Introduction
Chronic hepatitis B is a worldwide epidemic disease,
with China in particular representing a high hepatitis B virus
(HBV) epidemic area. In 2006, an epidemiological survey of HBV in
China revealed that the incidence of HBV surface antigen was 7.2%
in individuals aged 1–59 years old among the general population,
and that the incidence of HBV infection with glomerulonephritis was
6.8–20.0% (1). As such,
HBV-associated glomerulonephritis (HBV-GN) is an important cause of
chronic kidney disease (CKD) in China (2,3). While
Combes et al (4) first
reported on HBV-GN in 1971, the pathogenic mechanism is still yet
to be fully elucidated. The deposits of immune complexes formed by
HBV antigens and antibodies are the main causes of HBV-GN (5,6).
However, some recent studies have demonstrated that HBV induced
renal damage and may serve an important role in HBV-GN (7–9). The
pathological presentation of HBV-GN is varied, and includes
membranous nephropathy (MN), membranoproliferative
glomerulonephritis, mesangial proliferative glomerulonephritis,
minimal change disease and focal segmental glomerulosclerosis
(FSGS), though most clinical manifestations are of those classified
under nephrotic syndrome (10,11). As
is well established, the glomerular endothelial cells, glomerular
basal membrane and podocytes together constitute the glomerular
filtration barrier, and podocyte damage is considered to be among
the most critical factors resulting in proteinuria (12,13).
This is due to podocytes, as a highly differentiated cell type with
specific structure and biological function, being unrenewable
following sustained damage (12,14).
Previous research indicated that the number and density of
podocytes decreased significantly in patients with HBV-MN, with
this change accompanied by increases in urinary protein (15). Cell apoptosis, exfoliation and loss
of proliferative capacity are considered the main mechanisms
underlying podocyte reduction (15).
The hepatitis B virus X protein gene (HBx)
comprises the smallest open reading frame in the HBV genome, and
its product serves as the basic viral protein in the virus
infection cycle (16). With advances
in research, the X protein has been verified as a multifunctional
protein, which can activate various cell signaling pathways and
regulate apoptosis, among other effects (17). However, in different types of cell
and under different external conditions, the regulation of these
pathways by HBx is governed by differing mechanisms (18). Overexpression of HBx in
extracorporeal podocytes limits the proliferative capacity of the
cells through cell cycle regulation, and this mechanism may occur
due to upregulation of cyclin B1 and p21 (19). However, the mechanisms underlying
podocyte apoptosis induced by HBx are unclear. Therefore, the
current study examined the effect of HBx on the viability and
apoptosis of mouse podocyte clone 5 (MPC5) cells, and nephrin
protein expression was detected in an HBx transfection group to
investigate the possible mechanism involved.
Materials and methods
Cell culture
MPC5 cells were obtained from CHI Scientific, Inc.
(Maynard, MA, USA). After frozen MPC5 cells were recovered, they
were cultured in low sugar Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% heat-inactivated fetal calf serum (HyClone; GE
Healthcare, Little Chalfont, UK). The MPC5 cells were cultured and
expanded in this medium also containing 10 U/ml interferon-γ
(PeproTech, Inc., Rocky Hill, NJ, USA) at 33°C and 5%
CO2.
Podocyte transfection and
grouping
MPC5 cells were inoculated on 6-well plates at
5×104 cells/cm density, with each group assigned three
wells. When the cells reached 70% confluence, they were divided
into different groups according to transfection treatment. The
cells were transfected with pEX-HBx or pEX-neo plasmids (both
Shanghai GenePharma, Co., Ltd., Shanghai, China) using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The cells were divided
into three groups: A HBx transfection group (pEX-HBx group; treated
with 2 µl Lipofectamine 2000 + 20 pmol HBx-plasmid), a negative
control group (pEX-neo group; treated with 2 µl Lipofectamine 2000
+ 20 pmol empty plasmid) and a blank control group (MPC5 group;
treated with 2 µl Lipofectamine 2000 alone).
RNA isolation and real-time
quantitative PCR (qPCR)
The transcript levels of the HBx gene were
examined using qPCR. Following transfection of MPC5 cells for 12,
24, 48 and 72 h, the cells were digested with pancreatin (0.25%),
washed with phosphate-buffered saline and collected. Total RNA was
isolated from cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and reverse transcribed to cDNA using a
high-capacity cDNA archive kit (Takara Biotechnology, Co., Ltd.,
Dalian, China) according to the manufacturer's instructions. qPCR
was performed using an Applied Biosystems 7300 real-time PCR system
(Thermo Fisher Scientific, Inc.). The thermocycling conditions were
as follows: 94°C for 4 min, followed by 40 cycles at 94°C for 30
sec, 58°C for 30 sec and 72°C for 30 sec as well as 82°C for 30 sec
to collect fluorescence data. Primers were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China), the sequences of which are
listed in Table I. Expression of the
abelson murine leukemia viral oncogene homolog gene (ABL)
was used as a control. The relative expression of HBx was
calculated by the comparative 2−ΔΔCq method (20). In order to reduce the error, each
group was assayed three times.
 | Table I.Primer sequences and product
size. |
Table I.
Primer sequences and product
size.
| Primer | Oligonucleotide
sequence, 5′-3′ | Product size,
bp |
|---|
| HBx
sense |
TGCGGACGACCCTTCTCGGG | 195 |
| HBx
antisense |
GGGCAACATTCGGTGGGCGT |
|
| ABL
sense |
TCCTCCAGCTGTTATCTGGAAGA | 118 |
| ABL
antisense |
TCCAACGAGCGGCTTCAC |
|
MTT assay
The MTT method was used to detect the viability of
podocytes, using an MTT kit purchased from American Biomol
(Farmingdale, NY, USA). MPC5 cells in the exponential phase of
growth were trypsinized and seeded into 6-well plates at a density
of 4×105 cells per well, with three wells per group.
