Introduction
In recent years, the incidence and mortality of
cardiovascular disease (CVD) in China have been increasing. It has
been estimated that the number of patients with CVD was >290
million in China, 2017. Furthermore, the mortality rate of CVD has
increased from ~174 to 298 per 100,000 people from 1990 to 2015. It
has therefore become one of the major diseases threatening public
health in China and all over the world (1). Myocardial infarctions are a common
cause of increased morbidity and mortality in the population, and
the onset age is decreasing (2).
Early diagnosis and treatment of a myocardial infarction can
prevent or reduce myocardial ischemic injury, in addition to
preventing ventricular remodeling and heart failure following a
myocardial infarction (3).
MicroRNAs (miRs) are short, endogenous,
single-stranded, non-coding RNA fragments in animal eukaryotic
cells, with a length of 18–26 nucleotides, which can inhibit gene
expression at a post-transcriptional level (4). Therefore, miRs are considered to be an
important component of the cellular regulatory network (4). miRs that serve an important role in the
occurrence and development of cardiovascular diseases have been
reported since 2006, when they were first identified by van Rooij
et al (5). miRs associated
with the cardiovascular system are increasingly being identified
and studied, thus becoming a research hotspot (4–7). miR-21
is highly expressed in the cardiovascular system and is involved in
the pathophysiological mechanisms of various cardiovascular
diseases, particularly in myocardial infarctions; high levels of
miR-21 expression are associated with cardiovascular diseases
(6,7).
Programmed cell death protein 4 (PDCD4) has been
demonstrated to be the target protein of miR-21 in tumors and
numerous systems, including the circulatory and nervous systems;
its role in cellular apoptosis and cellular protection has been
increasing studied (8,9). There are two important α-helical
domains at the amino end of PDCD4, through which PDCD4 can bind to
eukaryotic initiation factor 4A, the initiation factor of
eukaryotic translation, and thereby promote cellular apoptosis by
inhibiting the formation of ribosome complexes and protein
synthesis (10).
Ginseng is one of the most popular herbal medicines
that have been used in China for thousands of years (11). Ginsenoside Rb1 (GS-Rb1) is the most
active and abundant monomer in ginseng (11). Although a number of studies have
demonstrated the protective effects of GS-Rb1 on the heart
(11–14) and recent studies have revealed that
GS-Rb1 could impact miR expression in hypoxia/ischemia-injured
cardiomyocytes (12,13), the miR targets involved and their
roles in the heart remain unknown. Therefore, in the current study,
the roles and mechanisms of miR-21 and its target gene, which
encodes PDCD4, in the protection of cardiomyocytes treated with
GS-Rb1 were studied by constructing an oxygen-glucose deprivation
(OGD) injury model in vitro.
Materials and methods
Culture of neonatal rat cardiomyocytes
(NRCMs) from heart tissues
Primary cultures of NRCMs from a total of 180 12–24
h-old male Sprague Dawley rats (weight range, 5–6 g; Laboratory
Animal Center, Jilin University, Changchun, China) were prepared
through gentle serial trypsinization, as described previously
(12–14). All animal protocols were approved by
the Animal Care Center and Use Committee of Jilin University
(Changchun, China). Briefly, rats were anesthetized without feed
and house, and the ventricular myocardium was removed and cut into
1–2-mm3 sections. The obtained ventricles were washed
three times in cold phosphate buffered saline (PBS) and digested
five times for 5 min each at 37°C with 0.18% (w/v) trypsin and
0.01% EDTA. Digestion was terminated by adding Dulbecco's modified
Eagle's medium (DMEM) containing 10% (v/v) fetal bovine serum (FBS;
both Thermo Fisher Scientific, Inc., Waltham, MA, USA). Then, the
cells were collected via centrifugation for 10 min at 1,000 × g at
room temperature and resuspended in DMEM with 10% (v/v) FBS for 90
min to facilitate the separation of ventricular myocytes from the
more adherent non-myocytes. NRCMs were subsequently collected and
plated in collagen-coated 96- or 6-well plates (at a density of
4×104/well and 6×106/well, respectively) and
maintained at 37°C in a 5% CO2/95% air humidified
incubator in DMEM containing 10% (v/v) FBS, 100 U/ml penicillin and
100 mg/ml streptomycin.
OGD treatment
Cells were divided into the following groups: A
control group, an OGD group and an OGD+GS-Rb1 (4, 8, 16, 32 and 64
µM) group. To generate the OGD group, culture medium was replaced
with serum-free DMEM (no glucose; Gibco; Thermo Fisher Scientific,
Inc.) and cultured in hypoxic conditions (5% O2 and 95%
N2) via an automatic injection of N2 into the
incubator at 37°C. The control plates were maintained under
normoxic conditions. NRCMs with or without GS-Rb1 (cat. no. 110704;
National Institutes for food and drug Control, Beijing, China) (0,
4, 8, 16, 32 and 64 µM) were then subjected to hypoxia at 37°C to
the indicated time (0, 6, 12, 18, 24, 36 and 48 h). The control
plates were maintained at 37°C in a 5% CO2/95% air
humidified incubator in DMEM containing 10% (v/v) FBS for the same
time with the OGD group.
Cell Counting Kit-8 (CCK-8) assay
Following OGD treatment (0, 6, 12, 18, 24, 36 and 48
h), cell viability was determined using CCK-8 (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) in accordance with the
manufacturer's protocol. A total of 10 µl CCK-8 solution was added
to each well of a 96-well plate (cell density,
4×104/well), followed by an incubation of 3.5 h at 37°C.
The optical density of each well was read at a wavelength of 450 nm
using a microplate reader (M200 Pro; Tecan Group, Ltd., Mannedorf,
Switzerland). The control group was maintained under normoxic
conditions for the corresponding times. The results are presented
as the fold-change relative to the control group.
Apoptosis assay with annexin
V-FITC/propidium iodide (PI) staining
NRCMs were treated with different concentrations of
GS-Rb1 (0, 16 and 32 µM) and exposed to 18 h OGD for 18 h and
harvested using 0.25% trypsin and 1 mM EDTA for 5 min at 37°C.
