Introduction
Since bone tissue engineering has provided an
efficient tool for bone regeneration, numerous studies have focused
on associated effects and the underlying mechanisms (1). Several different growth factors have
been indicated to stimulate bone growth (2), collagen synthesis (3) and fracture repair (4), and have thus attracted the attention of
scholars regarding their effects on bone regeneration.
Among the factors, bone morphogenetic proteins
(BMPs) have been proven to facilitate bone healing without bone
tissue transfer (5). Studies have
indicated that BMP-2, a protein of BMP family, has important roles
in bone generation-associated processes, including osteoblastic
differentiation (6), the healing
process of segmental bone defects (7) and the capacity of bone marrow stromal
cells (BMSCs) to undergo osteogenesis (8), and has been approved by the Food and
Drug Administration of the USA for clinical use in the orthopedic
and dental fields (9). However,
large amounts of BMP-2 are required to achieve clinically
significant bone regeneration and BMP-2 is easily inactivated by
dilution or interaction with enzymes in the blood if applied alone
by intravenous injection (10–12). To
enhance the bioavailability of BMP-2, numerous local delivery
systems have been assessed (13),
among which the Chitosan (CS) delivery system was proven efficient
and enhanced the osteogenic activity (14). Certain studies also reported that
BMP-2 promotes ectopic osteogenesis (15,16).
However, few studies have focused on the effects of BMP-2 delivery
systems, e.g. CS-based delivery systems, on processes of ectopic
osteogenesis.
To the best of our knowledge, it has remained
elusive whether the CS/human recombinant (rh)BMP-2 microsphere
delivery system influences the ectopic osteogenesis process. The
present study aimed to investigate the effect of rhBMP-2 delivered
by CS microspheres on ectopic osteogenesis in rats. It provides
basic data for future application of the CS/rhBMP-2 microsphere
delivery system and a deeper understanding of the roles of BMP-2 in
bone regeneration.
Materials and methods
Preparation of CS blank microspheres
and CS/rhBMP-2 microspheres
The preparation of CS blank microspheres and
CS/rhBMP-2 microspheres was performed using a procedure modified
from a previous study (17), namely
42 ml thiamine pyrophosphate solution was added instead of DS and
ZnSO4. In brief, to prepare CS blank microspheres, a
certain amount of CS (molecular weight, 50,000-190,000; Aladdin
Reagent Co. Ltd., Shanghai, China) was dissolved in 1% (v/v) acetic
acid and the pH was adjusted to 5.4 by addition of NaOH solution to
obtain a mixture with a CS concentration of 1.52 mg/ml. The mixture
was then filtered through a membrane with 0.22 µm pores and 100 ml
of the filtrate was stirred for 1 h at room temperature, followed
by addition of 42 ml thiamine pyrophosphate solution (0.5 mg/ml).
When the nanospheres formed, the solution turned from clear to an
emulsion and the reaction was continued until no alteration
appeared. After completion of the reaction, the mixture was stirred
for another 30 min at room temperature and centrifuged for 15 min
at 25,000 × g at room temperature. The precipitate was washed with
water and dried under cryogenic conditions with reduced
pressure.
To prepare the CS/rhBMP-2 microspheres, 100 mg dried
CS blank microspheres and 5 mg rhBMP-2 were added to 25 ml
double-distilled water, followed by stirring for 30 min at 4°C and
subsequent centrifugation for 15 min at 25,000 × g at room
temperature. The liquid supernatant was then collected and dried to
obtain CS/rhBMP-2 microspheres.
Characterization of CS/rhBMP-2
microspheres
The microsphere morphology and grain diameter were
analyzed using a scanning electron microscope (S-4800; Hitachi
Ltd., Tokyo, Japan) and a laser diffraction particle size analyzer
(N5; Beckman Coulter, Brea, CA, USA). The shape and size of
microspheres were determined and the grain diameter was calculated.
All experiments were performed in triplicate.
Determination of the entrapment
efficiency and drug loading ratio
As described previously (16), the drug loading ratio and entrapment
efficiency of CS/rhBMP-2 nanoparticles were calculated by using an
ELISA kit (cat. no ELH-BMP2-1; RayBiotech Inc., Norcross, CA, USA).
All experiments were performed in triplicate. The optical density
of the supernatant was determined at 450 nm according to the
protocol for the ELISA kit.
In vitro sustained-release profile and
degradation
The measurement of the in vitro
sustained-release profile was in accordance with that of a previous
study by our group and another study (17,18). In
brief, 50 mg CS/rhBMP-2 microspheres were immersed in 2 ml PBS (pH
7.4), followed by agitation in a water bath oscillator. The
solution was centrifuged for 15 min (25,000 × g) at room
temperature and 100 µl supernatant was taken every 3 days at 6, 12,
18, 24, 48 and 72 h. Each time the supernatant was obtained, 100 µl
PBS was added and the solution was agitated in a water bath
oscillator. The experiments lasted for 30 days. The content of
rhBMP-2 was measured using the ELISA kit as described above.
For measurement of the degradation of the CS/rhBMP-2
microspheres, 50 mg microspheres (weight, m0) was added
in a centrifuge tube with 2 ml PBS (pH 7.4), followed by agitation
in a water bath. Every 3 days, 3 tubes were randomly selected and
the microspheres in each tube were dried for determining the weight
(mt). The experiment lasted for 45 days and the in
vitro degradation was calculated as
D=(m0-mt)/m0 ×100%. All
experiments were performed in triplicate.
Animals and treatment
A total of 24 male Sprague Dawley (SD) rats were
provided by the Experimental Animal Center of Southern Medical
University (Guangzhou, China). The age of all rats was 6 weeks and
their weight was 130–160 g. The rats were kept under a 12-h
light/dark cycle and at a constant temperature (23-25°C) and
relative humidity (70%). All animals were housed in micro-isolator
cages with free access to food and water according to the Guide for
the Care and Use of Laboratory Animals of China (19). Any effort was made to avoid any
unnecessary pain for the animals. The protocol of the present study
was approved by the Institutional Animal Care Committee at General
Hospital of Southern Theater Command, People's Liberation Army
(Guangzhou, China).
All rats were divided into 4 groups with 6 rats in
each group: The CS/rhBMP-2 group, which was treated with
microspheres loaded with 1 mg rhBMP-2, the rhBMP-2 group in which 1
mg rhBMP-2 was directly implanted, the CS blank group, which was
treated with unloaded microspheres of the same size and the control
group, which was implanted with a gelatin sponge of the same size.
All microspheres were sterilized by irradiation of 3,000
Gy60Co and stored at 4°C prior to the experiments.
Ectopic osteogenesis experiment and
measurement
All SD rats were anaesthetized by intraperitoneal
injection of 10% chloral hydrate (400 mg/kg). A 1.5-cm incision was
made on the left leg of each rat and the muscle bag model of
quadriceps femoris was generated. The abovementioned materials
(microspheres or rhBMP-2) were implanted into the muscle bag and
the wound was then sutured. After the surgery, all rats were
intraperitoneally injected with 4×105 units of
penicillin every day for 3 days. The hardness of the tissue around
the implant was analyzed by palpation every day.
