Introduction
Hypertension refers to a chronic disease with
increased diastolic and/or systolic pressure, which endangers
kidneys, heart, brain and other organs and leads to organic damage
through injuring blood vessels, thereby threatening organ function
and life (1,2). With the changes in people's living
habits and the increase in psychological pressure, the incidence
rate of hypertension has also been increasing each year (3,4).
Hypertension is a long-term chronic disease, but
hypertension-induced myocardial infarction/cerebral infarction,
heart failure/renal failure and other target organ damage have
extremely high disability and fatality rates, placing great
psychological and economic burdens on individuals and families
(5). The heart is one of the
important organs directly damaged by hypertension, and long-term
cardiovascular hypertension leads to vascular remodeling, followed
by gradual cardiac remodeling due to response to such chronic
volume overload pressure, in which the left ventricular hypertrophy
is the most significant. Such persistent remodeling will result in
arrhythmia, and severe heart failure will cause sudden death
(6,7). More than 30% of hypertension patients
have ventricular remodeling in clinic. Therefore, it is necessary
to investigate the possibility of regulating cardiac function in
hypertension and prevent heart failure and sudden death in
hypertension patients.
Micro ribonucleic acid (miRNAs) is a kind of
endogenous non-coding inhibitor, which can bind to the target RNA
through complementary base pairing to interfere in the function of
target RNA and have an inhibitory effect on various types of tumors
(8,9). A number of studies have demonstrated
that the miR-29 family has a close correlation with fibrosis, and
it was found that miR-29 can inhibit fibrosis in the heart and
lungs (10). Widlansky et al
(11) found in the rat model of
diabetes mellitus type II that the miR-29 family is necessary for
the endothelial function in normal human and animal models, which
also has great therapeutic potential for cardiac metabolic
disturbance. Luo et al (12)
found in the mouse experiment that the downregulated miR-29b can
break the elastin, increase the collagen deposition inside the
blood vessel and also increase the degree of thoracic aortic
stiffness, thus leading to hypertension (13). In the present study, the possibility
of miR-29b in regulating blood pressure and cardiac function in the
rat model of hypertension was investigated, so as to provide a new
therapeutic target for hypertension patients and a potential marker
for diagnosing cardiac damage in hypertension.
Materials and methods
Laboratory animals
A total of 60 male specific pathogen-free rats with
spontaneous hypertension aged 3 months and with a weight of 260–290
g were purchased and fed for 1 week to adapt to the laboratory
environment (24°C, 12/12 light/dark cycles and humidity 60 ±10%)
with free access to water and food. The systolic pressure of rats
in quiet and waking conditions were measured via caudal artery
using the non-invasive blood pressure measurement and analysis
system (tail-cuff method) at about 10 a.m. after the tail was
heated for 5 min. It was measured every 5 min for 2 weeks until the
blood pressure became 150 mmHg. After feeding for 3 weeks, rats
were randomly divided into the lentivirus group (n=20), the
negative lentivirus group (n=20) and the control group (n=20).
The study was approved by the Ethics Committee of
Tianjin Hospital of ITCWM, Nankai Hospital (Nankai, China).
Reagents and materials
ZH-HX-Z non-invasive blood pressure measurement and
analysis system for spontaneously hypertensive rats (Anhui Zhenghua
Biological Instrument Equipment Co., Ltd., Huaibei, China),
lentivirus with miR-29b overexpression sequence and negative
control virus (Shanghai Zhonghong Boyuan Biological Technology Co.,
Ltd., Shanghai, China), high-efficiency lentivirus transfection
enhancement solution (Shanghai Umibio Science and Technology Co.,
Ltd., Shanghai, China), ultrasound diagnostic instrument (Henan
Enpusi Electronic Technology Co., Ltd., Henan, China), 10% chloral
hydrate (Shanghai Jianglai Biotechnology Co., Ltd., Shanghai,
China), TRIzol reagent, chloroform and isopropanol (Thermo Fisher
Scientific, Inc., Shanghai, China), reverse transcription reagent
and 2X All-in-One miRNA quantitative polymerase chain reaction
(PCR) kit (Wuhan MSK Biotechnology Co., Ltd., Wuhan, China).
Lentivirus transfection
Equal volumes of miR-29b inhibitor gene lentiviral
vector and negative control lentiviral vector were thawed via ice
bath, and diluted into 108 TU/ml with the
high-efficiency lentivirus transfection enhancement solution. Rats
in the lentivirus group were injected with 150 ml lentivirus
solution, and those in the negative lentivirus and control groups
were injected with the same amount of the negative control virus
solution and high-efficiency lentivirus transfection enhancement
solution, respectively.
