Introduction
Lung cancer is one of the most common cancer types
with the highest incidence and mortality rate of all cancer types
worldwide (1–5). Lung adenocarcinoma (LADC) accounts for
~75% of non-small cell lung cancer cases, which is the most common
subtype of lung cancer (6,7). LADC has a marked clinical, molecular
and histological heterogeneity. The five-year survival rate of LADC
patients in 2011 was <15% (8).
Therefore, it is essential to investigate the genes associated with
tumor cell invasion and metastasis in patients with LADC.
Furthermore, it is essential to identify the relevant molecular
mechanisms of LADC. With this information, scientists may be able
to develop better treatment methods for the diagnosis and prognosis
of LADC.
Fibronectin type III (FNIII) domain containing 3B
(FNDC3B), which is also known as factor for adipocyte
differentiation 104, is a member of the FN family. It contains 9
FNIII domains and one transmembrane domain (9). Furthermore, FNDC3B regulates the
differentiation of adipocytes and osteoblasts (10–12).
FNIII domains have an important role in cell adhesion and in growth
signaling pathways (13,14). The metastasis of hepatocellular
carcinoma has been reported to be promoted by overexpression of
FNDC3B, while knockdown of FNDC3B suppressed the proliferation and
metastatic capacity of tumor cells (15). It is therefore indicated that FNDC3B
is associated with the growth, invasion and metastasis of tumors.
However, only few studies have reported on the involvement of
FNDC3B in LADC.
Epithelial-mesenchymal transition (EMT) is a process
in which epithelial cells gradually transform into mesenchymal-like
cells, while losing their epithelial functionality and
characteristics (16). After
undergoing EMT, cells typically exhibit higher expression of the
proteins vimentin, N-cadherin and fibronectin; however, these cells
exhibit downregulated expression of E-cadherin and cytokeratins
(17). In patients with lung cancer,
the invasiveness and metastatic capacity of tumor cells is
characterized by the diagnostic marker vimentin (18). When a cell undergoes extensive EMT,
it exhibits a lack E-cadherin expression and an induced expression
of vimentin (19). Furthermore, EMT
is also induced by overexpression of FNDC3B. In different cell
types, including hepatocytes, mammary epithelial cells and renal
epithelial cells, the induced expression of the mesenchymal marker
vimentin and the suppression of E-cadherin occurs with the
transformation of FNDC3B (20).
However, it remains elusive whether the expression of FNDC3B is
associated with the EMT in LADC patients.
In the present study, it was revealed that FNDC3B
and EMT-associated markers were aberrantly expressed in LADC versus
normal tissues. The expression of FNDC3B and EMT markers was
demonstrated to be significantly associated with histological
differentiation, lymph node metastasis and TNM stage. When FNDC3B
was ectopically overexpressed, the protein expression of
EMT-associated genes was increased in the A549 LADC cell line.
Thus, FNDC3B acts as an oncogene in the development and progression
of LADC. Furthermore, the present results may suggest that the EMT
is regulated by FDNC3B.
Materials and methods
Patients and tissue samples
A total of 276 cases of LADC (Median age at
diagnosis, 62 years; age range, 39–77 years) and 82 normal lung
tissues adjacent to the LADC were collected from 276 patients that
were treated at the Affiliated Hospital of Nantong University in
China (Nantong, China). All of these patients were treated by
surgical resection between 2007 and 2011. A total of 125 females
were recruited, which accounted for 45.2% of the subjects included
in the present study. The clinical data of all patients included
were carefully recorded after the diagnosis of LADC was confirmed
by two pathologists. The pathological stage was determined by
according to the 8th Edition of the tumor-nodes-metastasis (TNM)
Classification for Lung Cancer (21). The follow-up was completed by June
30, 2014 and the median follow-up duration was 52 months.
Tissue microarray (TMA) construction
and immunohistochemistry (IHC)
The TMA was generated using the Quick-Ray system
(cat no. UT06; Unitma, Seoul, Korea). Core tissue biopsies (2 mm in
diameter) were taken from individual formalin-fixed and
paraffin-embedded tissue blocks and arranged in paraffin blocks.
TMA specimens were cut into 4-µm sections and placed on super
frost-charged glass microscope slides. Hematoxylin and eosin
staining was performed for 15 min at room temperature for TMA
analysis.
The EnVision method (22) was used for IHC staining. A total of
276 LADC samples and 82 normal lung tissues were included in the
TMA. The samples were incubated for 40 min at 70°C, dewaxed and
dehydrated in a graded series of ethanols. Using TMA in antigen
repairing 22 min at 99°C, dripping with 3% hydrogen peroxide for 20
min at room temperature. Samples were then blocked using 10% normal
goat serum (0060-01; Shanghai Haoran Biotechnology Co., Ltd.,
Shanghai, China) for 30 min at room temperature. Subsequently, the
samples were washed with PBS containing Triton X-100 three times,
and incubated with polyclonal rabbit anti human FNDC3B (1:150
dilution; cat. no. HPA007859; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), mouse anti-human E-cadherin (1:100 dilution; cat. no.
MAB-0738) and rabbit anti-human vimentin (1:100 dilution; RMA-0547)
(both from Maixin Biotech, Co. Ltd., Fuzhou, China) overnight at
4°C. Subsequently, the samples were incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:500
dilution; cat. no. sc-2004; SantaCruz Biotechnology, Inc., Dallas,
TX, USA) for 2 h at room temperature. Antibodies were visualized
using diaminobenzedene hydrochloride and PBS was used as a negative
control instead of the primary antibody.
FNDC3B staining was quantified by evaluating at
least 1,000 cells from at least five randomly selected fields of
view. Prior to performing univariate and multivariate analyses, the
intensity of immunostaining in each tumor section was classified as
follows: 3, strong; 2, moderate; 1, weak; and 0, negative.
Furthermore, the percentage of stained cells was determined using
the following semi-quantitative scale: 0, <5% of cells; 1,
5–25%; 2, 26–50%; 3, 51–75%; and 4, >75% of cells. The two
scores were multiplied and according to the final score obtained,
the samples were classified into two groups: i) Score of 0–6, low
expression and ii) score of 7–12, high expression (23,24). The
cut-off for E-cadherin expression was set at 70%. In certain tumor
samples, E-cadherin expression was >70%; a membranous staining
developed in the entire tumor containing these cells, indicating a
high expression of E-cadherin. Expression of vimentin was
frequently observed in the cytoplasm of the tumor cells. According
to cytoplasm staining, a high expression of vimentin was defined as
>20% of tumor cells (24–27).