X-ray imaging was performed at 4 weeks after the
surgery and micro-computed tomography (CT) examination was
performed at 1, 2, 3 and 4 weeks after the surgery to determine the
ectopic osteogenesis in the different groups of animals. The grafts
were evaluated using a micro-CT apparatus from GE Healthcare
(Little Chalfont, UK) with the following parameters: 80 kV, 0.6 mm,
80 µA and exposure time, 3,000 msec. The grafts were evaluated for
osteogenesis capacity based on the following morphometric indices:
Bone mineral density (BMD), tissue mineral density (TMD), tissue
mineral content (TMC) and bone volume fraction (BVF).
Histology
The animals were sacrificed at 4 weeks after the
surgery. Tissues around the implants were obtained and fixed in
formalin buffer. The samples were subjected to H&E staining and
the histologic morphology in each group was observed under an
inverted microscope.
Alkaline phosphatase (ALP) activity
analysis and determination of the calcium content
The implants were taken out at 4 weeks after the
surgery. For each animal, 0.5 g tissue around the implants was
obtained, ground and washed with deionized water. Cell Lysis
solution (RIPA lysis buffer; Beyotime Institute of Biotechnology,
Haimen, China) was added and the mixture was centrifuged at 16,000
× g for 30 min at 4°C. ALP activity was determined using an ALP
Detection Kit (cat. no. A059-2; Nanjing Jiancheng Biotechnology
Co., Ltd., Nanjing, China) according to manufacturer's protocols.
The calcium content of the tissues was determined using an Atomic
absorption spectrophotometer (i7500; Hitachi Ltd.) by using the
following formula: Calcium content=calcium content (µg)/sample wet
weight (mg).
Statistical analysis
The measurement data are expressed as the mean ±