High-frequency echocardiography
At 3 weeks after the injection of lentivirus, rats
were weighed and anesthetized with 10% chloral hydrate (200 mg/kg).
M-mode echocardiography was performed for all rats using the 7.5
MHz ultrasound diagnostic instrument to detect left ventricular
posterior wall thickness (LVPWT), interventricular septum thickness
(IVST), left ventricular end-diastolic diameter (LVEDD) and left
ventricular end-systolic diameter (LVESD), and left ventricular
ejection fraction (LVEF) was calculated using the Teichholtz
method.
Treatment of heart specimens of
rats
At 3 weeks after transfection (at 6 weeks of
feeding), rats were weighed and anesthetized with 10% chloral
hydrate (200 mg/kg). The chest was opened, and the heart was taken
and washed clean. The left ventricle, including interventricular
septum, was retained, and left ventricular mass (LVM) (mg) was
obtained. LVM index (LVMI) = LVM/body mass (BM). After the left
ventricle was weighed, it was stored in liquid nitrogen used for
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR).
Detection of miR-29b expression via
RT-qPCR
After 50 mg of myocardial tissue was taken and
ground in a mortar containing TRIzol reagent, RNA was extracted
with chloroform, and the upper aqueous phase was retained and added
with 0.5 volume of isopropanol to extract the total RNA. The
concentration and purity of RNA extracted were detected using an
ultraviolet spectrophotometer (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The absorbance A260/A280 of 1.8–2.0 indicated
the qualified purity. The reverse transcription was immediately
performed for extracted RNA using the 25 µl reaction system
prepared according to Table I (37°C
for 60 min and 85°C for 5 min). The synthesized complementary
deoxyribonucleic acid (cDNA) was diluted by 100 times, and 20 µl
amplification system was prepared according to Table I, followed by amplification on a PCR
instrument in accordance with the procedure in Table II, with U6 as an internal reference
(forward primer, 5′-CGCTTCGGCAGCACATATAC-3′ and reverse primer,
5′-TTCACGAATTTGCGTGTCAT-3′). miR-29b: forward primer,
5′-ACACTCCAGCTGGGTAGCACCATCTGAAA-3′ and reverse primer,
5′-CTCAACTGGTGTCGTGGA-3′. Results were statistically processed
using the 2−∆∆Cq method (14).
 | Table I.Preparation of reaction system. |
Table I.
Preparation of reaction system.
| Reverse
transcription |
| PCR |
|
|---|
| 2.5 U/µl Poly A
polymerase | 1 µl | miR-29b primer (10
µM) | 2 µl |
| Rtase mixture | 1 µl | Universal Adaptor PCR
Primer (2 µM) | 2 µl |
| 5X reaction
buffer | 5 µl | 2X All-in-One qPCR
Mix | 10 µl |
| Total RNA | 2 µg | cDNA | 2 µl |
| Add RNase free
H2O to 25 µl |
| Add RNase free
H2O to 20 µl |
|
 | Table II.PCR procedure. |
Table II.
PCR procedure.
| Step | Temperature (°C) | Time |
|---|
| Pre-denaturation | 95 | 10 min |
| 40 Ct |
|
|
|
Denaturation | 95 | 10 sec |
|
Annealing | 60 | 20 sec |
|
Extension | 72 | 32 sec |
Statistical analysis
Cardiac function parameters, blood pressure and PCR
results are presented as mean ± standard deviation (mean ± SD).
SPSS 20.0 (Asia Analytics, formerly SPSS China) software package
was used for data inspection and analysis. Repeated measures
analysis of variance was used for the comparison within the same
group before and after experiment according to the data
distribution characteristics. Analysis of variance was adopted for
the comparison among more than three groups with Least Significant
Difference test. t-test was used for pairwise comparisons in case
of difference. P<0.05 indicates that the difference was
statistically significant.
