Introduction
Osteoarthritis (OA) is recognized as a major public
health problem, characterized by joint pain, fatigue, functional
limitation, and decreased quality of life of the patient, leading
to increased use of healthcare services and consequent economic
burden (1). In older persons, the
knee is the joint most commonly affected by pain usually attributed
to OA. In a survey of adults 50 years of age and older, nearly half
of them reported having pain for a period of one year (2). Its incidence is rising due to
increasing obesity and the ageing of the population (3). The inability to walk due to symptomatic
knee OA has been associated with all-cause mortality (4). The high prevalence of such a condition
and its impact in terms of disability, mortality, and economics,
make the search for effective therapeutic alternatives of easy
implementation a priority.
Apart from education and exercise, the only
available nonsurgical treatments are directed at symptoms,
primarily to alleviate pain and enhance daily activities and
quality of life (5,6). In cases of advanced disease or
ineffective conservative therapy, a recommended option is total
joint arthroplasty (TJA), which consists of replacing the
articulation with a prosthesis. However, such surgical treatment is
costly (7–9), and there is frequently a long waiting
list for patients utilizing public healthcare systems.
A promising novel, bioactive cell-free formulation
(BIOF2) for articular cartilage regeneration, has recently been
tested in preclinical and clinical trials (10,11). The
intra-articular application of BIOF2 significantly increased
cartilage thickness (12–38%) in different OA animal models,
compared with articular cartilage treated with saline solution
(11). BIOF2 is a mixture whose main
components are a corticosteroid and organic acids (10). Corticosteroids are bioactive
substances that possibly facilitate tissue atrophy and joint
destruction when acting alone (12).
On the other hand, in in vitro trials with articular cells,
different organic acids, such as retinoic acid or ascorbic acid,
have been shown to increase the expression of genes related to
chondrogenesis (13,14) and osteogenesis (15). Even though it has been proven that
those acids promote differentiation into bone cells (15–18),
their capacity to generate, on their own, a morphologic
differentiation into cartilage cells is a topic of debate (19,20).
However, when those acids are combined with other co-factors, they
aid in the process of differentiation into chondrocytes (14,21–23).
According to previous reports of in vitro trials on animal
models and human patients (11,24), the
combination of the compounds present in BIOF2 act in synergy to
modify the intra-articular microenvironment and stimulate articular
regeneration by producing molecular and morphologic alterations in
synovial fluid cells and chondrocytes (11).
The results of a previous clinical trial showed the
intra-articular application of BIOF2 to be well-tolerated, with a
success rate similar to that of total arthroplasty for the
treatment of severe knee OA. Success was correlated with an average
22% increase in articular cartilage (24). However, the present study is the
first to evaluate treatment with BIOF2 in patients with severe knee
OA that are treated within the public healthcare system and receive
conservative ‘usual medical care’ (paracetamol/NSAIDs and general
care provided by the family physician) before entering into a TJA
program or other therapeutic option. In the general population,
there are subgroups of patients with comorbidities that exacerbate
knee OA, such as genu varum or genu valgum
malalignment greater than 20 degrees and/or class 2 obesity [body
mass index (BMI) of 35–39]. To determine the limits of this new
treatment, it is important to know whether BIOF2 is effective when
included as part of a conservative usual medical care regimen and
if its efficacy is affected in subgroups of patients with
comorbidities.
Therefore, the present study was designed to
randomly select patients undergoing usual medical care, for the
addition of treatment with BIOF2 and compare them with a control
group that only underwent usual medical care. The utilized regimen
was that most frequently carried out at the majority of public
healthcare centers in Mexico and other countries, which consisted
of nonsteroidal anti-inflammatory drugs (NSAIDs) and/or paracetamol
prescription. That is the therapy generally given to patients with
severe knee OA, while they wait for other therapeutic options.
Patients and methods
Study design
A prospective, single-blind, 2-arm, parallel group,
randomized phase III clinical trial was conducted between March
2016 and March 2018. The study was carried out according to the
‘CONSORT statement’ guidelines for randomized controlled
trials.
The present study was approved by the ethics
committee of the Cancerology State Institute of the Colima
State Health Services, Mexico, and written informed consent was
obtained from all the participants. The present clinical trial was
registered as ARTROTX–II/III: RPCEC00000277 in the Cuban Public
Registry of Clinical Trials (RPCEC) database.
Study subjects
The inclusion criteria were: Patients ≥40 years of
age, with a BMI ≤39 kg/m2 and knee OA, according to the
diagnostic criteria of the American College of Rheumatology
(25). The target knee was defined
as the more symptomatic knee (with a pain score of at least 5 on
the 0–10 Visual Analog Scale [VAS] for at least 6 months before
enrollment in the study). The patients had to be under usual
medical care, based on paracetamol/NSAIDs, prescribed by their
family physician. In short, they were patients with significant
symptoms and/or functional limitations associated with reduced
health-related quality of life. The exclusion criteria were: having
received some type of intra-articular treatment within the 12
months prior to the study, a history of knee surgery, inflammatory
polyarthritis, fibromyalgia, chronic fatigue syndrome,
thromboembolic disease, hemorrhagic blood disease, Hb <80 g/l,
neuromuscular disease, cancer, metabolic bone disease, alcoholism,
drug addiction, or class 3 obesity (BMI of 40 or higher) (26). Participants were recruited from
primary and secondary healthcare centers in the State of Colima,
Mexico. The efficacy evaluations and intra-articular BIOF2
application were performed at the Centro Hospitalario Unión
(a Secondary Healthcare Center) located in the State of Colima,
Mexico.
A total of 237 patients were randomly allocated to
the intra-articular BIOF2 group or the control group of usual
medical care (paracetamol/NSAIDs) prescribed by the family
physician. Randomization was performed using computer-generated
random allocation cards, and patients were assigned to one of the 2
groups. The process was conducted by researchers that did not
participate in the evaluation of the results. It should be made
clear that before their inclusion in the study, all the patients
were under the care of their family physician and receiving the
standard paracetamol/NSAID-based treatment for OA control. It was
explained to all the patients that they were candidates for other
established treatments, such as arthroplasty or
viscosupplementation, and could exit the present study at any time
to receive another treatment, whether through a government public
healthcare program or through private resources.
BIOF2 administration
BIOF2 is a patented formulation for cartilage
regeneration whose main components are a corticosteroid and organic
acids (10). The BIOF2 manufacturing
process was performed by Esteripharma Mexico (Mexico City, Mexico),
according to the GMP (Good Manufacturing Practices) for
pharmaceutical products for use in clinical trials.