standard deviation. Comparison between two groups was performed
using the Student's t-test. Comparison among three or more groups
was performed using one-way analysis of variance followed by
Tukey's post-hoc test. P<0.05 was considered to indicate
a statistically significant difference. All calculations were made
using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Characterization of CS/rhBMP-2
microspheres
First, the physicochemical properties of the
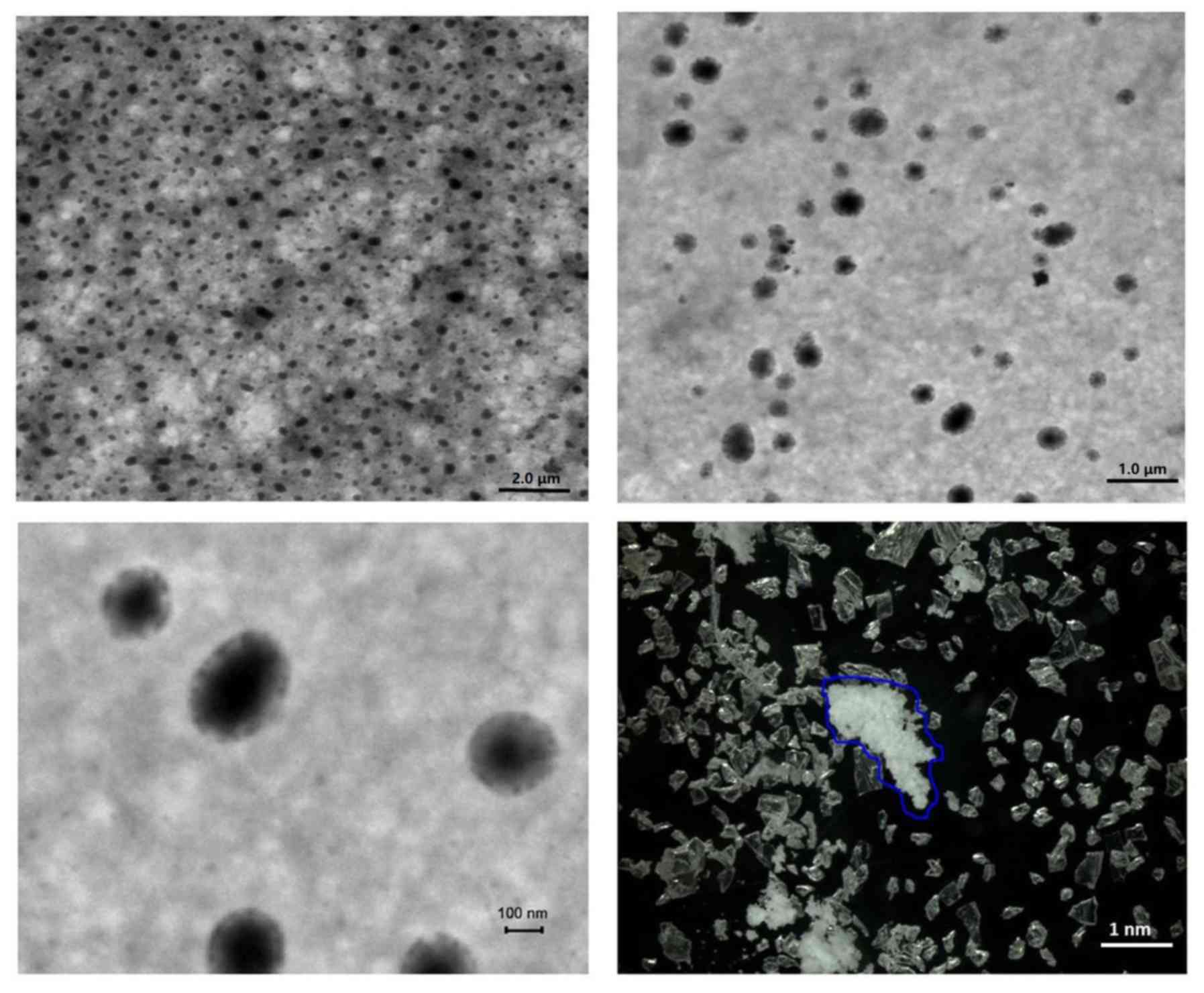
CS/rhBMP-2 microspheres were determined. As presented in Fig. 1, analysis of microsphere morphology
indicated that the CS/rhBMP-2 microspheres demonstrated spherical
regularity and the surface of the spheres was smooth and
unwrinkled, with rhBMP-2 contained in the spheres. The grain
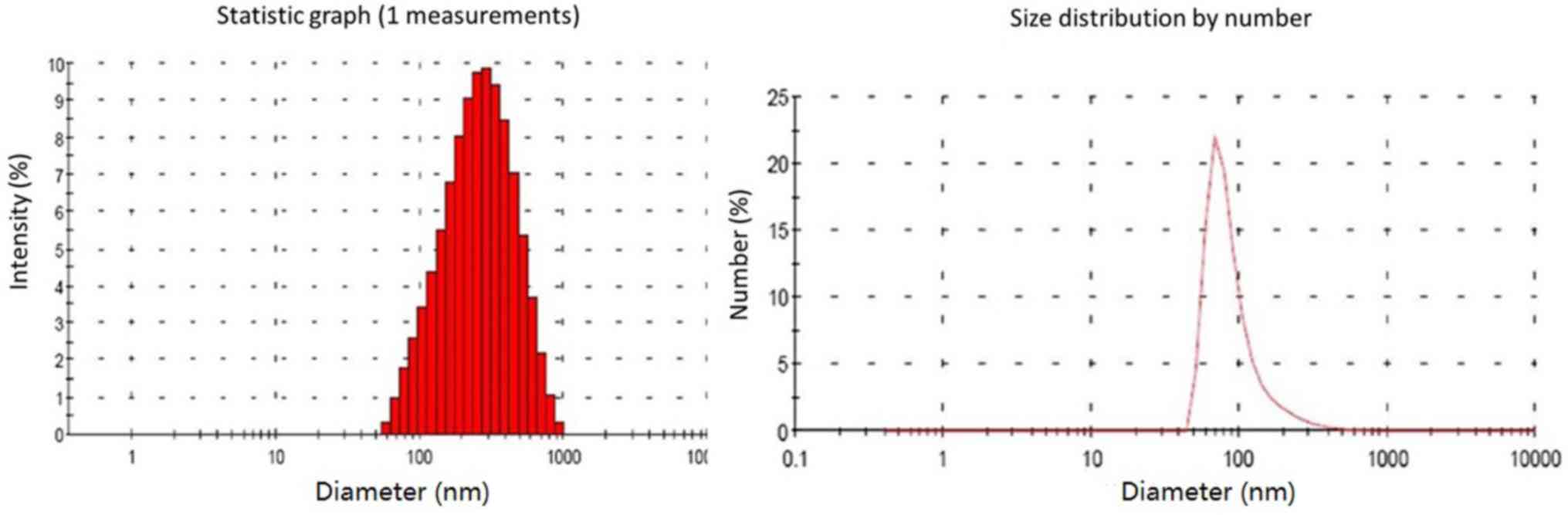
diameter analysis indicated that the grain diameter of CS/rhBMP-2
microspheres ranged from 58.8 to 955 nm, with the diameters being
mainly distributed within the 142–531 nm range (79.3%; Fig. 2). The mean diameter of the CS/rhBMP-2
microspheres was 230 nm. The entrapment efficiency for CS/rhBMP-2
microspheres was determined to be 66.9±4.6% and the drug loading
ratio was 33.4±2.3 µg/mg.
In vitro sustained-release profile and
degradation
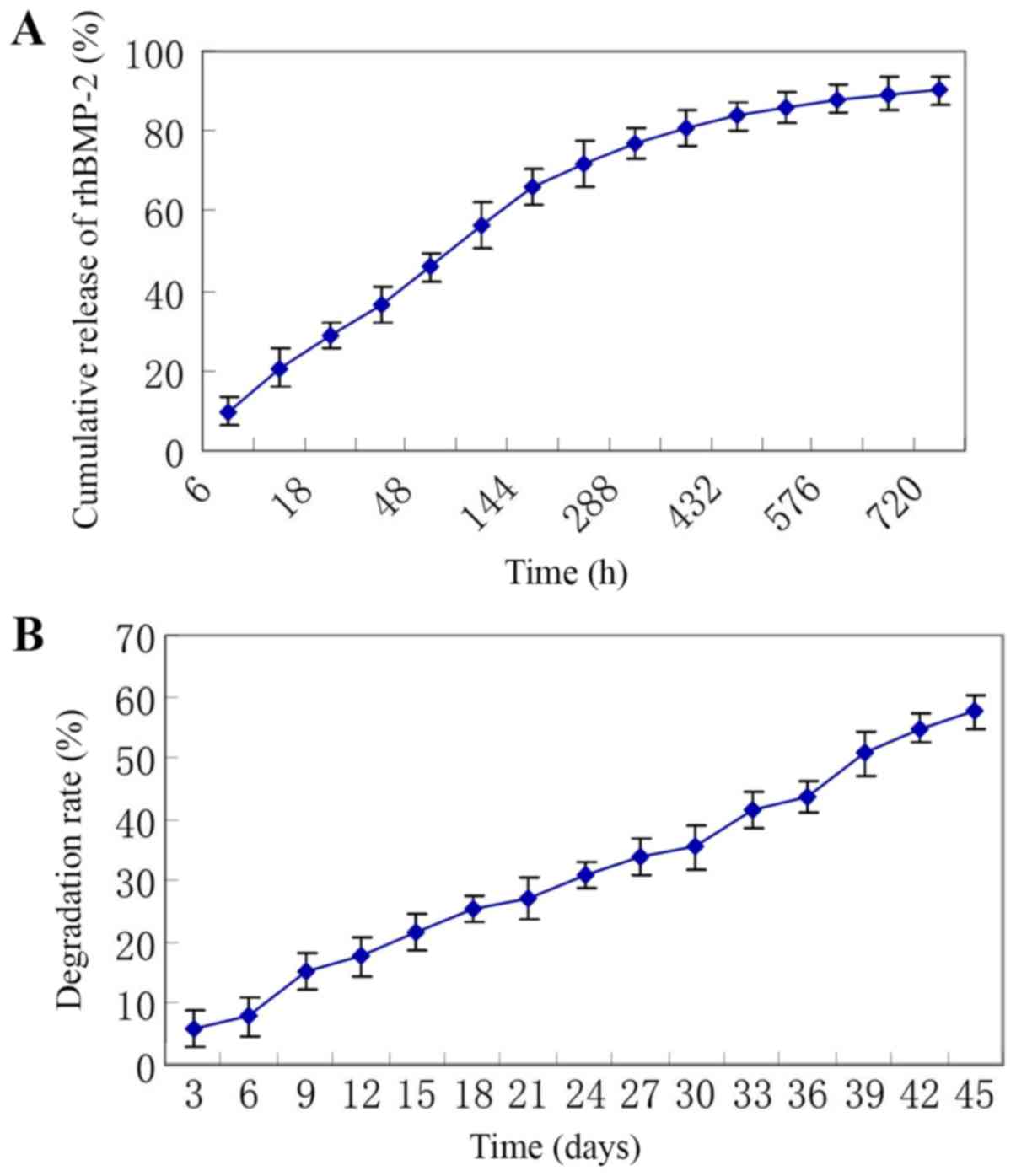
As presented in Fig.
3A, the release of rhBMP-2 lasted for 30 days and was in
consistency with biphasic dynamics. The initial phase was a fast
drug release phase with a cumulative drug release rate of 45.9±3.8%
at the first 48 h and a cumulative drug release rate of 66.0±4.4%
at the first 6 days. The posterior phase was a slow release phase
with a cumulative drug release rate of 80.8±4.8% at 15 days and a
cumulative drug release rate of 90.1±3.6% at 30 days.
