Introduction
Hypertensive intracerebral hemorrhage is a
non-traumatic brain parenchymal hemorrhage disease with the highest
incidence rate in the cold season. Since the cold climate induces
contraction of blood vessels in the human body, blood pressure
fluctuates greatly, which easily causes blood vessel rupture
resulting in hemorrhage (1).
However, the pathological mechanism of hypertensive intracerebral
hemorrhage is more complicated, which is related not only to the
cold climate factors, but also to the formation of intracranial
microaneurysms, intracranial arterial wall degeneration and
necrosis and cerebrovascular amyloidosis (2). The disability and mortality rates of
hypertensive intracerebral hemorrhage are high, so the improvement
of clinical efficacy of these methods has become a research hotspot
in recent years (3). At present,
minimally invasive puncture and small bone window craniotomy are
the main treatment methods for hypertensive intracerebral
hemorrhage, but their advantages and disadvantages are not yet
clear (4). In particular, there are
few studies and reports on the effects of the two surgical methods
on postoperative motor-evoked potentials (MEPs) of patients
(5). This study compared the
efficacy between minimally invasive puncture and small bone window
craniotomy in the treatment of hypertensive intracerebral
hemorrhage, and explored the effects of the above treatment methods
on MEPs and postoperative rehemorrhage, so as to provide a basis
for treating hypertensive intracerebral hemorrhage.
Patients and methods
General data
A total of 80 patients with hypertensive
intracerebral hemorrhage admitted and treated in Chengyang People's
Hospital (Qingdao, China) from March 2016 to December 2017 were
selected for the study. Inclusion criteria: i) patients definitely
diagnosed with hypertensive intracerebral hemorrhage (6); and ⅱ) patients undergoing surgical
treatment within 72 h of onset with postoperative follow-up period
≥3 months. Exclusion criteria: i) patients with cerebral hemorrhage
caused by trauma or other non-hypertensive factors; ⅱ) patients who
were in critical condition with no time for surgical treatment; ⅲ)
patients with diseases in the liver, kidney or other important
organs; ⅳ) psychopaths; or v) women in the gestation period. These
80 patients were randomly divided into the minimally invasive group
(n=40) and the craniotomy group (n=40). Comparisons of general data
between the two groups of patients are shown in Table I.
 | Table I.Comparison of general data between the
two groups of patients. |
Table I.
Comparison of general data between the
two groups of patients.
| Variables | Minimally invasive
group (n=40) | Craniotomy group
(n=40) | t/χ2 | P-value |
|---|
| Age (years) | 56.35±3.21 | 57.41±4.08 | 0.682 | 0.095 |
| Sex (n) |
| Male | 21 | 22 | 1.074 | 0.083 |
|
Female | 19 | 18 |
|
|
| Body mass index
(kg/m2) | 25.13±1.73 | 25.15±2.41 | 0.521 | 0.166 |
| Volume of hematoma
(ml) | 42.56±4.31 | 43.06±5.08 | 0.785 | 0.103 |
| Glasgow Coma Scale
score (points) | 10.11±1.54 | 10.24±1.69 | 1.092 | 0.065 |
| Operation timing
(n) |
| At 12 h
after onset | 20 | 21 | 1.336 | 0.072 |
| At 12–24
h after onset | 16 | 15 |
|
|
| At 24–72
h after onset | 4 | 4 |
|
|
This study was approved by the Ethics Committee of
Chengyang People's Hospital. Signed informed consents were obtained
from the patients or the guardians.
Treatment methods
The minimally invasive group was treated with
minimally invasive puncture and drainage for hematoma: hematoma
lesions were found by computed tomography (CT) before operation,
minimally invasive puncture points were marked on the patient's
scalp, and the direction and depth of minimally invasive needle
insertion were scientifically evaluated. The patients were locally
hypodermically injected with 1.0% lidocaine according to a method
described in a previous study (7).