Samples were then washed with PBS. The percentages of normal
nonapoptotic and apoptotic cells were measured via double
supravital staining with Annexin V and PI using an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (KGA
108; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Cells were
harvested with 0.25% trypsin and 1 mM EDTA for 5 min at 37°C. Cells
were then suspended in 500 µl of binding buffer (provided in the
aforementioned Apoptosis Detection kit) and stained with annexin
V-FITC and PI at room temperature for 5 min in the dark. Cells were
collected via centrifugation at 1,000 × g at room temperature for 3
min and the supernatant containing the unbound annexin V-FITC and
PI was aspirated. Then cells were resuspended in 400 µl binding
buffer. Flow cytometric analysis was conducted using a Cytomics
FC500 flow cytometer with CXP software (both Beckman Coulter, Inc.,
Brea, CA, USA); the operator was blind to the groups.
Fluorescent measurement of
intracellular reactive oxygen species (ROS)
The determination of ROS concentrations was based on
the oxidation of 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In
brief, the cells were collected using 0.25% trypsin and 1 mM EDTA
for 5 min at 37°C following OGD-injury, washed with serum-free DMEM
and incubated with DCFH-DA at 37°C for 20 min. Dichlorofluorescein
fluorescence intensity was detected at 488 nm excitation and 525 nm
emission using a M200 Pro microplate reader.
Detection of miR-21 expression using
poly(A) tailing SYBR green reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cell in the control group, OGD group (12–48 h), OGD
18 h + 32 µM GS-Rb1 group, miR-21 vehicle control group, miR-21
scramble control group, miR-21 inhibitor group and the miR-21
inhibitor + 32 µM GS-Rb1 group were lysed with 1 ml TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The reaction mixture
was then extracted with phenol/chloroform, precipitated with
isopropanol and resuspended in 25 µl diethylpyrocarbonate-treated
water. Total RNA (5 µg) was subsequently treated with DNase I
(Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at 22°C and
poly(A) polymerase (provided in the miRNA cDNA kit) at 37°C for 20
min. The poly(A)-tailed RNA (6 µl) was reverse-transcribed into
first-strand cDNA using an miRNA cDNA kit (cat. no. cw2141; Beijing
ComWin Biotech, Co., Ltd., Beijing, China) according to the
manufacturer's protocol. For qPCR analysis, 30 ng cDNA was employed
as a template in each reaction using the miRNA qPCR Assay kit (cat.
no. cw2142; Beijing ComWin Biotech, Co., Ltd.). The following
primers for miR-21 (mirBase no. MIMAT0000790) were utilized:
Forward, 5′-GCTAGCTTATCAGACTGATGTTGAAAA-3′ and reverse, provided in
the qPCR Assay kit (cat. no. cw2142; Beijing ComWin Biotech, Co.,
Ltd.). U6 small noncoding RNA was used as an internal control using
the following primers: 5′-CTCGCTTCGGCAGCACA-3′ (forward) and
5′-AACGCTTCACGAATTTGCGT-3′ (reverse). The thermocycling conditions
were as follows: Denaturation at 94°C for 20 sec, annealing at 60°C
for 45 sec followed by an extension at 72°C for 30 sec for 40
cycles. Gene expression was normalized to that of U6 and relative
fold changes were calculated using the 2−ΔΔCq method
(15).
Western blotting
NRCMs subjected to different conditions and
treatments were harvested and lysed using the Cell lysis buffer for
western and immunol precipitation IP kit (cat. no. P0013; Beyotime
Institute of Biotechnology, Haimen, China). For cytochrome c
(Cyt C) western blotting, preparation of the mitochondrial and
cytosolic protein fractions was conducted with a Cell Mitochondria
Isolation kit (Beyotime Institute of Biotechnology). Protein
concentrations were measured using a BCA protein assay kit. Equal
amounts of the sample lysate (30 µg) were separated via 12%
SDS-PAGE and then transferred through electroblotting to a
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). The
membrane was blocked with 5% non-fat milk in Tris-buffered saline
with Tween-20 (20 mM Tris-HCl, pH 7.4, 150 mM NaCl and 0.1%
Tween-20) overnight at 40°C. The following primary antibodies were
utilized: B-cell lymphoma (Bcl-2; 1:1,000; cat. no. 2872),
Bcl-2-associated X protein (Bax; 1:1,000; cat. no. 2772),
cytochrome c (1:1,000; cat. no. 4272), PDCD4 (1:1,000; cat. no.
9535) and GAPDH (1:1,000; cat. no. 2118; all, Cell Signaling
Technology, Inc., Danvers, MA, USA). The membrane was subsequently
incubated with the aforementioned primary antibodies for 2 h at
37°C and an immunoglobulin G horseradish peroxidase-conjugated
anti-rabbit antibody (1:2,000; cat. no. 7074; Cell Signaling
Technology, Inc.) for 1 h at room temperature. The resultant
signals were visualized using the SuperSignal West Femto Trial kit
(cat. no. 34095; Pierce; Thermo Fisher Scientific, Inc.) on a
Syngene G: BOX Chemi gel documentation system (Syngene Europe,
Cambridge, UK). The densitometric values were normalized using
GAPDH as an internal control. ImageJ software was also used for
densitomerty (version 1.6; National Institutes of Health, Bethesda,
MD, USA).
In vitro caspase-3 activity assay
Caspase-3 activity was measured with a Caspase-3
Activity Assay kit (Beyotime Institute of Biotechnology, Haimen,
China). Briefly, NRCM lysates were prepared following 18 h OGD
treatment with or without different doses of GS-Rb1 (4, 8, 16 and
32 µM). The assays were performed in 96-well micro plates by
incubating 10 µl protein cell lysate per sample in 80 µl reaction
buffer (provided by the Caspase-3 Activity Assay kit) containing 10
µl substrate (Asp-Glu-Val-Asp-p-nitroaniline). The lysates were
incubated at 37°C for 4–6 h. Samples were subsequently measured
with a M200 Pro microplate reader at an absorbance of 405 nm.