Western blot analysis
Cells were lysed in cell lysis solution containing
50 mM Tris-Cl, 1% (w/v) SDS, sodium pyrophosphate,
β-glycerophosphate, sodium orthovanadate, sodium fluoride, EDTA and
leupeptin (cat. no. P0013; Beyotime Institute of Biotechnology,
Haimen, China). A protease inhibitor cocktail (Roche Applied
Science, Penzburg, Germany) was supplemented prior to use. Protein
(50 µg) was separated using 10% SDS-PAGE gel and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Non-specific protein interactions were blocked by incubation
with 3% fat-free milk in Tris-buffered saline (150 mm NaCl and 50
mM Tris-HCl, pH 7.6) at 4°C for 1 h. Subsequently, the membrane was
incubated at 4°C for 2 h with polyclonal rabbit anti-human FNDC3B
(1:300 dilution; cat. no. HPA007859; Sigma-Aldrich; Merck KGaA),
mouse anti-human E-cadherin (1:1,000 dilution; cat. no. MAB-0738),
rabbit anti-human vimentin (1:1,000 dilution; cat. no. RMA-0547)
(both from Maixin Biotech, Co. Ltd.) and rabbit anti β-actin
antibody (1:2,000 dilution; cat. no. 4970; Santa Cruz
Biotechnology, Inc.). Following five washes with PBS with Tween-20
(5 min/wash), membranes were incubated with horseradish peroxidase
conjugated-polyclonal goat anti-mouse secondary antibodies
(1:10,000; cat. no. ab6789; Abcam, Cambridge, UK) for 1 h at room
temperature. Signals were detected with an enhanced
chemiluminescence system (Sigma-Aldrich; Merck KGaA) according to
the manufacturer's protocol. β-actin was used as the loading
control.
Cell culture
The A549 human LADC cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA). Cell culture
of A549 cells was performed in RPMI-1640 medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (Haoyang Biological Manufacture Co. Ltd., Tianjin, China) and
100 units penicillin-streptomycin in a humidified atmosphere with
5% CO2 at 37°C.
Construction of the pcDNA3.1-FNDC3B
vector
Based on the Genbank sequence (NM_022763.3),
upstream and downstream primers were synthesized for FNDC3B gene
amplification. The restriction enzyme sites for BamHI and
KpnI were added to the 5 end of each primer. Next, the
full-length FNDC3B gene was amplified by polymerase chain reaction
(PCR) from the complementary DNA template with the primers
containing the BamHI and KpnI restriction sites. The
PCR product was then ligated into the pGEM-T vector (Promega
Corporation, Madison, WI, USA), which was transformed into A549
cells that were screened for positive clones. After sequence
verification, the correct sequence was subcloned into the pcDNA3.1
expression vector (Shenzhen Zhonghong Boyuan Biological Technology
Co., Ltd., Shenzhen, China) to construct the pcDNA3.1-FNDC3B
recombinant expression vector.
Transfection of pcDNA3.1-FNDC3B or
FNDC3B small hairpin (sh)RNA into A549 cells
The A549 cells were transfected with either the
pcDNA3.1-FNDC3B eukaryotic expression vector or FNDC3B shRNA using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. After transfection, the
cells were diluted at a 1:16 ratio and re-seeded in 12-well plates
at a density of 2×105 cells per well. The media was
replaced with complete medium containing G418 (800 µg/ml).
Following isolation, cells were re-suspended and cultured in medium
containing G418 (cat. no. 10131035; Thermo Fisher Scientific, Inc.)
for subsequent experiments. The stable positive clones were
selected approximately 21 days later and protein expression was
evaluated via western blotting.
RNA extraction and reverse
transcription-quantitative (RTq)PCR
RTqPCR was performed according to a procedure
described in previous study (28).
The total RNA was extracted with TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to manufacturer's protocol. The
mRNA expression of FNDC3B and β-actin was determined with ABI7500
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The data were obtained by normalizing the
quantification cycle (Cq) values for FNDC3B to those of the
corresponding β-actin, followed by calculation of 2−ΔΔCq
(29). The primer sequences were as
follows: FNDC3B forward, 5′-GGGACAGACACCCGTTTTGA-3′ and reverse,
5′-GTGTTGCCCACGGTAATGCT-3′; β-actin forward,
5′-GCACCACACCTTCTACAATGAG-3′ and reverse,
5′-ACAGCCTGGATGGCTACGT-3′.
Invasion and migration assays
The invasion and migration assays were performed
according to a procedure described in a previous study (28).
Colony formation assay
A total of ~200 cells were seeded into six-well
plates in triplicate. The resultant cell culture was formed within
14 days in these plates. The cells were fixed with methanol for 15
min at room temperature and stained with 1% crystal violet for 30
min at room temperature. A colony was regarded as a cell aggregate
derived from a single cell containing >50 cells.
Statistical analysis
Statistical analysis of the RT-qPCR and western blot
data was performed using an unpaired Student's t-test when two
groups were compared. The comparisons multile groups were made
using a chi-square analysis or Fisher's exact test, if appropriate.
The Chi-square and Phi coefficient were used to confirm the
association between the expression of FNDC3B and EMT indicators.
Kaplan-Meier analysis with the log-rank test was used for
calculating and analyzing survival curves. Cox regression analysis
was performed for univariate and multivariate analyses. P<0.05
was considered to indicate a statistically significant difference.
The data were analyzed by using SPSS 22.0 software for Windows (IBM
Corporation, Chicago, IL, USA).
Results
FNDC3B protein is increased in LADC
tissues
IHC analysis indicated that FNDC3B protein was
highly expressed in LADC tumors; the high expression of FDNC3B was
determined to be 77.17% (213/276) in these tumors. By contrast, the
overexpression of FDNC3B was only 15.85% (13/82) in the adjacent
non-cancerous tissues. The expression of FNDC3B protein was
significantly upregulated in LADC patients (P<0.001; Table I).
 | Table I.FNDC3B immunohistochemical staining
of protein in normal lung tissues and LADC tissues. |
Table I.