After 48 h of incubation at 33°C, MTT reagent (5 mg/ml, 20 µl) was
added and the cells were incubated for another 4 h. Then, the
supernatant was discarded, 150 µl dimethyl sulfoxide (DMSO) was
added to each well, and the wells were agitated for 15 min. The
optical density (OD) of each well at 570 nm (OD570) was read with
an ELISA plate reader, and the cell viability rate was calculated
according to the following formula: Viability
rate=OD570treatment group/OD570control group
×100%. The experiment was repeated three times.
Flow cytometry
After transfection for 48 h, MPC5 cells in the
exponential phase of growth were trypsinized, centrifuged at 112 ×
g for 10 min at 4°C, and the cell pellet suspended in cell culture
medium. Then, 1×106 cells were resuspended in 200 µl of
1X Nexin buffer (Annexin V-FITC Apoptosis Detection Kit I; BD
Biosciences, San Jose, CA, USA). A total of 50 µl of the suspension
was transferred into a tube containing 5 µl propidium iodide and 5
µl Annexin V stain (BD Pharmingen; BD Biosciences), and incubated
for 30 min at room temperature in the dark. following addition of
250 µl 1X Nexin buffer into each tube, apoptotic cells were
detected by flow cytometry using a fluorescence-activated cell
sorter (FACSCalibur cytometer) and CELL Quest™ software
(version 3.3) (both from BD Biosciences).
Western blot analysis
The total protein of cells in each group was
extracted with radioimmunoprecipitation assay buffer and measured
using bicinchoninic assay reagents (Beyotime Institute of
Biotechnology, Haimen, China). Then, 60 µg protein per lane was
subjected to polyacrylamide gel electrophoresis in 6–12% gels, and
the resulting bands were transferred to polyvinylidene difluoride
membranes. The membranes were blocked at room temperature for 2 h
in Tris-buffered saline with Tween-20 (TBST; 0.2% Tween-20)
containing 5% skimmed milk, then incubated at 4°C overnight with
primary antibodies (1:1,000) against HBx, nephrin, signal
transducer and activator of transcription 3 (STAT3), phosphorylated
(p)-STAT3 and GAPDH (Abcam, Cambridge, UK; cat nos. ab157480,
ab58968, ab119352, ab76315 and ab8245, respectively). Following
washing with TBST, the appropiate horseradish-peroxidase-labeled
secondary antibody (1:5,000; cat no. A0208; Beyotime Institute of
Biotechnology) was added and incubated for 2 h at room temperature,
after which the membranes were washed three to five times with
TBST. Finally, an electrochemiluminescence kit (Sigma-Aldrich;
Merck KGaA) and gel imager were used to expose and visualize the
proteins, and protein grayscale values were measured with Quantity
One software (version 4.52; Bio-Rad Laboratories, Inc., Hercules,
CA, USA), and the actual grayscale value of the target protein=the
target protein average grayscale value/the GAPDH average grayscale
value.
Statistical analysis
All experiments were repeated at least three times.
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA) was used to generate charts and complete statistical analyses.
The data are presented as the mean ± standard deviation, and
t-tests were used to compare the data between two groups. The
comparisons between multiple groups were made by one-way analysis
of variance followed by post-hoc Tukey's tests. P<0.05 was
considered to indicate statistical significance.
Results
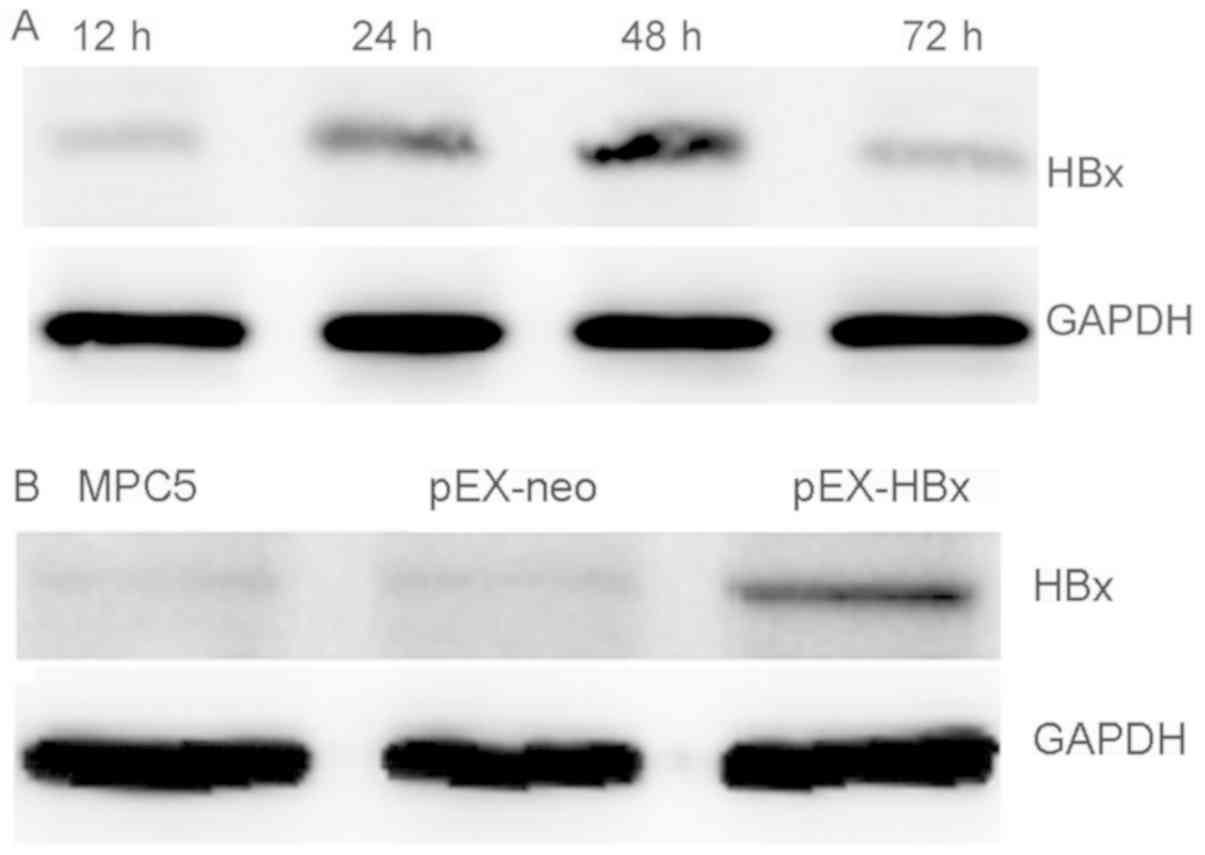
HBx is over-expression in the pEX-HBx
group
HBx mRNA in the pEX-HBx group was expressed
at the highest level at 48 h after transfection. HBx mRNA
expression was significantly higher compared with the MPC5 and
pEX-neo groups (P<0.01; Table
II). HBx expression was determined to be significantly
higher in the pEX-HBx group compared with that in the pEX-neo and
MPC5 groups, while there was no difference in expression between
the pEX-neo and MPC5 groups (P>0.05; Table II). Using western blotting to
examine the expression of HBx protein and verify the transfection
efficiency, corresponding results were obtained as those for qPCR
(Fig. 1A and B). Based on expression
increase, the 48-h post-transfection podocytes were used for the
follow-up experiments.