BIOF2 was administered on three occasions at 1-month
intervals (at month 0, 1, and 2). It was an outpatient procedure
performed at a traumatology or orthopedics consultation office, as
previously described. With the patient in a seated position and the
treatment knee flexed at 0 degrees, BIOF2 was injected into the
knee joint space, under sterile prep conditions. The area of
injection was inferior lateral to the patella, at the lateral level
of the joint line. The injection was performed with a 1.5-inch
20-gauge needle, passing through the fat pad to the firm surface of
the intercondylar notch. Following the withdrawal of the needle, a
cotton ball soaked in alcohol was placed with pressure at the
injection site, after which the site was covered with a sterile
dressing (BandAid). The patient could carry out his or her normal
activities immediately after the procedure, with no special
indications. All patients continued to be seen by their family
physician for general care, healthy lifestyle promotion, and if
necessary, continued taking the paracetamol/NSAID-based treatment
regimen, with no intervention from the researchers in relation to
drug prescription or lifestyle indications. In addition, the
patients were referred to the physiotherapy and rehabilitation
service. Those with genu varum or genu valgum
malalignment were prescribed a 6-mm external or internal insole,
respectively, as part of their treatment.
Usual medical care
That group of patients continued with the usual
treatment prescribed by their family physician. It consisted of
paracetamol/NSAID use and the promotion of a healthy lifestyle. The
researchers did not intervene in relation to drug prescription or
lifestyle indications. The patients were also referred to the
physiotherapy and rehabilitation service. A 6-mm external or
internal insole was prescribed to the patients with genu
varum or genu valgum malalignment, respectively, as part
of their treatment.
Outcome measures and follow-up
There were 3 co-primary endpoints, assessed as the
change from the baseline, or more exactly, the difference between
the values at enrollment and at 6 and 12 months. One endpoint was
the maximum pain upon movement during the week before the follow-up
visit, measured on the 0–10 Visual Analog Scale (VAS) (27). Intensity of joint pain was recorded,
from ‘no pain’ (score of 0) to ‘worst imaginable plain’ (score of
10). The VAS was selected because it is currently the validated
scale that best evaluates pain in diseases presenting with
arthralgia (28,29), and it has also been used as a primary
endpoint in other clinical trials on OA (30). Another endpoint was the number of
patients achieving minimal clinically important improvement (MCII),
defined as the smallest change in measurement that signifies
important improvement in a patient's symptom (27). It was calculated through a
dichotomous score per outcome, based on 30% improvement of pain
from the baseline, as previously described in different clinical
trials (27,31–34). The
third endpoint was the Patient Acceptable Symptom State (PASS),
defined as the value of symptoms the patient considers to be the
thresholds of well-being for pain and function. Our study
incorporated the most widely used anchoring question to identify
PASS cut-off points, which was: ‘Taking into account all your daily
activities, do you consider your current state satisfactory in
relation to pain level and functional impairment?’ The response
options were ‘Yes’ or ‘No’ (34).
Treatment success was defined as the MCII or PASS questionnaires
answered in the affirmative at month 12 of follow-up. The secondary
endpoints were change in daily NSAID use at month 12 of follow-up
and the register of all adverse events, monitored by the
researchers through anamnesis, and abnormal routine laboratory test
results.
Blinding
Only the researchers that evaluated treatment
effectiveness through the VAS, MCII, and PASS instruments answered
by the patients, those that carried out the anamnesis in relation
to NSAID consumption, and those that performed the statistical
analyses were blinded.
Sample size
Sample size was calculated based on a 100% increase
in the number of patients with MCII at 12 months in the BIOF2
group, compared with the control group (paracetamol/NSAIDs=20% vs.
BIOF2=40%). Eighty-one patients from each group were required to
reach the required power (0.8), when the statistical analysis was
performed at the level of the 2-tailed alpha (0.05). That
calculation was made using the Sample Size Calculator for two
independent study groups with binomial primary endpoints (ClinCalc
LLC; http://clincalc.com/stats/samplesize.aspx).
Statistical analysis
Data were presented as the mean ± standard deviation
(VAS) or percentages (MCII and PASS). For the inferential
statistics, normal data distribution was first determined using the
Kolmogorov-Smirnov test and the equality of variances was confirmed
using the Levene's test. The VAS pain quantification and other
numerical data (ΒΜI or age) were compared between groups using the
Student's t test. The categorical values were compared using the
Fisher's exact test. The relative risk (RR) and 95% confidence
interval were calculated to determine the probability of achieving
PASS or of habitually using paracetamol/NSAIDs (at least once a
day), comparing the usual medical care group vs. the BIOF2 group.
The statistical analyses were performed on the patient total and
compared between specific substrates to assess treatment efficacy
in patient subgroups [i.e., excluding patients with genu
varum or genu valgum malalignment greater than 20
degrees and/or class 2 obesity (BMI of 35–39)]. The statistical
analysis was carried out using the SPSS, version 20, software (IBM
Corp., Armonk, NY, USA), with the exception of the RR, which was
calculated using MedCalc v17.7.2 software (MedCalc Software bvba,
Ostend, Belgium) and the sample size, which was calculated using
the online ClinCalc software (ClinCalc LLC; http://clincalc.com/stats/samplesize.aspx). A 2-sided
P<0.05 was considered statistically significant.