Results
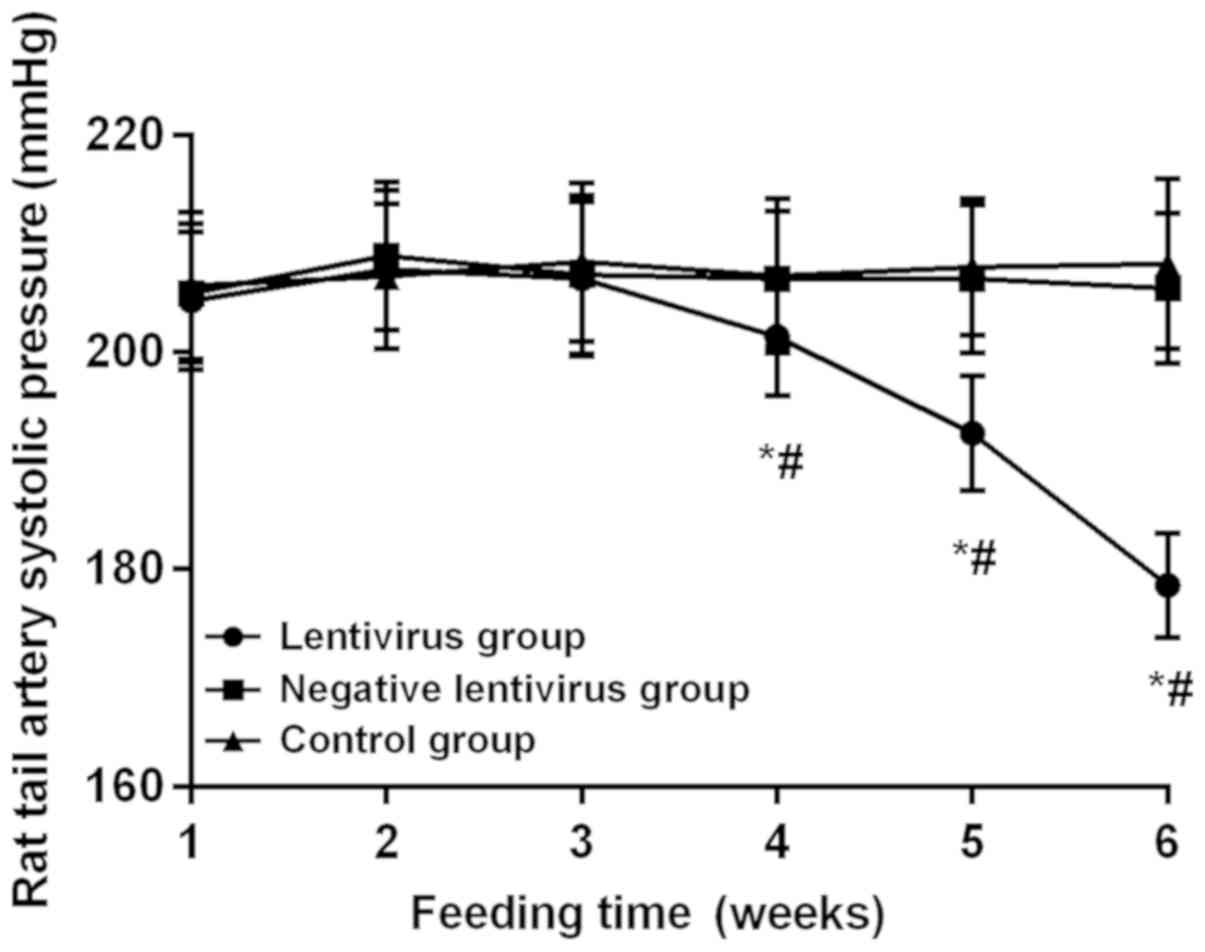
Blood pressure in rats
At 1, 2 and 3 weeks after feeding without virus
transfection, there were no statistically significant differences
in the blood pressure of rats among the groups, indicating that the
blood pressure is comparable after virus transfection. The blood
pressure in the lentivirus group began to drop at 3 weeks after
virus transfection, and it was significantly decreased at 4 weeks
(201.43±5.42 mmHg) compared with that at 3 weeks (206.76±7.12 mmHg)
significantly lower at 6 weeks (178.52±4.82 mmHg) than that at 5
weeks (192.52±5.25 mmHg) and also lower at 5 weeks than that at 4
weeks, displaying statistically significant differences (all
P<0.05). The blood pressure declined by 29.13 mmHg on average at
6 weeks after virus transfection compared with that before
transfection. There was no statistically significant difference in
the blood pressure between the negative lentivirus and control
groups during the monitoring for 6 weeks (P>0.05). The blood
pressure in the lentivirus group was obviously lower than that in
the negative lentivirus and control groups at 4, 5 and 6 weeks
(P<0.05) (Fig. 1).
