Introduction
Methotrexate (MTX) (amethopterin or
4-amino-N10-methyl pteroylglutamic acid) is a folic acid analog,
whose effects can be classified into anti-proliferative
[dihydrofolate reductase (DHFR)-mediated] and anti-inflammatory
effects (non-DHFR-mediated) (1).
In the center of the anti-inflammatory pathway is a
purine nucleoside known as adenosine, which has the capacity to
fight against the inflammatory process (2). The antiproliferative, antineoplastic,
and cytotoxic effects are based on decreased nucleic acid formation
in activated T cells and in keratinocytes (3).
The aim of the study was to synthesize the most
relevant information regarding the mechanism of action of MTX in
dermatological pathology, demonstrating each of them with
representative clinical cases.
MTX and MTX polyglutamates (MTXPGs) molecules have
the ability to inhibit a folate-dependent enzyme, involved in
purine nucleotides synthesis, termed 5-aminoimidazole-4-carboxamide
ribonucleotide (AICAR) transformylase (1,2,4–6).
This enzyme is involved in the transformation of
AICAR in formyl-AICAR, a purinic DNA precursor. Thus, in the
absence of the function of this transformylase, AICAR accumulates
within the cell, which results in the inhibition of adenosine
deaminase, leading to elevated levels of adenosine in the
extracellular space (1,2,4,5).
Adenosine is the key molecule regarding the
anti-inflammatory response of MTX (2). The anti-inflammatory effect is the
result of the interaction of adenosine with adenosine receptors on
the cell surface, a mechanism that inhibits leukocyte chemotaxis,
oxidative inflammation in neutrophils/monocytes and cytokine
synthesis from monocyte/macrophages (TNF-α, IL-6,-8,-10 and −12)
(1,2,4,5). Moreover, IL-1, IL-4, IL-13 and INF-γ
release is decreased (1,2,5).
Adenosine receptors, also called P1 receptors, can be divided into
A1, A2a, A2b and A3 (2). The A2a
receptor is associated with the greatest anti-inflammatory effect
(2,7). MTX promotes apoptosis in activated
CD4+ T lymphocyte and reduces neovascularization
(5). The combination of
malondialdehyde (MDA) and acetaldehyde (AA) can lead to
malondialdehyde-acetaldehyde (MAA)-protein-adduct, markers of
oxidative stress. It was previously shown that, by decreasing the
production of these compounds and by scavenging free radicals, MTX
could have an additional anti-inflammatory effect (8–12). The
anti-inflammatory effect of MTX was demonstrated in diseases such
as psoriasis (moderate to severe en plaque lesions, psoriatic
arthritis, erythrodermic and pustular forms), bullous diseases,
vasculitis, atopic dermatitis, lupus erythematosus, rheumatoid
arthritis and sclerodermia (3,5).
Case reports
Case 1: Chronic plaque psoriasis
vulgaris
A 57-year-old male patient presented for a
disseminated eruption involving the trunk and the limbs, including
the elbows and the knees, which was evolving for a few months. The
patient was diagnosed with psoriasis vulgaris for more than 30
years, with lesions affecting a small body surface (mainly elbows
and knees), for which he was treated with topical therapies
(vitamin D analogues, topical corticosteroids and emollients). The
evolution of the disease was chronic, with remissions and relapses
until a few months before, when the lesions became more
disseminated and severe.
Clinical examination revealed multiple erythematous,
well-demarcated large plaques, with a thick ivory-white scale
covering the lesions, mildly pruritic. Auspitz sign and wax candle
sign were present as well. The scalp and the genitalia were spared.
The nails of the hands had typical psoriatic signs, such as
thickening of the nail plate, distal onycholysis and a yellow
colour (oil spot sign). The patient did not have any arthralgia or
joint swelling and he was otherwise healthy. Evaluation of the
lesions concluded with a Psoriasis Area and Severity Index (PASI)
of 32.4 points.
Blood test evaluation (hemogram parameters,
transaminases, serum urea and creatinine, total and direct
bilirubin, alkaline phosphatase) was within normal values. The
tests for hepatitis B/C and HIV were negative. The patient was
prescribed oral MTX of 15 mg/week in a single dose, concomitant
with folic acid administration (5 mg/day, excepting the day of
administration of MTX).