The degradation assay indicated that the weight of
the microspheres gradually decreased and the degradation rate
increased with time, with a degradation rate of 21.5±3.0% at 15
days, 35.6±3.6% at 30 days and 57.6±2.8% at 45 days (Fig. 3B).
General observations in the ectopic
osteogenesis experiment
After the surgery, the rats were returned to their
cages with free access to food and water, and the surgical wounds
healed well in all animals. After 3 weeks, the area around the
implants became hard in the CS/rhBMP-2 group; however, the other
groups exhibited no obvious change. After 4 weeks, the area around
the implants became hard in the CS/rhBMP-2 group and in the rhBMP-2
group, and osteoid tissues were found in each of these two groups
at the area around the implants; however, no such osteoid tissues
were present in the CS blank and the control groups.
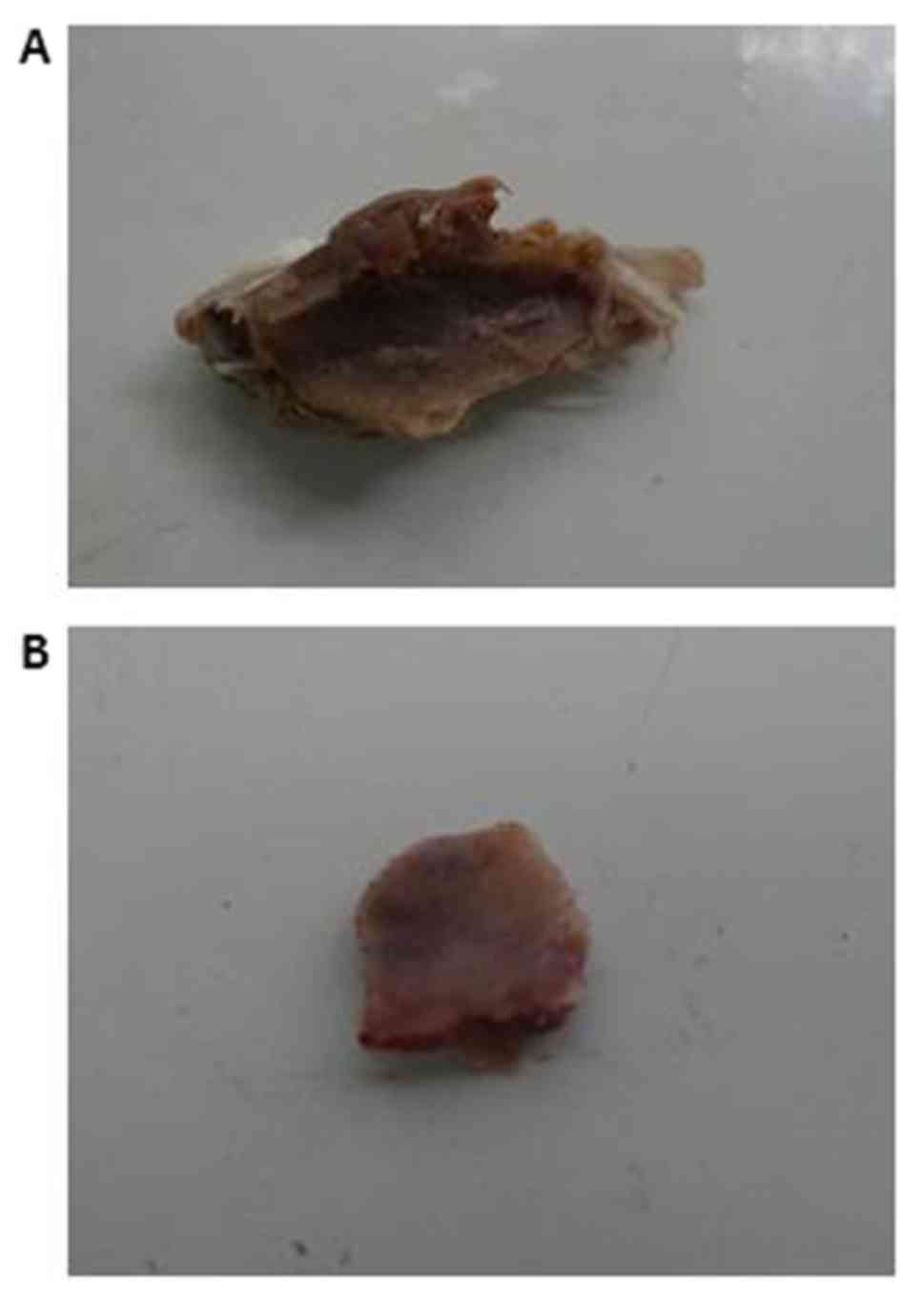
The mean diameter of the osteoid tissues was 1.1±0.3
cm (range, 0.8–1.4 cm) in the CS/rhBMP-2 group, which was
significantly bigger than that in the rhBMP-2 group (0.3±0.1;
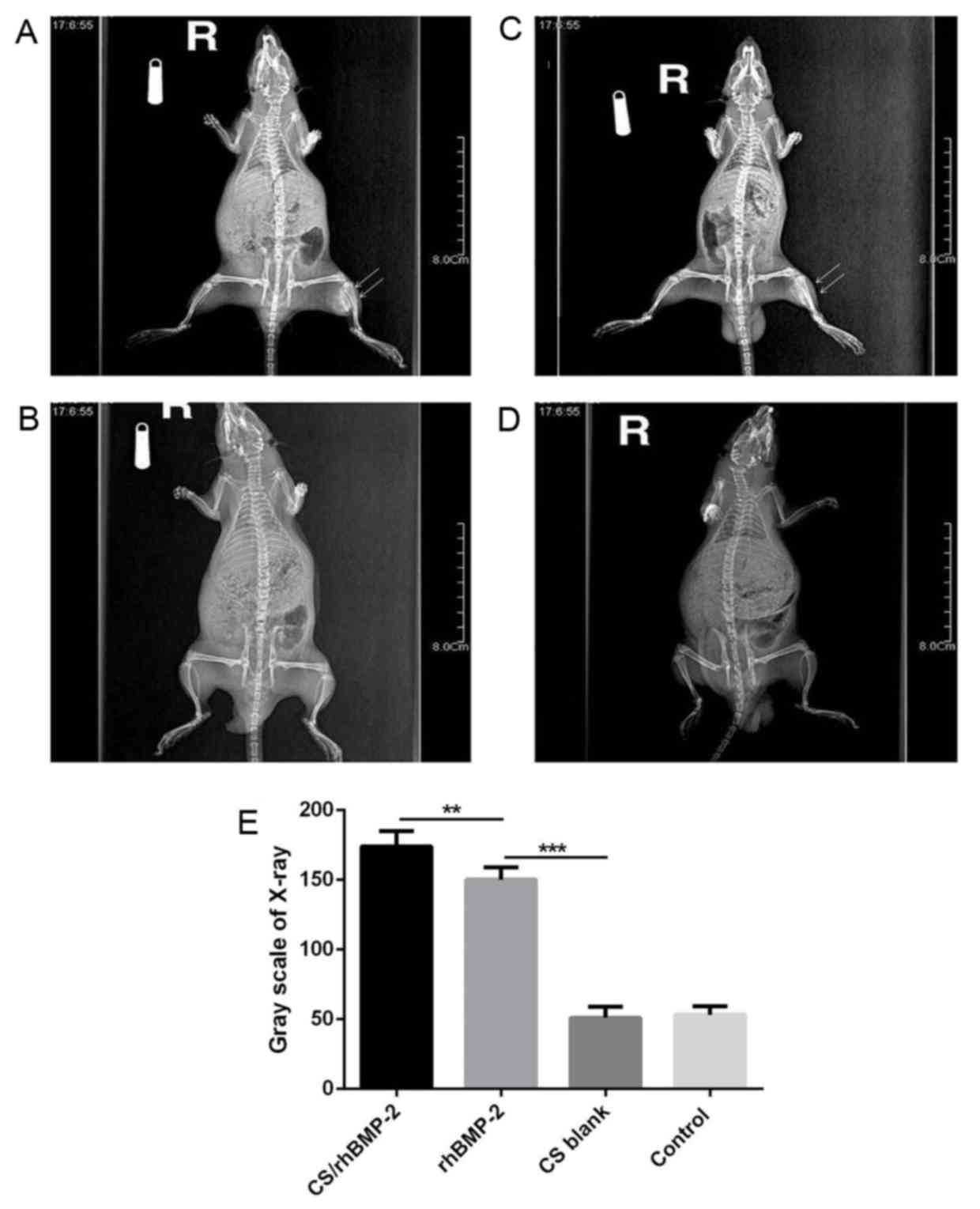
range, 0.1–0.4 cm; P=0.013; Fig. 4).