FNDC3B immunohistochemical staining
of protein in normal lung tissues and LADC tissues.
|
|
| FNDC3B
expression |
|
|---|
|
|
|
|
|
|---|
| Group | Cases (n) | Low, n (%) | High, n (%) | P-value |
|---|
| Normal lung
tissue | 82 | 69 (84.15) | 13 (15.85) | <0.001 |
| LADC | 276 | 63 (22.83) | 213 (77.17) |
|
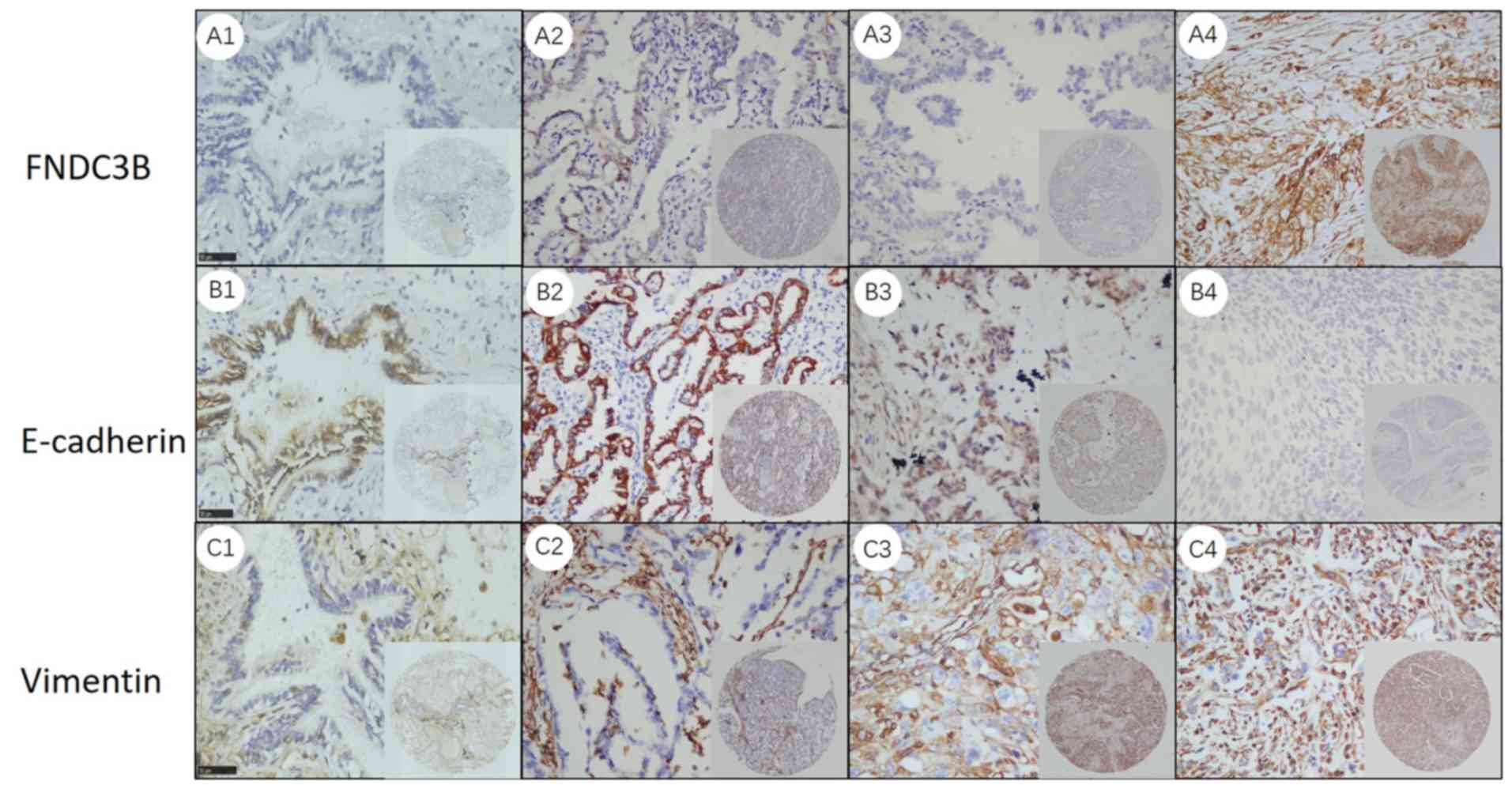
Representative IHC images for FNDC3B are presented
in Fig. 1A. In normal lung tissues,
the expression of FNDC3B protein was relatively low (Fig. 1A1). Furthermore, FNDC3B protein was
predominantly localized in the cytoplasm of tumor cells, as well as
in the membrane. The intensity of FNDC3B staining was increased
with the deterioration of the degree of differentiation of the
tumors (Fig. 1A2-4).
Association between the expression of
FNDC3B in LADC tissues and clinicopathological characteristics
Table II provides
the association of FNDC3B expression in LADC tissues and the
clinicopathological characteristics. The expression of FNDC3B (high
vs. low) was significantly associated with the histological
differentiation, lymph node metastasis and TNM stage (P<0.001);
however, it was not significantly associated with age, sex, smoking
history and the tumor maximum diameter (P>0.05).
 | Table II.Association of FNDC3B expression with
clinicopathological characteristics of lung adenocarcinoma
patients. |
Table II.
Association of FNDC3B expression with
clinicopathological characteristics of lung adenocarcinoma
patients.