During the treatment, the hemogram, the hepatic and
renal tests had normal values, including those made just after the
beginning of the therapy, with a good tolerance of the drug (only a
mild nausea). The drug was well-tolerated (only a mild nausea) and
a gradual improvement with regard to the cutaneous lesions was
observed. After 5 months of treatment, clinical evaluation revealed
a PASI of 5.0 points. The aspect of the nails remained stable,
without significant benefit. Although the renal and hepatic
functions were unaffected by the therapy, the medium corpuscular
volume (MCV) had an abnormally lower value than it had been before.
Taking into consideration the PASI 75 improvement, MTX
administration was interrupted, in order to prevent any possible
adverse events, related to bone marrow toxicity. The patient was
prescribed a topical combination of calcipotriol/betamethasone gel
for 1 month, resulting in a PASI reduction until 0.7 points.
Administration and pharmacokinetics of
MTX in psoriasis vulgaris
The route of administration can be oral,
intramuscular or subcutaneous, and on a weekly basis. The i.v.
route is also available for some products (MTX 100 mg/ml; Hospira
UK Ltd., SmPC, Maidenhead, UK), whereas other products are only for
s.c. use (Nordimet SmPC, Berkshire, UK).
In case of oral intake of the drug, the possibility
of dividing the dose into three equal parts taken every 12 h during
a 24-h period, may help to reduce digestive adverse events
(3). The parenteral administration
can also help the patient regarding digestive tolerance (4).
After administration, the highest serum
concentration is achieved in 1–2 h, being faster for the the
intramuscular pathway (<1 h) (3).
In the blood, the drug circulates into two forms
(MTX and its active form 7-OH MTX), bound to albumin at a
percentage of 50–70% (4,5). The serum half-life of the drug is 6–7
h, but longer for MTXPGs, which are the long-acting active
metabolites and which can last several months (4,5,13). Moreover, within 24 h after
administration, up to 80% of the amount of the molecule is
eliminated unchanged through renal system (4,5).
Patients taking salycilates, sulphonamides, tetracycline,
chloramphenicol and other drugs, are at great risk of toxicity,
considering the fact that albumin binding of MTX is reduced
(3). Moreover, other therapies such
as ciclosporin, and NSAIDs, reduce kidney excretion of MTX and may
increase its levels in the body and the risk of side effects
(3). For the majority of cutaneous
diseases treated using MTX, the usual dose can be between 10 and 25
mg/week, with an average dose of 15 mg/week (5). MTX therapy needs several weeks until
its efficacy is proved, considering the fact that it has a slow
action (3).
Adverse effects of MTX and management
regarding the therapy in psoriasis vulgaris
Hepatotoxicity
Hepatic toxicity refers to abnormal transaminases,
hepatic fibrosis and cirrhosis. The fibrosis can be reversible in
case of drug intake cessation (5,14).
Patients at risk are heavy drinkers, those with abnormal
transaminases, with hepatic disease, hepatitis B and C, those
taking other hepatotoxic therapies, obese or are diabetic (5,15).
Pretreatment assessment is based on transaminases, alkaline
phosphatase, bilirubin and albumin, as well as serologic tests for
hepatitis B and C. Hepatic test have to be repeated every month in
the first 6 months and then every 1–2 months (5).
Hepatotoxicity is dose-related; however, there is
controversy about hepatic biopsy. Subsequently, if hepatic risk
factors are absent, a hepatic biopsy is recommended after a
cumulative dose of 3.5–4 g of MTX (16,17).
Moreover, the SmPC for Nordimet (approved by the European Medicines
Agency) states that there is no evidence to support the idea of
performing a liver biopsy in evaluating liver toxicity in
rheumatological patients treated with MTX (18). This drug cannot be administered if
the patient is alcoholic, if hepatic function tests reveal
anomalies and in case of hepatitis B and C, diabetes mellitus,
obesity, or simultaneous intake of other drugs, which can affect
the liver.
At present, periodic evaluation of serum procollagen
type III N-terminal peptide (PIINP) in countries where it is
available and considering the fact that elevated levels can be
associated with fibrotic liver damage, are useful in avoiding
invasive liver biopsies (3).
Moreover, SmPC for Nordimet states that ‘further research is needed
to establish whether serial liver chemistry tests or propeptide of
type III collagen can detect hepatotoxicity sufficiently’ (18).
Haematologic toxicity
Bone marrow suppression induced by MTX can result in
leukocytopenia, trombocytopenia, pancytopenia and megaloblastic
anemia (4).
Patients that are more prone to developing
hematologic adverse events are those with renal impairment, a
decreased level of serum albumin, or those taking drugs that
interact with MTX, age above 65 years and patients suffering from
other systemic conditions and infections (5,19).