Results
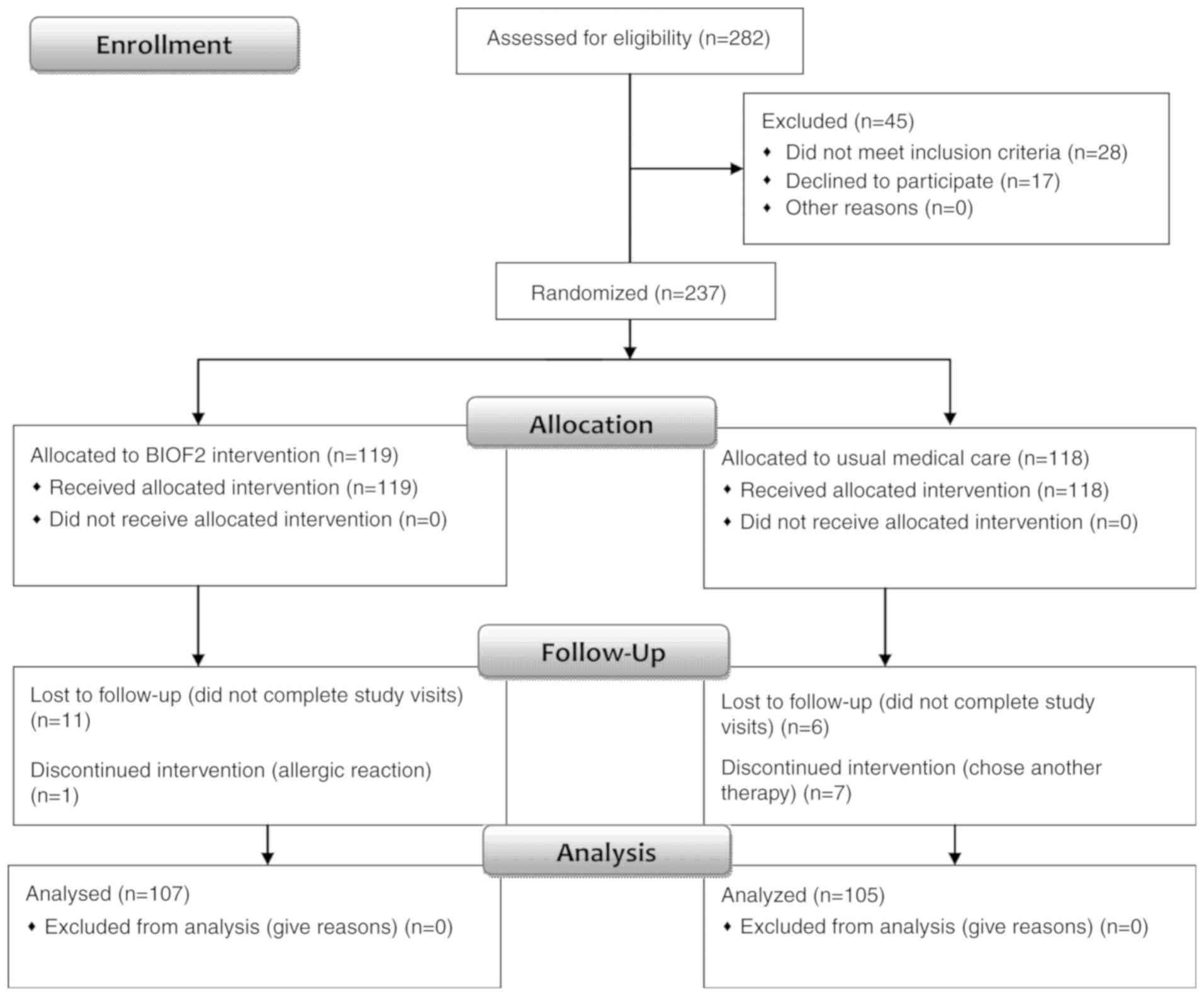
From the 282 patients screened, 237 were randomized
into one of the two study groups. A total of 107 patients in the
BIOF2 group and 105 patients in the usual medical care control
group completed the study and were analyzed (Fig. 1). The clinical characteristics of the
patients are shown in Table I.
 | Table I.Distribution of the main clinical
characteristics of the study subjects. |
Table I.
Distribution of the main clinical
characteristics of the study subjects.
| Clinical
characteristic | NSAIDs | BIOF2 | P-value |
|---|
| Women (%) | 60.0% | 58.0% | 0.43a |
| Age (years) | 61.5±8.2 | 60.7±6.7 | 0.41b |
| BMI | 31.9±4.0 | 32.7±3.3 | 0.12b |
|
Varus/valgusc | 39.0% | 32.7% | 0.20a |
| Diabetes | 22.8% | 25.2% | 0.40a |
| High blood
pressure | 32.4% | 29.9% | 0.40a |
Tables II–IV shows the clinical evaluations of knee
OA throughout the 12-month follow-up. Only 14% of the patients in
the usual medical care group (paracetamol/NSAIDs) achieved MCII in
12 months. In contrast, treatment with BIOF2 produced important
clinical improvement in 70% of the patients and >50% of the
patients achieved acceptable symptom state, which was significantly