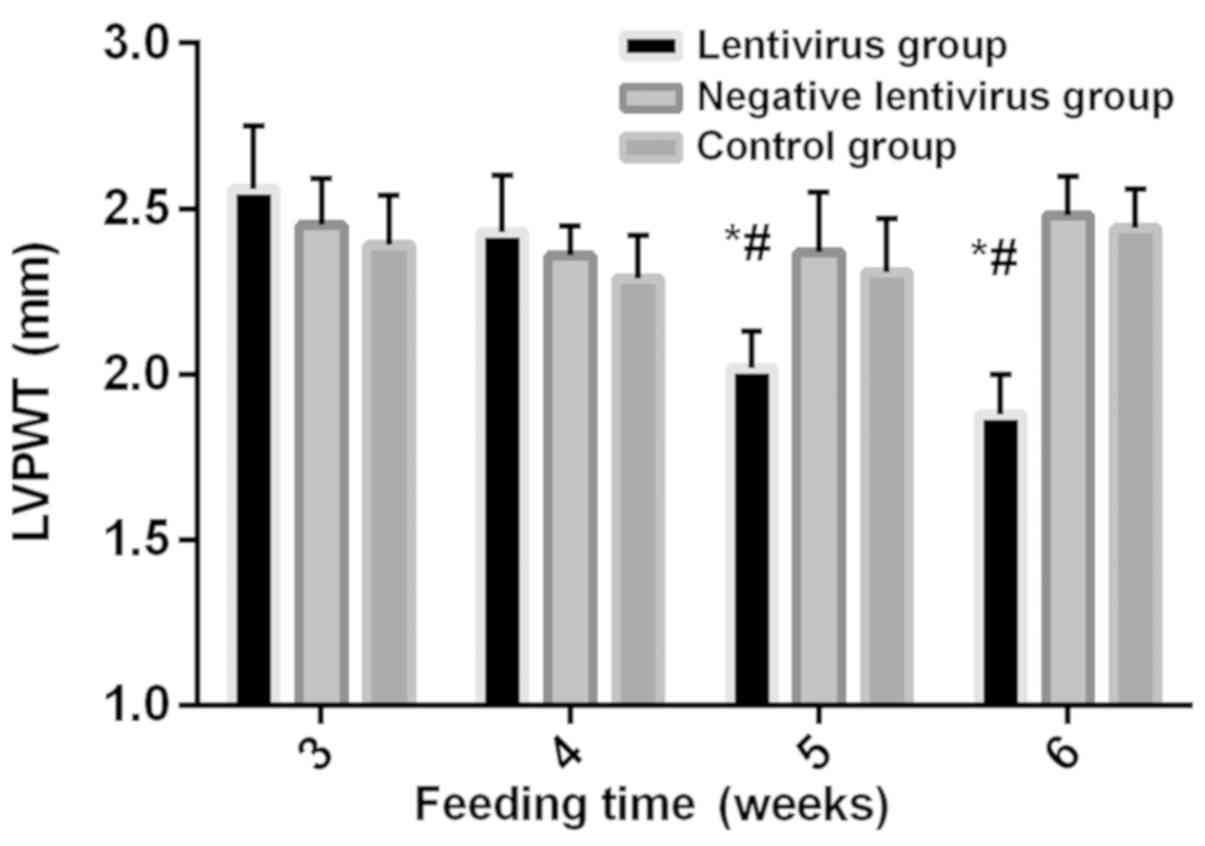
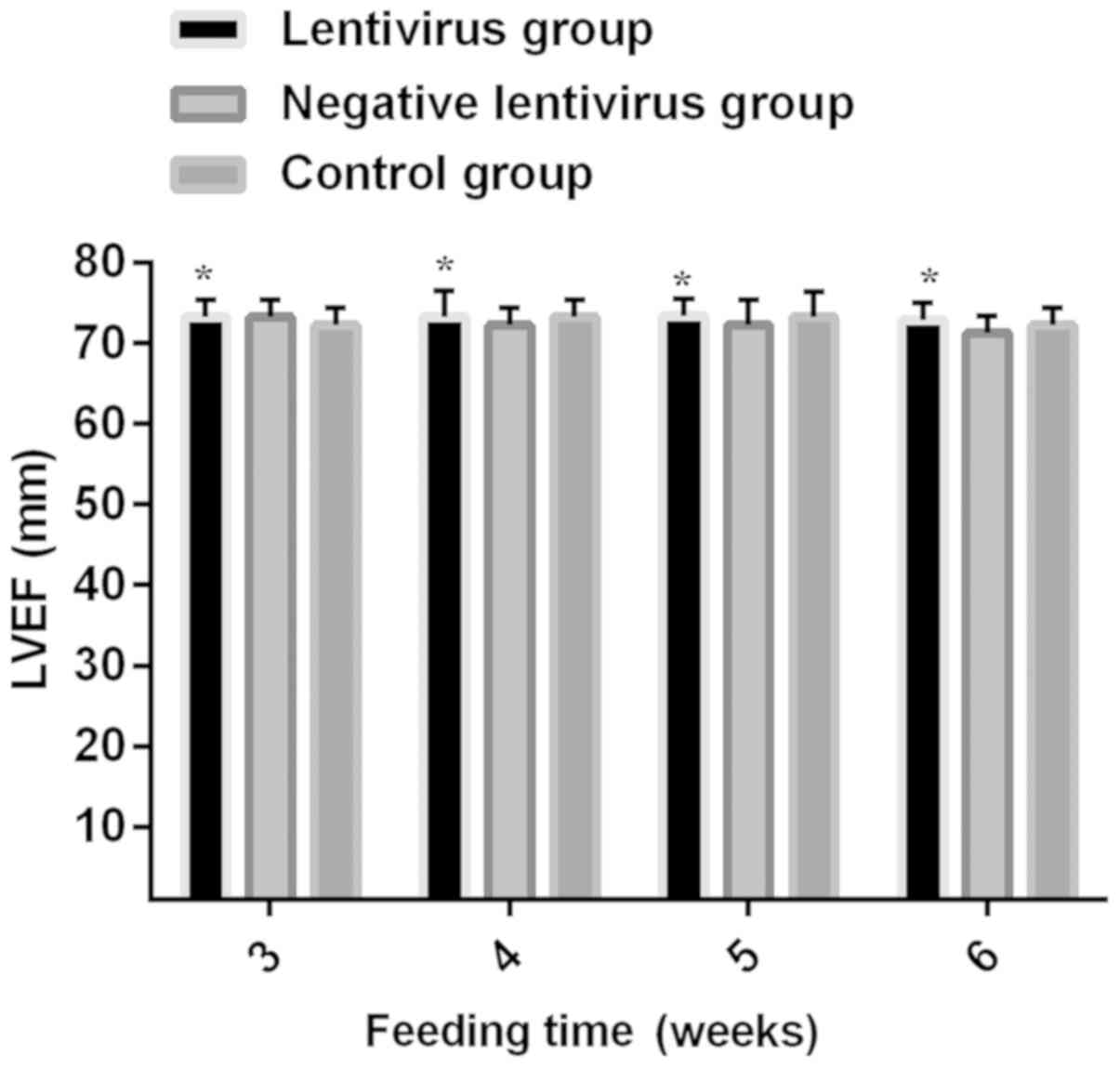
Comparison of echocardiographic
indexes among the three groups
At 3 weeks without virus transfection, no
statistically significant differences were found in LVPWT, IVST,
LVEDD, LVESD and LVEF among the three groups (P>0.05). Before
virus transfection, there were no statistically significant
differences in LVPWT among the three groups (P>0.05). After
virus transfection, LVPWT in the lentivirus group showed a
decreasing trend, and it was significantly decreased at 5 weeks and
6 weeks compared with that in the previous week, and significantly
lower than that in the negative lentivirus and control groups
during the same period (all P<0.05). Before virus transfection,
there were no statistically significant differences in LVEDD among
the three groups (P>0.05). After virus transfection, LVEDD in
the lentivirus group was gradually increased, and it was obviously
higher at 6 weeks than that at 5 weeks, and also higher than that
in the negative lentivirus and control groups during the same
period. There was no difference between the negative lentivirus and
control groups (all P<0.05). LVPWT, IVST, LVEDD, LVESD and LVEF
had no remarkable differences between the negative lentivirus and
control groups and at each time-point within the group (Figs. 2–6).
Comparison of LVMI
At 6 weeks, there was no significant difference in
body weight (BW) among the groups, but LVM and LVMI in the
lentivirus group were obviously lower than those in the negative
lentivirus and control groups (P<0.05) (Table III).
 | Table III.Comparison of LVMI. |
Table III.