Pretreatment evaluation of a patient includes complete blood cell
count, which is repeated after the first week of therapy, followed
by measurements every 2 weeks for the next 2 months, and then every
2–3 months (3,5). An HIV test is mandatory.
The drug cannot be administered in case of blood
test anomalies (leukocytes <3,500/mm3, platelets
<100,000/mm3), or immunodeficiency syndromes (HIV)
(5). Elevation of the value of MCV
is a reliable sign of bone marrow toxicity and requires a
supplementation of acid folic dosage and a decreased MTX dosage
(4). Moreover, mucositis is a
cutaneous disorder that predicts the risk of pancytopenia, which
can be prevented by folic acid supplementation (4,5). Other
suggestive signs of pancytopenia are cough, breathing difficulty,
bleeding, fever, nausea, and cyanosis (3). Myelosuppression usually occurs in case
of inadequate dose intake (daily dose instead of weekly); thus,
proper information regarding weekly administration of MTX should be
given to the patient (5).
Gastro-intestinal toxicity
Patients may experience nausea, vomiting, less often
diarrhea and mucositis (5).
Measures, such as dose-splitting, concomitant folic acid
administration or other route than orally can help curtail
digestive intolerance (4,20). The drug cannot be taken by
individuals with acute peptic ulcer (3).
Importance of renal function
Considering that MTX is excreted through kidney, an
abnormal renal function may result in high levels of MTX and thus,
several adverse effects. Baseline evaluation prior to treatment
should include blood urea nitrogen, serum creatinine, urinalysis
and determination of creatinine clearance (24-h urine or using
Cockrogt-Gault equation) (5).
Patients with severe renal insufficiency (Glomerular Filtration
Rate <10 ml/min) or those on dialysis cannot take MTX (5). A GFR >10 ml/min requires a dose
reduction (5). The recommendation is
to repeat the renal function tests (BUN, creatinine) every 2 months
(5).
Other possible adverse effects
A patient taking MTX can also experience fatigue,
headaches, dizziness, nausea, malaise, anorexia, alopecia, or
accentuation of sun-induced redness (4,5).
Pulmonary side effects are represented by pulmonary fibrosis and
acute pneumonitis (5). The drug is
contraindicated in pregnant women or during breastfeeding, because
of the potential teratogenicity, respectively, the risk of
secretion in the breast milk. The drug can induce spontaneous
abortion during the first trimester of pregnancy (5,21).
Men can be affected by reversible oligospermia and
possible genetic anomaly of the fetus may also occur (5,21).
Pretreatment evaluation includes pregnancy test, which should be
periodically repeated. Patients are advised to wait one ovulatory
cycle (in the case of a woman) or 3 months (if the patient is a
man) until conception (5,21).
Other important aspects regarding the
treatment
Folic and folinic acid supplementation during the
treatment with MTX has been controversial, with claims that it may
reduce the efficacy of MTX (22,23), as
well as claims that it is beneficial (24). A Cochrane review concluded that there
is some safety advantage in using folic or folinic supplementation
(25). It can decrease the risk of
myelosuppression, the risk of stomatitis, hepatic toxicity and
enhances gastro-intestinal tolerance. There are several approaches
regarding the administration regimen of folic acid, but usually the
dose ranges between 1 and 5 mg, with the possibility of increasing
the dose of folic acid in case of severe MTX-induced toxicity
(5).
In case of any overdose of MTX, the drug of choice
is folinic acid, a reduced folic compound, whose action is
independent of DHFR and which does not need activation (3). A Cochrane review published in 2014
concluded in the abstract that ‘It does not appear that
supplementation with either folic or folinic acid has a
statistically significant effect on the efficacy of MTX in treating
RA (as measured by RA disease activity parameters such as tender
and swollen joint counts, or physician's global assessment scores)’
(25). Other treatment options are
also available for psoriasis (26,27).
The antiproliferative effects
General information
As a folic acid analog, the molecule of the drug
suffers similar transformations as folates. MTX is a prodrug and it
needs the reduced folate carrier to facilitate its penetration into
the cell, the place where it is activated in MTXPGs through the
action of folylpolyglutamate synthetase, by adding glutamate groups
(1,3,28).
MTXPGs are long-acting metabolites that persist for months, which
explains the prolonged effect and the need for only weekly
administration (2,4). The substance is characterized with
affinity to fibroblast, red blood cells, myeloid cells, and liver
cells (5,13).
