Introduction
Bromophenols, a unique type of compound derived from
marine sources, are mainly isolated from marine fungi, marine
algae, sponges, ascidians, and bryozoans (1,2). It is
difficult to separate natural bromophenols from marine organisms,
and they often exhibit low biological activity (3–5).
However, bromophenol derivatives exhibit excellent biological
activity, including anti-oxidation, antibacterial and anti-tumor
activities (3,4). In our previous study, a series of
bromophenol compounds were designed. This series of compounds
exhibited excellent antitumor activities and could inhibit the
growth of a variety of tumor cells (6). One of these compounds,
3-(3-bromo-5-methoxy-4-(3-(piperidin-1-yl)propoxy)benzylidene)-N-(4-bromophenyl)-2-oxoindoline-5-sulfonamide
(BOS-93; Fig. 1A), exhibited
significant anti-tumor activities. Notably, in the present study,
it was demonstrated to induce autophagy in A549 cells, unlike the
mechanisms of the compounds we previously reported.
Apoptosis is the autonomous, orderly death of cells
controlled by genes to maintain homeostasis. It mainly comprises
two representative pathways: The mitochondrial pathway, which is
the major pathway, and the death receptor-mediated pathway
(7). Reactive oxygen species (ROS)
also serve a critical role in the mitochondrial pathway; generation
of ROS can induce mitochondrial membrane damage, releasing
cytochrome c from injured mitochondria and thereby inducing cell
apoptosis (8).
Autophagy, also known as type II cell death, is the
process by which cells use lysosomes to degrade their own damaged
organelles and macromolecules under the control of
autophagy-related genes (Atg) (9).
Apoptosis and autophagy are the molecular mechanisms by which cells
maintain organelles and homeostasis. Although autophagy can also
lead to cell death under certain conditions, it primarily maintains
a constant state in a cell through selective reutilization of
organelles and macromolecules (10,11).
Autophagy begins with the production of
double-membrane vacuoles (named autophagosomes) that entrap the
material to be degraded and eventually fused with lysosomes
(12). Autophagosomes are
characterized by the presence of the protein light chain 3 (LC3)
(derived from posttranslational modifications of a
microtubule-associated protein precursor) on their membranes.
Beclin-1, which was initially isolated as an interactor of the
oncogenic anti-apoptotic protein among many proteins that directly
or indirectly regulate the autophagy process (12,13).
In eukaryotes, autophagy is an important process
that is evolutionarily reserved for turnover of intracellular
material (13). The mammalian target
of rapamycin (mTOR) signaling pathway, and the phosphoinositide
3-kinase (PI3K)/protein kinase B (Akt)/mTOR pathway in particular,
is associated with most physiological processes, including cell
metabolism, proliferation and autophagy (14,15).
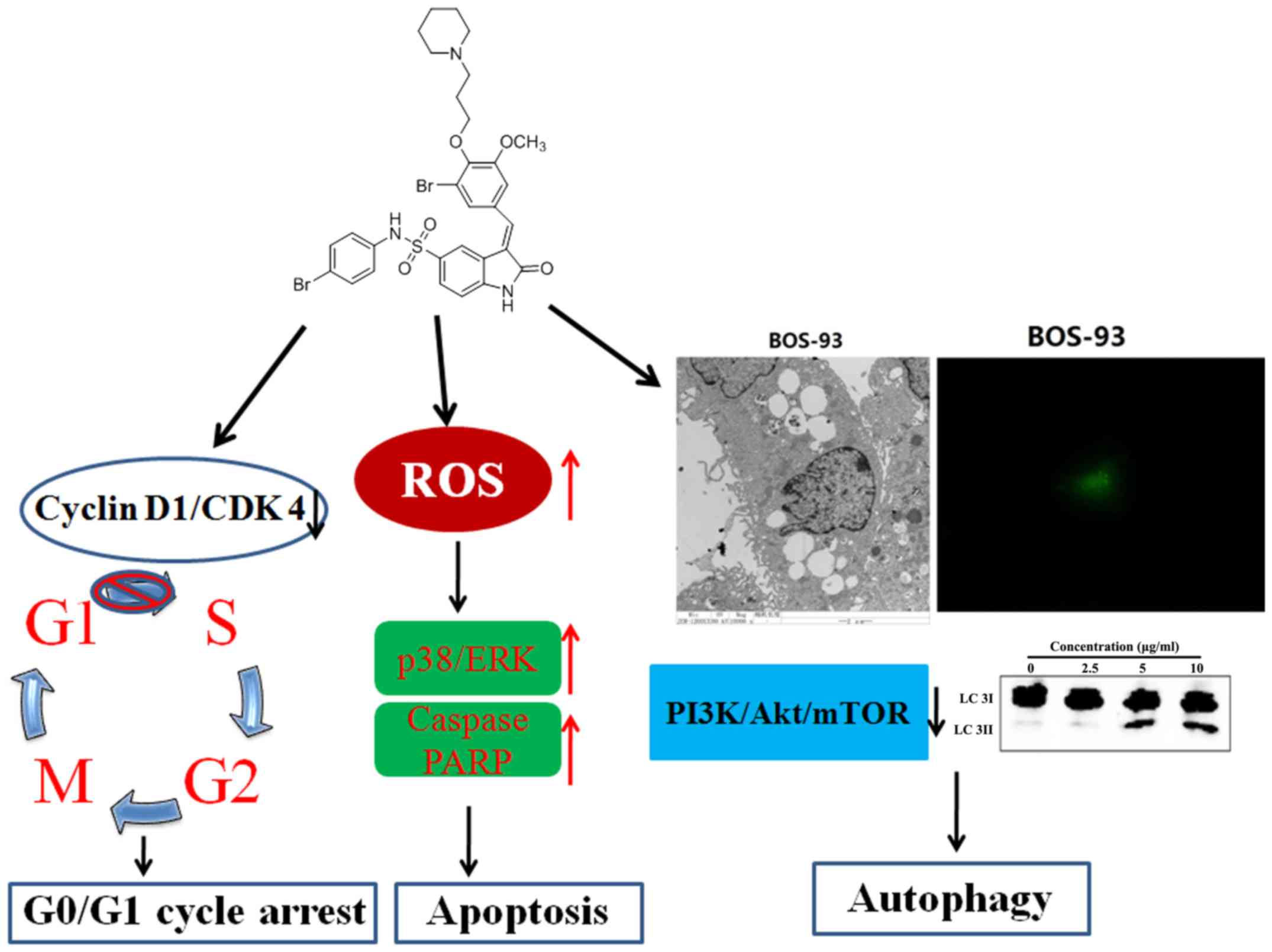
In the present study, the mechanism underlying the
anticancer effects of BOS-93 in A549 lung cells was investigated.
The results demonstrated that BOS-93 could induce apoptosis and
cause autophagy in A549 cells. In addition, the molecular
mechanisms underlying BOS-93-induced apoptotic and autophagic death
in A549 cells were investigated. Further studies indicated that the
PI3K/Akt/mTOR and p38/extracellular signal-regulated kinase
signaling pathways were associated with BOS-93-induced apoptosis
and autophagy. In summary, the present results indicated that
BOS-93 may be a promising anti-tumor drug against human lung
cancers.
Materials and methods
Reagents, chemicals and
antibodies
BOS-93 was synthesized by the CAS Key Laboratory of
Experimental Marine Biology, Institute of Oceanology, Chinese
Academy of Sciences, (Qingdao, China; purity >98%) (6). The purity of compound BOS-93 was
measured by high-performnce liquid chromatography (HPLC; Shimadzu
Corporation, Kyoto, Japan), carried out on a Shimadzu LC-20A system
(Shimadzu Corporation) equipped with a Shimadzu InertSustain C-18
reverse phase column (4.6×250 ×5 µm; Shimadzu Corporation) and
SPD-20A detector (Shimadzu Corporation). HPLC was conducted at 25°C
with 70% methanol and 50% acetonitrile mobile phases. The flow rate
was 1 ml/min and samples were 10 µl. The F-12K and RPMI-1640
mediums were obtained from Hyclone; GE Healthcare Life Sciences
(Logan, UT, USA), fetal bovine serum (FBS) was obtained from ExCell
Bio, Inc., (Shanghai, China), other culture reagents were purchased
from Invitrogen; Thermo Fisher Scientific, Inc., (Waltham, MA,
USA). Reactive oxygen species (ROS) assay kit, apoptosis assay kit,
Hoechst 33258 staining kit and adenovirus (Ad)-green fluorescent
protein (GFP)-LC3 were obtained from Beyotime Institute of
Biotechnology (Haimen, China). Autophagy inhibitor 3-MA was
obtained from Dalian Meilun Biotech Co., Ltd. (Dalian, China). The
antibodies against cyclin D1 (Rabbit mAb, cat. no. 2978; 1:1,000),
cyclin-dependent kinase (CDK)4 (Rabbit mAb cat. no. 12790;
1:1,000), cleaved-poly ADP ribose polymerase (PARP; Rabbit mAb cat.
no. 9532; 1:1,000), B cell lymphoma (Bcl)-2 (Mouse mAb cat. no.