 | Table II.Efficiency of HBx gene
transfection in MPC5 cells. |
Table II.
Efficiency of HBx gene
transfection in MPC5 cells.
|
| HBx
expression at different time points, h |
|---|
|
|
|
|---|
| Group | 12 | 24 | 48 | 72 |
|---|
| MPC5 | 0.48±0.14 | 0.51±0.12 | 0.54±0.12 | 0.44±0.10 |
| pEX-neo | 0.53±0.13 | 0.65±0.11 | 0.64±0.20 | 0.58±0.21 |
| pEX-HBx |
0.64±0.11a,c |
18.3±0.25b,d |
593.42±0.56b,d |
88.59±0.33b,d |
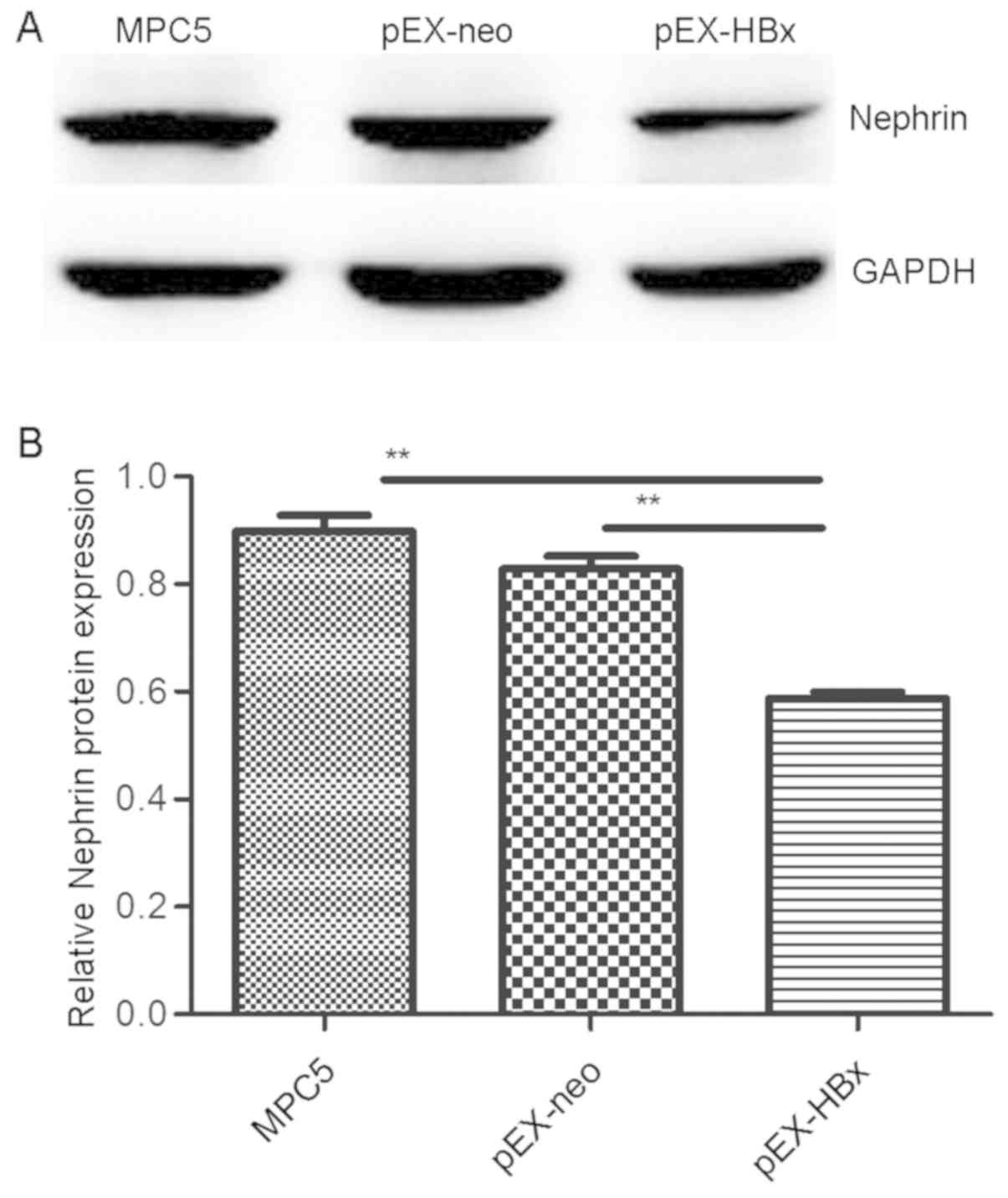
Nephrin expression is downregulated in
HBx-transfected podocytes
As expected, the expression of nephrin protein in
the pEX-HBx podocytes was lower than that in the pEX-neo and MPC5
groups (P<0.01 and P<0.01, respectively). Additionally, the
western blot results demonstrated that there was no difference in
nephrin protein expression between the pEX-neo and MPC5 groups
(P>0.05; Fig. 2).