MTX and MTXPGs derivatives inhibit cellular
replication by inhibiting folate acid-dependent enzymes. Those
involved in the pyrimidine production are DHFR and thymidylate
synthetase, whereas those related to purine synthesis are AICAR
transformylase and glycinamide ribonucleotide (GAR) transformylase.
Moreover, DNA methylation is affected by the inhibition of
methionine synthase, an enzyme involved in the transformation of
homocysteine in methionine (4,5)
DHFR is an important enzyme involved in the
activation of folate compounds, more specifically in the reduction
of DHFR in THF. DNA and RNA synthesis is based on purine and
pyrimidine nucleotide formation, which in turn requires the
presence of THF to act as a co-factor for many enzymatic reactions
(1,4,5).
Moreover, DNA synthesis can be impaired through the
reduction of thymidylic acid synthesis, through inhibition of
thymidylate synthetase. This pathway does not affect ARN synthesis,
because thymine is a pyrimidine nitrogenous base found only in the
DNA structure. The drug is characterized by a specificity regarding
the cell cycle, as it is active only in the S-phase (4,5,13).
An anticancer effect, as well as toxic effects, can
be associated with α-oxoaldehyde metabolism. Thus, MTX increases
the methylglyoxal level, which contributes to the glycation of
biomolecules, a pathway associated with antineoplastic results
(1,8). Moreover, increasing oxidative stress
within the cell leads to MTX promoting apoptosis and inhibiting
proliferation (1,29).
Thus, the antiproliferative, cytotoxic effect of MTX
was demonstrated in the treatment of keratoacanthoma.
Case 2: Keratoacanthoma of the
nose
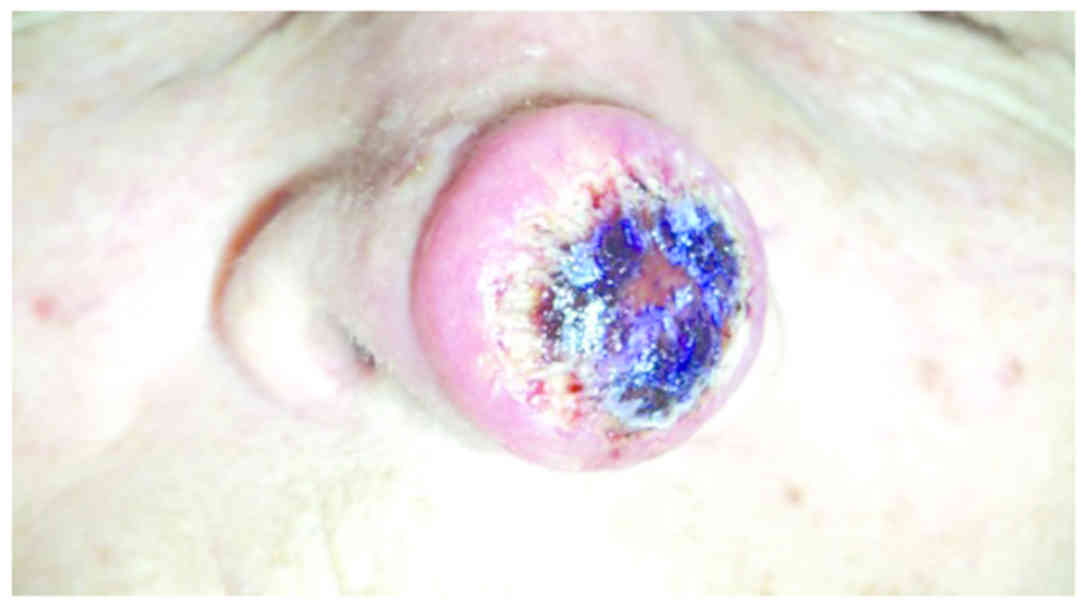
A 72-year-old female patient was referred for a
nodule located on the tip of the nose, rapidly evolving over the
past 3 weeks and whose clinical appearance was suggestive of
keratoacanthoma (Fig. 1). The
patient admitted having a similar lesion on the nose one year
before, which reappeared three times and which was treated each
time using electrocauterization. Clinical examination revealed an
erythematous, well-demarcated nodule, 2.5 cm in diameter, with a
crateriform hyperkeratotic core.
