonecrosis
of the jaw, in mice. J Bone Miner Metab. 28:165–175. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Valavanidis A, Vlachogianni T and Fiotakis
C: 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of
oxidative stress and carcinogenesis. J Environ Sci Health C Environ
Carcinog Ecotoxicol Rev. 27:120–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jaiprakash A, Prasadam I, Feng JQ, Liu Y,
Crawford R and Xiao Y: Phenotypic characterization of
osteoarthritic osteocytes from the sclerotic zones: A possible
pathological role in subchondral bone sclerosis. Int J Biol Sci.
8:406–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawamoto T and Kawamoto K: Preparation of
thin frozen sections from nonfixed and undecalcified hard tissues
using Kawamoto's film method (2012). Methods Mol Biol.
1130:149–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dempster DW, Compston JE, Drezner MK,
Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR
and Parfitt AM: Standardized nomenclature, symbols and units for
bone histomorphometry: A 2012 update of the report of the ASBMR
Histomorphometry Nomenclature Committee. J Bone Miner Res. 28:2–17.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huja SS and Beck FM: Bone remodeling in
maxilla, mandible, and femur of young dogs. Anat Rec (Hoboken).
291:1–5. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hughes DE, Wright KR, Uy HL, Sasaki A,
Yoneda T, Roodman GD, Mundy GR and Boyce BF: Bisphosphonates
promote apoptosis in murine osteoclasts in vitro and in vivo. J
Bone Miner Res. 10:1478–1487. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Selander KS, Mönkkönen J, Karhukorpi EK,
Härkönen P, Hannuniemi R and Väänänen HK: Characteristics of
clodronate-induced apoptosis in osteoclasts and macrophages. Mol
Pharmacol. 50:1127–1138. 1996.PubMed/NCBI
|
|
44
|
Hiroi-Furuya E, Kameda T, Hiura K, Mano H,
Miyazawa K, Nakamaru Y, Watanabe-Mano M, Okuda N, Shimada J,
Yamamoto Y, et al: Etidronate (EHDP) inhibits osteoclastic-bone
resorption, promotes apoptosis and disrupts actin rings in
isolate-mature osteoclasts. Calcif Tissue Int. 64:219–223. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Santini D, Vespasiani Gentilucci U,
Vincenzi B, Picardi A, Vasaturo F, La Cesa A, Onori N, Scarpa S and
Tonini G: The antineoplastic role of bisphosphonates: From basic
research to clinical evidence. Ann Oncol. 14:1468–1476. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ruggiero SL, Dodson TB, Fantasia J,
Goodday R, Aghaloo T, Mehrotra B and O'Ryan F: American Association
of Oral and Maxillofacial Surgeons: American Association of Oral
and Maxillofacial Surgeons position paper on medication-related
osteonecrosis of the jaw-2014 update. J Oral Maxillofac Surg.
72:1938–1956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bonewald LF: The amazing osteocyte. J Bone
Miner Res. 26:229–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Busse B, Djonic D, Milovanovic P, Hahn M,
Püschel K, Ritchie RO, Djuric M and Amling M: Decrease in the
osteocyte lacunar density accompanied by hypermineralized lacunar
occlusion reveals failure and delay of remodeling in aged human
bone. Aging Cell. 9:1065–1075. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dunstan CR, Somers NM and Evans RA:
Osteocyte death and hip fracture. Calcif Tissue Int. 53 Suppl
1:S113–S117. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kobayashi K, Nojiri H, Saita Y, Morikawa
D, Ozawa Y, Watanabe K, Koike M, Asou Y, Shirasawa T, Yokote K, et
al: Mitochondrial superoxide in osteocytes perturbs canalicular
networks in the setting of age-related osteoporosis. Sci Rep.
5:91482015. View Article : Google Scholar : PubMed/NCBI
|