Electric drill was applied to gradually insert the puncture needle
into the patient's intracranial hematoma lesions. After the needle
was pulled out, the hematoma aspiration was performed. When the
hematoma volume reached ~50%, the aspiration was stopped.
The craniotomy group underwent small bone marrow
craniotomy: the patients were generally anesthetized using the
procedure and were examined via CT before operation in order to
view the lesions that were away from important blood vessels and
functional areas of the brain. Generally, bone windows with a
diameter of ≤4 cm were selected at the closest point near the
hematoma lesions. After the patient's endocranium was incised, the
hematomas were aspirated with aspiration volume of 60–92%. After
hemostasis, a drainage tube was placed on the patient's hematoma
cavity. Urokinases were injected into the patient's hematoma cavity
postoperatively, and if there were no symptoms of intracranial
hypertension, open drainage was conducted 4 h after injection once
a day. The patients were subjected to regular cranium CT
re-examination after operation. If the hematoma volume was <10
ml, the drainage tube could be removed. Both groups of patients
were treated with routine treatments such as anti-infective and
neurotrophic treatments after operation.
Observation indexes
Operation-related indexes
The operation time, drainage tube removal time,
length of hospital stay and the total expenses of hospitalization
of patients were recorded and compared.
Neurological function
Before operation and at 28 days after operation, the
Chinese scale of clinical neurological deficit of stroke patients
(CSS) (8) was adopted to evaluate
the neurological deficit scores of the two groups of patients.
Ability to daily living
activities
Barthel index (9) was
employed to assess the ability to daily living activities of the
two groups of patients at 3 months after operation.
MEPs
Neurotic electrophysiology was utilized to detect
MEPs in the two groups of patients (10).
Serum S-100β
Changes in the level of serum S-100β in the two
groups of patients before operation and at 1, 3, 7, 14, 21 and 28
days after operation were determined via the enzyme-linked
immunosorbent assay (ELISA).
Complications
After 3 months of follow-up, postoperative
complications such as postoperative rehemorrhage or new
intracranial hemorrhage, multiple organ failure and pulmonary
embolism were observed and recorded.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
21.0 software was used for data analysis. Enumeration data were
also expressed as percentage (%). Chi-square test was used for
intergroup comparisons. Measurement data were expressed as mean ±
standard deviation (SD), and the t-test was applied for intragroup
and intragroup comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of postoperative
complications
After 3 months of follow-up, there were no
significant differences in the incidence rate of postoperative
complications such as rehemorrhage or new intracranial hemorrhage
and mortality rate (p>0.05) (Table
II).
 | Table II.Comparison of postoperative
complications between the two groups [n (%)]. |
Table II.
Comparison of postoperative
complications between the two groups [n (%)].
| Group | n | Stress ulcer | Rehemorrhage or new
intracranial hemorrhage | Multiple organ
failure | Pulmonary
embolism | Mortality |
|---|
| Minimally invasive
group | 40 | 3 (7.50) | 3
(7.50) | 2 (5.00) | 1 (2.50) | 6 (15.00) |
| Craniotomy group | 40 | 2 (5.00) | 3
(7.50) | 1 (2.50) | 1 (2.50) | 5 (12.50) |
| χ2 |
| 0.962 | 0.856 | 1.008 | 1.145 | 0.697 |
| P-value |
| 0.154 | 0.097 | 0.133 | 0.098 | 0.086 |
Comparison of the CSS score
Before operation, there was no significant
difference in the CSS score between the two groups (p>0.05). At
28 days after operation, the CSS score in the minimally invasive
group (20.04±3.51 points) was lower than that in the craniotomy
group (22.63±4.77 points) (p<0.05) (Table III).
 | Table III.Comparison of the CSS score between
the two groups (mean ± SD, points). |
Table III.