Caspase-3 activity was expressed as the percentage relative to the
control group.
NRCMs transfection
A micrOFF™ rno-miR-21 inhibitor (cat. no.
miR20000790-1-5; 10 nM) or the micrOFF™ inhibitor Negative Control
(cat. no. miR02201-1-5; 5 nM; each, RiboBio, Guangzhou, China) was
transfected into NRCMs using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
Subsequent GS-Rb1 treatment, and total RNA and protein extraction
were performed 48 h post-transfection for qPCR and western blot
analyses, respectively.
Statistical analysis
The data were expressed as the mean ± standard
deviation of at least three independent experiments. The group
results were analyzed for variance using one-way analysis of
variance followed by a post-hoc Bonferroni test. GraphPad Prism 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA) was employed
for all the analyses. P<0.05 indicated that the difference
between groups was statistically significant.
Results
GS-Rb1 protects NRCMs from OGD
impairment in a dose-dependent manner
As indicated in Fig.
1, cell activity was detected after 6, 12, 18, 24, 36 and 48 h
of cultivation, and it was determined that cell viability gradually
decreased as the duration of OGD treatment was prolonged (Fig. 1A). OGD for 18 h reduced cell activity
by ~50%; therefore, 18 h OGD was used to induce cell damage in
subsequent experiments (Fig. 1A and
B). When GS-Rb1 was applied at different concentrations (4–64
µM) and the protective effect of GS-Rb1 was examined using a cell
viability assay, the effect of GS-Rb1 at 32 µM was revealed to be
the most significant in OGD-damaged cells compared with the OGD
group (Fig. 1C). Annexin V-FITC/PI
double staining was used to detect apoptosis and the results
demonstrated that the cell percentage of living cells in the OGD 18
h-damaged group was ~40.2%, while the apoptosis rate, which
included early and late apoptosis, reached about 58.8% compared
with the control group (Fig. 1D and
E). When GS-Rb1 was applied at 32 µM in the model group, the
percentage of living cells significantly increased compared with
the OGD group (Fig. 1E), whereas the
percentage of apoptotic cells were decreased.
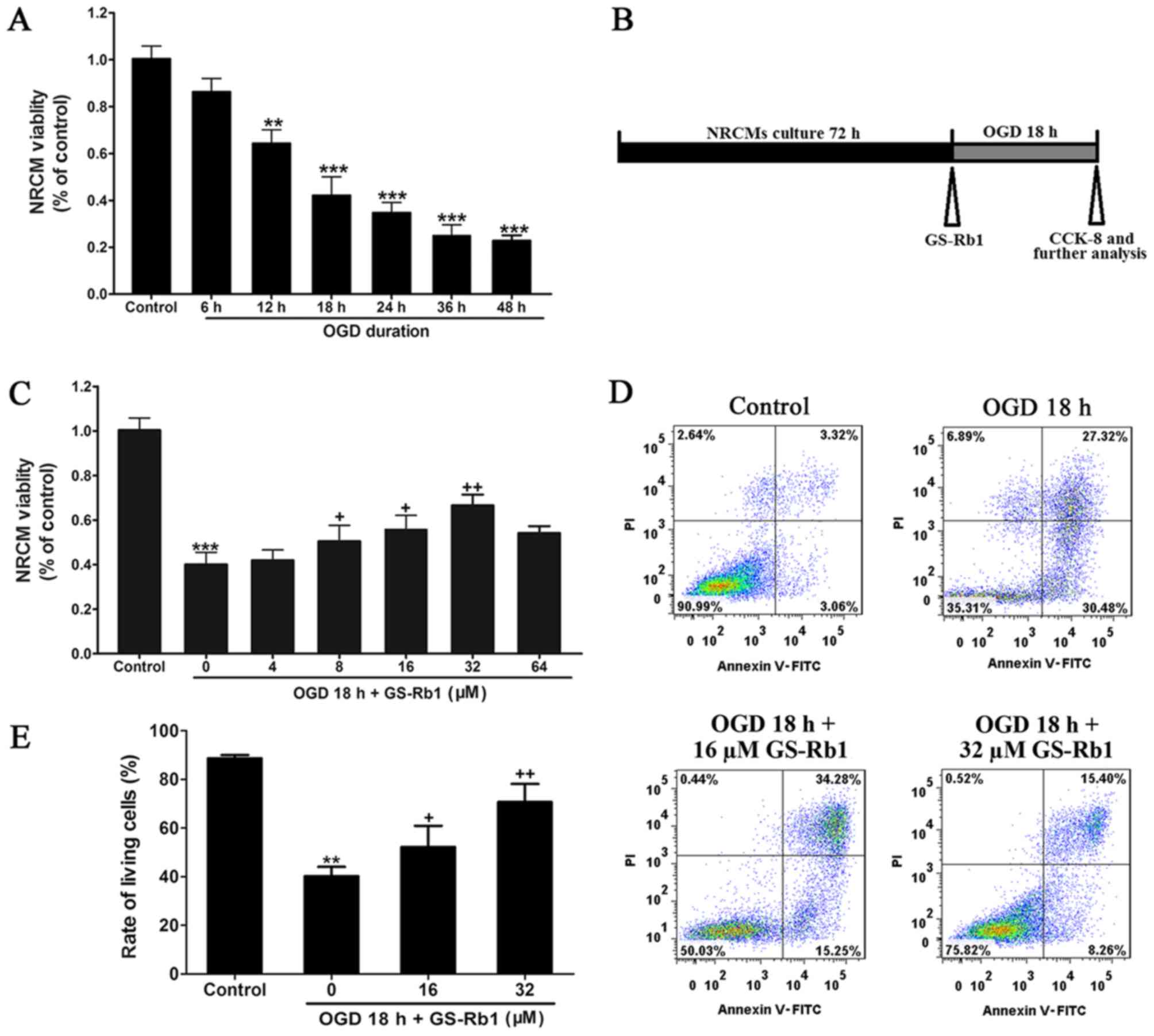 | Figure 1.GS-Rb1 protects NRCMs from OGD
impairment in a dose-dependent manner. (A) Cell viability of NRCMs
after 6, 12, 18, 24, 36 and 48 h of OGD cultivation. (B) For the
experimental procedure, NRCMs were cultured for 72 h and subjected
to the hypoxia conditions with or without GS-Rb1 for 18 h (95%
N2 and 5% O2), then cells were collected for
further analysis. (C) Cell viability of NRCMs following OGD and
GS-Rb1 treatment at different concentrations. (D) Effect of GS-Rb1
on OGD-damaged NRCMs detected via flow cytometry. (E)
Quantification of the percentage of apoptotic and living cells in
each group. Data is represented as the mean ± standard deviation.