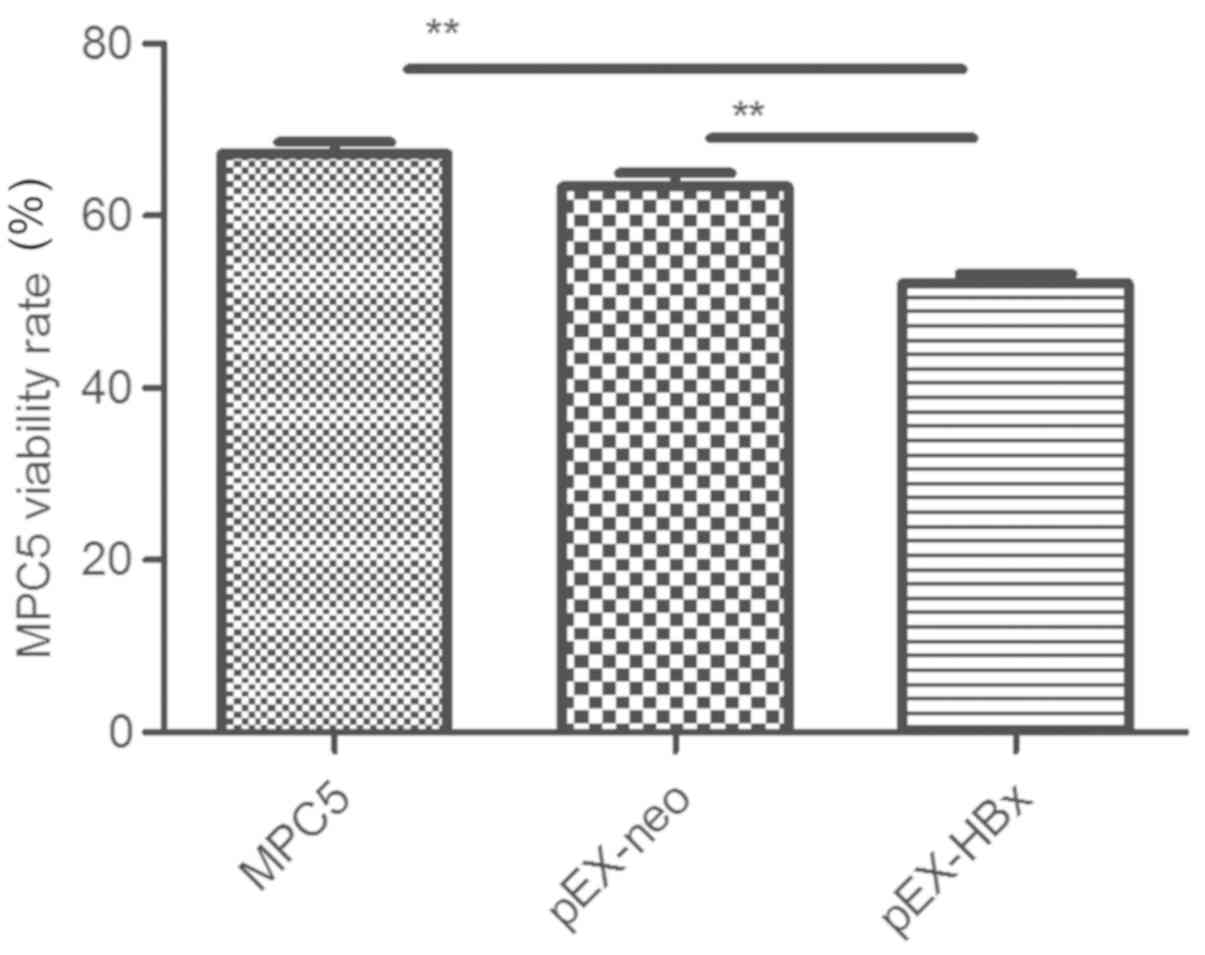
Overexpression of the HBx gene
suppresses podocyte viability
The viable cell rate of the pEX-HBx podocytes was
52.2±2.4%, which was the lowest rate observed compared with that of
the MPC5 (67.2±3.0%) and pEX-neo (63.4±3.4%) groups (P<0.01 and
P<0.01, respectively). No significant difference was identified
between the rates of viable cells in the pEX-neo and MPC5 groups
(P>0.05; Fig. 3).