Comparison of the CSS score between
the two groups (mean ± SD, points).
| Group | No. of survived
cases | Before operation | At 28 days after
operation | t | P-value |
|---|
| Minimally invasion
group | 34 | 40.96±6.15 | 20.04±3.51 | 21.095 | <0.001 |
| Craniotomy group | 35 | 40.87±5.32 | 22.63±4.77 | 17.126 | <0.001 |
| t |
| 1.091 | 1.352 |
|
|
| P-value |
| 0.174 | 0.026 |
|
|
Comparison of the Barthel score
At 3 months after operation, there were no
significant differences in the scores of self-care ability, mild
dysfunction, moderate dysfunction and severe dysfunction between
the two groups (p>0.05) (Table
IV).
 | Table IV.Comparison of the Barthel score
between the two groups at 3 months after operation [n (%)]. |
Table IV.
Comparison of the Barthel score
between the two groups at 3 months after operation [n (%)].
| Group | No. of survived
cases | Self-care ability
(100 points) | Mild dysfunction
(60–99 points) | Moderate dysfunction
(41–60 points) | Severe dysfunction
(≤40 points) |
|---|
| Minimally invasion
group | 34 | 6 (17.65) | 22 (64.71) | 5 (14.71) | 1 (2.94) |
| Craniotomy group | 35 | 7 (20.00) | 21 (60.00) | 5 (14.29) | 2 (5.71) |
| χ2 |
| 0.541 | 0.762 | 0.994 | 0.805 |
| P-value |
| 0.372 | 0.248 | 0.116 | 0.129 |
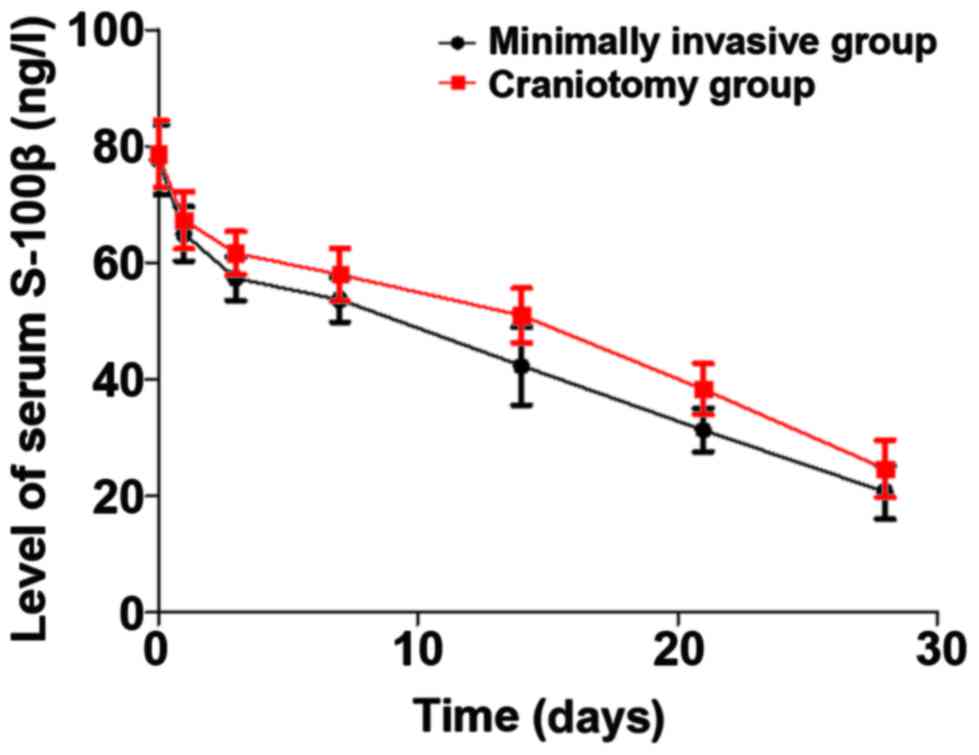
Comparison of serum S-100β
Through treatment, the levels of serum S-100β in
both groups displayed a gradual decreasing trend. The level of
S-100β in the minimally invasive group was remarkably lower than
that in the craniotomy group at 28 days after operation (p<0.05)
(Fig. 1).