Comparison of LVMI.
| Groups | LVM (mg) | BW (g) | LVMI (mg/g) |
|---|
| Lentivirus |
720.41±40.23a | 251.41±7.17 | 2.87±5.61 |
| Negative
lentivirus | 890.92±88.81 | 248.51±7.32 | 3.59±12.13 |
| Control | 910.18±90.44 | 253.71±7.54 | 3.59±12.00 |
| F | 16.66 | 1.13 | 1.46 |
| P-value | <0.001 | 0.39 | 0.26 |
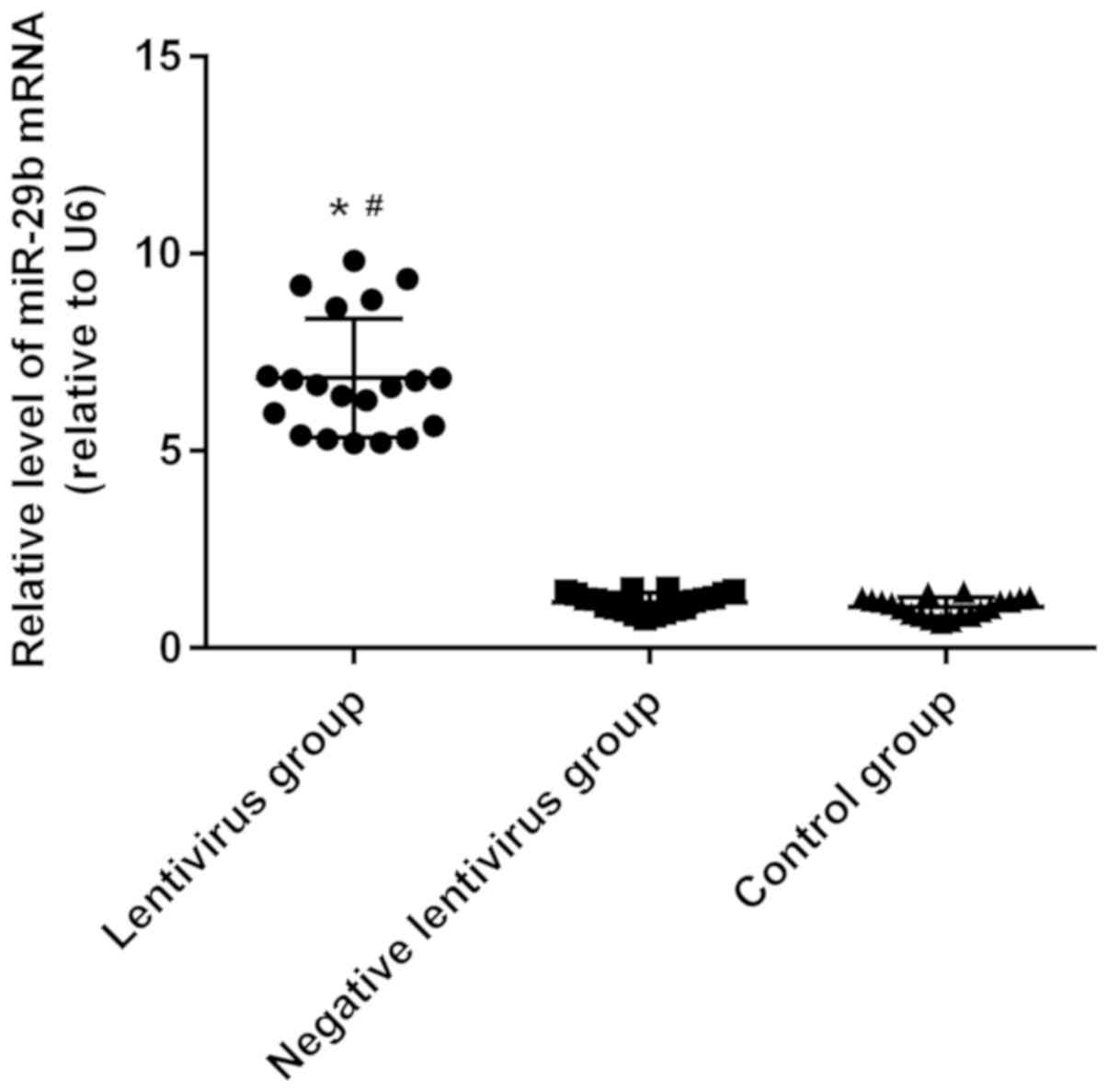
Detection of miR-29b expression via
RT-qPCR
According to results of RT-qPCR analysis of miR-29b
expression in left ventricular tissues of rats, the expression of
miR-29b in the lentivirus group was significantly higher than those
in the negative lentivirus and control groups (P<0.05), and it
had no statistically significant difference between the negative
lentivirus and control groups (Fig.