15071; 1:1,000), Bcl-2-associated X protein (Bax; Rabbit cat. no.
2774; 1:1,000), cleaved-caspase-3 (Rabbit; cat. no. 9661; 1:1,000),
phosphorylated (p)-PI3K (Rabbit, cat. no. 4228; 1:1,000), PI3K
(Rabbit mAb cat. no. 4257; 1:1,000), p-Akt (Rabbit mAb cat. no.
4060; 1:2,000), Akt (Mouse mAb cat. no. 2920; 1:2,000), p-p38
mitogen-activated protein kinase (MAPK; Rabbit mAb cat. no. 4511;
1:1,000), p38 MAPK (Rabbit mAb cat. no. 8690; 1:1,000), p-ERK1/2
(Rabbit mAb cat. no. 4370; 1:2,000), ERK1/2 (Mouse mAb cat. no.
4696; 1:2,000), LC3 (Rabbit mAb cat. no. 3868; western blotting,
1:1,000; immunohistochemistry, 1:3,200), beclin-1 (Rabbit mAb cat.
no. 3495, 1:1,000) and Ki-67 [Rabbit mAb (IHC Specific) cat. no.
9027; 1:400] were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Antibodies against GAPDH (Rabbit, ab128915;
1:10,000), p-mTOR (Rabbit, ab109268; 1:5,000) and mTOR (Rabbit,
ab32028; 1:5,000) were purchased from Abcam (Cambridge, UK).
Antibody against Atg14 (Rabbit, WL02420; 1:1,000) was purchased
from Wanleibio Co., Ltd. (Shanghai, China).
Cell line and culture
Cells (A549, 95D and NCI-H460) were purchased from
Cell Bank, Chinese Academy of Sciences (Shanghai, China). A549
cells were maintained in F-12K medium, whereas 95D cells and
NCI-H460 cells were maintained in RPMI-1640 medium, and all mediums
were supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin. Cells were cultured at 37°C in a humidified
CO2 (5%) atmosphere.
Determination of cell viability
Cell viability was determined via MTT assay. Cells
in growth phase were plated into 96-well plates at a density of
3×103 cells per well and incubated for 24 h. Then, cells
were exposed to 0, 2.5, 5, 10 and 20 µg/ml BOS-93 for 48 h.
Following treatment, cells were further incubated with MTT for 4 h.
Then the formazan was dissolved by dimethyl sulfoxide and measured
using a microplate reader at 490 nm. The 50% inhibitory
concentration (IC50) was defined as the concentration
that reduced the absorbance of the untreated wells by 50% of the
vehicle in the MTT assay.
Colony formation assay
A549 cells were harvested and 500 cells/well were
seeded in 6-well plates and allowed to settle overnight at 37°C.
Then cells were treated with BOS-93 (0, 2.5, 5 and 10 µg/ml) at
37°C for 10 days. Following treatment, cells were washed with PBS
and fixed with 4% paraformaldehyde at room temperature for 10 min.
Cells were washed with PBS and finally stained with crystal violet
at room temperature for 10 min. Cells were scored under light
microscopy (magnification, ×100) and >50 cells clustered
together were scored as colonies (16).
Apoptosis assay by flow cytometry
Apoptosis was determined by flow cytometry. Briefly,
cells (1×105 cells/well) were seeded in 6-well plates
and allowed to settle overnight at 37°C, and then cells were
treated with BOS-93 (0, 2.5, 5 and 10 µg/ml) for 48 h. Cells were
harvested and stained with Annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (PI; Beyotime Institute of
Biotechnology, Haimen, China) at room temperature for 15 min, then
cells were analyzed using a flow cytometer (BD FACSCalibur) and
CellQuest Pro software (FACSstation 6.0; BD Biosciences, Franklin
Lakes, NJ, USA).
Cell cycle analysis
Cell cycle analysis was determined by flow
cytometry. Briefly, A549 cells (1×105 cells/well) were
harvested and seeded in 6-well plates and allowed to settle
overnight at 37°C. Cells were treated with BOS-93 (0, 2.5, 5 and 10
µg/ml) at 37°C. Following 48 h treatment, cells were harvested and
fixed in 7% ethanol at −20°C overnight. Cells were washed twice
with PBS and stained with PI solution containing 20 µg/ml RNaseA
and 50 µg/ml PI (both Beyotime Institute of Biotechnology) at 37°C
for 30 min. Then cells were analyzed using flow cytometry and
CellQuest Pro software.
Hoechst 33258 staining
The cell nucleus shape was observed by fluorescence
microscopy following Hoechst 33258 staining. Briefly, cells
(1×105 cells/well) were seeded in 6-well plates and
allowed to settle overnight at 37°C, and then cells were treated
with BOS-93 (0, 2.5, 5 and 10 µg/ml) at 37°C for 48 h. Following
treatment, cells were stained with Hoechst dye 33258 for 5 min at
room temperature and assessed by fluorescence microscopy
(magnification, ×400; Olympus Corporation, Tokyo, Japan).
Transmission electron microscopy
A549 cells were exposed to BOS-93 (10 µg/ml) for 48
h at 37°C and cells were fixed in 2.5% glutaraldehyde (Shanghai
Yuanye Biotechnology Co., Ltd., Shanghai, China) overnight at 4°C
and postfixed with 1% osmium tetroxide (OsO4) and
kaliumhexacyanoferrate [K3FE(CN)6] in
cacodylate buffer. Subsequently, the cells were dehydrated in an
alcohol series and embedded in resin overnight at room temperature.
Ultrathin sections (200 nm) were collected on formvar-coated grids,
counterstained with uranil acetate and lead citrate at room
temperature for 10 min, and visualized with transmission electron
microscopy (TEM; JEM-1200EX, JEOL Ltd., Akishima, Japan;
magnification, ×8,000).
Analysis of cells with GFP-LC3
To detect GFP-LC3 translocation, A549 cells
(1×105 cells/ml) were grown on glass coverslips at 37°C
for 24 h and then infected with Ad-GFP-LC3 (Beyotime Institute of
Biotechnology). Following overnight culture at 37°C, cells were
treated with BOS-93 (10 µg/ml) at 37°C for 48 h. Then cells were
fixed with 4% paraformaldehyde at 37°C for 20 min and examined
under a fluorescence microscope (magnification, ×400).
Analysis of autoghagy with 3-MA
The autophagy inhibitor 3-MA was also used to block
autophagy at the final concentration of 5 mM. Briefly, A549 cells
(1×105 cells/well) in a 6-well plate were pretreated
with 3-MA (5 mM) at 37°C for 1 h, and then cells were treated with
BOS-93 (0, 2.5, 5 and 10 µg/ml) at 37°C for 48 h. Cell lysates were
detected by western blotting.
Measurement of intracellular ROS
The measurement of ROS was determined by flow
cytometry. A549 cells (1×105 cells/well) were harvested
and seeded in 6-well plates and allowed to settle overnight at
37°C. Cells were treated with BOS-93 (0, 2.5, 5 and 10 µg/ml) for
48 h at 37°C. The medium was removed and cells were co-incubated
with 10 µM 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Beyotime
Institute of Biotechnology) at 37°C for 30 min. Then cells were
harvested and analyzed by flow cytometry and CellQuest Pro
software.
Western blot analysis
A549 cells were harvested and seeded in 6-well
plates (1×105 cells/well) and allowed to settle
overnight at 37°C. Cells were treated with BOS-93 (0, 2.5, 5 and 10
µg/ml) for 48 h at 37°C. Proteins were harvested by RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) and separated by SDS-PAGE on a 10% gel and transferred onto
polyvinylidene difluoride membranes. Membranes were blocked in
blocking solution (containing 5% non-fat milk) at room temperature
for 1 h and subsequently probed with primary antibodies at 4°C
overnight. Following 15 min washes in TBS-Tween 20, the membranes
were incubated with horseradish peroxidase (HRP)-conjugated
secondary antibodies (cat. no. 7074; 1:10,000; Cell Signaling
Technology, Inc.) for 1 h at room temperature. The bands were
detected using an enhanced chemiluminescence system BeyoECL Plus
(Beyotime Institute of Biotechnology).