**P<0.01 and ***P<0.001 vs. the control group;
+P<0.05 and ++P<0.01 vs. the OGD group
(n=3). GS-Rb1, ginsenoside Rb1; NRCMs, neonatal rat cardiomyocytes;
OGD, oxygen-glucose deprivation; PI, propidium iodide; FITC,
fluorescein isothiocyanate; CCK-8, Cell Counting Kit-8. |
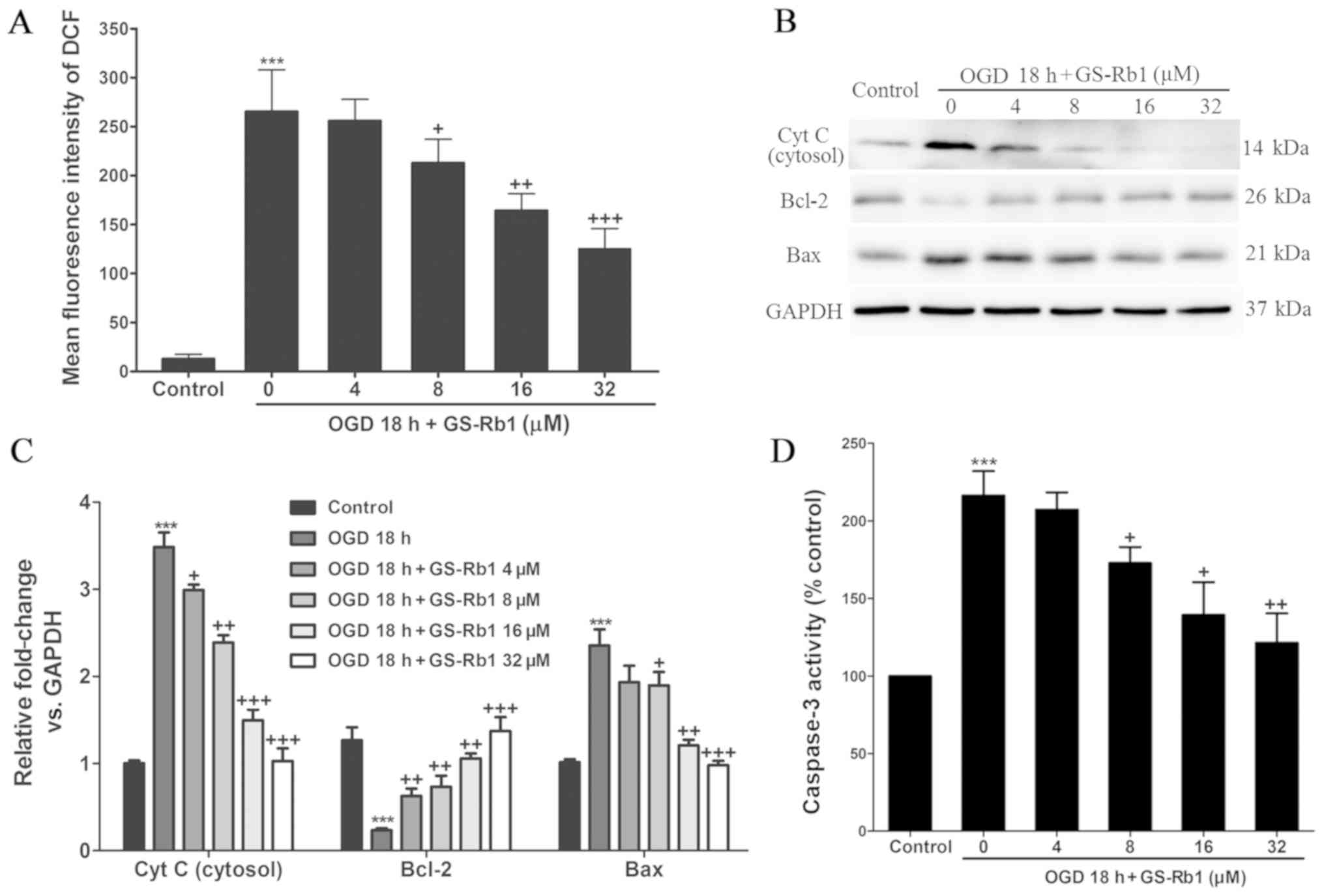
Anti-apoptotic signaling pathway
activities in OGD-injured NRCMs are increased by GS-Rb1
treatment
Furthermore, the apoptotic signaling pathways in
OGD-injured NRCMs were evaluated following GS-Rb1 treatment. OGD
treatment significantly increased the intracellular level of ROS,
with an >10-fold increase in the fluorescence intensity of DCF.