higher than that found in the usual medical care group. Treatment
with BIOF2, in relation to usual medical care, was associated with
a 5-fold increased probability of achieving MCII (RR=4.90, 95% CI:
3.0–7.9, P<0.001), and an 11-fold increased probability of
achieving PASS (RR=11.18, 95% CI: 4.6–26.7, P<0.001).
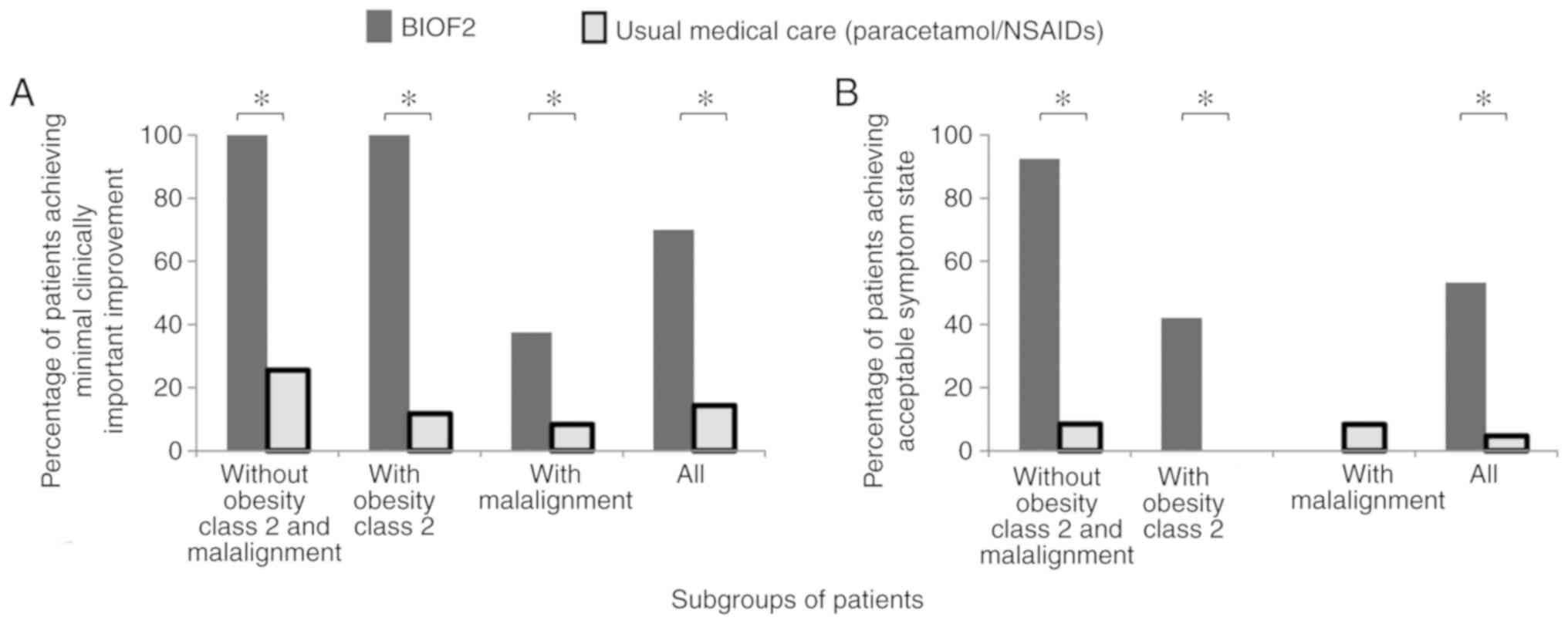
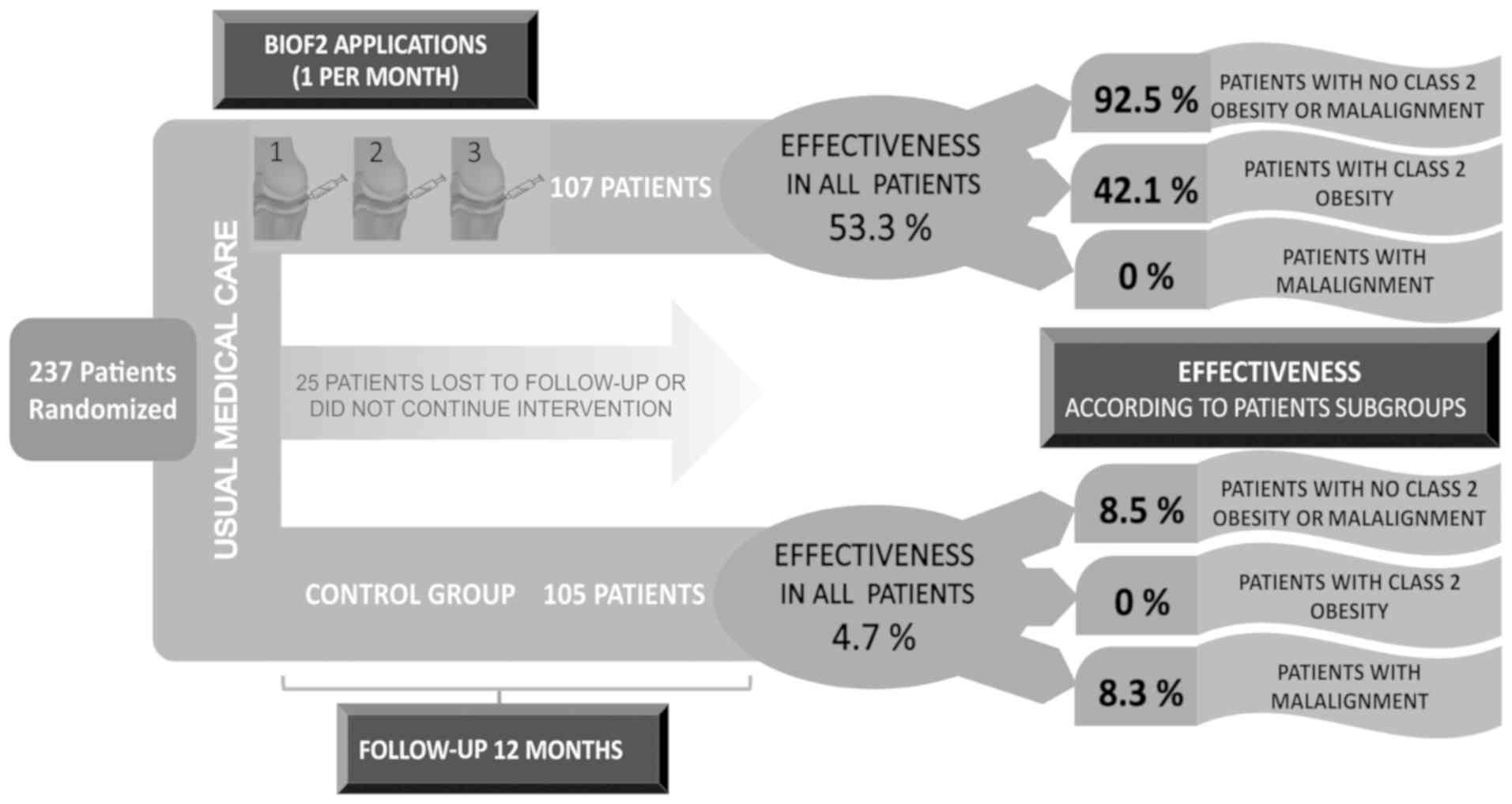
Furthermore, there was greater therapeutic success with BIOF2 when
patients with class 2 obesity and genu varum or genu
valgum malalignment greater than 20 degrees were excluded,
resulting in MCII in 100% of the PASS in 90% (Figs. 2 and 3). Even though BIOF2 treatment
significantly reduced pain in the patients with class 2 obesity and
genu varum or genu valgum malalignment, its efficacy
in those subgroups was drastically reduced, given that none of the
BIOF2 group patients with malalignment achieved acceptable symptom
state and only 42% of the patients with class 2 obesity treated
with BIOF2 did (Fig. 2).
 | Table II.Comparison of VAS scores between
patients treated with NSAIDs and BIOF2 at 6 and 12 months following
the intervention. |
Table II.
Comparison of VAS scores between
patients treated with NSAIDs and BIOF2 at 6 and 12 months following
the intervention.