Comparisons of various MEP
indexes
At 1 week after operation, 35 patients in the
minimally invasive group were able to elicit MEP waveforms, and
only 7 patients in the craniotomy group were able to elicit
positive waveforms. At 2 weeks after operation, 40 patients in the
minimally invasive and 20 patients in the craniotomy group could
elicit MEP waveforms. The incubation period, central motor
conduction time, and amplitude in the former were significantly
better than those in the latter (p<0.05) (Table V).
 | Table V.Comparison of various MEP indexes
between the two groups at 2 weeks after operation. |
Table V.
Comparison of various MEP indexes
between the two groups at 2 weeks after operation.
| Group | No. of survived
cases | Incubation period
(msec) | Central motor
conduction time (msec) | Amplitude (mV) |
|---|
| Minimally invasion
group | 40 | 21.74±2.68 |
8.78±1.35 | 9.68±5.44 |
| Craniotomy
group | 40 | 42.15±3.27 | 13.76±1.92 | 3.42±1.75 |
| t |
| 6.941 | 5.432 | 6.017 |
| P-value |
| 0.024 | 0.033 | 0.021 |
Comparison of operation-related
indexes
There was no significant difference in the drainage
tube removal time between the two groups (p>0.05). The operation
time and length of hospital stay were shorter with more total
expenses of hospitalization in the minimally invasive group
compared to those in the craniotomy group (p<0.05) (Table VI).
 | Table VI.Comparison of operation-related
indexes between the two groups (mean ± SD). |
Table VI.
Comparison of operation-related
indexes between the two groups (mean ± SD).
| Group | No. of survived
cases | Operation time
(min) | Drainage tube
removal time (days) | Length of hospital
stay (days) | Total expenses of
hospitalization (RMB ¥ × 104) |
|---|
| Minimally invasion
group | 34 |
71.69±15.42 | 5.65±1.64 | 20.57±4.32 | 17.96±2.89 |
| Craniotomy
group | 35 | 104.85±26.44 | 4.91±1.53 | 25.34±5.45 | 12.55±1.95 |
| t |
| 7.276 | 1.341 | 8.125 | 7.954 |
| P-value |
| 0.017 | 0.088 | 0.012 | 0.029 |
Discussion
The efficacy of conservative treatments for
hypertensive intracerebral hemorrhage is currently not ideal, and
surgical treatment is an important method to treat this kind of
disease. Relevant data have indicated (11,12) that
the application of traditional craniotomy evacuation of hematomas
is limited in clinical practice due to iatrogenic injury. Small
bone window craniotomy is an improved technique based on
traditional craniotomy, which is currently applied in the treatment
of intracranial superficial hematomas. However, hematomas in the
deep site increase the damage to brain tissues and reduce the
significance of cerebral hemorrhage treatment (13,14). In
recent years, as the understanding of the minimally invasive
techniques continues to deepen, the application of minimally
invasive puncture and drainage for hematomas in the treatment of
cerebral hemorrhage has gradually drawn attention (15). The principle of minimally invasive
puncture and drainage for hematomas is to pierce a puncture needle
into the patient's hematoma lesion via an electric drill to
construct a channel for hematoma evacuation. Subsequently, the
biochemical enzyme technique is adopted to liquefy and extract
hematomas, thus achieving hematoma evacuation (16,17). The
results of the present study manifested that at 28 days after
operation, the CSS score was 20.04±3.51 points in the minimally
invasive group, which was lower than that in the craniotomy group
(22.63±4.77 points) (p<0.05), in consistency with other research
findings (18).
After 3 months of follow-up, there were no
significant differences in the incidence rate of postoperative
complications such as rehemorrhage or new intracranial hemorrhage,
the mortality rate, and the Barthel score (p>0.05), suggesting
that the efficacies of the two treatment options were identical in
the aspects of complications, mortality rate and the ability to
daily living activities. The operation time and length of hospital
stay were shorter with more total expenses of hospitalization in
the minimally invasive group compared to those in the craniotomy
group (p<0.05), which is in agreement with the literature
(19) and may be related to factors
such as the operation procedures and anesthetic methods of the
above two operations.