Co-treatment with GS-Rb1 significantly inhibited the increase in
the intracellular concentration of ROS induced by OGD (Fig. 2A). Western blotting revealed the
cytosolic Cyt C and Bcl-2/Bax expression levels (Fig. 2B). GS-Rb1 significantly reduced Bax
(a pro-apoptosis protein) expression and the release of Cyt C from
the nucleus to cytosol, while increasing Bcl-2 (an anti-apoptosis
protein) expression, compared with the OGD group (Fig. 2C). In addition, OGD significantly
increased the activity of caspase-3 to 216.24% compared with the
control group (Fig. 2D). When
co-treated with different doses of GS-Rb1, the activity of
caspase-3 was attenuated from 207.3 to 121.4%. These results
indicated that GS-Rb1 inhibited OGD-induced apoptosis by regulating
apoptotic signaling pathways.
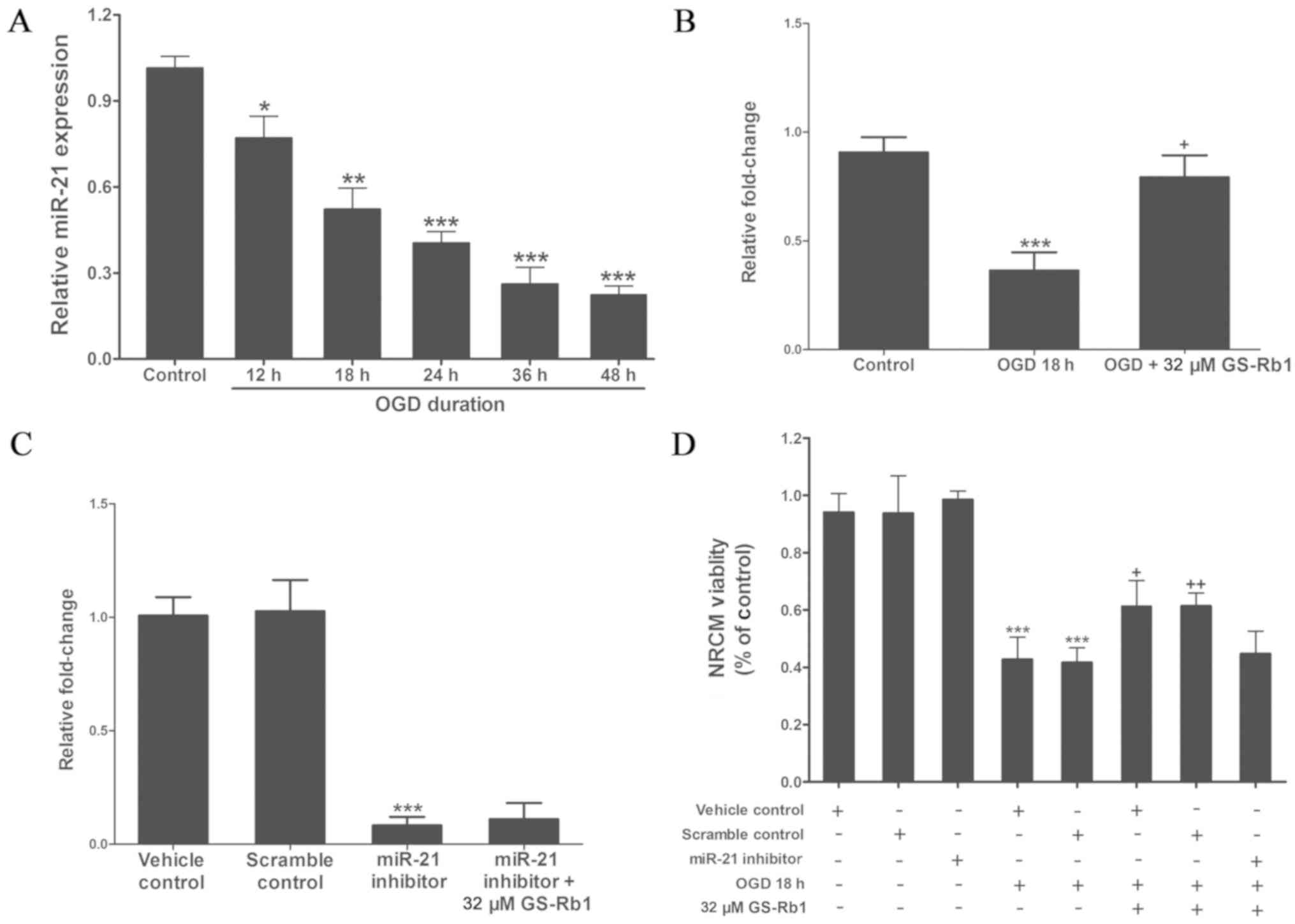
GS-Rb1 treatment increases miR-21
expression and cell viability in OGD-injured NRCMs
In the current study, RT-qPCR analysis was used to
detect the expression of miR-21 in various groups. Compared with
the control group, as the OGD duration increased, the expression of
miR-21 continuously declined (Fig.
3A). When the duration of OGD treatment reached 18 h, miR-21
expression in cardiac muscle cells was only ~45% of the control
group. Following treatment with 32 µM GS-Rb1, the expression of
miR-21 significantly increased compared with the OGD 18 h group
(Fig. 3B). Following the
transfection of NRCMs with a miR-21 inhibitor, the expression of
miR-21 was significantly reduced compared to the vehicle control
group and the increase in miR-21 expression caused by GS-Rb1 was
inhibited by the miR-21 inhibitor (Fig.
3C). Further analysis of cell viability demonstrated that the
inhibition of miR-21 weakened the protective effect of GS-Rb1 on
NRCMs damaged by OGD, which demonstrated that miR-21 is likely to
act as a target of GS-Rb1 in the protection of cardiac muscle cells
(Fig. 3D).