In vivo tumor model
Female congenital athymic BALB/c nude (nu/nu) mice
(n=12) were purchased from Model Animal Research Center Of Nanjing
University (Nanjing, China). Mice were housed in a specific
pathogen-free room with controlled temperature (24±2°C), humidity
(60-80%) and lighting (12 h light/dark cycle) with ad
libitum access to water and food. All experiments with mice
were approved by Institute of Oceanology, Chinese Academy of
Sciences Laboratory Animal Care and Ethics Committee (Qingdao,
China) in accordance with the animal care and use guidelines.
Efforts were made to minimize animal suffering. All experiments
were carried out using 6–8-week-old mice weighting 18–22 g. Mice
were given 1 week to acclimatize to the housing conditions prior to
experiments. In vitro cultured A549 cancer cells
(1×107) were injected subcutaneously into the back of
mice. When the tumor reached 150 mm3 in volume, animals
were divided randomly into two groups (n=6 each) and administered
with 50 mg/kg intraperitoneal BOS-93 (dissolved in 0.5%
carboxymethyl cellulose-Na) for 21 days (once a day). Tumor volumes
and body weight were measured every 3 days. Tumor volumes were
calculated according to the following equation: Length ×
(width)2/2. Body weight was measured every three days
and clinical symptoms were observed daily. Following treatment,
mice were anaesthetized with isoflurane (inhalation anesthesia;
Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China) and
sacrificed by decapitation and tumor tissues were collected for
immunohistochemistry, and haematoxylin and eosin (H&E)
analysis.
Immunohistochemistry and H&E
staining
Tumor tissues were obtained, immediately fixed in
10% neutral formaldehyde at room temperature for 24 h and later
embedded in paraffin wax. The paraffin-embedded tissue sections (4
µm) were treated with heat-induced antigen retrieval buffer (pH
6.0; citrate buffer; Beyotime Institute of Biotechnology) and
blocked using 5% bovine serum albumin (Beijing Solarbio Science
& Technology Co., Ltd.) at room temperature for 1 h.
For immunohistochemistry, samples were then
incubated with rabbit anti-Ki-67 (cat. no. 9027; 1:400) or
anti-LC3B (cat. no. 12741; 1:500; Cell Signaling Technology, Inc.)
antibodies overnight at 4°C. Tissue was then incubated with
Equilibrate SignalStain® Boost IHC Detection Reagent
(HRP, Rabbit; cat. no. 8114; Cell Signaling Technology, Inc.) for
30 min at room temperature and developed using a DAB kit (cat. no.
8059; Cell Signaling Technology, Inc.) at room temperature for 1
min. Samples were then counterstained with hematoxylin for 30 sec
at room temperature and then observed under a light microscope
(magnification, ×200).
For H&E staining, samples were stained with
hematoxylin for 10 min at room temperature. Samples were washed
with water for 10 min at room temperature and then stained with
eosin for 2 min at room temperature. Samples were observed under a
light microscope (magnification, ×200).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). All data
are presented as mean + standard deviation. Differences were
analysed with one-way analysis of variance followed by Tukey's post
hoc test. The difference between the control and model groups was
analysed using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
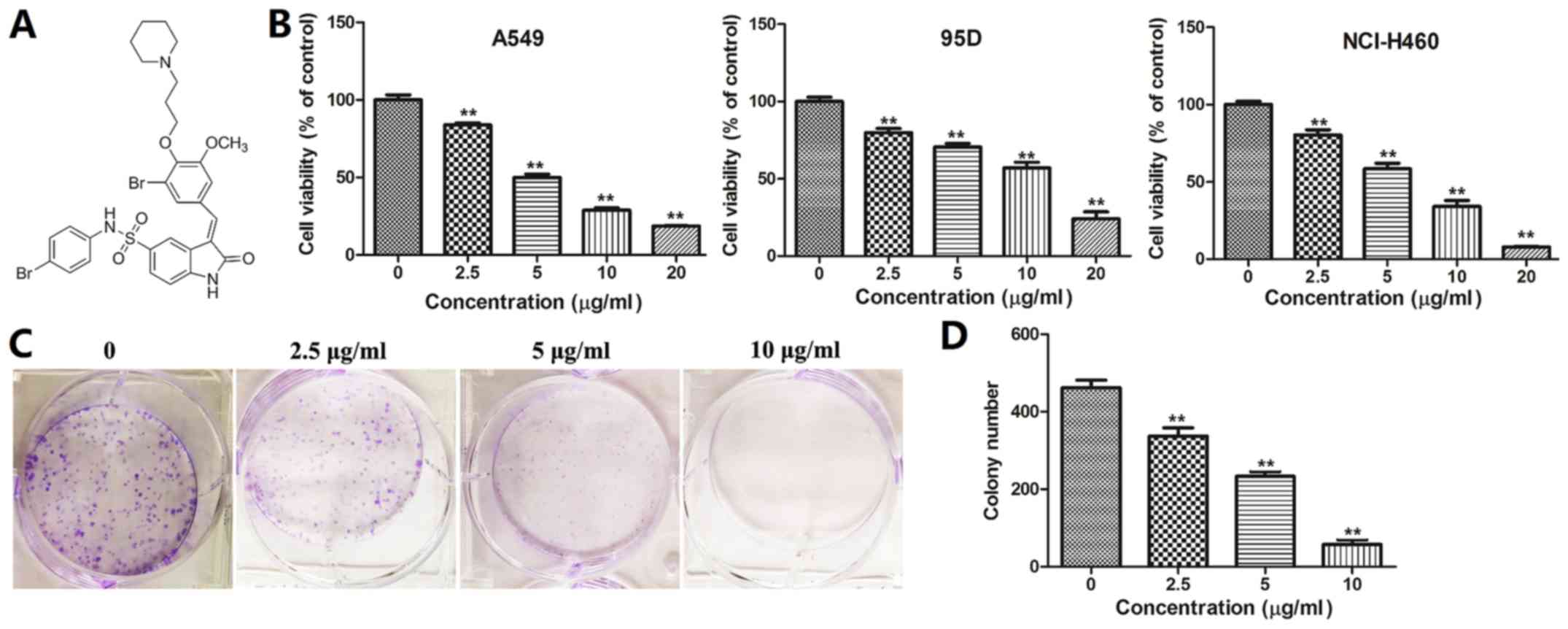
BOS-93 inhibits cell
proliferation
Cell viability was detected by MTT assay. As
presented in Fig. 1B, BOS-93 had a
dose-dependent inhibitory effect on three human lung cancer cells
including A549, 95D and NCI-H460 cells. The IC50 value
of BOS-93 on the three cells was 4.78±0.56, 9.99±1.81 and 6.14±0.60
µg/ml, respectively. The effect of BOS-93 on the relative colony
formation ability of A549 cells was also investigated. As presented
in Fig. 1C and D, the clonogenicity
of A549 cells was reduced in a dose-dependent manner following
exposure to BOS-93.