According to relevant data (20), serum S-100β is involved in the
occurrence and development of cerebral hemorrhage, and the
prognosis of patients with cerebral hemorrhage can be evaluated by
measuring the level of serum S-100β. This study revealed that the
level of S-100β in the minimally invasive group was notably lower
than that in the craniotomy group at 28 days after operation
(p<0.05), suggesting that the minimally invasive puncture and
drainage for hematomas can reduce more effectively the expression
of serum S-100β than the small bone marrow craniotomy evacuation of
hematomas. The reason may be that the minimally invasive puncture
and drainage for hematomas does not aggravate brain and systemic
injuries in patients. The evacuation of hematomas can reduce the
mechanical compression of them in patients, and the inflammatory
damage mediated by them can be decreased through reducing the
content of serum S-100β.
Relevant data have manifested (21) that MEPs can be used to evaluate motor
function (pyramidal beam function). Clinically, MEP is generally
employed for the recovery of motor function after stroke. The
results of this study demonstrated that at 1 week after operation,
35 patients in the minimally invasive group were able to elicit MEP
waveforms, and only 7 patients in the craniotomy group were able to
elicit positive waveforms. At 2 weeks after surgery, 40 patients in
the minimally invasive group could elicit MEP waveforms, and 20
patients in the craniotomy group could elicit MEP waveforms. The
incubation period, central motor conduction time and amplitude in
the minimally invasive group were significantly better than those
in the craniotomy group (p<0.05). In the craniotomy group, 20
patients were not able to elicit positive MEP waveforms at 2 weeks
postoperatively, suggesting that the patient's motor center cannot
produce nerve impulses at this time. It is worth mentioning that
MEP can indicate the neurological function. Besides, the lack and
absence of MEPs suggest that the neurological function may not be
well restored (22). The above
evaluation results of MEPs further suggest that the minimally
invasive puncture and drainage for the clinical treatment of
hypertensive cerebral hemorrhage has better clinical efficacy.
In summary, compared with the small bone window
craniotomy, the minimally invasive puncture reduces the content of
serum S-100β. Its advantages are obvious, so it is worthy of
promotion and application.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL wrote and revised the manuscript. LL, HS and ML
treated the patients and were also involved in the conception of
the study. LL and HS recorded and analyzed the operation-related
indexes. GL interpreted the neurological function. WP was
responsible for serum S-100β detection. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Chengyang People's Hospital (Qingdao, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen G, Ping L, Zhou S, Liu W, Liu L,
Zhang D, Li Z, Tian Y and Chen Z: Early prediction of death in
acute hypertensive intracerebral hemorrhage. Exp Ther Med.
11:83–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Doden T, Sato H, Sasahara E, Murata T,
Yako T, Kitazawa K, Higuchi K, Kobayashi S and Hashimoto T:
Clinico-radiological characteristics and pathological diagnosis of
cerebral amyloid angiopathy-related intracerebral hemorrhage. J
Stroke Cerebrovasc Dis. 25:1736–1745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fouda AY, Artham S, El-Remessy AB and
Fagan SC: Renin-angiotensin system as a potential therapeutic
target in stroke and retinopathy: Experimental and clinical
evidence. Clin Sci (Lond). 130:221–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu R and Feng H: Lenticulostriate artery
and lenticulostriate-artery neural complex: New concept for
intracerebral hemorrhage. Curr Pharm Des. 23:2206–2211. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsumoto K, Sakaki S, Abekura M and
Yoshimine T: Co-existence of unruptured cerebral aneurysms in
patients with hypertensive intracerebral hemorrhage. Acta Neurochir
(Wien). 146:1085–1089; discussion 1089. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian X, Shu H, Zhang H, Wang H and Guo L:
Intracranial hemorrhage due to rupture of an anterior communicating
artery aneurysm in a patient with pituitary adenoma. J Craniofac
Surg. 26:e154–e155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yiannakopoulos CK: Carpal ligament
decompression under local anaesthesia: The effect of lidocaine
warming and alkalinisation on infiltration pain. J Hand Surg Br.