The patients received 3 injections with
intralesional MTX every 2 weeks, with a total dose of 60 mg. The
tumor was injected in four quadrants with 2 ml of MTX (a
concentration of 10 mg/ml) until blanching was achieved. During the
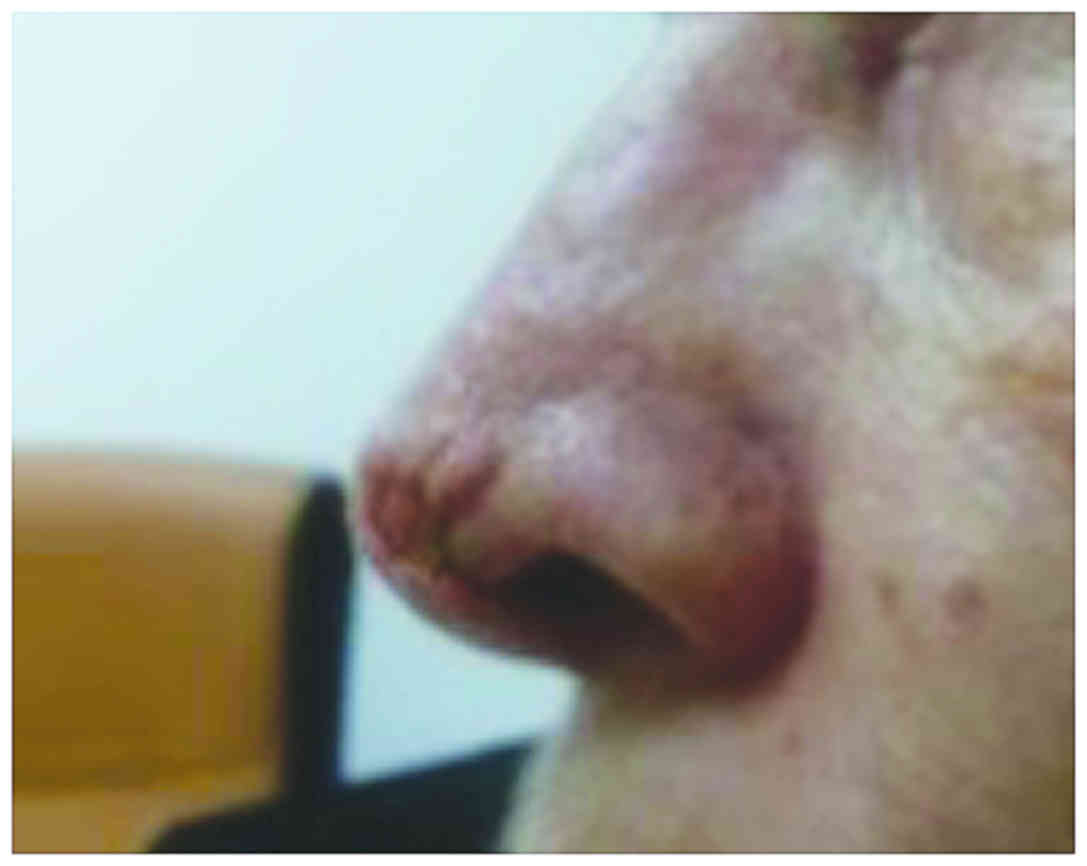
treatment, a progressive regression of the lesion was evident. A
complete remission was obtained after the third injection, with a
good tolerance of the drug and without adverse effects. At the end
of the therapy, only a minor defect was present on the nose
(Fig. 2); thus, the patient was sent
to the Department of Plastic Surgery, in order to evaluate the
esthetic result, the oncologic safety of the lesion and
histopathological evaluation.