X-ray analysis also demonstrated that the area of high-density
tissues was largest in the CS/rhBMP-2 group, followed by the
rhBMP-2 group. However, no high-density tissue was observed in the
CS blank and the control group (Fig.
5).
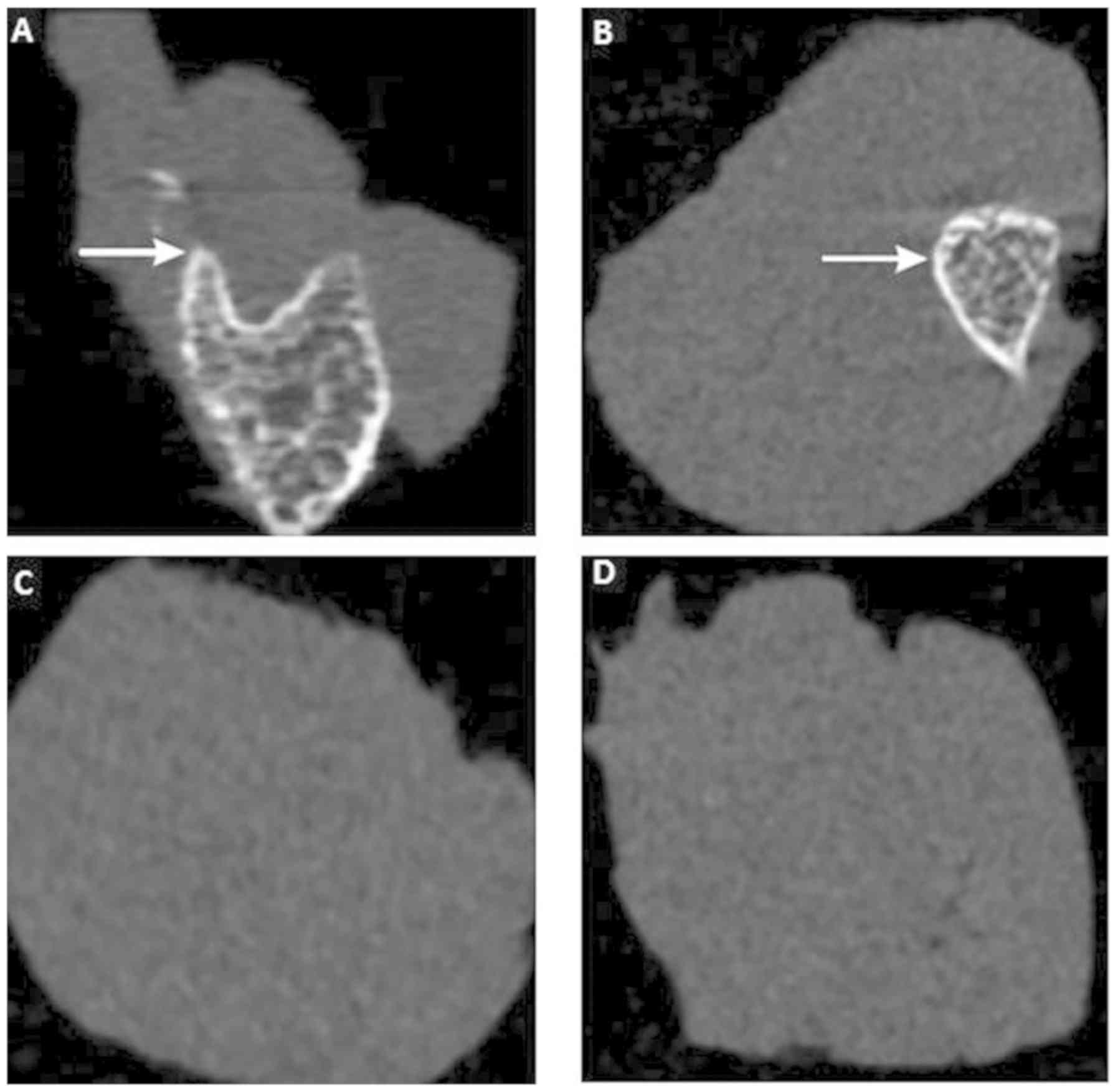
Micro-CT scan and 3-dimensional (3D)
reconstruction
To further investigate the effect of CS/rhBMP-2 on
ectopic osteogenesis, micro-CT scan and 3D reconstruction were
performed. As presented in Fig. 6,
no ectopic osteogenesis was observed in the CS blank and the
control group. However, in the CS/rhBMP-2 and rhBMP-2 groups,
obvious ectopic osteogenesis was observed with a higher bone
density at the edge and a lower bone density at the center.