29:32–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Liu B, Liu Z, Wang Y, Zhao H, Zi
M, Wang L, Liu H, Chen Z and Xie Y: Development of a
disease-specific health-related quality of life questionnaire for
patients with post-stroke spasticity. J Tradit Chin Med.
32:674–678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lue YJ, Lin RF, Chen SS and Lu YM:
Measurement of the functional status of patients with different
types of muscular dystrophy. Kaohsiung J Med Sci. 25:325–333. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kenning TJ, Dalfino JC, German JW, Drazin
D and Adamo MA: Analysis of the subdural evacuating port system for
the treatment of subacute and chronic subdural hematomas. J
Neurosurg. 113:1004–1010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Southwell DG, Hervey-Jumper SL, Perry DW
and Berger MS: Intraoperative mapping during repeat awake
craniotomy reveals the functional plasticity of adult cortex. J
Neurosurg. 124:1460–1469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Velnar T and Bunc G: Iatrogenic metastasis
of a benign meningioma to the periosteum at the site of previous
craniotomy: A case report. Wien Klin Wochenschr. 120:766–769. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chi FL, Lang TC, Sun SJ, Tang XJ, Xu SY,
Zheng HB and Zhao HS: Relationship between different surgical
methods, hemorrhage position, hemorrhage volume, surgical timing,
and treatment outcome of hypertensive intracerebral hemorrhage.
World J Emerg Med. 5:203–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morotti A, Paciaroni M, Zini A,
Silvestrelli G, Del Zotto E, Caso V, Dell'Acqua ML, Simone AM,
Lanari A, Costa P, et al: Risk profile of symptomatic lacunar
stroke versus nonlobar intracerebral hemorrhage. Stroke.
47:2141–2143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han WY, Tao YQ, Xu F, Zhang YQ, Li ZY and
Liang GB: The short- and long-term efficacy analysis of
stereotactic surgery combined external ventricular drainage in the
treatment of the secondary intraventricular hemorrhage. Brain
Behav. 7:e008642017. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guldberg-Kjär T and Johansson B: ADHD
symptoms across the lifespan: A comparison of symptoms captured by
the Wender and Barkley Scales and DSM-IV criteria in a
population-based Swedish sample aged 65 to 80. J Atten Disord.
19:390–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sonni S, Lioutas VA and Selim MH: New
avenues for treatment of intracranial hemorrhage. Curr Treat
Options Cardiovasc Med. 16:2772014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang WZ, Jiang B, Liu HM, Li D, Lu CZ,
Zhao YD and Sander JW: Minimally invasive craniopuncture therapy
vs. conservative treatment for spontaneous intracerebral
hemorrhage: Results from a randomized clinical trial in China. Int
J Stroke. 4:11–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
James ML, Blessing R, Phillips-Bute BG,
Bennett E and Laskowitz DT: S100B and brain natriuretic peptide
predict functional neurological outcome after intracerebral
haemorrhage. Biomarkers. 14:388–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikedo T, Nakamura K, Sano N, Nagata M,
Okada Y, Terakawa Y and Murata T: Intraoperative transcranial
motor-evoked potentials predict motor function outcome in
intracerebral hemorrhage surgery. World Neurosurg. 90:518–523.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Voulgaris S, Karagiorgiadis D, Alexiou GA,
Mihos E, Zigouris A, Fotakopoulos G, Drosos D and Pahaturidis D:
Continuous intraoperative electromyographic and transcranial motor
evoked potential recordings in spinal stenosis surgery. J Clin
Neurosci. 17:274–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bestmann S and Krakauer JW: The uses and
interpretations of the motor-evoked potential for understanding
behaviour. Exp Brain Res. 233:679–689. 2015. View Article : Google Scholar : PubMed/NCBI
|