| A, All
patients |
|---|
|
|---|
| Timepoint | NSAID, n=105 | BIOF2, n=107 | P-value |
|---|
| Baseline | 9.0+1.0 | 9.0+1.0 |
0.893 |
| Month 6 | 8.5+1.2 | 3.9+3.3 | <0.001 |
| Month 12 | 8.7+1.4 | 4.1+3.5 | <0.001 |
|
| B, Patients with
no class 2 obesity or malalignment |
|
|
Timepoint | NSAID,
n=47 | BIOF2,
n=53 | P-value |
|
| Baseline | 8.8+1.2 | 9.1+1.1 |
0.211 |
| Month 6 | 8.0+1.4 | 1.3+1.6 | <0.001 |
| Month 12 | 8.2+1.7 | 1.4+1.6 | <0.001 |
|
| C, Patients with
class 2 obesity and malalignment |
|
|
Timepoint | NSAID,
n=29 | BIOF2,
n=27 | P-value |
|
| Baseline | 9.1+0.7 | 8.9+0.8 |
0.230 |
| Month 6 | 8.8+0.7 | 8.3+0.7 |
0.016 |
| Month 12 | 9.2+0.6 | 8.4+0.6 | <0.001 |
|
| D, Patients with
class 2 obesity and no malalignment |
|
|
Timepoint | NSAID,
n=17 | BIOF2,
n+19 | P-value |
|
| Baseline | 9.2+0.9 | 9.3+0.9 |
0.814 |
| Month 6 | 8.8+1.0 | 4.1+2.5 | <0.001 |
| Month 12 | 8.7+0.9 | 4.4+3.4 | <0.001 |
|
| E, Patients with
malalignment and no class 2 obesity |
|
|
Timepoint | NSAID,
n=12 | BIOF2,
n=8 | P-value |
|
| Baseline | 9.3+0.9 | 8.3+1.4 |
0.089 |
| Month 6 | 9.0+1.1 | 6.1+1.2 | <0.001 |
| Month 12 | 9.2+1.4 | 7.6+0.5 |
0.007 |
 | Table IV.Comparison of the percentage of
patients reaching PASS among patients treated with NSAIDs and BIOF2
at 6 and 12 months following the intervention. |
Table IV.
Comparison of the percentage of
patients reaching PASS among patients treated with NSAIDs and BIOF2
at 6 and 12 months following the intervention.
| A, All
patients |
|---|
|
|---|
| Timepoint | NSAID, n=105
(%) | BIOF2, n=107
(%) | P-value |
|---|
| Baseline | 0.0 |
0.0 | – |
| Month 6 | 4.7 | 52.3 | <0.001 |
| Month 12 | 4.7 | 53.3 | <0.001 |
|
| B, Patients with
no class 2 obesity or malalignment |
|
|
Timepoint | NSAID, n=47
(%) | BIOF2, n=53
(%) | P-value |
|
| Baseline | 0.0 |
0.0 | – |
| Month 6 | 8.5 | 90.5 | <0.001 |
| Month 12 | 8.5 | 92.5 | <0.001 |
|
| C, Patients with
class 2 obesity and malalignment |
|
|
Timepoint | NSAID, n=29
(%) | BIOF2, n=27
(%) | P-value |
|
| Baseline | 0.0 | 0.0 | – |
| Month 6 | 0.0 | 0.0 | – |
| Month 12 | 0.0 | 0.0 | – |
|
| D, Patients with
class 2 obesity and no malalignment |
|
|
Timepoint | NSAID, n=17
(%) | BIOF2, n=19
(%) | P-value |
|
| Baseline | 0.0 |
0.0 | – |
| Month 6 | 0.0 | 42.1 | 0.002 |
| Month 12 | 0.0 | 42.1 | 0.002 |
|
| E, Patients with
malalignment and no class 2 obesity |
|
|
Timepoint | NSAID, n=12
(%) | BIOF2, n=8
(%) | P-value |
|
| Baseline | 0.0 | 0.0 | – |
| Month 6 | 8.3 | 0.0 | 0.638 |
| Month 12 | 8.3 | 0.0 | 0.638 |
At the beginning of the study, all the patients
required daily paracetamol/NSAID use. The drug most frequently used
by each patient was distributed as follows: 40% diclofenac, 32%
naproxen, 12% ketorolac, 9% paracetamol, and 7% celecoxib. 11% of
the patients combined one of those drugs with tramadol. At the
12-month follow-up, treatment with BIOF2 significantly reduced
daily NSAID use (RR=0.42, 95% CI 0.34–0.53, P<0.001), compared
with the usual medical care group. Upon study completion, only 42%
of the BIOF2 group required habitual NSAID use, whereas 100% of the
patients in the usual medical care group required paracetamol/NSAID
use daily. Only 13% of the patients in the subgroup that had no
class 2 obesity and no malalignment that were treated with BIOF2
required daily paracetamol/NSAID use at the end of the study.
With respect to adverse effects, 90% of the patients
treated with BIOF2 presented with local joint pain of 8.0±0.9
intensity (0 to 10 on the visual analogue scale) after BIOF2
application. It lasted 98±45 sec and subsided spontaneously. In
some cases, pain radiated to the pelvis. One patient had an
allergic reaction to BIOF2, which was resolved with the use of oral
antihistamines. That patient was eliminated from the study after
the first application. Routine laboratory testing identified no
significant abnormalities in either group. Abdominal
pain/discomfort was another frequently reported adverse event
(74.3% in the usual medical care group and 17.7% in the BIOF2
group), for which the family physician of the majority of the
patients added H2-blockers or proton pump inhibitors to prevent
severe acute NSAID-related gastroduodenal damage.
Discussion
In patients with severe knee OA that were
conservatively treated with usual medical care based on
paracetamol/NSAIDs, the intra-articular application of a cell-free
bioactive formulation, BIOF2, produced clinically important and
statistically significant benefits. At 12 months, 70% of the
patients treated with BIOF2 achieved MCII, and in patients with no
lower limb malalignment, that figure was 100%. The best PASS result
was produced in 92% of the patients treated with BIOF2 that had no
class 2 obesity or malalignment. BIOF2 efficacy was reduced in the
patients with class 2 obesity, with PASS achieved in only 42%. None
of the patients with genu varum or genu valgum
malalignment achieved a state of well-being.
The results of the present study are congruent with
those of a previous clinical trial demonstrating a similar success
rate of BIOF2 treatment to that of TJA (75%) at one year of
treatment (24). Prior preclinical
and clinical trials showed that BIOF2 was capable of increasing
articular cartilage and simultaneously reducing the histologic
abnormalities caused by OA (11).
Joint cartilage was increased through the elevated expression of
SOX9, a transcription factor that is essential for chondrocyte
differentiation and cartilage formation (11). The present study produced new data
with respect to the subgroup of patients that most benefitted from
treatment with BIOF2 and the limitations in patients with
OA-exacerbating comorbidities, such as obesity and lower limb
malalignment. With such data, family physicians can have a better
idea of treatment outcome and inform patients of the expectations
in relation to BIOF2 treatment.
Obesity has been associated with greater pain and
articular damage, due to a metabolic-inflammatory process and a
mechanical effect (35). Obesity
produces increased proinflammatory cytokine and collagenase
production in cartilage, which is related to a systemic increase of
leptin in obese individuals (36,37).
Leptin and its receptor have been identified in human chondrocytes,
osteophytes, synovium, and infrapatellar fat pad, and may affect
growth factor synthesis and anabolism (38–40).