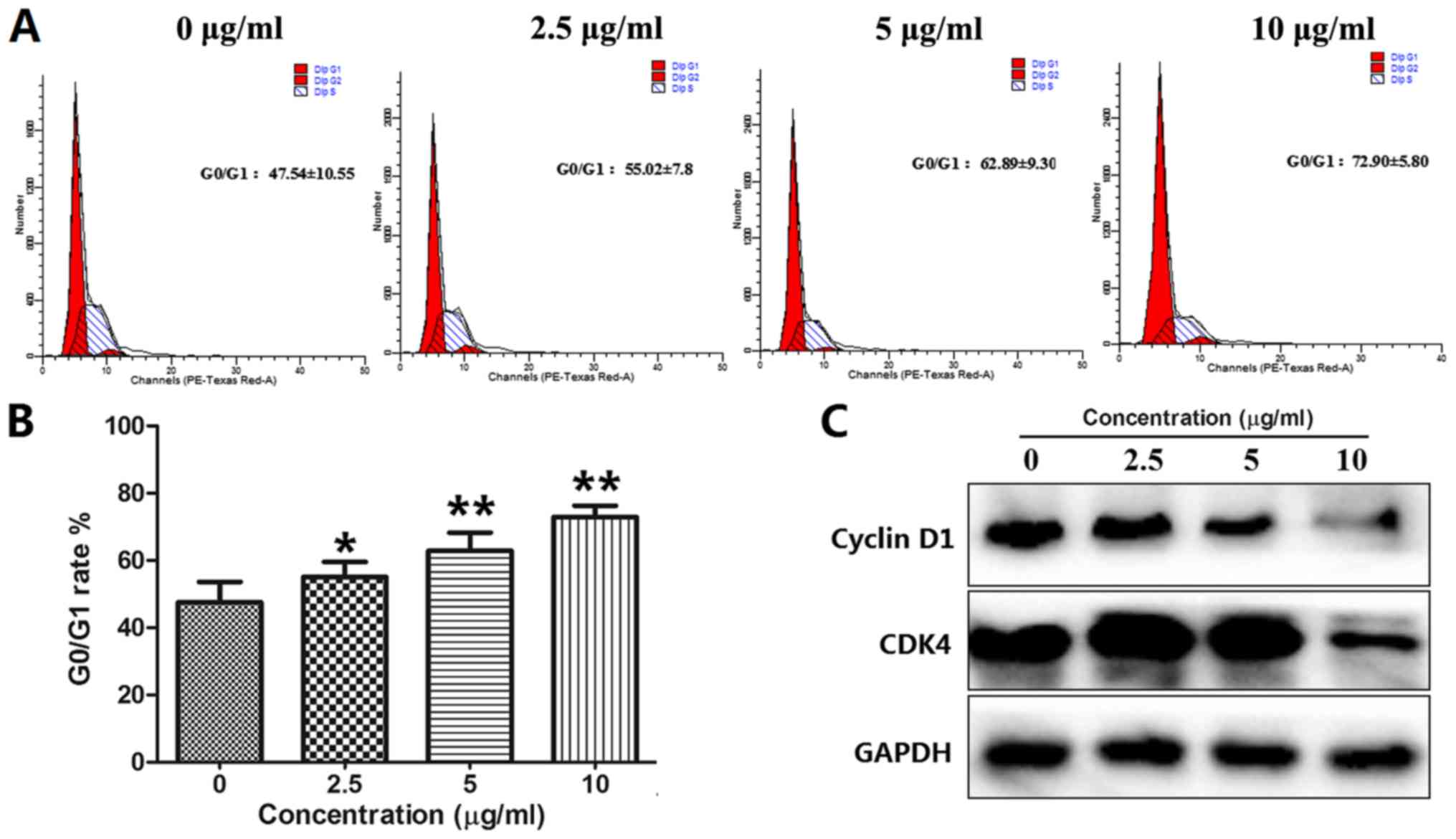
BOS-93 induces G0/G1 cell cycle
arrest
The cell cycle progression of A549 cells was
analyzed via flow cytometry. A549 cells were analyzed by flow
cytometry following treatment with BOS-93 (0, 2.5, 5 and 10 µg/ml)
for 48 h. As presented in Fig. 2A and
B, following treatment with BOS-93, the accumulation of cells
in the G0/G1 phase was increased in a dose-dependent manner. The
percentage of cells in the 0, 2.5, 5 and 10 µg/ml groups at the
G0/G1 phase was significantly enhanced from 47.54±10.55 to
55.02±7.8, 62.89±9.30 and 72.90±5.80%, respectively.
Western blotting was used to analyze cell cycle
associated proteins. As presented in Fig. 2C, following treatment with BOS-93,
protein levels of cyclin D1 and CDK4 were decreased, these data
indicated that BOS-93-mediated cell cycle arrest at the G0/G1 phase
may inhibit the formation of CDK/cyclin complexes via
downregulation of cyclin D1 and CDK4.
BOS-93 induces A549 apoptosis
Apoptosis is a major form of cell death induced by
chemotherapeutic agents (17). In
the present study, A549 cells were treated with BOS-93 for 48 h.
Cells were stained with Annexin-V-FITC/PI and analyzed by flow
cytometry. The results indicated a dose-dependent increase in the
proportion of cells in which apoptosis was induced by BOS-93. The
apoptotic cell rate in the 0, 2.5, 5 and 10 µg/ml groups were
increased from 10.67±1.96 to 17.66±1.72, 31.66±7.16 and
46.63±8.34%, respectively (Fig. 3A and
B).
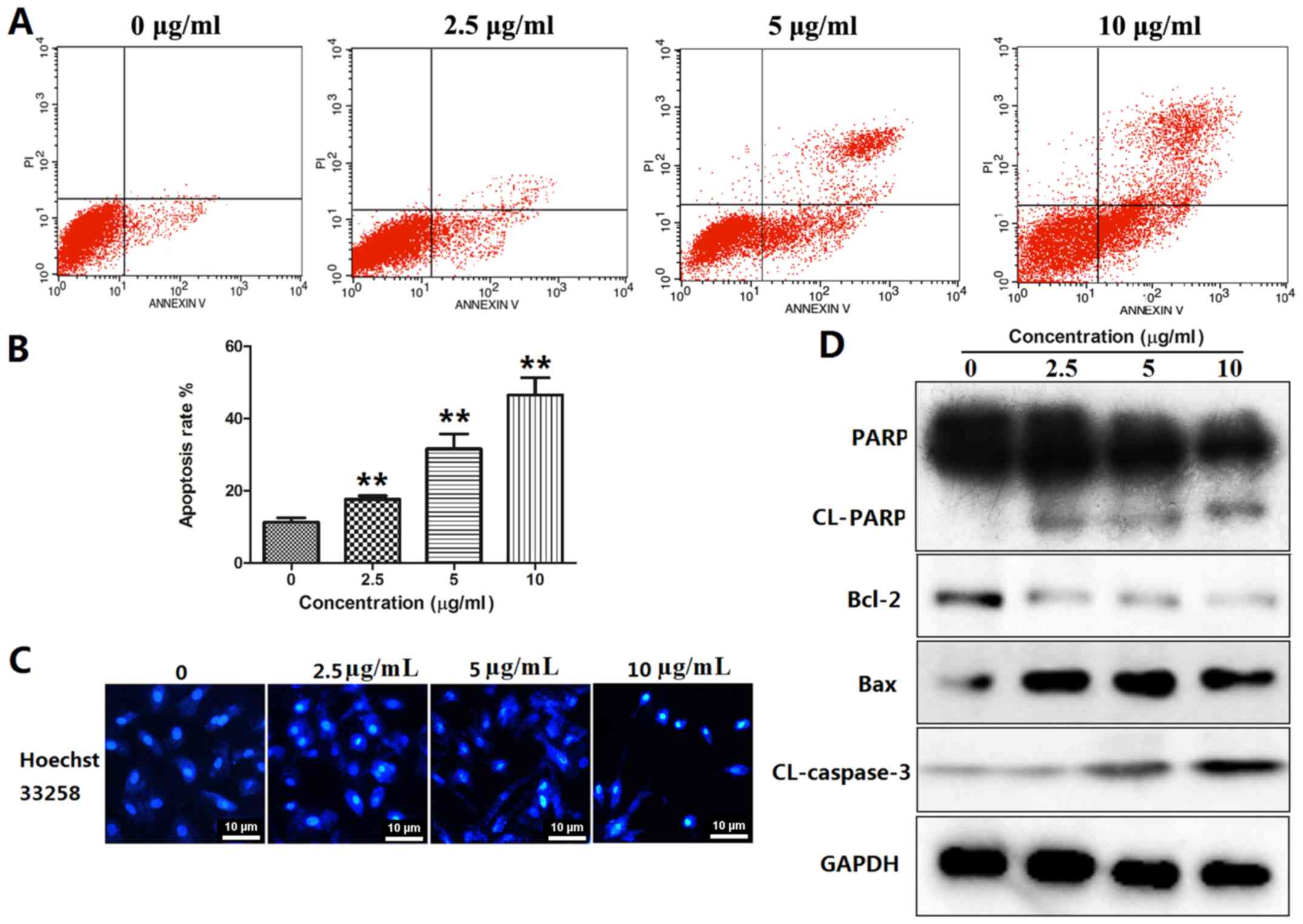 | Figure 3.BOS-93 induces cell apoptosis. (A and
B) A549 cells were treated with BOS-93 for 48 h, and then cells
were stained with Annexin V/PI and analyzed by flow cytometry. (C)
A549 cells were treated with BOS-93 for 48 h, and then cells were
stained with Hoechst 33258 and photographed using fluorescence
microscopy (scale bar, 50 µm). (D) A549 cells were treated with
BOS-93 for 48 h and then apoptosis-associated proteins, including
cleaved-PARP, Bcl-2, Bax and cleaved-caspase-3 were analyzed using
western blotting. Data are expressed as mean + standard deviation
(n=3). **P<0.01 vs. control group. BOS-93,
3-(3-bromo-5-methoxy-4-(3-(piperidin-1-yl)propoxy)benzylidene)-N-(4-bromophenyl)-2-oxoindoline-5-sulfonamide;
PI, propidium iodide; PARP, poly ADP ribose polymerase; Bcl-2, B
cell lymphoma-2; Bax, Bcl-2-associated X protein; CL, cleaved. |
Morphological changes in A549 cells were also
detected with the Hoechst 33258 staining method. Apoptotic features
such as nuclear shrinkage and chromatin condensation were observed,
as presented in Fig. 3C. These
results demonstrated that BOS-93 could induce apoptosis in A549
cells.