Furthermore, the bone volume in the 3D reconstruction for the
CS/rhBMP-2 group was obviously higher than those in the rhBMP-2
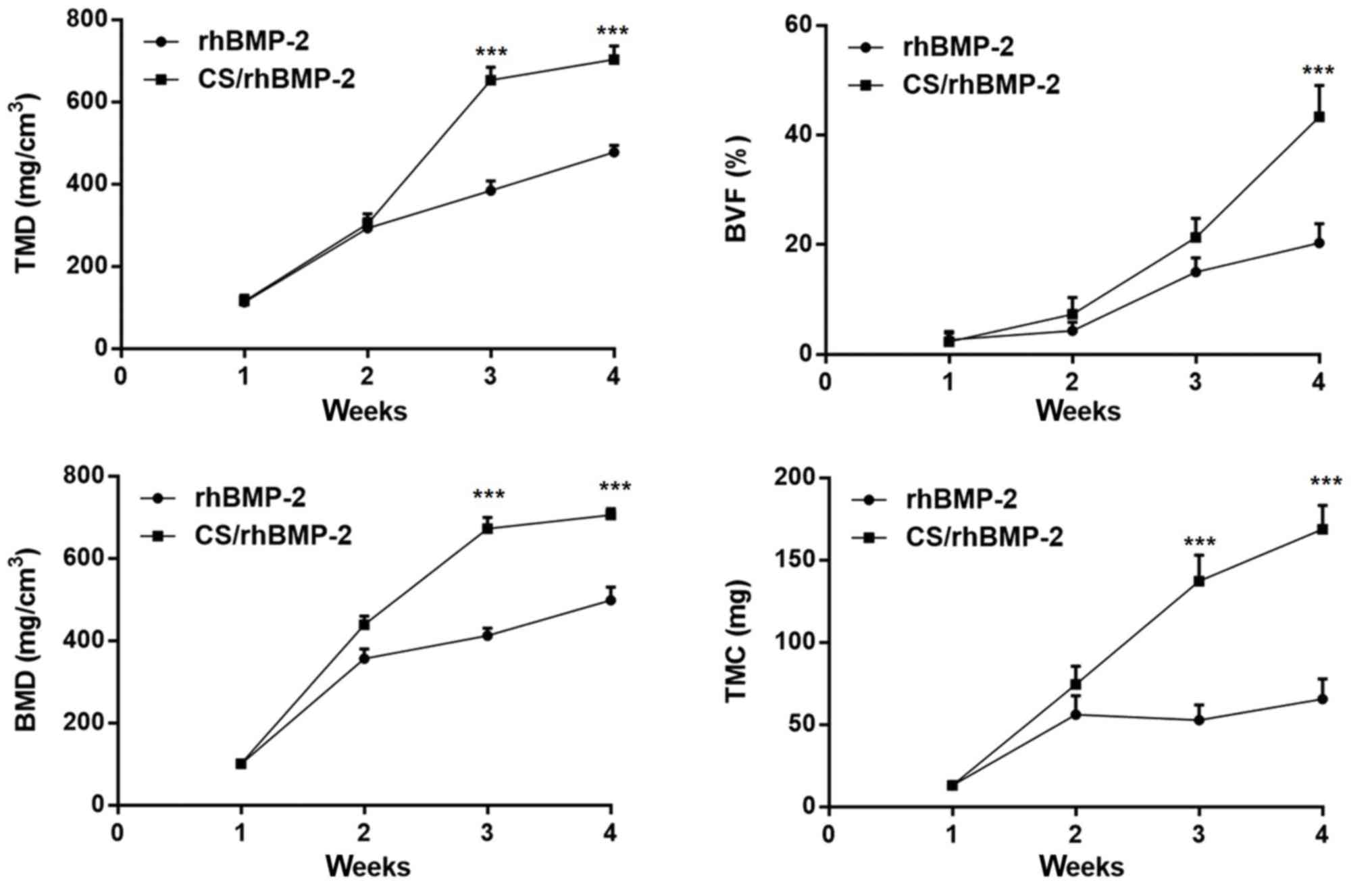
group. In addition, analysis of the micro-CT bone parameters
revealed that at 3 weeks after surgery, all parameters, namely the
TMD, BVF, BMD and TMC, were significantly higher in the CS/rhBMP-2
group compared with those in the rhBMP-2 group (P<0.05; Fig. 7).
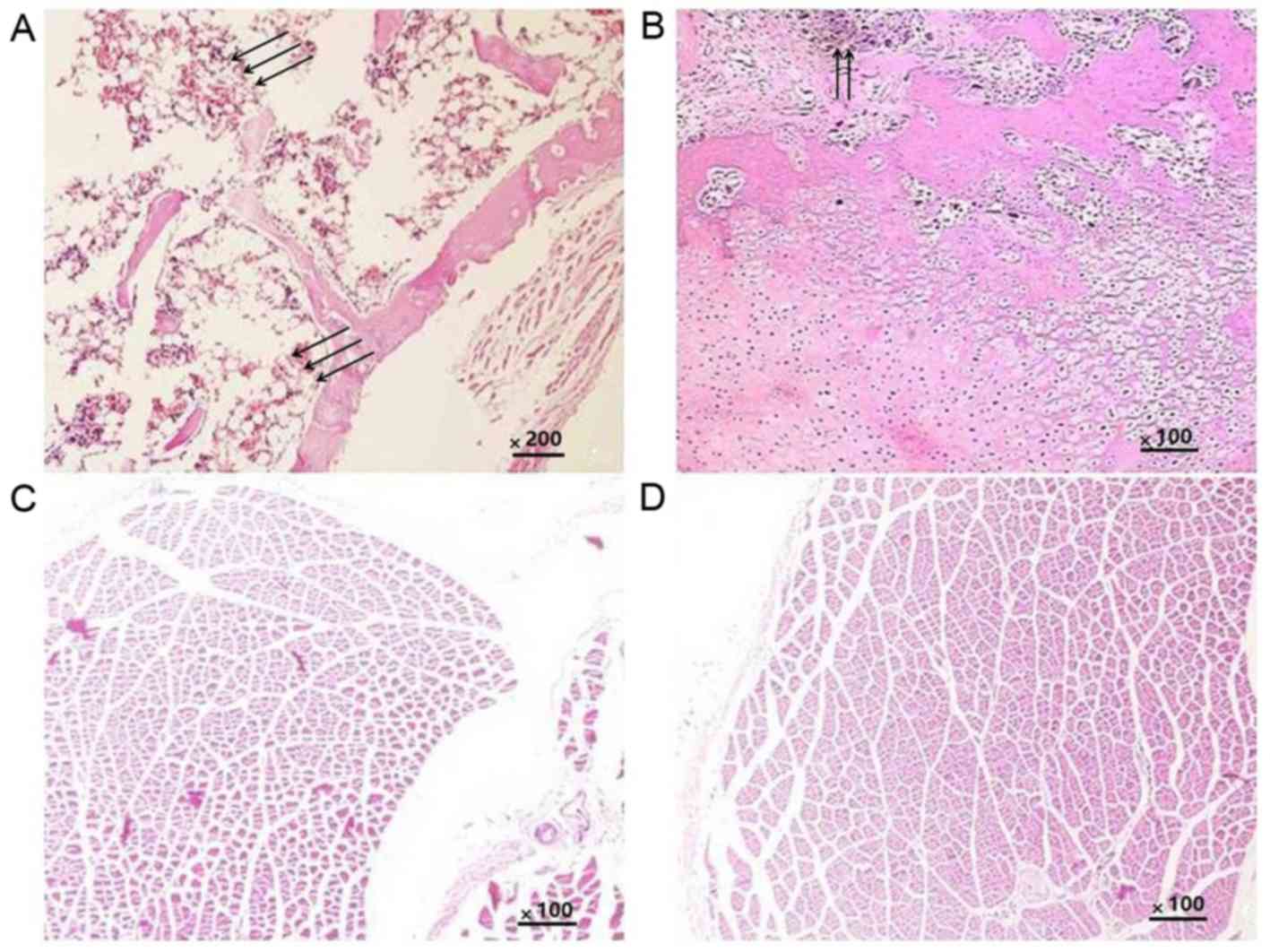
Histology
Histological analysis was performed to further
confirm the ectopic osteogenesis in the different groups. The
results indicated that in all groups, the implants were completely
absorbed and no inflammatory or granulomatous tissues were present.