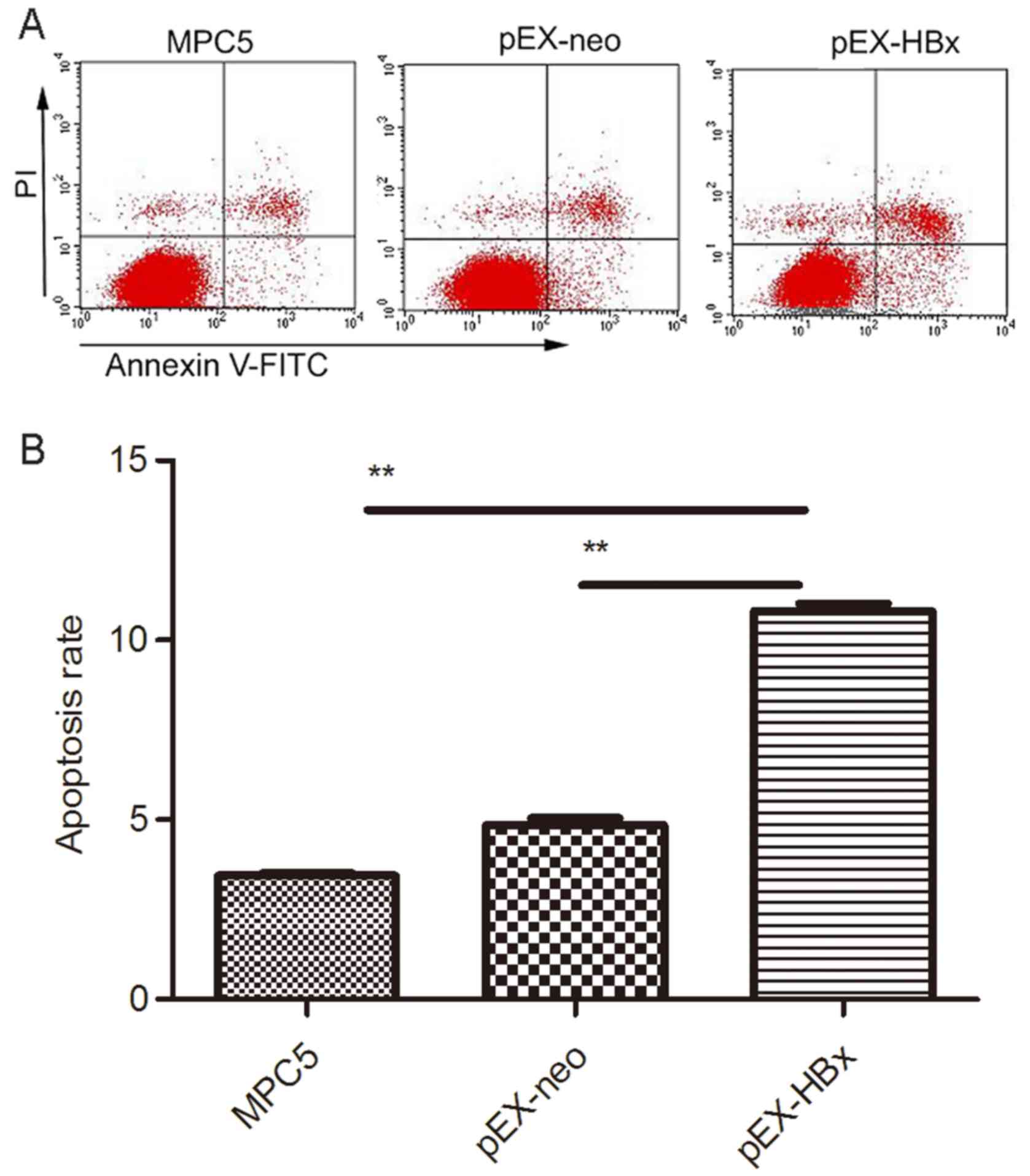
Overexpression of HBx increases
podocyte apoptosis
The rate of cell apoptosis in the pEX-HBx group was
significantly higher than that in the MPC5 and pEX-neo groups
(P<0.01 and P<0.01, respectively); the ratio of apoptotic
cells in each of the MPC5, pEX-neo and pEX-HBx groups was
3.46±0.17, 4.86±0.55 and 10.82±0.45, respectively. There was no
difference between the MPC5 and pEX-neo groups (P>0.05; Fig. 4).
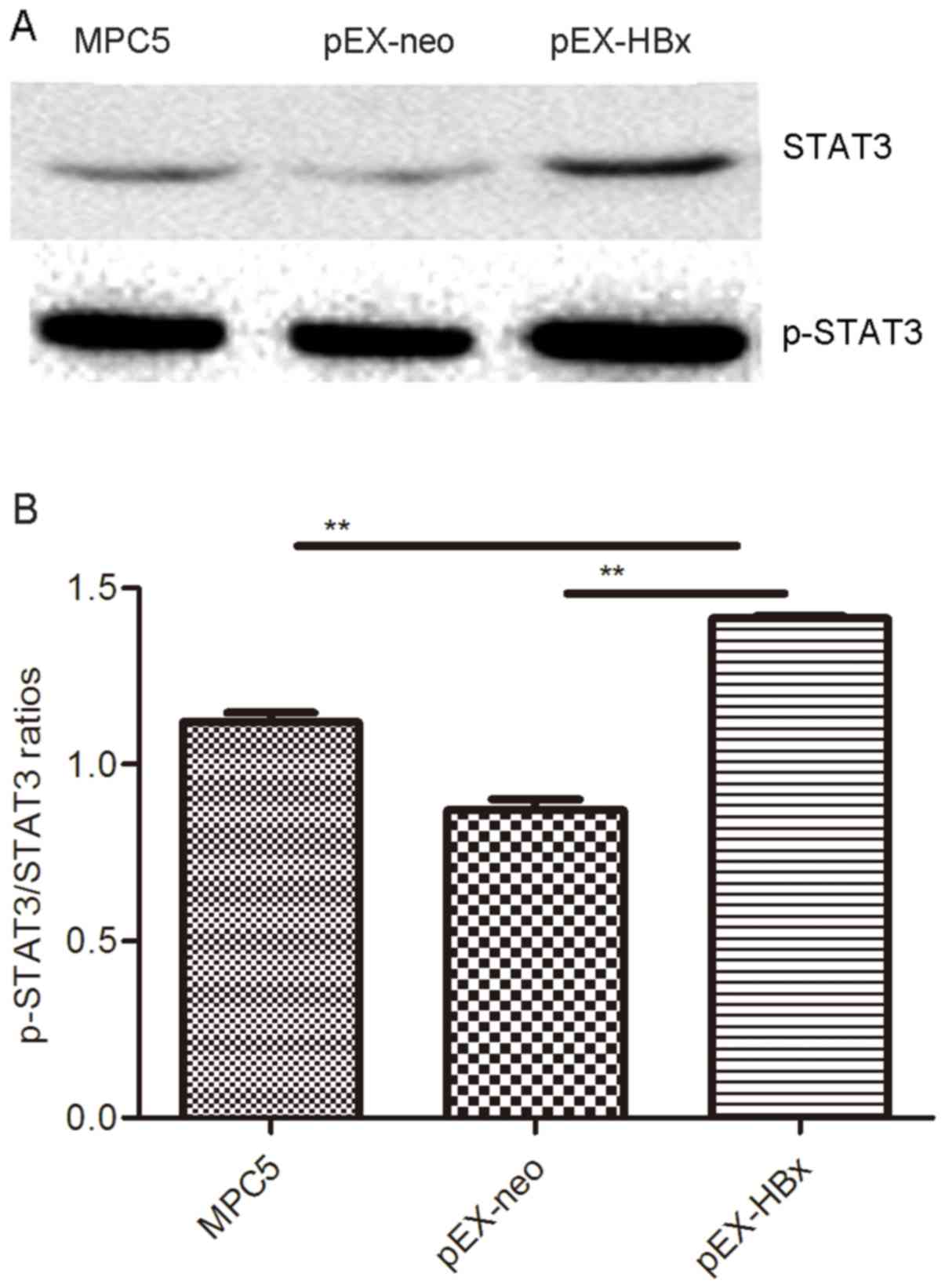
HBx stimulates STAT3 production in
MPC5 cells
The expression of STAT3 and p-STAT3 was highest in
the pEX-HBx group; no difference was observed in the expression
levels between the MPC5 and pEX-neo groups (P>0.05; Fig. 5). p-STAT3/STAT3 ratios in the MPC5,
pEX-neo and pEX-HBx groups were 1.160±0.017, 0.877±0.014 and
1.411±0.008, respectively (Table
III). The proportion of STAT3 phosphorylation in the pEX-HBx
group was significantly increased compared with that in the other
two groups (P<0.01 and P<0.01, respectively; Fig. 5; Table
III).