Several classic markers of apoptosis (Bax, Bcl-2,
cleaved-caspase3 and cleaved-PARP) were detected by western blot
analysis. The results demonstrated that BOS-93 could induce
apoptosis via mitochondrial pathways. As presented in Fig. 3D, the expression levels of Bax were
increased in BOS-93 treated cells, whereas expression levels of
Bcl-2 were decreased. Furthermore, as the concentration of BOS-93
increased, the levels of cleaved caspase-3 and cleaved-PARP were
increased (Fig. 3D).
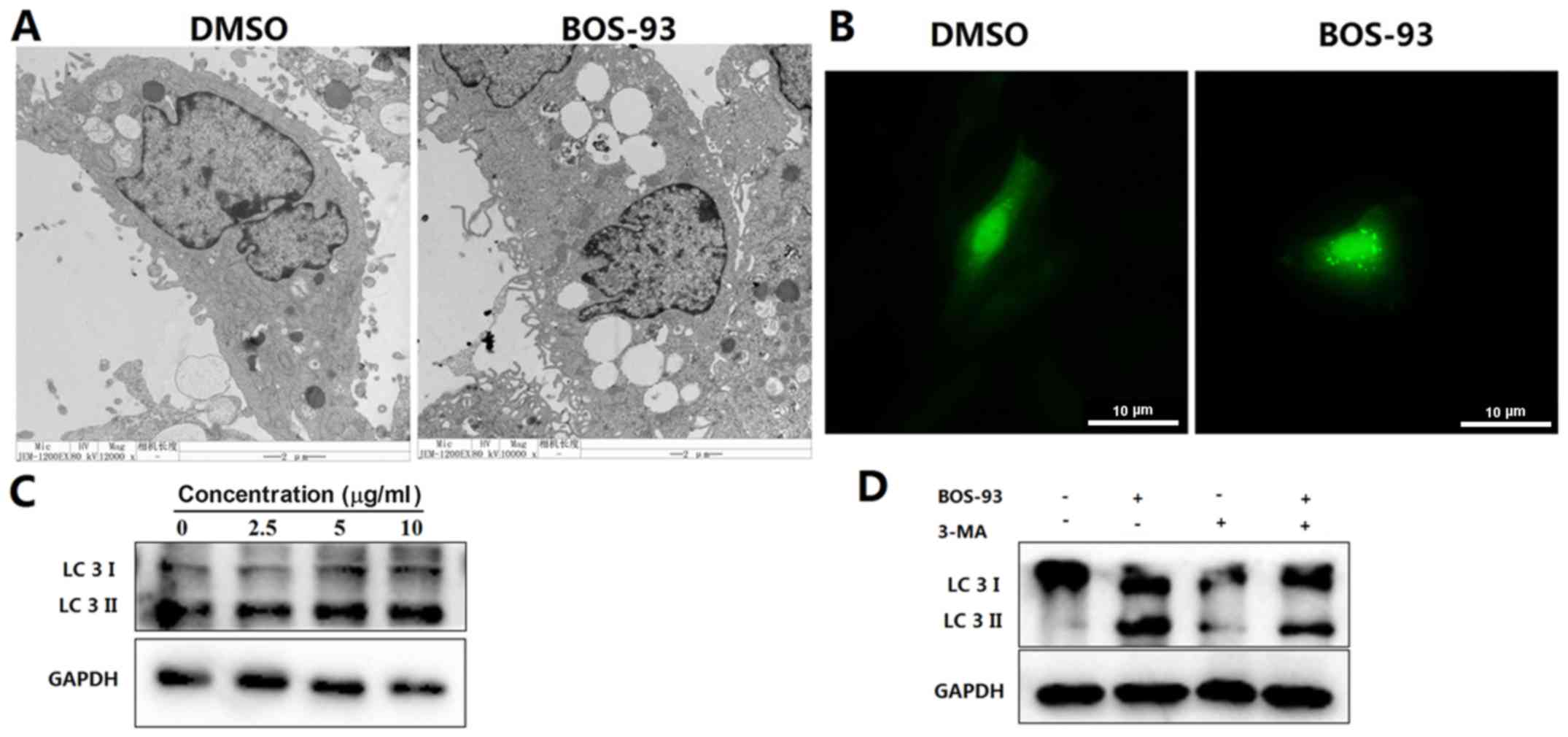
BOS-93 induces autophagy in A549
cells
Following observing that BOS-93 induced apoptosis
(Fig. 3A), it was determined that
BOS-93 could induce autophagy in A549 cells. A549 cells were
treated with BOS-93 for 48 h and evaluated by transmission electron
microscopy. As presented in Fig. 4A,
marked autophagic characteristics appeared in A549 cells following
treatment with BOS-93. Autophagic vacuoles were detected by
transmission electron microscopy. GFP-LC3 was used to detect the
formation of autophagic vesicles. A549 cells were infected with
recombinant adenoviral vector carrying GFP-tagged LC3, and then
cells were examined under a fluorescence microscope. As presented
in Fig. 4B, GFP-LC3 exhibited
diffuse expression in untreated cells, whereas high punctate
expression was observed in cells treated with BOS-93.
Another notable hallmark of autophagy is the
conversion of LC3; specifically, the conversion of LC3-I (18 kDa)
to LC3-II (16 kDa), which can be measured by western blot analysis
(14). As presented in Fig. 4B, an increasing ratio of LC3-II/LC3-I
was detected following treatment of BOS-93 for 48 h (Fig. 4C). Notably, the LC3-II/LC3-I ratio
was decreased in the presence of the autophagy inhibitor, 3-MA
(Fig. 4D).
Western blot analysis was used to examine the
expression of autophagy-related proteins. As presented in Fig. 5A, the expressions of Atg14 and
beclin-1 were increased in a concentration-dependent manner
following treatment of BOS-93 for 48 h. Furthermore, the content of
these proteins was decreased in the presence of 3-MA (Fig. 5B). These results indicated that A549
cells treated with BOS-93 were undergoing autophagy.
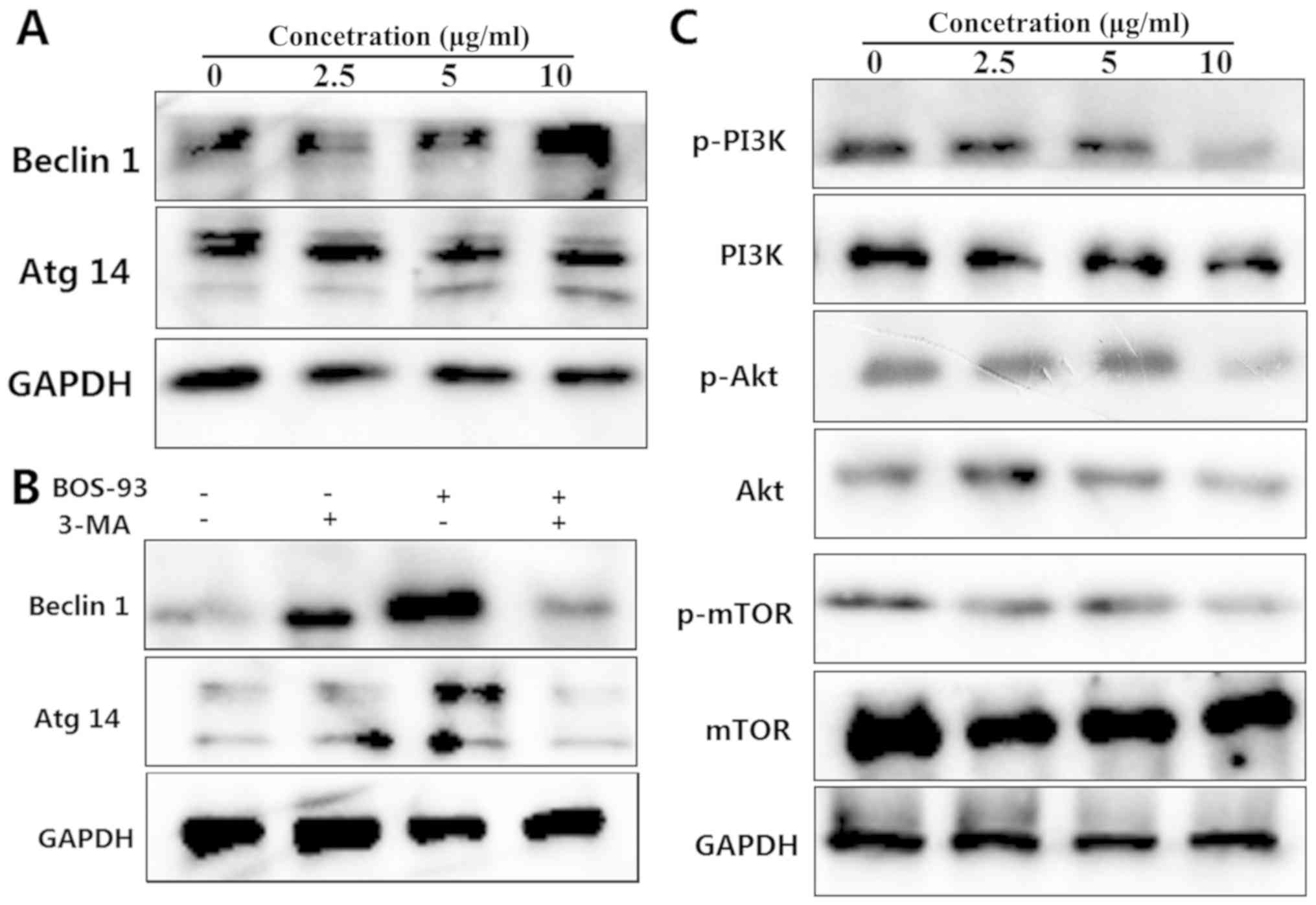 | Figure 5.(A) Effects of BOS-93 on the levels
of beclin-1 and Atg14 proteins. A549 cells were treated with BOS-93
for 48 h and then autophagy-related proteins, including beclin-1
and Atg14 were analyzed using western blotting. (B) A549 cells were
pretreated for 1 h in the presence or absence of 3-MA (5 mM) prior
to addition of BOS-93. Western blot analysis of beclin-1 and Atg14
was performed following addition of 10 µg/ml BOS-93 for 48 h. (C)
A549 cells were treated with BOS-93 (0, 2.5, 5 or 10 µg/ml) for 48
h. The expressions of PI3K, Akt, mTOR, p-PI3K, p-Akt and p-mTOR
were assessed by western blot analysis. GAPDH was used to normalize
protein content. All data were representative of three independent
experiments. BOS-93,
3-(3-bromo-5-methoxy-4-(3-(piperidin-1-yl)propoxy)benzylidene)-N-(4-bromophenyl)-2-oxoindoline-5-sulfonamide;
Atg14, autophagy-related gene 14; PI3K, phosphoinositide 3-kinase;
Akt, protein kinase B; mTOR, mechanistic target of rapamycin; p,
phosphorylated. |
It has been reported that the PI3K/Akt/mTOR
signaling pathway serves a major role in regulating autophagy in
cancer cells (15). A549 cells were
treated with BOS-93 (2.5, 5, and 10 µg/ml) for 48 h, and the
associated proteins were analyzed using western blot analysis. As
presented in Fig. 5C, treating A549
cells with BOS-93 for 48 h resulted in decreased phosphorylation of
PI3k, Akt and mTOR.