In the CS/rhBMP-2 group, mature bone tissue was formed, and the
bone cells, bone marrow and bone trabeculae had been converted into
mature bone; the formation of a bone marrow cavity and myeloid
material between the trabeculae was also observed (Fig. 8A). In the rhBMP-2 group, osteoid
tissues were also present (Fig. 8B);
however, they were not as mature as those in the CS/rhBMP-2 group.
No osteoid tissues were observed in the blank and control groups
(Fig. 8C and D).
ALP activity and calcium content
At last, the ALP and Ca2+ content was
determined in each group. As presented in Table I, the ALP activity and the calcium
content were significantly higher in the CS/rhBMP-2 and rhBMP-2
groups compared with those in the other 2 groups (P<0.05). Of
note, the ALP activity and calcium content were also significantly
higher in the CS/rhBMP-2 group compared with the rhBMP-2 group
(P<0.05), indicating that the CS/rhBMP-2 system had the greatest
ability to induce osteoblast differentiation.
 | Table I.ALP and Ca2+ content in
each group (n=6). |
Table I.
ALP and Ca2+ content in
each group (n=6).
| Group | ALP activity
(katal/g) | Ca2+
contents (µg/mg) |
|---|
| CS/rhBMP-2 |
1.94±0.35a–c |
5.20±1.42a–c |
| rhBMP-2 |
1.48±0.56b,c |
3.80±1.40b,c |
| CS blank | 0.20±0.07 | 0.19±0.08 |
| Control | 0.18±0.06 | 0.20±0.08 |
Discussion
In the field of bone tissue engineering, BMP-2 is a
growth factor known to enhance osteogenesis. However, the
circulation half-life of BMP-2 is rather short and the bone
formation does not increase dose-dependently with increasing BMP-2
concentrations. To overcome the insufficiency of direct injection
of BMP-2, several novel BMP-2 local delivery systems have been
reported, including injectable sonication-induced silk hydrogel
(20), polyethylene glycol-coated
albumin nanoparticles (21) and
CS-based 3D constructs (22).
Studies have demonstrated that collagen sponges may be used as an
adequate matrix to prolong the duration of BMP-2 residing in the
tissue to facilitate bone regeneration (23). Bhakta et al (24) reported that collagen and hyaluronan
matrices may be used for delivery of BMP-2, which may help abrogate
the adverse clinical effects associated with high-dose growth
factor use.
Among the delivery systems, CS-based systems have
attracted a large amount of attention. Yilgor et al
(25) developed a sequential
BMP-2/BMP-7 delivery system with CS-based scaffolds for bone tissue
engineering, with which an enhanced ALP activity was achieved. Gan
et al (26) designed a
CS-based delivery system for BMP-2 and reported that it enhanced
bone regeneration. However, whether the CS/rhBMP-2 microspheres
delivery system is able to initiate ectopic osteogenesis has
remained elusive. The present study aimed to investigate the effect
of rhBMP-2 delivered by CS microspheres on ectopic osteogenesis of
rats.
First, CS/rhBMP-2 microspheres were successfully
prepared and their physicochemical properties were demonstrated.
Subsequently, by using several analysis methods, including X-ray
analysis, micro-CT scan and 3D reconstruction, histological
analysis, ALP activity analysis and calcium content analysis, it
was demonstrated that rhBMP induced ectopic osteogenesis in rats,
and promoted osteoblast differentiation by enhancing ALP activity
and the calcium content. Of note, the CS/rhBMP-2 microsphere
delivery system significantly enhanced the induction of
osteogenesis compared with that achieved with rhBMP-2 alone.
Several associated studies have focused on BMP-2 in
processes of osteogenesis. Lee et al (15) studied the effect of dual treatment of
stromal cell-derived factor (SDF)-1 and BMP-2 on ectopic and
orthotopic bone formation and identified that BMP-2 induced ectopic
and orthotopic bone regeneration; however, SDF-1 treatment did not
enhance the effect. Ma et al (27) demonstrated that rhBMP-2 and basic
fibroblast growth factor had a synergistic effect on ectopic
osteogenesis in mice. Lai et al (28) indicated that application of rhBMP-2
sustained-release nanocapsules significantly promoted ectopic
osteogenesis compared with the direct use of rhBMP-2. All of these
studies were consistent with the present results.
In conclusion, in the present study, a CS/rhBMP-2
microsphere delivery system was prepared and used to investigate
the effect of rhBMP-2 delivered by CS microspheres on ectopic
osteogenesis in rats. The results demonstrated that rhBMP induced
ectopic osteogenesis in rats, and promoted osteoblast
differentiation by enhancing ALP activity and the calcium content.