Leptin expression has been directly associated with the degree of
cartilage degeneration (38). In
addition, excess weight contributes to greater mechanical load on
the joint (35). Those aspects may
be the cause of the lower BIOF2 effectiveness in patients with
class 2 obesity found in the present study. It is important to
mention that all the patients with class 2 obesity had relevant
clinical improvement, even though less than half achieved PASS.
Most likely, patient weight reduction and/or a greater number of
BIOF2 applications could increase the therapeutic response in that
subgroup of patients, which is an aspect that should be analyzed in
future studies.
In patients with important malalignment, BIOF2
application produced MCII in 75% of the patients at 6 months. It
was reduced to 35% at 12 months, and none of those patients
achieved PASS. Malalignment is a potent predictor of disease
progression in patients with OA, and is a local mechanical factor
in the knee that can mediate symptoms (41). The beneficial effect of BIOF2 in that
subgroup of patients appears to be temporary and does not
completely resolve the patient's complaints. Surgical correction of
the malalignment, followed by treatment with BIOF2, could be a
therapeutic strategy to be evaluated in future studies.
A relevant characteristic of the present study is
that it assessed the use of BIOF2 in patients receiving usual
medical care. The term ‘usual care’ describes the care commonly
given by practitioners in a community. For more than a decade, the
usefulness of evaluating new treatments against background
conditions of medical practices has been postulated, considering
that it is often essential, for scientific and ethical reasons, to
have a usual care comparison arm in the study of a new drug
(42). The use of NSAIDs and/or
paracetamol has been shown in clinical trials to improve knee OA
symptomatology. However, its effectiveness varies, depending on the
drug used, dose (17), baseline
pain, and radiologic features (43–46).
Oral NSAIDs or paracetamol are the agents most frequently utilized
in the treatment of arthrosis (43).
However, neither the patients nor the physicians that prescribe the
drugs are satisfied with their results, given that in general,
adequate health states are not achieved through their therapeutic
use (43). Despite that fact, the
use of those drugs, together with the promotion of healthy
lifestyles and rehabilitation techniques, is the usual medical care
given for the treatment of knee OA in the majority of public
healthcare systems in Mexico and other countries. With respect to
severe knee OA, the treatment of choice could be TJA, but that
option is often not available in the short term for patients within
the public healthcare system and the wait for said treatment can be
years. As those patients wait, the common usual medical care is the
prescription of paracetamol/NSAIDs.
The low level of efficacy of paracetamol/NSAID
prescription found in the present study does not concur with the
good or moderate success rates reported in other studies on OA
(44,45). There are several possible
explanations for that. The high OA severity in the patients upon
entering the present study (mean VAS for pain of 9, 0–10 scale)
could have affected the results. With respect to the drugs used, it
was reported in other studies that etoricoxib, celecoxib, and
aceclofenac had the highest rankings for improvement, whereas in
our study celecoxib was used in only 7% of the patients. In
previous trials, evaluations were carried out only during active
treatment. Our study reflected habitual NSAID use of the patients
in the community and therefore it is likely that drug dose and
treatment adherence varied considerably over a one-year period.
Discontinuation rates of prescription NSAIDs have been reported to
exceed 85% within six months of their use (46). Nevertheless, our results coincided
with those of a study that analyzed the effect of prescription
NSAIDs on knee OA. Those authors reported that NSAID prescription
was not associated with MCII in the patient-reported symptoms of
pain, stiffness, and function (46)
in evaluations of one and two years. Therefore, we believe that our
results reflect the real-life occurrence in a community of patients
with knee OA receiving long-term treatment with
paracetamol/NSAIDs.
The addition of BIOF2 to the usual medical care
significantly increased the well-being indicators analyzed in the
patients and significantly reduced NSAID use. Prolonged NSAID use
can cause adverse effects, especially that of kidney damage
(47). Thus, treatment with BIOF2
could also aid in reducing the risks caused by long-term NSAID
intake. Other clinical trials have evaluated strategies for
articular cartilage regeneration through cellular therapy or
implants utilizing novel biomaterials. However, those procedures
are complex, costly, and difficult to implement in medical centers.
Therefore, we consider treatment with the new BIOF2 to be a
promising and readily implemented option for the treatment of OA
that can be incorporated into the usual medical care of patients
with knee OA at a public or private healthcare center with ease.
BIOF2 can be applied as an outpatient procedure in routine medical
consultations, taking the customary precautions utilized in any
intra-articular injection. The only adverse effect detected was
pain upon application, which, albeit intense, spontaneously
remitted within sec.
In conclusion, the intra-articular application of a
new BIOF2, was safe and well-tolerated and resulted in a success
rate above 90% in patients with no class 2 obesity and no
malalignment. At 12 months, its effect was limited in the patients
with class 2 obesity and was close to null in the patients with
malalignment. BIOF2 is a safe and easily implemented therapeutic
alternative in patients receiving usual medical care for knee
OA.
Acknowledgements
The authors would like to thank Ms. Herminia
Farias-Lopez and Mrs. Ana Maria Blancas-Alcaraz (Department of
Traumatology, Union Hospital Center, Villa de Alvarez, Mexico) for
their excellent assistance in placing the clinical data in the
different platforms for their analysis.
Funding
The present study was completed using equipment
resources obtained through grant no. 270485 from the
2016-INFRAESTRUCTURA-CONACYT (author ADSH) and grant no. 272792
from the 2016-FOSISS-CONACYT (author IDE). Esteripharma Mexico
provided support in the form of salaries for author BPM. The
specific role of this author is described in the ‘author
contributions’ section.
Availability of data and materials
All relevant data appear in the present study. The
datasets used and/or analyzed during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ID, AOC, ADS, JD and IPR designed the study,
performed the analyses and drafted the manuscript. BP and JPG
conceived the novel bioactive cell-free formulation. JV, MMH, JPR,
JLC and JG participated in the clinical evaluation of the patients.
MLM, CEB and AC participated in the design of the statistical
analysis. JD was the clinical trial administrative coordinator. All
the authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Cancerology State Institute of the Colima State
Health Services, Mexico (reference number:
CEICANCL061115-OSTEOART10), and written informed consent was
obtained from all the participants. All procedures performed in
this protocol were in accordance with the Declaration of Helsinki.
The present clinical trial was registered as ARTROTX–II/III:
RPCEC00000277 in the Cuban Public Registry of Clinical Trials
(RPCEC) database.
Competing interests
Dr Juan Paz-Garcia and Dr Brenda Paz-Michel declare
that they are the inventors of the experimental formulation (BIOF2)
used in the present study (patent no. US9089580 B1). These authors
did not have a role in the study design, data collection, or the
analyses. The other authors declare that they have no competing
interests.