Case 3: Keratoacanthoma of the
nose
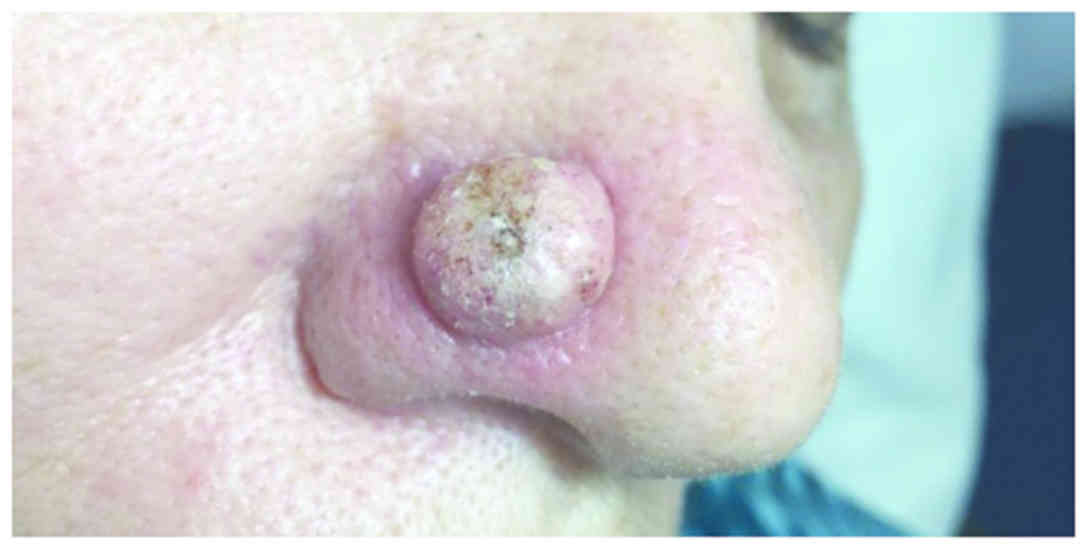
A 69-year-old female patient presented with a red
dome-shaped nodule, 1.5 cm in diameter, near the alar crease of the
nose, with a fast evolution within 2 months (Fig. 3). Clinical examination was consistent
with the diagnosis of keratoacanthoma.
The therapeutic approach was based on only one
injection with intralesional MTX (1 ml of a 12.5 mg/ml) injected in
4 quadrants and at the base of the tumor, until a whitish color was
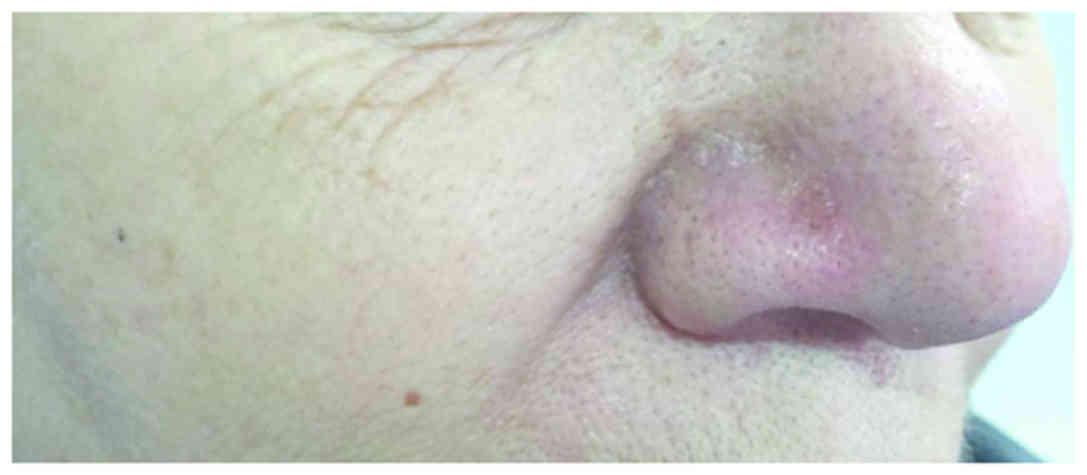
observed. After 2 weeks, the nodule began to shrink and to develop
a central necrosis, followed by spontaneous rupture from the base
and a complete remission (Fig. 4).
The patient did not experience any adverse effect during the
therapy and no recurrences were evident within the first year of
follow-up.
Discussion
Administration in keratoacanthoma
Keratoacanthoma is a cutaneous disorder affecting
mainly the sun-exposed parts of the body of older patients,
clinically presenting as an erythematous round nodule with sharp
demarcation, which has a typical crateriform keratotic core. In the
literature, this tumor is considered either a sub-type of
well-differentiated squamous cell carcinoma, or a squamous lesion
that can spontaneous involute. Even though complete surgical
excision is the treatment of choice, intralesional MTX has proven
its benefits in various cases of large keratoacanthoma of the face,
significantly reducing the tumor size before surgical excision,
with superior aesthetic results (30,31).
The therapy of keratoacanthoma with intralesional
administration of MTX is an ‘off-label’ indication, being based on
a number of cases reported in the literature. The dose is 1 ml of
solution with different concentrations, depending on tumor
dimension (5, 12.5 and 25 mg/mg) injected into four quadrants,
until blanching. The reduction depends on every single case, but
usually 1–4 injections are needed in order to achieve an optimal
result. There is no fixed recommendation regarding the interval
between doses; thus, the sessions can be repeated every 7–49 days
(an average of 2–3 weeks). The complete response rate was 92% based
on a series of 38 cases (more than half from the literature), but
it might be influenced to a certain extent by publication bias
(30,31).