7).
Discussion
With the development of medical science in recent
years, the diagnostic criteria for hypertension have been
constantly improving, the detection rate of hypertension has been
increasing continuously and the therapeutic regimen has also been
continuously perfecting. However, there is still a big gap in China
compared with developed countries (15). The prevalence rate of hypertension is
still increasing, and it is conservatively estimated that there are
at least 200 million people with hypertension in China currently
(16). At present, therapeutic drugs
for hypertension need to be taken for a long time, there will be a
rebound easily after drug withdrawal, the blood pressure can be
maintained for a short time, and they have such toxic and side
effects as increased serum potassium (17). miR-29b is necessary for normal
endothelial function, which can resist fibrosis and cardiac
metabolic disturbance (18).
Therefore, the mechanism of hypertension in cardiovascular diseases
was explored in this study, so as to provide new ideas for primary
hypertension and cardiovascular and cerebrovascular diseases to
develop new molecular therapy.
It was found in the detection of miR-29b expression
in left ventricular tissues of rats via RT-qPCR that the expression
of miR-29b in the lentivirus group was significantly higher than
that in the negative lentivirus and control groups. Results of this
study revealed that there was no significant difference in systolic
pressure among groups before transfection, and it began to decline
gradually in the lentivirus group after transfection with miR-29b
and was always lower than that in the negative lentivirus and
control groups, suggesting that miR-29b inhibits the increase in
systolic pressure of rats and alleviates hypertension. After
transfection with miR-29b, LVPWT and IVST in the lentivirus group
displayed decreasing trends, LVEDD and LVESD showed increasing
trends, and LVEF remained essentially unchanged. After
transfection, BW had no significant difference among groups, but
LVM and LVMI in the lentivirus group were remarkably lower than
those in the negative lentivirus and control groups, indicating
that the overexpression of miR-29b reverses the left ventricular
hypertrophy. Liu et al (19)
found that Smad3 reverses the downregulation of miR-29b in renal
tissues in hypertensive nephropathy through nuclear factor-κB
(NF-κB), thus, suppressing hypertensive nephropathy. Zhu et
al (20) studied the preventive
effect of berberine on cardiovasculzar diseases, and they also
found that it relieves cardiac remodeling, promotes angiogenesis
and reduces infarct size through significantly increasing the
expression level of miR-29b, thus, improving cardiac function.
Moreover, the expression of miR-29b also significantly declines in
model rats with pulmonary hypertension (13). It was found in this study that the
overexpression of miR-29b in the rat model of hypertension could
improve cardiac function, indicating that miR-29b possesses the
therapeutic potential for hypertension and left ventricular
hypertrophy. miR-29b also exerts a protective effect on the heart
in ventricular remodeling involving AngII.
The rat model of spontaneous hypertension was used
in the present study, and it is more representative for the
spontaneous occurrence of hypertension than induced rat model, with
a probability of hypertension of 100% (20). However, there were also shortcomings
in this study; the occurrence and development of hypertension
lasted for a long time, there were various influencing factors, the
mechanism was complex involving a wide range, it was difficult to
simulate and restore in vitro, and the rat model of
spontaneous hypertension constructed could not fully represent the
actual situation. In the subsequent study, therefore, relevant
clinical data should be collected, and the correlation between
actual expression and hypertension should be analyzed, so as to
further verify the conclusion in this study. Besides, it was found
in this study that miR-29b could indeed reduce blood pressure and
improve cardiac function, but through which target protein it
regulates and through which signaling pathway it causes its effects
need further experiments. It is suggested that TargetScan and other
online software be used to predict the target protein of miR-29b,
and western blotting be performed for verification.
In conclusion, the overexpression of miR-29b can
improve cardiac function, and inhibiting the miR-29b expression can
reduce blood pressure and obviously improve the cardiac function in
hypertension rats.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH and CW assisted with the design of the rat model.