|
| FNDC3B
expression |
|
|
|---|
|
|
|
|
|
|---|
| Characteristic | Low, n (%) | High, n (%) | χ2
test | P-value |
|---|
| Sex |
|
Male | 66 (54.55) | 85 (54.55) | 0.002 | 0.961 |
|
Female | 55 (45.45) | 70 (45.45) |
|
|
| Age (years) |
|
≤60 | 56 (46.28) | 69 (46.28) | 0.085 | 0.77 |
|
>60 | 65 (53.72) | 86 (53.72) |
|
|
| Smoking
history |
| No | 79 (65.29) | 97 (65.29) | 0.216 | 0.642 |
|
Yes | 42 (34.71) | 58 (34.71) |
|
|
| Maximum tumor
diameter (cm) |
| ≤3 | 57 (47.11) | 86 (47.11) | 1.91 | 0.167 |
|
>3 | 64 (52.89) | 69 (52.89) |
|
|
| Histological
differentiation |
|
Well | 32 (26.45) | 4 (26.45) | 54.176 | <0.001 |
|
Moderate | 65 (53.72) | 64 (53.72) |
|
|
|
Poor | 24 (19.83) | 87 (19.83) |
|
|
| Lymph node
metastasis |
| No | 107 (88.43) | 34 (88.43) | 120.238 | <0.001 |
|
Yes | 14 (11.57) | 121 (11.57) |
|
|
| TNM stage |
| I | 105 (86.78) | 30 (86.78) | 127.668 | <0.001 |
| II | 13 (10.74) | 50 (10.74) |
|
|
|
III | 3 (2.48) | 66 (2.48) |
|
|
| IV | 0 (0.00) | 9 (0.00) |
|
|
Overexpression of FNDC3B indicates
poor prognosis
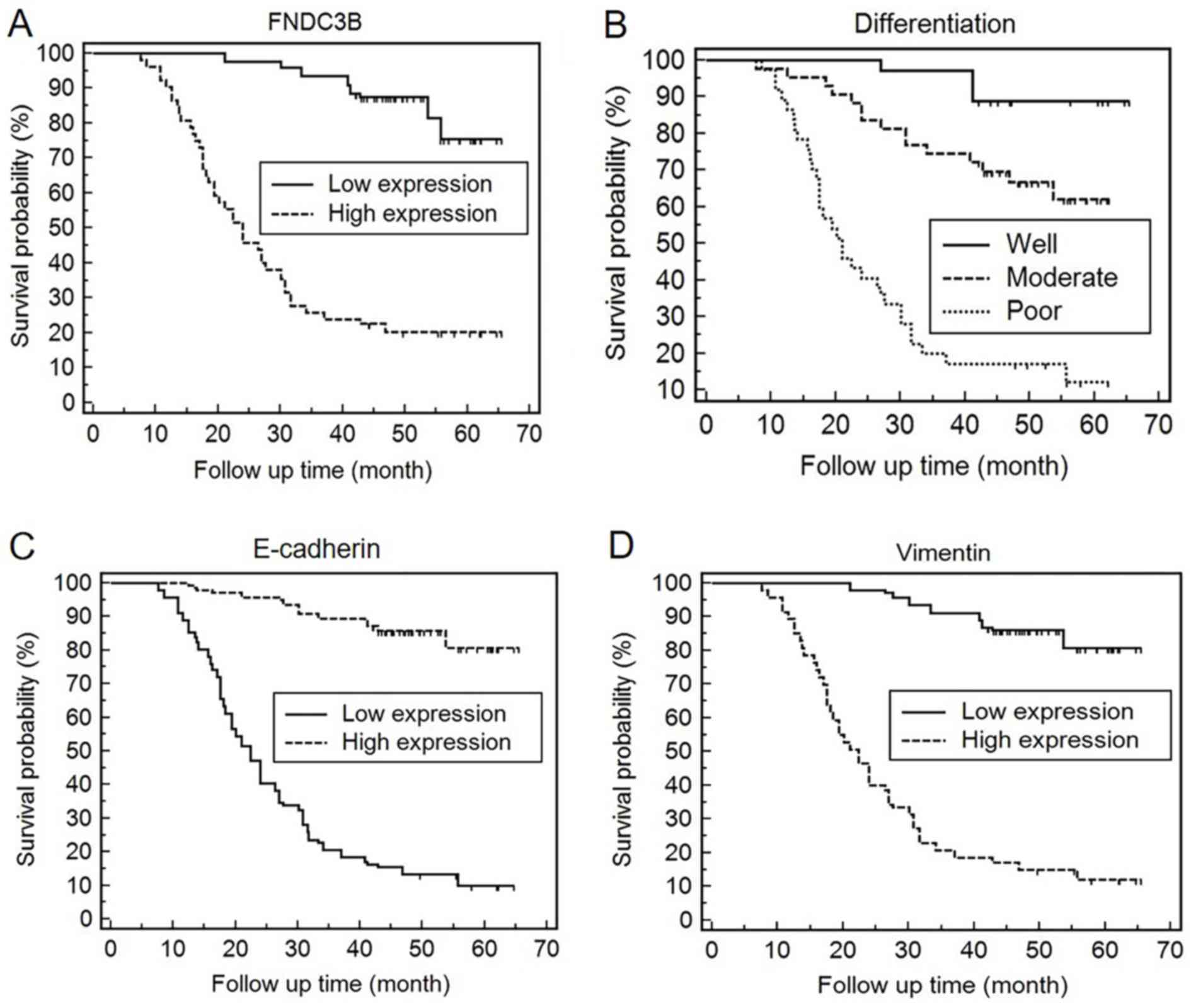
Kaplan-Meier survival curves indicated that the
expression of FNDC3B may be used to predict the prognosis of LADC
patients (χ2=122.2695, P<0.0001). The survival rate
of patients with high protein expression of FNDC3B in LADC tissues
was significantly lower than that with a low expression (Fig. 2A). In these LADC patients, the
expression of FNDC3B was an independent prognostic factor as
indicated by logistic regression analysis using Cox's proportional
hazards model (P=0.007; Table
III). Kaplan-Meier survival analysis was also used to evaluate
the impact of the histological degree of differentiation on patient
survival (Fig. 2B). The histological
differentiation was revealed to be an independent prognostic factor
in LADC patients (P<0.001), which was also confirmed by Cox
regression analysis (Table
III).
 | Table III.Univariate and multivariate analyses
of various prognosis parameters in 276 patients with lung
adenocarcinoma using Cox regression model. |
Table III.