 | Table III.Effect of HBx on the protein
expression of STAT3 and p-STAT3. |
Table III.
Effect of HBx on the protein
expression of STAT3 and p-STAT3.
| Group | STAT3 | p-STAT3 | p-STAT3/STAT3 |
|---|
| MPC5 | 0.312±0.012 | 0.362±0.015 | 1.160±0.017 |
| pEX-neo | 0.342±0.018 | 0.301±0.011 | 0.877±0.014 |
| pEX-HBx |
0.680±0.036a,b |
0.960±0.015a,b |
1.411±0.008a,b |
Discussion
HBV-GN is generally considered to be caused by
immune complex deposition, as well as HBV replication and direct
virus infection, accompanied by the renal and immune dysfunction
caused by the infection, which is also considered a main pathogenic
mechanism underlying HBV-GN (21).
To date, little information has been established regarding the
potential effects of HBx protein in terms of the damage and
dysfunction observed in renal podocytes during chronic HBV
infection. HBx is considered the most important determinant in
viral pathogenesis (22,23); it is a necessary transcription factor
for HBV replication, having an trans-activation effect, which can
promote viral replication and induce apoptosis directly, and
subsequently induce the occurrence of an inflammatory reaction and
serve an important role in cell transformation and proliferation
(24,25). Due to the widely trans-activation
properties of the HBx gene and its effect on glomerular foot
cells, mesangial cells and renal tubule epithelial cells (26–29), it
is considered to have an important role in the pathogenesis of
HBV-GN (28,29). A previous study by Zhang et al
(15) demonstrated that podocyte
number was significantly decreased in the glomeruli of children
with HBV-GN, while HBx protein was visualized within the glomerulus
in 71% children with HBV-GN, where the expression of HBx protein
was localized mainly in the cytoplasm of podocytes, with lower
expression in the nuclei of the podocytes (19). Therefore, in the present study it was
examined whether HBx could affect the apoptosis and proliferation
of renal podocytes and change the expression of nephrin.
In the current study, the highest level of
HBx mRNA expression in podocytes occurred at 48 h post-HBx
transfection. Thus, these cells at 48 h were selected to detect the
viability of podocytes, and the data indicated that the viability
of the HBx group was significantly lower than that of the blank
control and negative control groups. This indicated that
overexpression of HBx may supress podocyte viability, which
is consistent with the report of Zhang et al (19). Nephrin is a main marker of foot cell
damage; previous research has confirmed it serves an important role
in recovering membrane integrity and in cytoskeletal remodelling
(30). Furthermore, nephrin
downregulation is considered a main mechanism involved in
proteinuria (31). We therefore
studied the relationship between nephrin and HBx protein, and
observed that the expression of nephrin protein in the HBx
transfection group was decreased to a greater extent than that in
the negative and blank control groups, suggesting that the
HBx gene may induce proteinuria through downregulation of
nephrin protein.
Proteinuria is a common symptom of glomerular
filtration membrane damage, and podocytes are highly differentiated
epithelial cells that serve a key role in preventing the urinary
leakage of plasma proteins; thus, podocyte injury or loss leads to
proteinuria (12). To date, a number
of studies have demonstrated that podocyte injury, loss and
dysfunction serve an important role in diseases including FSGS and
MN, among others (32–34). Therefore, maintenance of the
integrity of podocyte structure and function, and protection of
foot cells from injury have become potential therapeutic methods
for the treatment of proteinuria. Apoptosis is a major method
involved in podocyte decline (35,36), and
the present study confirmed that HBx could increase the apoptosis
of podocytes. Recently, studies have investigated the mechanisms
underlying HBx-induced apoptosis of renal tubular cells (29,37), and
He et al (28) suggested that
HBx could reduce podocyte adhesion via downregulation of α3β1
integrin.
STATs are transcription factors located in the
cytoplasm, where they can become activated by extracellular
stimuli. Activated STATs, through regulation of gene expression,
are able to regulate a series of biological processes, including
cell proliferation, survival, apoptosis and differentiation, among
others, and thus serve an important regulatory role in
physiological and pathological reactions in cells (38–40).