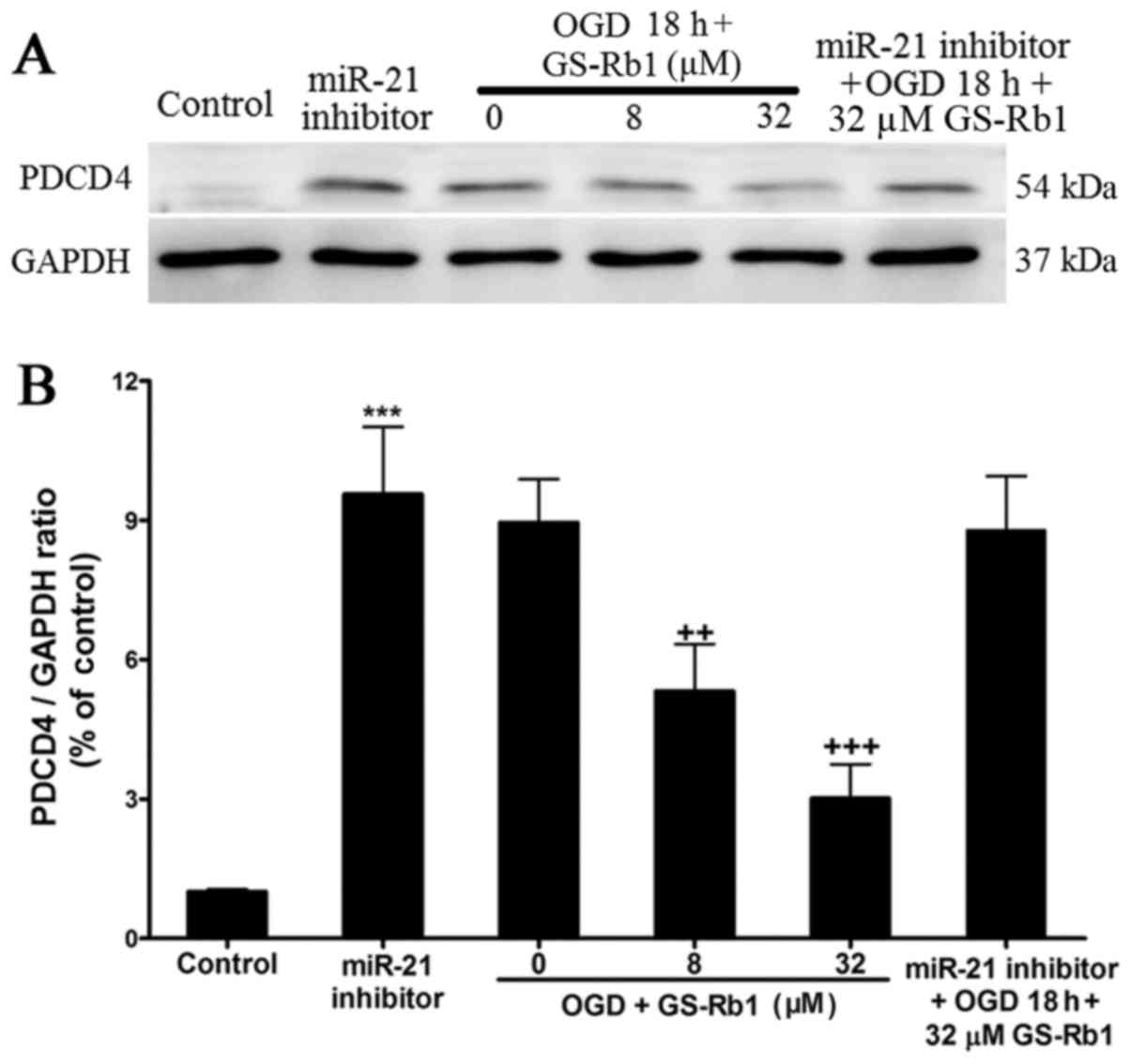
GS-Rb1 decreases PDCD4 in NRCMs
damaged by OGD
The PDCD4 downstream target protein of miR-21 was
evaluated using western blot analysis (Fig. 4A). In the control group, PDCD4
exhibited almost no expression; however, PDCD4 expression was
higher in cardiac muscle cells damaged by OGD (Fig. 4B). Increasing GS-Rb1 concentration
gradually decreased the expression of PDCD4. When the expression of
miR-21 was inhibited by the miR-21 inhibitor, the expression of
PDCD4 was significantly increased compared with the control group,
while OGD and GS-Rb1 treatment had no significant effect on the
increased PDCD4 expression induced by miR-21 expression
inhibition.
Discussion
Previous studies have only identified differentially
expressed miRNAs in OGD-injured NRCMs with GS-Rb1 treatment
(12,13). The present study is the first to
verify the miRNA target of GS-Rb1 in the OGD induced cardiomyocytes
apoptosis. Through inhibition of cardiac muscle cell apoptosis,
GS-Rb1 may greatly alleviate cardiac muscle cell reduction and
relieve myocardial damage (16).
In the current study, cardiac muscle cell damage
following a myocardial infarction was simulated by establishing a
model of OGD damage in NRCMs. GS-Rb1 was then added to the cells at
different concentrations to determine the optimum dose of GS-Rb1
for the protection of cardiac muscle cells, which appeared to be
dose dependent. Through the detection of apoptosis-associated
proteins, it was determined that GS-Rb1 may reduce OGD-induced
intracellular ROS contents, and decrease the expression of Cyt C in
the cytoplasm and Bax. Simultaneously, GS-Rb1 may increase the
expression of Bcl-2 and inhibit the activity of caspase-3,
ultimately inhibiting the activation of the apoptosis signaling
pathway and contributing to myocardial preservation. Further
experiments revealed that GS-Rb1 may increase miR-21 expression
following OGD damage, thereby reducing the expression of PDCD4 and
limiting the activation of the apoptosis signaling pathway, which
ultimately decreased apoptosis caused by OGD.
Studies on the regulatory effect of miR-21 on
myocardial infarctions and apoptosis have been increasing. Numerous
studies have verified the overexpression of miR-21 protects the
heart (17,18). The investigation of a model of
ischemia-reperfusion injury in mice and rats demonstrated that the
expression of miR-21 in ischemic regions of the myocardium was
lower when compared with the control group, miR-21 overexpression
may reduce myocardial ischemia-reperfusion injury and a miR-21
inhibitor can eliminate the protective effect of a miR-21 stimulant
(19,20). Studies on ischemic cardiomyopathy
revealed that the expression levels of miR-21 in the heart ischemia
border zone and non-ischemic regions were higher, while miR-21
expression in ischemic regions was lower compared with the normal
tissues (7,21). Furthermore, it was demonstrated that
the overexpression of miR-21 through a virus transfection method
could reduce the ischemic area and relieve congestive heart failure
after 2 weeks (21).