Adverse effects of MTX in
keratoacanthoma
Even though there is no consensus regarding the
evaluation before intralesional therapy as the one in psoriasis
vulgaris, it is recommended to exclude those patients in whom the
treatment might be contraindicated. Usually, the intralesional
therapy is well-tolerated, without significant adverse effects,
compared to those encountered in the systemic administration of
MTX. Nevertheless, pancytopenia occurred in 2 patients who had
severe renal impairment (32,33)
The immunosupresive effect
MTX effects are related to inhibition of two types
of immune responses: humoral immune response (antibody-mediated)
and cellular immune response (lymphocyte mediated), by reducing
lymphocites migration in the skin, and modulating intra- and
intercelluar signaling. By decreasing the level of cytokines such
as TNF-α, IL-10, IL-12, it can be effective in several
immune-mediated skin disorders (4).
This study highlights the various mechanisms of
action of MTX in different skin diseases. We explain both the
therapeutic and possible side effects of MTX in intralesional and
non-intralesional routes of administration. In our opinion,
illustrating with clinical cases from our professional experience
may be explanatory for the intricate pathways through which MTX
exerts its anti-inflammatory and anti-proliferative actions.
Acknowledgements
Not applicable.
Funding
This study was partially supported by a grant from
the Ministry of Research and Innovation, CNCS-UEFISCDI (project no.
PN-III-P4-ID-PCE-2016-0641) within PNCDI–III; and from the Romanian
Ministry of Research and Innovation, CCCDI-UEFISCDI (project no.
61PCCDI⁄2018 PN-III-P1-1.2-PCCDI-2017-0341) within PNCDI–III.
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
RIN, MB, GT, AB, MA, AH, AC, RP, CMP were
contributed to the conception of the study, acquisition, analysis,
interpretation of data, and drafting the manuscript. DAI, AIB, CGP,
MC, LN, SP, CD, CB, DP, SAZ were responsible for the conception and
design of the study, and revising it critically for important
intellectual content. All authors approved the final version to be
published and agreed to be accountable for all aspects of the study
in ensuring that questions related to the accuracy or integrity of
any part of the study are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Patient consent for the images was obtained from
patients included in the sudy.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sramek M, Neradil J and Veselska R: Much
more than you expected: the non-DHFR-mediated effects of
methotrexate. Biochim Biophys Acta Gen Subj. 1861:499–503. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan ESL and Cronstein BN: Molecular
action of methotrexate in inflammatory diseases. Arthritis Res.
4:266–273. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pathirana D, Ormerod AD, Saiag P, Smith C,
Spuls PI, Nast A, Barker J, Bos JD, Burmester GR, Chimenti S, et
al: European S3-guidelines on the systemic treatment of psoriasis
vulgaris. J Eur Acad Dermatol Venereol. 23:1–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Belgi G and Friedmann PS: Traditional
therapies: glucocorticoids, azathioprine, methotrexate,
hydroxyurea. Clin Exp Dermatol. 27:546–554. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bangert CA and Costner MI: Methotrexate in
dermatology. Dermatol Ther. 20:216–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Negrei C, Ginghina O, Caruntu C,
Burcea-Dragomiroiu GTA, Jinescu G and Boda D: Investigation
relevance of methotrexate polyglutamates in biological systems by
high performance liquid chromatography. Rev Chim. 66:766–768.
2015.
|
|
7
|
Strober BE and Menon K: Folate
supplementation during methotrexate therapy for patients with
psoriasis. J Am Acad Dermatol. 53:652–659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zimmerman MC, Clemens DL, Duryee MJ,
Sarmiento C, Chiou A, Hunter CD, Tian J, Klassen LW, O'Dell JR,
Thiele GM, et al: Direct antioxidant properties of methotrexate:
inhibition of malondialdehyde-acetaldehyde-protein adduct formation
and superoxide scavenging. Redox Biol. 13:588–593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Negrei C, Caruntu C, Ginghina O,
Burcea-Dragomiroiu GTA, Toderescu CD and Boda D: Qualitative and
quantitative determination of methotrexate polyglutamates in
erythrocytes by high performance liquid chromatography. Rev Chim.