YL and ZJ performed the PCR. BZ and YD interpreted the
high-frequency echocardiography result. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Tianjin Hospital of ITCWM, Nankai Hospital (Nankai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li P, Guo W, Du L, Zhao J, Wang Y, Liu L,
Hu Y and Hou Y: microRNA-29b contributes to pre-eclampsia through
its effects on apoptosis, invasion and angiogenesis of trophoblast
cells. Clin Sci (Lond). 124:27–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takase S, Lerond L, Bergan JJ and
Schmid-Schönbein GW: The inflammatory reaction during venous
hypertension in the rat. Microcirculation. 7:41–52. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Volmink J, Bradley H, Maroney R, Maroney
R, Mbewu A, Opie LH and Volmink J: Betablockers for hypertension.
Cochrane Database Syst Rev. 1:CD0020032007.
|
|
4
|
Liu JQ, Zelko IN, Erbynn EM, Sham JS and
Folz RJ: Hypoxic pulmonary hypertension: Role of superoxide and
NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol.
290:L2–L10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sung YK and Chung L: Connective tissue
disease-associated pulmonary arterial hypertension. Rheum Dis Clin
North Am. 41:295–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rich S and Rabinovitch M: Diagnosis and
treatment of secondary (non-category 1) pulmonary hypertension.
Circulation. 118:2190–2199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scherrer JF, Xian H, Bucholz KK, Eisen SA,
Lyons MJ, Goldberg J, Tsuang M and True WR: A twin study of
depression symptoms, hypertension, and heart disease in middle-aged
men. Psychosom Med. 65:548–557. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan B, Guo Q, Fu FJ, Wang Z, Yin Z, Wei YB
and Yang JR: The role of miR-29b in cancer: Regulation, function,
and signaling. Onco Targets Ther. 8:539–548. 2015.PubMed/NCBI
|
|
9
|
Chaturvedi P, Kalani A, Medina I,
Familtseva A and Tyagi SC: Cardiosome mediated regulation of MMP9
in diabetic heart: Role of mir29b and mir455 in exercise. J Cell
Mol Med. 19:2153–2161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM
and Lan HY: miR-29b as a therapeutic agent for angiotensin
II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol
Ther. 22:974–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Widlansky ME, Jensen DM, Wang J, Liu Y,
Geurts AM, Kriegel AJ, Liu P, Ying R, Zhang G, Casati M, et al:
miR-29 contributes to normal endothelial function and can restore
it in cardiometabolic disorders. EMBO Mol Med. 10:e80462018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo Y, Dong HY, Zhang B, Feng Z, Liu Y,
Gao YQ, Dong MQ and Li ZC: miR-29a-3p attenuates hypoxic pulmonary
hypertension by inhibiting pulmonary adventitial fibroblast
activation. Hypertension. 65:414–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Li Y, Li Y, Xie L, Wang J, Zhang Y
and Xiao T: Effect of miR-29b on the proliferation and apoptosis of
pulmonary artery smooth muscle cells by targeting Mcl-1 and CCND2.
Biomed Res Int. 2018:60514072018.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coghlan JG, Denton CP, Grünig E, Bonderman
D, Distler O, Khanna D, Müller-Ladner U, Pope JE, Vonk MC, Doelberg
M, et al DETECT study group, : Evidence-based detection of
pulmonary arterial hypertension in systemic sclerosis: The DETECT
study. Ann Rheum Dis. 73:1340–1349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosenkranz S, Gibbs JS, Wachter R, De
Marco T, Vonk-Noordegraaf A and Vachiéry JL: Left ventricular heart
failure and pulmonary hypertension. Eur Heart J. 37:942–954. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taichman DB, Ornelas J, Chung L, Klinger
JR, Lewis S, Mandel J, Palevsky HI, Rich S, Sood N, Rosenzweig EB,
et al: Pharmacologic therapy for pulmonary arterial hypertension in
adults: CHEST guideline and expert panel report. Chest.
146:449–475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steele R, Mott JL and Ray RB: MBP-1
upregulates miR-29b that represses Mcl-1, collagens, and
matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer.
1:381–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu GX, Li YQ, Huang XR, Wei LH, Zhang Y,
Feng M, Meng XM, Chen HY, Shi YJ and Lan HY: Smad7 inhibits
AngII-mediated hypertensive nephropathy in a mouse model of
hypertension. Clin Sci (Lond). 127:195–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu ML, Yin YL, Ping S, Yu HY, Wan GR,
Jian X and Li P: Berberine promotes ischemia-induced angiogenesis
in mice heart via upregulation of microRNA-29b. Clin Exp Hypertens.
39:672–679. 2017. View Article : Google Scholar : PubMed/NCBI
|