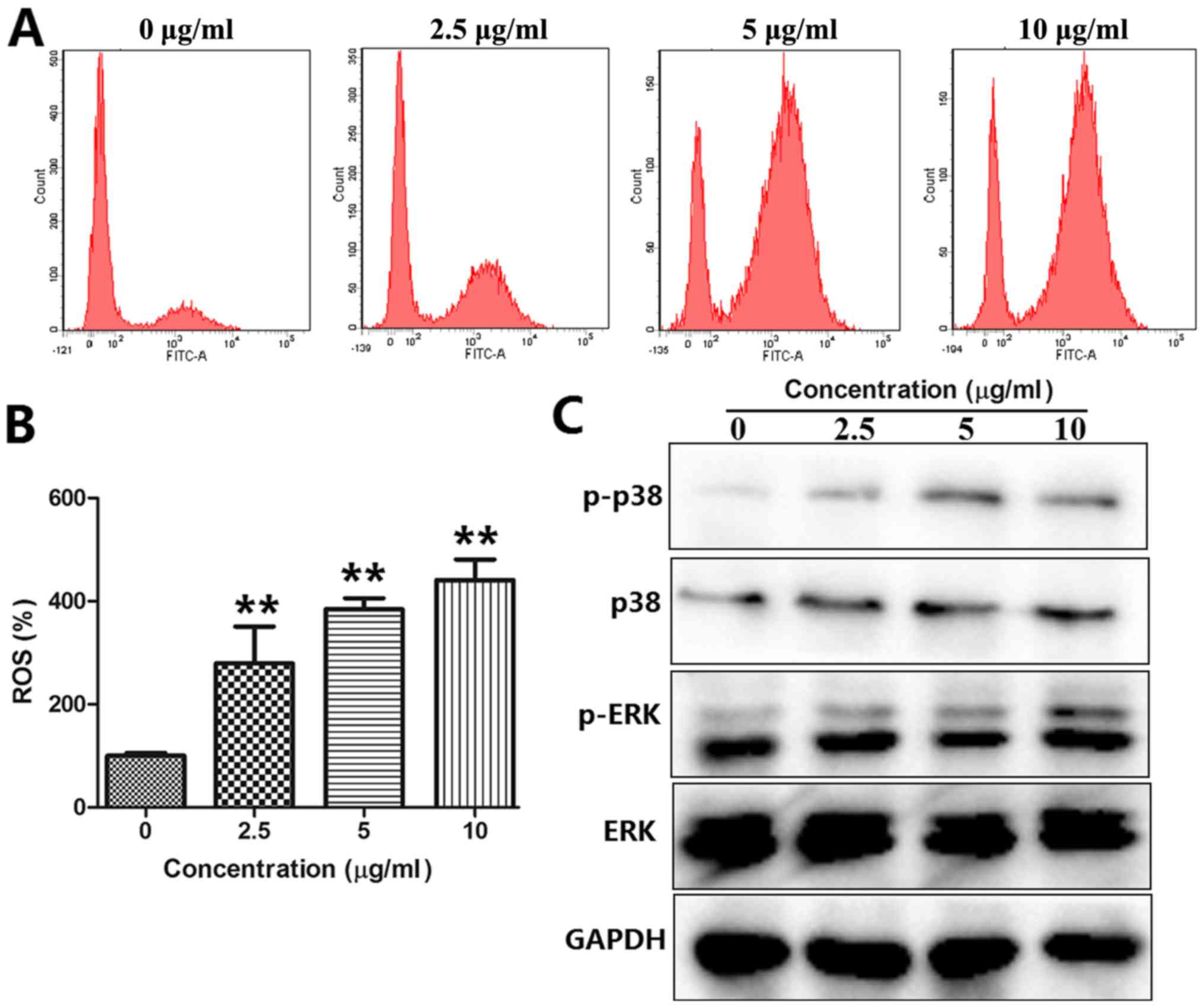
BOS-93 stimulates ROS generation in
A549 cells
The level of intracellular ROS was quantitatively
estimated using a peroxide-sensitive fluorescent probe, DCFH-DA,
and analyzed by flow cytometry. Compared with the control group,
ROS was rapidly produced following exposure to A549 cells with
BOS-93 (Fig. 6A and B). When treated
with BOS-93 for 48 h, the mean DCF fluorescence in the 2.5, 5 and
10 µg/ml groups was increased by 279.38±71.23, 384.01±21.60 and
440.80±40.04%, respectively.
BOS-93 induces autophagy and apoptosis
in A549 cells through ROS-dependent ERK and P38 MAPK
activation
It has been reported that the activation of MAPKs,
such as ERK and p38 MAPK, is associated with regulation of
apoptosis and autophagy (15). A549
cells were treated with BOS-93 (2.5, 5, and 10 µg/ml) for 48 h, and
the expression levels of ERK and p38 MAPK were analyzed by western
blot analysis. As presented in Fig.
6C, following treatment of BOS-93, the phosphorylation of p-p38
and p-ERK was increased. These findings indicated that BOS-93 could
induce apoptosis and autophagy in A549 cells via the ROS-mediated
PI3K/Akt/mTOR and MAPK signaling pathways.