66:607–610. 2015.
|
|
10
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomark Med. 9:513–528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI
|
|
12
|
Boda CBD, Negrei C and Nicolescu F:
Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
13
|
Kremer JM: Toward a better understanding
of methotrexate. Arthritis Rheum. 50:1370–1382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Newman M, Auerbach R, Feiner H, Holzman
RS, Shupack J, Migdal P, Culubret M, Camuto P and Tobias H: The
role of liver biopsies in psoriatic patients receiving long-term
methotrexate treatment. Improvement in liver abnormalities after
cessation of treatment. Arch Dermatol. 125:1218–1224. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roenigk HH Jr, Auerbach R, Maibach H,
Weinstein G and Lebwohl M: Methotrexate in psoriasis: Consensus
conference. J Am Acad Dermatol. 38:478–485. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu S, Papp KA, Lebwohl MG, Bagel J,
Blauvelt A, Duffin KC, Crowley J, Eichenfield LF, Feldman SR,
Fiorentino DF, et al National Psoriasis Foundation Medical Board, :
Consensus guidelines for the management of plaque psoriasis. Arch
Dermatol. 148:95–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kalb RE, Strober B, Weinstein G and
Lebwohl M: Methotrexate and psoriasis: 2009 National Psoriasis
Foundation Consensus Conference. J Am Acad Dermatol. 60:824–837.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Summary of Product Characteristics: Annex
I. https://www.ema.europa.eu/documents/product-information/nordimet-epar-product-information_en.pdf
|
|
19
|
Lim AYN, Gaffney K and Scott DGI:
Methotrexate-induced pancytopenia: Serious and under-reported? Our
experience of 25 cases in 5 years. Rheumatology (Oxford).
44:1051–1055. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duhra P: Treatment of gastrointestinal
symptoms associated with methotrexate therapy for psoriasis. J Am
Acad Dermatol. 28:466–469. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Temprano KK, Bandlamudi R and Moore TL:
Antirheumatic drugs in pregnancy and lactation. Semin Arthritis
Rheum. 35:112–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manna R, Verrecchia E, Diaco M, Montalto
M, Cammarota G and Gasbarrini G: Folic acid supplementation during
methotrexate treatment: Nonsense? Rheumatology (Oxford).
44:563–564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murphy R: Folic acid reduces the efficacy
of methotrexate. Nat Clin Pract Rheumatol. 2:42006. View Article : Google Scholar
|
|
24
|
Whittle SL and Hughes RA: Folate
supplementation and methotrexate treatment in rheumatoid arthritis:
a review. Rheumatology (Oxford). 43:267–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lopez-Olivo MA, Siddhanamatha HR, Shea B,
Tugwell P, Wells GA and Suarez-Almazor ME: Methotrexate for
treating rheumatoid arthritis. Cochrane Database Syst Rev.
10:CD0009572014.
|
|
26
|
Olteanu R, Zota A and Constantin M:
Biosimilars: an update on clinical trials (review of published and
ongoing studies). Acta Dermatovenerol Croat. 25:57–66.
2017.PubMed/NCBI
|
|
27
|
Rodica O, Constantin MM, Zota A, Dorobantu
DM, Constantin T, Serban ED, Balanescu P, Mihele D and Solovastru
LG: Original clinical experience and approach to treatment study
with interleukine 12/23 inhibitor in moderate-to-severe psoriasis
patients. Farmacia. 64:918–921. 2016.
|
|
28
|
Tian H and Cronstein BN: Understanding the
mechanisms of action of methotrexate: Implications for the
treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis.
65:168–173. 2007.PubMed/NCBI
|
|
29
|
Phillips DC, Woollard KJ and Griffiths HR:
The anti-inflammatory actions of methotrexate are critically
dependent upon the production of reactive oxygen species. Br J
Pharmacol. 138:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Annest NM, VanBeek MJ, Arpey CJ and
Whitaker DC: Intralesional methotrexate treatment for
keratoacanthoma tumors: a retrospective study and review of the
literature. J Am Acad Dermatol. 56:989–993. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoo MG and Kim IH: Intralesional
methotrexate for the treatment of keratoacanthoma: Retrospective
study and review of the korean literature. Ann Dermatol.
26:172–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goebeler M, Lurz C, Kolve-Goebeler ME and
Bröcker EB: Pancytopenia after treatment of keratoacanthoma by
single lesional methotrexate infiltration. Arch Dermatol.
137:1104–1105. 2001.PubMed/NCBI
|
|
33
|
Cohen PR, Schulze KE and Nelson BR:
Pancytopenia after a single intradermal infiltration of
methotrexate. J Drugs Dermatol. 4:648–651. 2005.PubMed/NCBI
|