Univariate and multivariate analyses
of various prognosis parameters in 276 patients with lung
adenocarcinoma using Cox regression model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Covariate | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 0.929 | (0.669–1.288) | 0.657 | 0.952 | (0.676–1.341) | 0.779 |
| Sex | 1.002 | (0.722–1.391) | 0.991 |
|
|
|
| Smoking
history | 1.344 | (0.964–1.875) | 0.081 |
|
|
|
| Maximum tumor
diameter | 1.026 | (0.739–1.424) | 0.878 |
|
|
|
| Histological
differentiation | 4.597 | (3.349–6.310) | <0.001 | 2.663 | (1.899–3.736) | <0.001 |
| Lymph node
metastasis | 7.009 | (4.664–10.534) | <0.001 | 0.776 | (0.36–1.672) | 0.517 |
| TNM stage | 2.974 | (2.434–3.633) | <0.001 | 1.278 | (0.89–1.835) | 0.184 |
| FNDC3B | 8.967 | (5.616–14.317) | <0.001 | 2.311 | (1.255–4.254) | 0.007 |
| E-cadherin | 0.086 | (0.054–0.135) | <0.001 | 0.428 | (0.218–0.842) | 0.014 |
| Vimentin | 11.707 | (7.384–18.561) | <0.001 | 2.428 | (1.247–4.728) | 0.009 |
EMT markers are aberrantly expressed
in LADC tissues
The high expression rates of E-cadherin and vimentin
were 20.29% (56/276) and 74.28% (205/276), respectively (Table IV). According to the IHC results,
E-cadherin was mainly expressed in the cell membrane of LADC
tissues. Furthermore, vimentin was mainly expressed in the
cytoplasm. In normal lung tissues, vimentin expression was low,
while that of E-cadherin was high (Fig.
1B1 and C1). In LADC tissues, the intensity of IHC staining for
E-cadherin declined with the deterioration of the degree of
differentiation (Fig. 1B2-4), while
the opposite trend was observed for vimentin (Fig. 1C2-4).
 | Table IV.Immunohistochemical analysis of
epithelial-mesenchymal transition markers in normal lung tissues
and LADC tissues. |
Table IV.
Immunohistochemical analysis of
epithelial-mesenchymal transition markers in normal lung tissues
and LADC tissues.
|
|
| E-cadherin
expression |
| Vimentin
expression |
|
|---|
|
|
|
|
|
|
|
|---|
| Tissue sample | Cases (n) | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value |
|---|
| Normal lung
tissue | 82 | 12 (14.63) | 70 (85.37) | <0.001 | 71 (86.59) | 11 (13.41) | <0.001 |
| LADC | 276 | 220 (79.71) | 56 (20.29) |
| 71 (25.72) | 205 (74.28) |
|
Association between the expression of
EMT markers in LADC tissues and clinicopathological
characteristics
Table V presents the
association of EMT marker expression in LADC tissues (high vs. low)
with clinicopathological parameters. An aberrant expression of EMT
markers was significantly associated with histological
differentiation, metastasis and TNM stage (P<0.001); however, it
was not significantly associated with age, sex, smoking history and
the tumor maximum diameter (P>0.05).
 | Table V.Association between markers of the
epithelial-mesenchymal transition and clinicopathological
characteristics. |
Table V.
Association between markers of the
epithelial-mesenchymal transition and clinicopathological
characteristics.
|
| E-cadherin
expression |
|
| Vimentin |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Characteristic | Low | High | χ2
test | P-value | Low | High | χ2
test | P-value |
|---|
| Sex |
|
Male | 74 | 77 | 0.01 | 0.922 | 72 | 79 | 0.339 | 0.561 |
|
Female | 62 | 63 |
|
| 64 | 61 |
|
|
| Age (years) |
|
≤60 | 65 | 60 | 0.679 | 0.41 | 62 | 63 | 0.01 | 0.922 |
|
>60 | 71 | 80 |
|
| 74 | 77 |
|
|
| Smoking
history |
| No | 80 | 96 | 2.837 | 0.092 | 93 | 83 | 2.471 | 0.116 |
|
Yes | 56 | 44 |
|
| 43 | 57 |
|
|
| Maximum tumor
diameter (cm) |
| ≤3 | 70 | 73 | 0.012 | 0.911 | 70 | 73 | 0.012 | 0.911 |
|
>3 | 66 | 67 |
|
| 66 | 67 |
|
|
| Histological
differentiation |
|
Well | 4 | 32 | 59.444 | <0.001 | 32 | 4 | 64.94 | <0.001 |
|
Moderate | 48 | 81 |
|
| 80 | 49 |
|
|
|
Poor | 84 | 27 |
|
| 24 | 87 |
|
|
| Lymph node
metastasis |
| No | 23 | 118 | 125.316 | <0.001 | 120 | 21 | 148.069 | <0.001 |
|
Yes | 113 | 22 |
|
| 16 | 119 |
|
|
| TNM stage |
| I | 22 | 113 | 120.939 | <0.001 | 115 | 20 | 146.977 | <0.001 |
| II | 44 | 19 |
|
| 19 | 44 |
|
|
|
III | 61 | 8 |
|
| 2 | 67 |
|
|
| IV | 9 | 0 |
|
| 0 | 9 |
|
|
Abnormally expressed EMT markers
indicate poor prognosis
Kaplan-Meier survival analysis indicated that the
expression of E-cadherin and vimentin was associated with the OS of
LADC patients (χ2=173.7963, P<0.0001;
χ2=170.3951, P<0.0001). In LADC patients, an
increased expression of vimentin indicated a lower OS rate. By
contrast, a high expression of E-cadherin indicated that the
probability of survival for LADC patients at any given time-point
was significantly higher compared with that for patients with low
E-cadherin expression (Fig. 2C and
D). Multivariate Cox regression analyses further revealed that
E-cadherin and vimentin may be used as independent prognostic
markers for determining the prognosis of LADC patients (P=0.014;
0.009; Table III).
Association between the expression of
FNDC3B and markers of EMT
The association between the expression of FNDC3B and
the two EMT markers determined by IHC was calculated and presented
in Table VI. The results indicate
that the expression of FNDC3B was positively associated with
expression of vimentin (P<0.001) but negatively associated with
the levels of E-cadherin levels in LADC tissues (P<0.001).
 | Table VI.Association between the expression of
FNDC3B and EMT marker proteins detected by
immunohistochemistry. |
Table VI.