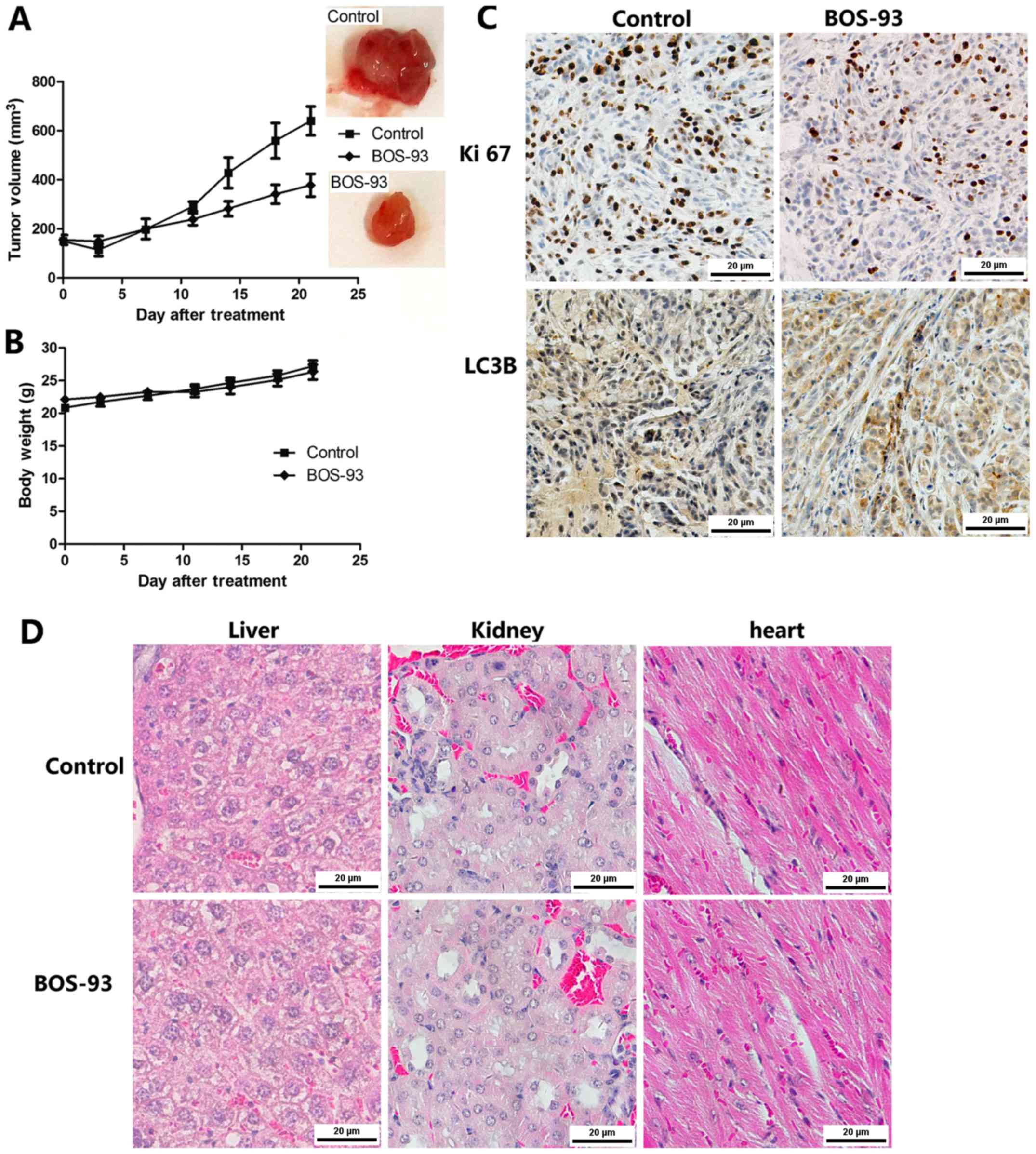
BOS-93 inhibits tumor growth in the in
vivo A549 cell xenograft model
In order to evaluate the anti-tumor properties of
BOS-93 in vivo, an A549 ×enografted athymic mice mode was
established in the present study. As presented in Fig. 7A, the tumor growth was markedly
inhibited in the BOS-93 treated group at an inhibition ratio of
47.93%. Furthermore, there was no significant change in mice body
weight during the experiment (Fig.
7B). For histological analysis, the heart, spleen and kidney
were stained with hematoxylin and eosin, as presented in Fig. 7C, and there were no marked
histological changes in these tissues following treatment with
BOS-93, these results may suggest that the anti-tumor activity of
BOS-93 has low toxicity on athymic mice.
It was further demonstrated that BOS-93 induced
apoptosis and autophagy in vivo. Immunohistochemistry with
Ki 67 (a cell proliferation marker) and LC3B (a cell autophagy
marker) were examined in paraffin-embedded tumor sections. As
presented in Fig. 7D, BOS-93
treatment markedly decreased the expression of Ki 67 and increased
the expression of LC 3B compared with the control group.
Discussion
A novel bromophenol, BOS-93, was synthesized in the
CAS Key Laboratory of Experimental Marine Biology, Institute of
Oceanology, Chinese Academy of Sciences (6). In the present study, it was
demonstrated that BOS-93 could lead to growth inhibition of human
A549 lung cancer cells in association with apoptosis and autophagy.
It was demonstrated that BOS-93 induced the loss of viability on
human lung cancer cells, and the IC50 value on A549
cells was 4.78±0.56 µg/ml. The present results also indicated that
BOS-93 could significantly inhibit colony formation in A549 cells.
Furthermore, BOS-93 inhibited tumor growth without causing apparent
toxicity effect in A549 cell xenograft model.
Regulation of cell growth and proliferation of
mammalian cells are mediated through cell cycle progression
(18). In the present study, cell
cycle analysis demonstrated that BOS-93 could induce G0/G1 cell
cycle arrest in A549 cells. It is well known that cyclin-dependent
kinases serve an important role in cell cycle regulation (19). Additionally, BOS-93 induces G0/G1
cell cycle arrest by inhibiting the activity of cyclin-dependent
kinases cyclin D1 and CDK 4 (20).
The present study indicated that BOS-93 could exert its
anti-proliferative effect via the modulation of cyclin-CDK
machinery-mediated cell-cycle arrest.
Apoptosis serves a key role in the regulation of
cells. It mainly comprised two apoptotic pathways: The death
receptor-mediated apoptosis pathway and the mitochondria-mediated
apoptosis pathway (21). In the
latter, proteins from the Bcl-2 family, such as Bax and Bcl-2, are
the main components that regulate mitochondrial permeability. The
implementation of apoptosis is achieved through a proteolysis chain
reaction comprising, for example, the caspase family (22). In the present study, it was
demonstrated that BOS-93 treatment could increase the ratio of
Bax/Bcl-2 as well as activate caspase-3 and PARP. These results
indicated that BOS-93 could activate the mitochondrial apoptotic
pathway in A549 cells. In addition, it has been reported that ROS
serves a key role in the process of mitochondria-mediated apoptosis
(23). In the present study, an
increase in ROS generation was observed in cells treated with
BOS-93, indicating that ROS serves an important role in
BOS-93-induced apoptosis.
In research on chemotherapeutic drugs, apoptosis is
commonly considered to be a prevailing anti-cancer mechanism
(24–26). However, apoptosis is not the only
such mechanism; other forms of cell death, such as autophagy, can
also occur (27). When autophagy
occurs, certain morphological characteristics, such as
autophagosomes, can be observed by TEM (28). Notably, TEM also revealed that BOS-93
induces cell autophagy.
Microtubule-associated protein LC3 is a cytoplasmic
protein that is quickly cleaved to form LC3-I (18 kDa) (29). It is activated by lipidation with
phosphatidylethanolamine to form LC3-II (16 kDa), and the amount of
LC3-II is correlated with the number of autophagosomes (29). The conversion of LC3-I (18 kDa) to
LC3-II (16 kDa) typically corresponds to an increase in the rate of
autophagy (28,30). In the present study, TEM was used to
detect the effect of BOS-93 on the ultrastructure of A549 cells.
Following BOS-93 treatment, the A549 cells displayed morphological
characteristics associated with cell autophagy. Ad-GFP-LC3 was used
to detect GFP-LC3 translocation in A549 cells. GFP-LC3 exhibited
diffuse expression in untreated cells but high punctate expression
in BOS-93-treated cells. Western blot analysis revealed
accumulation of autophagosome-bound LC3-II. This phenomenon was
reversed when cells were treated with 3-MA, an autophagy inhibitor,
which indicated that BOS-93 induces autophagy of A549 cells.
It has been reported that the molecular machinery of
autophagy is largely directed by a number of Atgs. For example,
previous studies have demonstrated that Atg 14 and beclin-1 are
essential for the formation of autophagosomal precursor (9,14). In
the present study, the expression of Atg14 and beclin-1 were
determined using western blot analysis. the data demonstrated that
BOS-93 treatment induced upregulation of Atg14 and beclin1.
Furthermore, combining 3-MA with BOS-93 resulted in a significant
decrease in the expression of Atg14 and beclin-1 compared with
BOS-93 cultured alone, suggesting that BOS-93 induced autophagy in
A549 cells.
Given that both apoptosis and autophagy could be
induced by BOS-93 in A549 cells, their mutual association was
investigated. Several studies have reported that chemotherapeutic
drugs can induce both apoptosis and autophagy in cancer cells
(10,17). Autophagosome formation is regulated
by the mTOR pathway, a major nutrient sensor (9). The present study demonstrated that,
upon BOS-93 treatment, PI3K, Akt and mTOR phosphorylation was
reduced in A549 cells, indicating downregulation of the
PI3K/Akt/mTOR pathway.