References
|
1
|
Hafez AR, Alenazi AM, Kachanathu SJ,
Alroumi AM and Mohamed ES: Knee osteoarthritis: A review of
literature. Phys Med Rehabil Int. 1:1–8. 2014.
|
|
2
|
Jinks C, Jordan K, Ong BN and Croft P: A
brief screening tool for knee pain in primary care (KNEST). 2.
Results from a survey in the general population aged 50 and over.
Rheumatology (Oxford). 43:55–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson VL and Hunter DJ: The epidemiology
of osteoarthritis. Best Pract Res Clin Rheumatol. 28:5–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Q, Niu J, Li H, Ke Y, Li R, Zhang Y
and Lin J: Knee symptomatic osteoarthritis, walking disability,
NSAIDs use and all-cause mortality: Population-based wuchuan
osteoarthritis study. Sci Rep. 7:33092017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis AM: Osteoarthritis year in review:
Rehabilitation and outcomes. Osteoarthritis Cartilage. 20:201–206.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bannuru RR, Osani M, Vaysbrot EE and
McAlindon TE: Comparative safety profile of hyaluronic acid
products for knee osteoarthritis: A systematic review and network
meta-analysis. Osteoarthritis Cartilage. 24:2022–2041. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herrera-Espiñeira C, Escobar A,
Navarro-Espigares JL, Castillo Jde D, García-Pérez L and
Godoy-Montijano A: Total knee and hip prosthesis: Variables
associated with costs. Cir Cir. 81:207–213. 2013.(In Spanish).
PubMed/NCBI
|
|
8
|
Ethgen O, Bruyère O, Richy F, Dardennes C
and Reginster JY: Health-related quality of life in total hip and
total knee arthroplasty. A qualitative and systematic review of the
literature. J Bone Joint Surg Am 86-A. 963–974. 2004. View Article : Google Scholar
|
|
9
|
Robinson JC, Pozen A, Tseng S and Bozic
KJ: Variability in costs associated with total hip and knee
replacement implants. J Bone Joint Surg Am. 94:1693–1698. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia JP and Paz Michel BA: Formulation
for regeneration of bone, cartilage, teeth, and periodontium and
treatment of tumors and cysts. US Patent No. 9089580 B1. 2015.
|
|
11
|
Delgado-Enciso I, Paz-Garcia J,
Rodriguez-Hernandez A, Madrigal-Perez VM, Cabrera-Licona A,
Garcia-Rivera A, Soriano-Hernandez AD, Cortes-Bazan JL,
Galvan-Salazar HR, Valtierra-Alvarez J, et al: A promising novel
formulation for articular cartilage regeneration: Preclinical
evaluation of a treatment that produces SOX9 overexpression in
human synovial fluid cells. Mol Med Rep. 17:3503–3510.
2018.PubMed/NCBI
|
|
12
|
Farkas B, Kvell K, Czömpöly T, Illés T and
Bárdos T: Increased chondrocyte death after steroid and local
anesthetic combination. Clin Orthop Relat Res. 468:3112–3120. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sekiya I, Tsuji K, Koopman P, Watanabe H,
Yamada Y, Shinomiya K, Nifuji A and Noda M: SOX9 enhances aggrecan
gene promoter/enhancer activity and is up-regulated by retinoic
acid in a cartilage-derived cell line, TC6. J Biol Chem.
275:10738–10744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reiter I, Tzukerman M and Maor G:
Spontaneous differentiating primary chondrocytic tissue culture: A
model for endochondral ossification. Bone. 31:333–339. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hadzir SN, Ibrahim SN, Abdul Wahab RM,
Zainol Abidin IZ, Senafi S, Ariffin ZZ, Abdul Razak M and Zainal
Ariffin SH: Ascorbic acid induces osteoblast differentiation of
human suspension mononuclear cells. Cytotherapy. 16:674–682. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogston N, Harrison AJ, Cheung HF, Ashton
BA and Hampson G: Dexamethasone and retinoic acid differentially
regulate growth and differentiation in an immortalised human clonal
bone marrow stromal cell line with osteoblastic characteristics.
Steroids. 67:895–906. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gentili C, Bianco P, Neri M, Malpeli M,
Campanile G, Castagnola P, Cancedda R and Cancedda FD: Cell
proliferation, extracellular matrix mineralization, and
ovotransferrin transient expression during in vitro differentiation
of chick hypertrophic chondrocytes into osteoblast-like cells. J
Cell Biol. 122:703–712. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skillington J, Choy L and Derynck R: Bone
morphogenetic protein and retinoic acid signaling cooperate to
induce osteoblast differentiation of preadipocytes. J Cell Biol.
159:135–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benya PD, Brown PD and Padilla SR:
Microfilament modification by dihydrocytochalasin B causes retinoic
acid-modulated chondrocytes to reexpress the differentiated
collagen phenotype without a change in shape. J Cell Biol.
106:161–170. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Temu TM, Wu KY, Gruppuso PA and
Phornphutkul C: The mechanism of ascorbic acid-induced
differentiation of ATDC5 chondrogenic cells. Am J Physiol
Endocrinol Metab. 299:E325–E334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zur Nieden NI, Kempka G, Rancourt DE and
Ahr HJ: Induction of chondro-, osteo- and adipogenesis in embryonic
stem cells by bone morphogenetic protein-2: Effect of cofactors on
differentiating lineages. BMC Dev Biol. 5:12005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen H, Wang H, Li B, Feng B, He X, Fu W,
Yuan H and Xu Z: Enhanced chondrogenic differentiation of human
mesenchymal stems cells on citric acid-modified chitosan hydrogel
for tracheal cartilage regeneration applications. RSC Adv.