Association between the expression of
FNDC3B and EMT marker proteins detected by
immunohistochemistry.
|
| FNDC3B
expression |
|
|
|---|
|
|
|
|
|
|---|
| EMT marker
expression | Low | High | Phi
coefficient | P-value |
|---|
| E-cadherin |
|
Low | 9 | 127 | −0.739 | <0.001 |
|
High | 112 | 28 |
|
|
| Vimentin |
|
Low | 110 | 26 | 0.736 | <0.001 |
|
High | 11 | 129 |
|
|
FNDC3B has an oncogenic role in
LADC
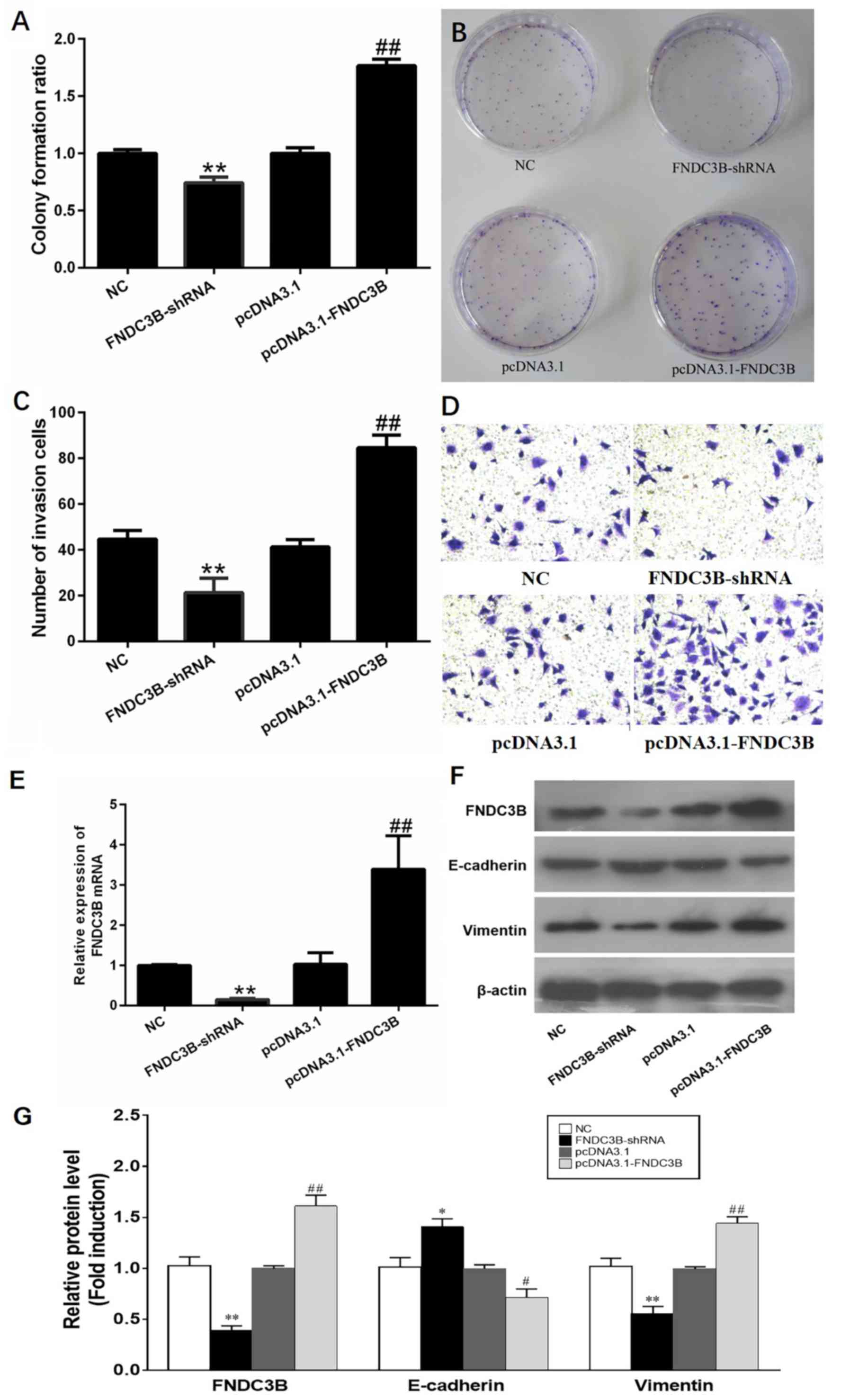
By performing colony formation assays using
transfected A549 cells, it was revealed that LADC cells
proliferated rapidly when the levels of FNDC3B were increased,
while it was suppressed following knockdown of FNDC3B (Fig. 3A and B). In a Transwell system, an
increased expression of FNDC3B significantly promoted the migration
and invasion of A549 cells. By contrast, the migration and invasion
of LADC cells was reduced following knockdown of FNDC3B (Fig. 3C and D). In the present study, the
A549 cell line was transfected with pcDNA3.1-FNDC3B or shFNDC3B,
and the efficacy of ectopic overexpression or knockdown of FNDC3B
was determined by RT-qPCR (Fig. 3E).
Overexpression of the FNDC3B protein resulted in downregulation of
E-cadherin levels but upregulation of vimentin levels, while the
opposite effect was obtained with FNDC3B knockdown (Fig. 3F and G). These results indicate that
FNDC3B may have an oncogenic role in LADC.
Discussion
The present study suggested that FNDC3B may be used
as a prognostic marker in patients with LADC. Compared with that in
normal lung tissues, the expression of FNDC3B was significantly
higher in LADC tissues. A high expression of FNDC3B was detected in
TMAs of LADC specimens by using IHC, and it was identified to be
significantly associated with histological differentiation, lymph
node metastasis and TNM stage. Furthermore, a high expression of
FNDC3B was associated with a poor prognosis in terms of OS. Several
studies have reported that FNDC3B is an oncogene, which promotes
cell proliferation in the following types of malignancies:
Esophageal squamous cell carcinoma, glioblastoma and hepatocellular
carcinoma (20,30). A previous study has also indicated
that the metastatic potential of prostate cancer cells may be
enhanced by modulating the expression of FNDC3B (31). This result further supports the
notion that FNDC3B may be an important oncogenic driver gene
(20). In line with this, the
results of the present study indicate that FNDC3B protein was
overexpressed in LADC tissues.
Metastasis is one of the most decisive factors that
affect the prognosis of patients. The detection of EMT is widely
used to determine tumor metastasis (32). A lack of E-cadherin expression, which
also leads to an upregulation of vimentin expression, is regarded
to be a hallmark of EMT (33). The
EMT significantly increases the invasive potential of tumor cells
(34). Tumor metastasis
significantly impairs the long-term survival of patients;
therefore, it is important to identify the molecular markers
associated with metastasis. These molecular markers may be used to
predict the prognosis of patients with lung cancer (35). An abnormal expression of EMT markers
indicates the presence of invasive and metastatic tumor cells in
patients with lung cancer (18).