MAPK signal pathways (including p38 MAPK and ERK1/2)
are associated with the transduction of extracellular stimuli
signals into cells and their nuclei and initiation of cellular
biological reactions such as cell proliferation, differentiation,
transformation and apoptosis (31,32). ROS
can act as a second messenger to activate the MAPK signaling
pathway (23). In addition, it is
well known that p38 is closely associated with the initiation of
apoptosis and the cell cycle (33).
ERK1/2 is mainly activated by phosphorylation of various growth
factors and oxidative stress, and it is closely associated with
cell proliferation and differentiation (34). In the present study, it was
demonstrated that BOS-93 treatment triggered ROS generation and
increased phosphorylation of p38 and ERK, suggesting that the
p38/ERK signaling pathway was associated with BOS-93 induced
apoptosis and autophagy in A549 cells.
In conclusion, the present study demonstrated that
BOS-93 could induce G0/G1 arrest, apoptosis and autophagy in A549
cells, and that BOS-93 could inhibit PI3K/Akt/mTOR and activate the
MAPK pathway (Fig. 8). Furthermore,
BOS-93 could also exert anti-tumor activity in vivo.
Therefore, the findings indicated that BOS-93 may display excellent
effects on human lung cancer cells and act as a multi-target
anti-cancer drug due to its ability of leading to apoptosis and
autophagy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81773586 and
81703354) and Key Research Program of Frontier Sciences, CAS (grant
no. QYZDB-SSW-DQC014), and the Project of Discovery, Evaluation and
Transformation of Active Natural Compounds, Strategic Biological
Resources Service Network Programme of Chinese Academy of Sciences
(grant no. ZSTH-026), and Shandong Provincial Natural Science
Foundation for Distinguished Young Scholars (grant no. JQ201722),
and the National Program for Support of Top-notch Young
Professionals, and Taishan Scholar Youth Project of Shandong
Province.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CG, LW and DS conceived and designed the present
study. CG, LW, YZ, BJ and JL performed the experiments. CG and LW
analyzed experimental data. CG wrote and revised the paper. All
authors reviewed the manuscript.
Ethics approval and consent to
participate
All studies in mice were approved by Institute of
Oceanology, Chinese Academy of Sciences Laboratory Animal Care and
Ethics Committee in accordance with the animal care and use
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blunt JW, Copp BR, Hu WP, Munro MH,
Northcote PT and Prinsep MR: Marine natural products. Nat Prod Rep.
24:31–86. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang BG, Gloer JB, Ji NY and Zhao JC:
Halogenated organic molecules of Rhodomelaceae origin: Chemistry
and biology. Chem Rev. 113:3632–3685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu M, Hansen PE and Lin XK: Bromophenols
in marine algae and their bioactivities. Marine Drugs. 9:1273–1292.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oztaskin N, Cetinkaya Y, Taslimi P, Göksu
S and Gülçin I: Antioxidant and acetylcholinesterase inhibition
properties of novel bromophenol derivatives. Bioorg Chem. 60:49–57.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi D, Guo S, Jiang B, Guo C, Wang T,
Zhang L and Li J: HPN, a synthetic analogue of bromophenol from red
alga Rhodomela confervoides: Synthesis and anti-diabetic effects in
C57BL/KsJ-db/db mice. Mar Drugs. 11:350–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang LJ, Guo CL, Li XQ, Wang SY, Jiang B,
Zhao Y, Luo J, Xu K, Liu H, Guo SJ, et al: Discovery of novel
bromophenol hybrids as potential anticancer agents through the
Ros-mediated apoptotic pathway: Design, synthesis and biological
evaluation. Mar Drugs. 15(pii): E3432017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki M, Endo M, Shinohara F, Echigo S
and Rikiishi H: Rapamycin suppresses ROS-dependent apoptosis caused
by selenomethionine in A549 lung carcinoma cells. Cancer Chemother
Pharmacol. 67:1129–1136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lalaoui N, Lindqvist LM, Sandow JJ and
Ekert PG: The molecular relationships between apoptosis, autophagy
and necroptosis. Semin Cell Dev Biol. 39:63–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar D, Shankar S and Srivastava RK:
Rottlerin induces autophagy and apoptosis in prostate cancer stem
cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett.
343:179–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan P, Hu C, Quan C, Yu T, Zhou W, Yuan
M, Shi Y and Yang K: 4-Nonylphenol induces apoptosis, autophagy and
necrosis in Sertoli cells: Involvement of ROS-mediated
AMPK/AKT-mTOR and JNK pathways. Toxicology. 341:28–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kliosnky D, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Russell RC, Yuan HX and Guan KL: Autophagy
regulation by nutrient signaling. Cell Res. 24:42–57. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu JJ, Yang C, Guo C, Li X, Yang N, Zhao
L, Hang H, Liu S, Chu P, Sun Z, et al: SZC015, a synthetic
oleanolic acid derivative, induces both apoptosis and autophagy in
MCF-7 breast cancer cells. Chem Biol Interact. 244:94–104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shinojima N, Yokoyama T, Kondo Y and Kondo
S: Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in
curcumin-induced autophagy. Autophagy. 3:635–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang Y, Wang X and Hu D: Furanodienone
induces G0/G1 arrest and causes apoptosis via the
ROS/MAPKs-mediated caspase-dependent pathway in human colorectal
cancer cells: A study in vitro and in vivo. Cell Death Dis.
8:e28152017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie ZZ, Li MM, Deng PF, Wang S, Wang L, Lu
XP, Hu LB, Chen Z, Jie HY, Wang YF, et al: Paris saponin-induced
autophagy promotes breast cancer cell apoptosis via the Akt/mTOR
signaling pathway. Chem Biol Interact. 264:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang R, Zhang Q, Peng X, Zhou C, Zhong Y,
Chen X, Qiu Y, Jin M, Gong M and Kong D: Stellettin B induces G1
arrest, apoptosis and autophagy in human non-small cell lung cancer
A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep.
6:270712016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gupta S: Molecular steps of death receptor
and mitochondrial pathways of apoptosis. Life Sci. 69:2957–2964.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo CL, Wang LJ, Zhao Y, Liu H, Li XQ,
Jiang B, Luo J, Guo SJ, Wu N and Shi DY: A novel bromophenol
derivative BOS-102 induces cell cycle arrest and apoptosis in human
A549 lung cancer cells via ROS-mediated PI3K/Akt and the MAPK
signaling pathway. Mar Drugs. 16(pii): E432018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu CC and Bratton SB: Regulation of the
intrinsic apoptosis pathway by reactive oxygen species. Antioxid
Redox Signal. 19:546–558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, Chang F, Li F, Fu H, Wang J, Zhang
S, Zhao J and Yin D: Palmitate promotes autophagy and apoptosis
through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun.
463:262–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim R, Emi M, Tanabe K, Uchida Y and Toge
T: The role of fas ligand and transforming growth factor beta in
tumor progression: Molecular mechanisms of immune privilege via
Fas-mediated apoptosis and potential targets for cancer therapy.
Cancer. 100:2281–2291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burz C, Berindan-Neagoe I, Balacescu O and
Irimie A: Apoptosis in cancer: Key molecular signaling pathways and
therapy targets. Acta Oncol. 48:811–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okada H and Mak TW: Pathways of apoptotic
and non-apoptotic death in tumour cells. Nat Rev Cancer. 4:592–603.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lockshin RA and Zakeri Z: Apoptosis,
autophagy, and more. Int J Biochem Cell Biol. 36:2405–2419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuma A, Matsui M and Mizushima N: LC3, an
autophagosome marker, can be incorporated into protein aggregates
independent of autophagy: Caution in the interpretation of LC3
localization. Autophagy. 3:323–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen SD, Wu CL, Hwang WC and Yang DI: More
insight into BDNF against neurodegeneration: Anti-apoptosis,
Anti-oxidation, and suppression of autophagy. Int J Mol Sci.
18(pii): E5452017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ki YW, Park JH, Lee JE, Shin IC and Koh
HC: JNK and p38 MAPK regulate oxidative stress and the inflammatory
response in chlorpyrifos-induced apoptosis. Toxicol Lett.
218:235–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang H, Cong L, Zhi Y, Xu H, Jia X and
Peng S: T-2 toxin inhibits murine ES cells cardiac differentiation
and mitochondrial biogenesis by ROS and p-38 MAPK-mediated pathway.
Toxicol Lett. 258:259–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bulavin DV, Phillips C, Nannenga B,
Timofeev O, Donehower LA, Anderson CW, Appella E and Fornace AJ Jr:
Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis
through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf)
pathway. Nat Genet. 36:343–350. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoon S and Seger R: The extracellular
signal-regulated kinase: Multiple substrates regulate diverse
cellular functions. Growth Factors. 24:21–44. 2006. View Article : Google Scholar : PubMed/NCBI
|