8:16910–16917. 2018. View Article : Google Scholar
|
|
23
|
Mahmod SA, Snigh S, Djordjevic I, Yee YM,
Yusof R, Ramasamy TS and Rothan HA: Phytoestrogen (Daidzein)
promotes chondrogenic phenotype of human chondrocytes in 2D and 3D
culture systems. Tissue Eng Regen Med. 14:103–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delgado-Enciso I, Paz-Garcia J,
Valtierra-Alvarez J, Preciado-Ramirez J, Almeida-Trinidad R,
Guzman-Esquivel J, Mendoza-Hernandez MA, Garcia-Vega A,
Soriano-Hernandez AD, Cortes-Bazan JL, et al: A phase I–II
controlled randomized trial using a promising novel cell-free
formulation for articular cartilage regeneration as treatment of
severe osteoarthritis of the knee. Eur J Med Res. 23:522018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ringdahl E and Pandit S: Treatment of knee
osteoarthritis. Am Fam Physician. 83:1287–1292. 2011.PubMed/NCBI
|
|
26
|
Center for Disease Control and Prevention,
. Defining adult overweight and obesity. Centers Dis Control Prev.
pp8–9. 2016.
|
|
27
|
Tubach F, Ravaud P, Baron G, Falissard B,
Logeart I, Bellamy N, Bombardier C, Felson D, Hochberg M, van der
Heijde D and Dougados M: Evaluation of clinically relevant changes
in patient reported outcomes in knee and hip osteoarthritis: The
minimal clinically important improvement. Ann Rheum Dis. 64:29–33.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Englbrecht M, Tarner IH, van der Heijde
DM, Manger B, Bombardier C and Müller-Ladner U: Measuring pain and
efficacy of pain treatment in inflammatory arthritis: A systematic
literature review. J Rheumatol Suppl. 90:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Delgado-Enciso I, Paz-Michel B, Melnikov
V, Guzman-Esquivel J, Espinoza-Gomez F, Soriano-Hernandez AD,
Rodriguez-Sanchez IP, Martinez-Fierro ML, Ceja-Espiritu G,
Olmedo-Buenrostro BA, et al: Smoking and female sex as key risk
factors associated with severe arthralgia in acute and chronic
phases of chikungunya virus infection. Exp Ther Med. 15:2634–2642.
2018.PubMed/NCBI
|
|
30
|
Reginster JY, Dudler J, Blicharski T and
Pavelka K: Pharmaceutical-grade Chondroitin sulfate is as effective
as celecoxib and superior to placebo in symptomatic knee
osteoarthritis: The ChONdroitin versus CElecoxib versus placebo
trial (CONCEPT). Ann Rheum Dis. 76:1537–1543. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van der Wees PJ, Wammes JJ, Akkermans RP,
Koetsenruijter J, Westert GP, van Kampen A, Hannink G, de
Waal-Malefijt M and Schreurs BW: Patient-reported health outcomes
after total hip and knee surgery in a Dutch University Hospital
Setting: Results of twenty years clinical registry. BMC
Musculoskelet Disord. 18:972017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Revicki D, Hays RD, Cella D and Sloan J:
Recommended methods for determining responsiveness and minimally
important differences for patient-reported outcomes. J Clin
Epidemiol. 61:102–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Escobar A, García Pérez L,
Herrera-Espiñeira C, Aizpuru F, Sarasqueta C, Gonzalez Sáenz de
Tejada M, Quintana JM and Bilbao A: Total knee replacement; minimal
clinically important differences and responders. Osteoarthritis
Cartilage. 21:2006–2012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kvien TK, Heiberg T and Hagen KB: Minimal
clinically important improvement/difference (MCII/MCID) and patient
acceptable symptom state (PASS): what do these concepts mean? Ann
Rheum Dis. 66 (Suppl 3):iii40–iii41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sowers MR and Karvonen-Gutierrez CA: The
evolving role of obesity in knee osteoarthritis. Curr Opin
Rheumatol. 22:533–537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koskinen A, Vuolteenaho K, Nieminen R,
Moilanen T and Moilanen E: 011 Proinflammatory and catabolic role
of leptin in osteoarthritis. Correlation with IL-6, MMP-1 and MMP-3
in synovial fluid and effects in human OA cartilage. Osteoarthritis
Cartilage. 18:S13–S14. 2010. View Article : Google Scholar
|
|
37
|
Lago R, Gomez R, Otero M, Lago F, Gallego
R, Dieguez C, Gomez-Reino JJ and Gualillo O: A new player in
cartilage homeostasis: Adiponectin induces nitric oxide synthase
type II and pro-inflammatory cytokines in chondrocytes.
Osteoarthritis Cartilage. 16:1101–1109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Simopoulou T, Malizos KN, Iliopoulos D,
Stefanou N, Papatheodorou L, Ioannou M and Tsezou A: Differential
expression of leptin and leptin's receptor isoform (Ob-Rb) mRNA
between advanced and minimally affected osteoarthritic cartilage;
effect on cartilage metabolism. Osteoarthritis Cartilage.
15:872–883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Toussirot E, Streit G and Wendling D: The
contribution of adipose tissue and adipokines to inflammation in
joint diseases. Curr Med Chem. 14:1095–1100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Presle N, Pottie P, Dumond H, Guillaume C,
Lapicque F, Pallu S, Mainard D, Netter P and Terlain B:
Differential distribution of adipokines between serum and synovial
fluid in patients with osteoarthritis. Contribution of joint
tissues to their articular production. Osteoarthritis Cartilage.
14:690–695. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hunter DJ, Sharma L and Skaife T:
Alignment and osteoarthritis of the knee. J Bone Joint Surg Am. 91
(Suppl 1):S85–S89. 2009. View Article : Google Scholar
|
|
42
|
Dawson L, Zarin DA, Emanuel EJ, Friedman
LM, Chaudhari B and Goodman SN: Considering usual medical care in
clinical trial design. PLoS Med. 6:e10001112009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arboleya LR, DE LA Figuera E, García Ms
and Aragón B; Grupo De Estudio Vicoxx, : Tratamiento sintomático de
la artrosis: Patrón de utilización de antiinflamatorios no
esteroides en los centros de salud españoles. Rev Española
Reumatol. 29:300–307. 2002.
|
|
44
|
Da Costa BR, Reichenbach S, Keller N,
Nartey L, Wandel S, Jüni P and Trelle S: RETRACTED: Effectiveness
of non-steroidal anti-inflammatory drugs for the treatment of pain
in knee and hip osteoarthritis: A network meta-analysis. Lancet.
387:2093–2105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bannuru RR, Schmid CH, Kent DM, Vaysbrot
EE, Wong JB and McAlindon TE: Comparative effectiveness of
pharmacologic interventions for knee osteoarthritis: A systematic
review and network meta-analysis. Ann Intern Med. 162:46–54. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lapane KL, Yang S, Driban JB, Liu SH, Dubé
CE, McAlindon TE and Eaton CB: Effects of prescription nonsteroidal
antiinflammatory drugs on symptoms and disease progression among
patients with knee osteoarthritis. Arthritis Rheumatol. 67:724–732.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yaxley J and Litfin T: Non-steroidal
anti-inflammatories and the development of analgesic nephropathy: A
systematic review. Ren Fail. 38:1328–1334. 2016. View Article : Google Scholar : PubMed/NCBI
|