Therefore, EMT markers may be considered as independent diagnostic
markers of lung cancer. In the present study, an aberrant
expression of molecular markers of the EMT was associated with the
TNM stage, metastasis and prognosis of lung cancer patients. A high
expression of vimentin in LADC tissues was associated with a
significantly decreased probability of OS. However, a high
expression of E-cadherin in LADC tissues was associated with a
significantly increased probability of OS. By performing Cox
regression analysis, E-cadherin and vimentin were identified as
independent prognostic factors for LADC. In LADC tissues, FNDC3B
expression was positively associated with vimentin levels but
negatively associated with E-cadherin levels. The IHC results
indicated that the intensity of FNDC3B and vimentin staining in
LADC tissues was increased with deteriorating degree of
differentiation; however, the opposite effect was observed for
E-cadherin. In the present study, it was concluded that in LADC
tissues, FNDC3B expression was positively associated with vimentin
levels (r=0.736, P<0.01) but negatively associated with
E-cadherin levels (r=−0.739, P<0.001).
Overexpression of FNDC3B protein may not only
induces EMT but also activate several pathways associated with the
development of cancer, including phosphoinositide-3 kinase/Akt,
retinoblastoma 1 and transforming growth factor (TGF)-β signaling
(20). A recent study demonstrated
that FNDC3B is a novel suppressor of TGF-β signalling and represses
the TGF-β-mediated EMT in cervical cancer cells (36). The present study indicated that
FNDC3B is an oncogene that promotes the invasion and migration of
A549 LADC cells. In the in vitro experiments of the present
study, overexpression of FNDC3B protein not only enhanced the
expression of vimentin but also reduced the expression of
E-cadherin. These results allow for the following conclusion: In
LADC tissues, FNDC3B may instigate the process of EMT to promote
the invasion and metastasis of tumor cells.
Despite these significant results, the present study
has several limitations. First, it was a retrospective research
study, which may have led to selection bias and the availability of
clinical data was limited. Consequently, it is required to confirm
the present results in future studies including a larger number of
LADC cases. Furthermore, the present study only included LADC
patients; therefore, the results are restricted to lung cancer
only. In addition, the present study only identified the function
of FNDC3B in A549 cells. In future studies, the function of FNDC3B
will be verified in another LADC cell line. Furthermore, EMT
markers not only include the decreased expression of E-cadherin and
increased expression of vimentin, but also other markers, e.g.
N-cadherin, Snail and Slug. Therefore, the association between
FNDC3B and those other EMT proteins will be examined in cancer
specimens and in vitro in a subsequent study. Finally, the
present study did not examine the mechanism through which FNDC3B
regulates the EMT in LADC. A follow-up study will determine whether
FNDC3B is regulated by the classical pathway of the EMT to promote
the progression of LADC.
In conclusion, the present study indicated that
FNDC3B is overexpressed in LADC tissues and is an independent
prognostic factor of an inferior outcome. Furthermore, FNDC3B
functions as an oncogene in LADC, and its high expression may
promote the invasion and metastasis of tumor cells. Furthermore,
the present results indicate that FNDC3B has an important role in
the regulation of genes associated with the EMT in LADC tissues.
FNDC3B may be utilized as a novel molecular marker in LADC and may
serve as target for drug development.
Acknowledgements
Not applicable.
Funding
This study was funded by grants from the Six Talent
Peaks Project of Jiangsu Province (grant no. WSN-059), the Science
Foundation of Nantong City (grant nos. MS12015007 and MS22016032),
Scientific Research Topic of Jiangsu Provincial Health and Family
Planning Commission (grant no. H201626), Youth Scientific Research
Fund of Nantong Health and Family Planning Commission (grant nos.
WQ2016072 and WQ2016031) and the Nantong Science and Technology
Program (guidance; grant no. GJZ17009).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TB, LZ, YL and SY conceived and designed the present
study. JL, JZ, JF, QZ, LQ and HQ collected the data and performed
the experiments. DJ performed the data analysis and interpretation.
TB and YL were involved in the preparation of manuscript. All the
authors have read and approved the final manuscript.
Ethical approval and consent to
participate
Written informed consent was obtained from all study
participants, and the study protocol was approved by the Human
Research Ethics Committee of the Affiliated Hospital of Nantong
University in China (Nantong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cha MJ, Lee HY, Lee KS, Jeong JY, Han J,
Shim YM and Hwang HS: Micropapillary and solid subtypes of invasive
lung adenocarcinoma: Clinical predictors of histopathology and
outcome. J Thorac Cardiovasc Surg. 147:921–928.e2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ujiie H, Kadota K, Chaft JE, Buitrago D,
Sima CS, Lee MC, Huang J, Travis WD, Rizk NP, Rudin CM, et al:
Solid predominant histologic subtype in resected stage I lung
adenocarcinoma is an independent predictor of early, extrathoracic,
multisite recurrence and of poor postrecurrence survival. J Clin
Oncol. 33:2877–2884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu S, Xi J, Jiang W, Lu S and Wang Q:
Solid Component and Tumor Size Correlate With Prognosis of Stage IB
Lung Adenocarcinoma. Ann Thorac Surg. 99:961–967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang X, Liu Y, Lian F, Guo L, Wen P, Liu
XY and Lin DM: Lepidic and micropapillary growth pattern and
expression of Napsin A can stratify patients of stage I lung
adenocarcinoma into different prognostic subgroup. Int J Clin Exp
Pathol. 7:1459–1468. 2014.PubMed/NCBI
|
|
5
|
Zhang Y, Wang R, Cai D, Li Y, Pan Y, Hu H,
Wang L, Li H, Ye T, Luo X, et al: A comprehensive investigation of
molecular features and prognosis of lung adenocarcinoma with
micropapillary component. J Thorac Oncol. 9:1772–1778. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 1:10–29. 2012. View Article : Google Scholar
|
|
7
|
Lortettieulent J, Soerjomataram I, Ferlay
J, Rutherford M, Weiderpass E and Bray F: International trends in
lung cancer incidence by histological subtype: adenocarcinoma
stabilizing in men but still increasing in women. Lung Cancer.
84:13–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith CB, Kelley AS and Meier DE: Evidence
for new standard of care in non-small cell lung cancer patients.