The results of the current study demonstrated that
after 18 h of OGD, the miR-21 expression level was decreased, while
apoptosis was increased. Additionally, GS-Rb1 enhanced miR-21
expression while reducing NRCM apoptosis, demonstrating that miR-21
may be a miR target through which GS-Rb1 protects the myocardium.
Although the effect of miR-21 mimics on OGD-injured NRCMs was
evaluated with and without GS-Rb1, and miR-21 mimics upregulated
miR-21 expression, NRCM viability did not change with culturing
under OGD conditions or GS-Rb1 treatment (data not shown). It was
hypothesized that the stagnation of cell viability may be
associated with the NRCM injury model (e.g., OGD/reoxygenation
model or the oxidative stress injury model). Previous studies
revealed that miR-21 overexpression could protect against
ischemia-reperfusion- and doxorubicin-induced cardiac cell death
(18,22). However, the results of miR-21
overexpression in the OGD model require further study. In addition,
future study should focus on other signaling pathways and miRs
involved in the process.
miRs can regulate gene expression at the
post-transcription level. The major mechanism of action of miRs
involves the binding of the ‘seed sequence’ of the miR to the
3′-untranslated region of the target mRNA to inhibit the
translation of the target mRNA or promote its degradation, thereby
restricting gene and protein expression (23). Previous studies have highlighted that
miR-21-specific targets include phosphatidylinositol
3,4,5-trisphosphate 3-phosphatase and dual-specificity protein
phosphatase PTEN (PTEN) (24),
reversion-inducing cysteine-rich protein with Kazal motifs (RECK)
(25) and PDCD4 (26–28).
However, positive results were not obtained in western blotting for
PTEN and RECK in the present study (data not shown). The PDCD4 gene
is a cancer suppressor gene that may directly regulate the
apoptosis of cells (29). A number
of studies have verified that apoptosis in a tumor can be regulated
by PDCD4, a target gene of miR-21 (30,31). In
the cardiovascular system, it has been demonstrated that the
upregulation of miR-21 expression can inhibit the expression of
PDCD4 to protect cardiomyocytes (26–28). The
present study also verified that treatment with GS-Rb1 in
vitro can protect OGD-damaged NRCMs through the upregulation of
miR-21 and the inhibition of PDCD4 expression.
Myocardial apoptosis caused by OGD is one of the key
characteristics of congestive heart failure following a myocardial
infarction (32). The identification
of a method for reducing the apoptosis of cardiac muscle cells
under OGD conditions is an important research direction in regard
to myocardial infarction treatment.
Although the cause-effect association between GS-Rb1
and miR-21/PDCD4 requires further clarification, to the best of our
knowledge, the in vitro experiments in the current study
revealed for the first time that the protective effect of GS-Rb1
may act directly via miR-21 and its target gene, PDCD4. Future
in vivo studies to investigate the effects of GS-Rb1
treatment and role of miR-21 in other ischemic cardiovascular
diseases will be performed. To conclude, the current study not only
provides new options and concepts for cardiovascular
disease-associated medicines involving miRs as targets, but also
provides direct laboratory data for the clinical application of
GS-Rb1 in the treatment of myocardial infarction.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY performed the western blot analysis and analyzed
the data. BL performed the cell biology experiments. YSL performed
the transfections into the neonatal rat cardiomyocytes and reverse
transcription-quantitative polymerase chain reaction analysis. YX
designed the current study and was a major contributor in writing
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal protocols in current research were
approved by the Animal Care Center and Use Committee of Jilin
University.
Patient consent to publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GS-Rb1
|
ginsenoside Rb1
|
|
OGD
|
oxygen-glucose deprivation
|
|
miR-21
|
microRNA-21
|
|
PDCD4
|
programmed cell death protein 4
|
|
Cyt C
|
cytochrome C
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
CCK-8
|
Cell Counting Kit-8
|
|
DCFH
|
2,7-dichlorodihydrofluorescein
|
|
PTEN
|
phosphatidylinositol
3,4,5-trisphosphate 3-phosphatase and dual-specificity protein
phosphatase PTEN
|
|
RECK
|
reversion-inducing cysteine-rich
protein with Kazal motifs
|
References
|
1
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al: Heart disease and stroke statistics-2017 update: A report
from the american heart association. Circulation. 135:e146–e603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andersson C and Vasan RS: Epidemiology of
cardiovascular disease in young individuals. Nat Rev Cardiol.
15:230–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fordyce CB, Gersh BJ, Stone GW and Granger
CB: Novel therapeutics in myocardial infarction: Targeting
microvascular dysfunction and reperfusion injury. Trends Pharmacol
Sci. 36:605–616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao L, Zhou QS, Wang L and Hou G:
MicroRNA-182-5p protects H9c2 cardiomyocytes from hypoxia-induced
apoptosis by down-regulation of PTEN. Int J Clin Exp Pathol.
10:5220–5226. 2017.
|
|
5
|
van Rooij E, Sutherland LB, Liu N,
Williams AH, McAnally J, Gerard RD, Richardson JA and Olson EN: A
signature pattern of stress-responsive microRNAs that can evoke
cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA.