STAT3 is an important member of the STAT family; it is widely
expressed in different tissue and cell types, and is responsible
for transferring extracellular signals to the nucleus and for
inducing the transcriptional expression of target genes, which
involves tyrosine phosphorylation of STAT3, for instance at
tyrosine 705 near the SH2 domain, to establish the activated form
of STAT3 (p-STAT3) (41,42). The proportion of STAT3
phosphorylation in the pEX-HBx group was higher than that in the
control groups), indicating HBx overexpression may activate the
STAT3 protein. Although previous literature suggests that
activation of STAT3 is associated with inhibition of apoptosis,
particularly in cancers (43–45), in
the present study, the activation of STAT3 was concomitant with
podocyte apoptosis; a finding consistent with a study by He et
al (29) in renal tubular
epithelial cells. Therefore, it may be speculated that the
apoptosis of podocytes in HBV-GN is associated with STAT3
activation, although the specific mechanism is unclear and requires
further investigation; in future research, our group will conduct
immunoprecipitation to screen protein interactions and provide
further information about HBx in inducing HBV-GN via STAT signaling
pathways.
In conclusion, these findings supported that HBx was
involved in the pathogenic mechanism that HBV directly damages
nephridial tissue. Additionally, find STAT3 related signal pathway
and use specific inhibitors may be useful as new therapautics for
the treatment of HBV-GN.
Acknowledgements
Not applicable.
Funding
The current study was funded by the National Natural
Science Foundation of China (grant no. 81760133), the National
Natural Science Foundation of Gansu Province (grant no.
1606RJ2A107) and the Science Foundation of Gansu Province People's
Hospital (grant no. 17GSSY1-1).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XYL and XXC designed the study, analyzed the data
and wrote the manuscript. YHSun and MDG conducted the experiments.
XXH and YHSuo assisted with the technical performance of
experiments and contributed to the writing of the manuscript. All
authors read and approved the final manuscript. XYL and XXC
contributed equally to the present study as co-first authors. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HBV
|
hepatitis B virus
|
|
HBx
|
hepatitis B virus X protein
|
|
HBV-GN
|
hepatitis B virus-associated
glomerulonephritis
|
|
CKD
|
chronic kidney disease
|
|
MPC5
|
mouse podocyte clone 5
|
|
MN
|
membranous nephropathy
|
|
FSGS
|
focal segmental glomerulosclerosis
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
p-
|
phosphorylated
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
OD
|
optical density
|
|
TBST
|
Tris-buffered saline with Tween-20
|
References
|
1
|
Liang X, Bi S, Yang W, Wang L, Cui G, Cui
F, Zhang Y, Liu J, Gong X, Chen Y, et al: Epidemiological
serosurvey of hepatitis hepatitis B in China-declining HBV
prevalence due to hepatitis B vaccination. Vaccine. 27:6550–6557.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu P, Zhou FD and Zhao MH: The renal
histopathology spectrum of elderly patients with kidney diseases: A
study of 430 patients in a single chinese center. Medicine
(Baltimore). 93:e2262014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Zhang Z, Zhuo L, Chen DP and Li
WG: The spectrum of biopsy-proven glomerular disease in China: A
systematic review. Chin Med J (Engl). 131:731–735. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Combes B, Shorey J, Barrera A, Stastny P,
Eigenbrodt EH, Hull AR and Carter NW: Glomerulonephritis with
deposition of Australia antigen-antibody complexes in glomerular
basement membrane. Lancet. 2:234–237. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lai WL, Yeh TH, Chen PM, Chan CK, Chiang
WC, Chen YM, Wu KD and Tsai TJ: Membranous nephropathy: A review on
the pathogenesis, diagnosis, and treatment. J Formos Med Assoc.
114:102–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dettmar AK and Oh J: Infection-related
focal segmental glomerulosclerosis in children. Biomed Res Int.
2016:73519642016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakai K, Morito N, Usui J, Hagiwara M,
Hiwatashi A, Fukuda K, Nanmoku T, Toda T, Matsui N, Nagata M and
Yamagata K: Focal segmental glomerulosclerosis as a complication of
hepatitis B virus infection. Nephrol Dial Transplant. 26:371–373.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He P, Zhou G, Qu D, Zhang B, Wang Y and Li
D: HBx inhibits proliferation and induces apoptosis via Fas/FasL
upregulation in rat renal tubular epithelial cells. J Nephrol.
26:1033–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong L, Zhang J, Min J, Lu J, Li F, Li H,
Guo S and Li Q: A role for MHBst167/HBx in hepatitis B
virus-induced renal tubular cell apoptosis. Nephrol Dial
Transplant. 25:2125–2133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun YH, Lei XY, Sai YP, Chen JH, Sun YC
and Gao X: Relationship between genotypes and clinical
manifestation, pathology, and cccDNA in Chinese children with
hepatitis B virus-associated glomerulonephritis. World J Pediatr.
12:347–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Souza LO, Perez RM, Carvalho-Filho RJ,
Matos CA, Moutinho RS, Silva IS, Medina-Pestana JO, Silva AE and
Ferraz ML: Unexpected distribution of hepatitis B genotypes in
patients with kidney disease: Comparison with immunocompetent
subjects. J Med Virol. 84:1548–1552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pavenstadt H, Kriz W and Kretzler M: Cell
biology of the glomerular podocyte. Physiol Rev. 83:253–307. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Zhang B, Chai Y, Xu Y, Xing C and
Wang X: Fluvastatin attenuated the effect of expression of beta1
integrin in PAN-treated podocytes by inhibiting reactive oxygen
species. Mol Cell Biochem. 398:207–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shankland SJ and Al'Douahji M: Cell cycle
regulatory proteins in glomerular disease. Exp Nephrol. 7:207–211.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Zhou JH and Wang HT: Podocyte
depletion in children with hepatitis B virus-associated membranous
nephropathy. Zhonghua Er Ke Za Zhi. 45:344–348. 2007.(In Chinese).
PubMed/NCBI
|
|
16
|
Seeger V and Mason WS: Hepatitis B virus
biology. Microbiol Mol Biol Rev. 64:51–68. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clippinger AJ, Gearhart TL and Bouchard
MJ: Hepatitis B virus X protein modulates apoptosis in primary rat
hepatocytes by regulating both NF-kappaB and the mitochondrial
permeability transition pore. J Virol. 83:4718–4731. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moolla N, Kew M and Arbuthnot P: Regulator
elements of hepatitis B virus transcription. J Viral Hepat.