Semin Thorac Cardiovasc Surg. 22:193–194. 2011. View Article : Google Scholar
|
|
9
|
Kishimoto K, Nishizuka M, Ueda T, Kajita
K, Ugawa S, Shimada S, Osada S and Imagawa M: Indispensable role of
factor for adipocyte differentiation 104 (fad104) in lung
maturation. Exp Cell Res. 317:2110–2123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tominaga K, Kondo C, Johmura Y, Nishizuka
M and Imagawa M: The novel gene fad104, containing a fibronectin
type III domain, has a significant role in adipogenesis. FEBS Lett.
577:49–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishizuka M, Kishimoto K, Kato A, Ikawa M,
Okabe M, Sato R, Niida H, Nakanishi M, Osada S and Imagawa M:
Disruption of the novel gene fad104 causes rapid postnatal death
and attenuation of cell proliferation, adhesion, spreading and
migration. Exp Cell Res. 315:809–819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kishimoto K, Kato AS, Nishizuka M and
Imagawa M: Fad104, a positive regulator of adipogenesis, negatively
regulates osteoblast differentiation. Biochem Biophys Res Commun.
397:187–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin F, Ren XD, Pan Z, Macri L, Zong WX,
Tonnesen MG, Rafailovich M, Bar-Sagi D and Clark RA: Fibronectin
growth factor-binding domains are required for fibroblast survival.
J Invest Dermatol. 131:84–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Obara M, Sakuma T and Fujikawa K: The
third type III module of human fibronectin mediates cell adhesion
and migration. J Biochem. 147:327–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin CH, Lin YW, Chen YC, Liao CC, Jou YS,
Hsu MT and Chen CF: FNDC3B promotes cell migration and tumor
metastasis in hepatocellular carcinoma. Oncotarget. 7:49498–49508.
2016.PubMed/NCBI
|
|
16
|
Sung WJ, Kim H and Park KK: The biological
role of epithelial-mesenchymal transition in lung cancer (Review).
Oncol Rep. 36:1199–1206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weber CE, Li NY, Wai PY and Kuo PC:
Epithelial-mesenchymal transition, TGF-Î2, and osteopontin in wound
healing and tissue remodeling after injury. J Burn Care Res.
33:311–318-. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geiger TR and Peeper DS: Metastasis
mechanisms. Biochim Biophys Acta. 1796:293–308. 2009.PubMed/NCBI
|
|
19
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428-.
2015. View
Article : Google Scholar
|
|
20
|
Cai C, Rajaram M, Zhou X, Liu Q, Marchica
J, Li J and Powers RS: Activation of multiple cancer pathways and
tumor maintenance function of the 3q amplified oncogene FNDC3B.
Cell Cycle. 11:1773–1781. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sabattini E, Bisgaard K, Ascani S, Poggi
S, Piccioli M, Ceccarelli C, Pieri F, Fraternali-Orcioni G and
Pileri SA: The EnVision++ system: a new immunohistochemical method
for diagnostics and research. Critical comparison with the APAAP,
ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol.
51:506–511. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui Y, Liu J, Yin HB, Liu YF and Liu JH:
Fibulin-1 functions as a prognostic factor in lung adenocarcinoma.
Jpn J Clin Oncol. 45:854–859. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Gai L, Liu J, Cui Y, Zhang Y and
Feng J: Expression of poly(C)-binding protein 1 (PCBP1) in NSCLC as
a negative regulator of EMT and its clinical value. Int J Clin Exp
Pathol. 8:7165–7172. 2015.PubMed/NCBI
|
|
25
|
Brzozowska A, Sodolski T, Duma D,
Mazurkiewicz T and Mazurkiewicz M: Evaluation of prognostic
parameters of E-cadherin status in breast cancer treatment. Ann
Agric Environ Med. 19:541–546. 2012.PubMed/NCBI
|
|
26
|
Xu B, Jin DY, Lou WH and Wang DS:
Lipocalin-2 is associated with a good prognosis and reversing
epithelial-to-mesenchymal transition in pancreatic cancer. World J
Surg. 37:1892–1900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu MM, Mao GX, Liu J, Li JC, Huang H, Liu
YF and Liu JH: Low expression of the FoxO4 gene may contribute to
the phenomenon of EMT in non-small cell lung cancer. Asian Pac J
Cancer Prev. 15:4013–4018-. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bian T, Jiang D, Liu J, Yuan X, Feng J, Li
Q, Zhang Q, Li X, Liu Y and Zhang J: miR-1236-3p suppresses the
migration and invasion by targeting KLF8 in lung adenocarcinoma
A549 cells. Biochem Biophys Res Commun. 492:461–467. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen CF, Hsu EC, Lin KT, Tu PH, Chang HW,
Lin CH, Chen YJ, Gu DL, Lin CH, Wu JY, et al: Overlapping
high-resolution copy number alterations in cancer genomes
identified putative cancer genes in hepatocellular carcinoma.
Hepatology. 52:1690–1701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan X, Xu C, Deng W, Zhong G, Cai Q and
Lin T: Upregulated microRNA-143 in cancer stem cells
differentiation promotes prostate cancer cells metastasis by
modulating FNDC3B expression. BMC Cancer. 13:612013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shan ZZ, Yan XB, Yan LL, Tian Y, Meng QC,
Qiu WW, Zhang Z and Jin ZM: Overexpression of Tbx3 is correlated
with Epithelial-Mesenchymal Transition phenotype and predicts poor
prognosis of colorectal cancer. Am J Cancer Res. 5:344–353.
2015.PubMed/NCBI
|
|
33
|
Koren A, Motaln H and Cufer T: Lung cancer
stem cells: a biological and clinical perspective. Cell Oncol
(Dordr). 36:265–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial|[ndash]|mesenchymal transitions. Nat Rev Mol
Cell Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi Y, Wu H, Zhang M, Lei D, Meng F and
Fan X: Expression of the epithelial-mesenchymal transition-related
proteins and their clinical significance in lung adenocarcinoma.
Diagnostic Pathology. 8:89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goto M, Osada S, Imagawa M and Nishizuka
M: FAD104, a regulator of adipogenesis, is a novel suppressor of
TGF-β mediated EMT in cervical cancer cells. Sci Rep. 7:163652017.
View Article : Google Scholar : PubMed/NCBI
|