103:18255–18260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang K, Jiang Z, Webster KA, Chen J, Hu H,
Zhou Y, Zhao J, Wang L, Wang Y, Zhong Z, et al: Enhanced
cardioprotection by human endometrium mesenchymal stem cells driven
by exosomal MicroRNA-21. Stem Cells Transl Med. 6:209–222. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng Y and Zhang C: MicroRNA-21 in
cardiovascular disease. J Cardiovasc Transl Res. 3:251–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krichevsky AM and Gabriely G: miR-21: A
small multi-faceted RNA. J Cell Mol Med. 13:39–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu XN, Chen Y, Xu Z, Liang X, Wang XH,
Zhang Y, Yuan M, Ni YP, Liu HM and Li GP: MiR-21 suppresses
ox-LDL-induced HUVECs apoptosis by targeting PDCD4. Int J Clin Exp
Pathol. 10:10075–10084. 2017.
|
|
10
|
Suzuki C, Garces RG, Edmonds KA, Hiller S,
Hyberts SG, Marintchev A and Wagner G: PDCD4 inhibits translation
initiation by binding to eIF4A using both its MA3 domains. Proc
Natl Acad Sci USA. 105:3274–3279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee CH and Kim JH: A review on the
medicinal potentials of ginseng and ginsenosides on cardiovascular
diseases. J Ginseng Res. 38:161–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan X, Xue J, Wu H, Wang S, Liu Y, Zheng
S, Zhang C and Yang C: Ginsenoside-Rb1 protects Hypoxic- and
ischemic-damaged cardiomyocytes by regulating expression of miRNAs.
Evid Based Complement Alternat Med. 2015:1713062015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan X, Liu J, Wu H, Liu Y, Zheng S, Zhang
C and Yang C: Impact of miR-208 and its target gene Nemo-like
kinase on the protective effect of ginsenoside Rb1 in
Hypoxia/Ischemia injuried cardiomyocytes. Cell Physiol Biochem.
39:1187–1195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan X, Tian J, Wu H, Liu Y, Ren J, Zheng
S, Zhang C, Yang C, Li Y and Wang S: Ginsenoside rb1 protects
neonatal rat cardiomyocytes from hypoxia/ischemia induced apoptosis
and inhibits activation of the mitochondrial apoptotic pathway.
Evid Based Complement Alternat Med. 2014:1491952014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown DI and Griendling KK: Regulation of
signal transduction by reactive oxygen species in the
cardiovascular system. Circ Res. 116:531–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duygu B and Da Costa Martins PA: miR-21: A
star player in cardiac hypertrophy. Cardiovasc Res. 105:235–237.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong Z, Jiang B, Wu Y, Liu Y, Li Y, Gao M,
Jiang Y, Lv Q and Xiao X: MiR-21 Protected cardiomyocytes against
doxorubicin-induced apoptosis by targeting BTG2. Int J Mol Sc.
16:14511–14525. 2015. View Article : Google Scholar
|
|
19
|
Mukhopadhyay P, Mukherjee S, Ahsan K,
Bagchi A, Pacher P and Das DK: Restoration of altered microRNA
expression in the ischemic heart with resveratrol. PLoS One.
5:e157052010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fasanaro P, D'Alessandra Y, Magenta A,
Pompilio G and Capogrossi MC: microRNAs: Promising biomarkers and
therapeutic targets of acute myocardial ischemia. Curr Vasc
Pharmacol. 13:305–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong SM, Cheng Y, Yang J, Li J, Liu X,
Wang X, Wang D, Krall TJ, Delphin ES and Zhang C: MicroRNA
expression signature and the role of MicroRNA-21 in the early phase
of acute myocardial infarction. J Biol Chem. 284:29514–29525. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin Y, Yu Y, Dong H, Bian X, Guo X and
Dong S: MicroRNA 21 inhibits left ventricular remodeling in the
early phase of rat model with ischemia-reperfusion injury by
suppressing cell apoptosis. Int J Med Sci. 9:413–423. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015.doi: 10.7554/eLife.05005. View Article : Google Scholar
|
|
24
|
Tu YF, Wan L, Fan YH, Wang K, Bu L, Huang
T, Cheng Z and Shen BZ: Ischemic Postconditioning-mediated miRNA-21
protects against cardiac ischemia/reperfusion Injury via PTEN/Akt
pathway. PLoS One. 8:e758722013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han L, Yue X, Zhou X, Lan FM, You G, Zhang
W, Zhang KL, Zhang CZ, Cheng JQ, Yu SZ, et al: MicroRNA-21
expression is regulated by β-catenin/STAT3 pathway and promotes
glioma cell invasion by direct targeting RECK. CNS Neurosci Ther.
18:573–583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng Y, Zhu P, Yang J, Liu X, Dong S,
Wang X, Chun B, Zhuang J and Zhang C: Ischaemic
preconditioning-regulated miR-21 protects heart against
ischaemia/reperfusion injury via anti-apoptosis through its target
PDCD4. Cardiovasc Res. 87:431–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao J, Pan Y, Li XH, Yang XY, Feng YL,
Tan HH, Jiang L, Feng J and Yu XY: Cardiac progenitor cell-derived
exosomes prevent cardiomyocytes apoptosis through exosomal miR-21
by targeting PDCD4. Cell Death Dis. 7:e22772016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei C, Li L, Kim IK, Sun P and Gupta S:
NF-κB mediated miR-21 regulation in cardiomyocytes apoptosis under
oxidative stress. Free Radic Res. 48:282–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Q and Yang HS: The role of Pdcd4 in
tumour suppression and protein translation. Biol Cell. May
28–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang LH, Ge MH, Hou XX, Cao J, Hu SS, Lu
XX, Han J, Wu YC, Liu X, Zhu X, et al: miR-21 regulates tumor
progression through the miR-21-PDCD4-Stat3 pathway in human
salivary adenoid cystic carcinoma. Lab Invest. 95:1398–1408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rodrigues PM, Afonso MB, Simão AL,
Borralho PM, Rodrigues CMP and Castro RE: Inhibition of NF-κB by
deoxycholic acid induces miR-21/PDCD4-dependent hepatocellular
apoptosis. Sci Rep. 5:175282015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Uriel N, Sayer G, Annamalai S, Kapur NK
and Burkhoff D: Mechanical Unloading in heart failure. J Am Coll
Cardiol. 72:569–580. 2018. View Article : Google Scholar : PubMed/NCBI
|