9:323–331. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Chen Y, Yang F and Zhou J: HBx
transfection limits proliferative capacity of podocytes through
cell cycle regulation. Acta Biochim Biophys Sin (Shanghai).
46:1016–1023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren J, Wang L, Chen Z, Ma ZM, Zhu HG, Yang
DL, Li XY, Wang BI, Fei J, Wang ZG and Wen YM: Gene expression
profile of transgenic mouse kidney reveals pathogenesis of
hepatitis B virus associated nephropathy. J Med Virol. 78:551–560.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murakami S: Hepatitis B virus X protein: A
multifunctional viral regulator. J Gastroenterol. 36:651–660. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bouchard MJ and Schneider RJ: The
enigmatic X gene of hepatitis B virus. J Virol. 78:12725–12734.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu J, Yun X, Jiang J, Wei Y, Wu Y, Zhang
W, Liu Y, Wang W, Wen Y and Gu J: Hepatitis B virus X protein
blunts senescence-like growth arrest of human hepatocellular
carcinoma by reducing Notch1 cleavage. Hepatology. 52:142–154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui H, Li QL, Chen J, Na Q and Liu CX:
Hepatitis B virus X protein modifies invasion, proliferation and
the inflammatory response in an HTR-8/SVneo cell model. Oncol Rep.
34:2090–2098. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu HZ and Zhou JH: Hepatitis B virus X
protein up-regulates tumor necrosis factor-α expression in cultured
mesangial cells via ERKs and NF-KB pathways. Asian Pac J Trop
Biomed. 3:217–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu H and Zhou J: HBV X gene transfection
upregulates IL-1beta and IL-6 gene expression and induces rat
glomerular mesangial cell proliferation. J Huazhong Univ Sci
Technolog Med Sci. 28:247–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He P, Liu D, Zhang B, Zhou G, Su X, Wang
Y, Li D and Yang X: Hepatitis B virus X protein reduces podocyte
adhesion via downregulation of α3β1 Integrin. Cell Physiol Biochem.
41:689–700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He P, Zhang D, Li H, Yang X, Li D, Zhai Y,
Ma L and Feng G: Hepatitis B virus X protein modulates apoptosis in
human renal proximal tubular epithelial cells by activating the
JAK2/STAT3 signaling pathway. Int J Mol Med. 31:1017–1029. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma Y, Yang Q, Zhong Z, Liang W, Zhang L,
Yang Y and Ding G: Role of c-Abl and nephrin in podocyte
cytoskeletal remodeling induced by angiotensin II. Cell Death Dis.
9:1852018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kato T, Mizuno S and Kamimoto M: The
decreases of nephrin and nuclear WTl in podocytes may cause
albuminuria during the experimental sepsis in mice. Biomed Res.
31:363–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu C, Xuan X, Che R, Ding G, Zhao M, Bai
M, Jia Z, Huang S and Zhang A: Dysfunction of the
PGC-1α-mitochondria axis confers adriamycin-induced podocyte
injury. Am J Physiol Renal Physiol. 306:F1410–F1417. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maezawa Y, Onay T, Scott RP, Keir LS,
Dimke H, Li C, Eremina V, Maezawa Y, Jeansson M, Shan J, et al:
Loss of the podocyte expressed transcription factor Tcf21/Pod1
results in podocyte differentiation defects and FSGS. J Am Soc
Nephrol. 25:2459–2470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagata M: Podocyte injury and its
consequences. Kidney Int. 89:1221–1230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mundel P and Shankland SJ: Podocyte
biology and response to injury. J Am Soc Nephrol. 13:3005–3015.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marshall CB and Shankland SJ: Cell cycle
regulatory proteins in podocyte health and disease. Nephron Exp
Nephrol. 106:e51–e59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y, Wang X, Zhang Y and Yuan W:
Hepatitis B virus X protein and proinflammatory cytokines synergize
to enhance TRAIL-induced apoptosis of renal tubular cells by
upregulation of DR4. Int J Biochem Cell Biol. 97:62–72. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rawlings JS, Rosler IM and Harrison DA:
The JAK/STAT signaling pathway. J Cell Sci. 117:1281–1283. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aittomäki S and Pesu M: Therapeutic
targeting of the Jak/STAT pathway. Basic Clin Pharmacol Toxicol.
114:18–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cai B, Cai JP, Luo YL, Chen C and Zhang S:
The specific roles of JAK/STAT signaling pathway in sepsis.
Inflammation. 38:1599–1608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferguson SD, Srinivasan VM and Heimberger
AB: The role of STAT3 in tumor-mediated immune suppression. J
Neurooncol. 123:385–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Demaria M, Misale S, Giorgi C, Miano V,
Camporeale A, Campisi J, Pinton P and Poli V: STAT3 can serve as a
hit in the process of malignant transformation of primary cells.
Cell Death Differ. 19:1390–1397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang G, Huang X, Liu M, Hua Y, Deng B,
Jin W, Yan W, Tan Z, Wu Y, Liu B and Zhou Y: Secoisolariciresinol
diglucoside prevents the oxidative stress-induced apoptosis of
myocardial cells through activation of the JAK2/STAT3 signaling
pathway. Int J Mol Med. 41:3570–3576. 2018.PubMed/NCBI
|
|
44
|
Liu K, Gao H, Wang Q, Wang L, Zhang B, Han
Z, Chen X, Han M and Gao M: Hispidulin suppresses cell growth and
metastasis by targeting PIM1 through JAK2/STAT3 signaling in
colorectal cancer. Cancer Sci. 109:1369–1381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu GQ, Du X, Li YJ, Gao XQ, Chen BQ and Yu
L: Inhibition of cerebral ischemia/reperfusion injury-induced
apoptosis: Nicotilforin and JAK2/STAT3 pathway. Neural Regen Res.
12:96–102. 2017. View Article : Google Scholar : PubMed/NCBI
|