Of note, the CS/rhBMP-2 microsphere delivery system significantly
enhanced the ectopic osteogenesis compared with the effects of
rhBMP-2 on its own. The present study may provide basic data for
the future application of the CS/rhBMP-2 microsphere delivery
system and provide a deeper understanding of the roles of BMP-2 in
bone regeneration.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Guangzhou
Science and Technology project (grant no. 201804010136).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJX and WW conducted the experiments and analyzed
the data. YJX wrote the manuscript and revised the manuscript. HX,
QSY and XY conducted the experiments. LHL and JHW collected the
data. YZ analyzed the data, revised the manuscript and approved the
submission.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Institutional Animal Care Committee at General Hospital of
Southern Theater Command, PLA (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Henkel J, Woodruff MA, Epari DR, Steck R,
Glatt V, Dickinson IC, Choong PF, Schuetz MA and Hutmacher DW: Bone
regeneration based on tissue engineering conceptions-a 21st century
perspective. Bone Res. 1:216–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nauth A, Ristevski B, Li R and Schemitsch
EH: Growth factors and bone regeneration: How much bone can we
expect? Injury. 42:574–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu SZ, Ni PY, Wang BY, Chu B, Zheng L, Luo
F, Luo J and Qian Z: Injectable and thermo-sensitive PEG-PCL-PEG
copolymer/collagen/n-HA hydrogel composite for guided bone
regeneration. Biomaterials. 33:4801–4809. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Majidinia M, Sadeghpour A and Yousefi B:
The roles of signaling pathways in bone repair and regeneration. J
Cell Physiol. 233:2937–2948. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krishnakumar GS, Roffi A, Reale D, Kon E
and Filardo G: Clinical application of bone morphogenetic proteins
for bone healing: A systematic review. Int Orthop. 41:1073–1083.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan X, Kang D, Pan J, Jiang C, Lin Y and
Qi S: Osteoblastic differentiation and cell calcification of
adamantinomatous craniopharyngioma induced by bone morphogenetic
protein-2. Cancer Biomark. 18:191–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujita N, Matsushita T, Ishida K, Sasaki
K, Kubo S, Matsumoto T, Kurosaka M, Tabata Y and Kuroda R: An
analysis of bone regeneration at a segmental bone defect by
controlled release of bone morphogenetic protein 2 from a
biodegradable sponge composed of gelatin and β-tricalcium
phosphate. J Tissue Eng Regen Med. 6:291–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chao Q, Zhu C, Yu W, Jiang X, Zhang F and
Jian S: Bone morphogenetic protein 2 promotes osteogenesis of bone
marrow stromal cells in type 2 diabetic rats via the Wnt signaling
pathway. Int J Biochem Cell Biol. 80:143–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carreira AC, Lojudice FH, Halcsik E,
Navarro RD, Sogayar MC and Granjeiro JM: Bone morphogenetic
proteins: Facts, challenges, and future perspectives. J Dent Res.
93:335–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohajel N, Najafabadi AR, Azadmanesh K,
Amini M, Vatanara A, Moazeni E, Rahimi A and Gilani K: Drying of a
plasmid containing formulation: Chitosan as a protecting agent.
Daru. 20:222012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jun SH, Lee EJ, Jang TS, Kim HE, Jang JH
and Koh YH: Bone morphogenic protein-2 (BMP-2) loaded hybrid
coating on porous hydroxyapatite scaffolds for bone tissue
engineering. J Mater Sci Mater Med. 24:773–782. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walker DH and Wright NM: Bone
morphogenetic proteins and spinal fusion. Neurosurg Focus.
13:e32002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Segredo-Morales E, García-García P, Évora
C and Delgado A: BMP delivery systems for bone regeneration:
Healthy vs osteoporotic population. Review. J Drug Delivery Sci
Technol. 42:107–118. 2017. View Article : Google Scholar
|
|
14
|
Chung RJ, Ou KL, Tseng WK and Liu HL:
Controlled release of BMP-2 by chitosan/γ-PGA polyelectrolyte
multilayers coating on titanium alloy promotes osteogenic
differentiation in rat bone-marrow mesenchymal stem cells. Surface
Coatings Technol. 303:283–288. 2016. View Article : Google Scholar
|
|
15
|
Lee CH, Jin MU, Jung HM, Lee JT and Kwon
TG: Effect of dual treatment with SDF-1 and BMP-2 on ectopic and
orthotopic bone formation. PLoS One. 10:e01200512015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dudarić L, Cvek SZ, Cvijanović O, Santić
V, Marić I, Crncević-Orlić Z and Bobinac D: Expression of the
BMP-2, −4 and −7 and their antagonists gremlin, chordin, noggin and
follistatin during ectopic osteogenesis. Coll Antropol.
37:1291–1298. 2013.PubMed/NCBI
|
|
17
|
Xia YJ, Xia H, Chen L, Ying QS, Yu X, Li
LH, Wang JH and Zhang Y: Efficient delivery of recombinant human
bone morphogenetic protein (rhBMP-2) with dextran sulfate-chitosan
microspheres. Exp Ther Med. 15:3265–3272. 2018.PubMed/NCBI
|
|
18
|
Özbaş-Turan S, Akbuǧa J and Aral C:
Controlled release of interleukin-2 from chitosan microspheres. J
Pharm Sci. 91:1245–1251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan MA: Guide for the care and use of
laboratory animals. Sichuan Animal. 19:29–30. 1989.
|
|
20
|
Zhang W, Kaplan DL and Jiang X, Zhao J, Xu
L, Zhu C, Zeng D, Chen J, Zhang Z, Kaplan DL and Jiang X: The use
of injectable sonication-induced silk hydrogel for VEGF(165) and
BMP-2 delivery for elevation of the maxillary sinus floor.
Biomaterials. 32:9415–9424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Wang G, Lin X, Chatzinikolaidou
M, Jennissen HP, Laub M and Uludağ H: Polyethylenimine-coated
albumin nanoparticles for BMP-2 delivery. Biotechnol Prog.
24:945–956. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan J, Park H, Lee MK, Bezouglaia O,
Fartash A, Kim J, Aghaloo T and Lee M: Adipose-derived stem cells
and BMP-2 delivery in chitosan-based 3D constructs to enhance bone
regeneration in a rat mandibular defect model. Tissue Eng Part A.
20:2169–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Geiger M, Li RH and Friess W: Collagen
sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev.
55:1613–1629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhakta G, Lim ZX, Rai B, Lin T, Hui JH,
Prestwich GD, van Wijnen AJ, Nurcombe V and Cool SM: The influence
of collagen and hyaluronan matrices on the delivery and bioactivity
of bone morphogenetic protein-2 and ectopic bone formation. Acta
Biomater. 9:9098–9106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yilgor P, Tuzlakoglu K, Rui LR, Hasirci N
and Hasirci V: Incorporation of a sequential BMP-2/BMP-7 delivery
system into chitosan-based scaffolds for bone tissue engineering.
Biomaterials. 30:3551–3559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gan Q, Zhu J, Yuan Y, Honglai Liu, Qian J,
Lib Y and Liu C: A dual-delivery system of pH-responsive
chitosan-functionalized mesoporous silica nanoparticles bearing
BMP-2 and dexamethasone for enhanced bone regeneration. J Materials
Chemistry B. 3:2056–2066. 2015. View Article : Google Scholar
|
|
27
|
Ma SY, Feng ZQ, Lai RF, Zhou ZY and Yin
ZD: Synergistic effect of RhBMP-2 and bFGF on ectopic osteogenesis
in mice. Asian Pac J Trop Med. 8:53–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai RF, Li ZJ, Zhou ZY, Feng ZQ and Zhao
QT: Effect of rhBMP-2 sustained-release nanocapsules on the ectopic
osteogenesis process in Sprague-Dawley rats. Asian Pac J Trop Med.
6:884–888. 2013. View Article : Google Scholar : PubMed/NCBI
|