Introduction
The pathogenesis of psoriasis vulgaris, a chronic,
inflammatory skin disorder, is far from being elucidated. It is
considered a multifactorial disease, involving the interaction of
immune mediated processes, genetic background and environmental
factors (1,2). In response, a series of changes will
appear: dilated capillaries in the dermis due to enhanced
angiogenesis, hyperproliferation and impaired differentiation of
keratinocytes, increased level of inflammatory cells predominantly
with dermal infiltration. A major role is played by T lymphocytes
and their corresponding cytokines, among them tumor necrosis
factor-α (TNF-α), which is a targeted molecule for several
biological treatments in psoriatic patients (3,4). Drug
treatment in psoriasis is long-lasting, designed to control the
disease without providing complete healing. For moderate or severe
forms with no response to conventional treatments (acitretin,
methotrexate), the only therapeutic option remains biological
treatment, a costly variant whose long-term adverse effects are not
yet fully known (5–7).
In earlier phases of psoriasis, it seems that
proliferation of endothelial cells and angiogenesis are caused by
the vascular endothelial growth factor (VEGF) synthetized at
keratinocyte level under the influence of TNF-α (8). Also, the other chemokines produced will
lead to the infiltration of neutrophils in the epidermis. In
chronic lesions, the proliferation of keratinocytes is increased,
due to the enhanced production of cytokeratin (CK6, CK16 and CK17)
(9). Prolactin (PRL), a polypeptide
hormone and a member of type I cytokines has been previously
mentioned as having a role in psoriasis pathogenesis: it has a
stimulatory effect upon keratinocyte proliferation, it may promote
angiogenesis and the infiltration of psoriatic plaque with Th1
cells (10,11).
The study had a 2-fold purpose. First, to
investigate the expression of TNF-α, vascular endothelial growth
factor receptor 2 (VEGFR2) and prolactin receptor (PRLR) in
psoriatic lesion and perilesional skin of untreated patients by
immunohistochemical analysis. Second, to assess the correlation of
these factors with psoriasis duration and severity.
Patients and methods
This cross-sectional study included 19 psoriasis
vulgaris patients, age range 18–80 years, with no topical or
systemic treatment for the last 3 months. The study protocol was
approved by the Ethics Committee of ‘Iuliu Haţieganu’ University of
Medicine and Pharmacy (300/28.07.2014; Cluj-Napoca, Romania), and
all the patients signed the informed consent prior to enrolment.
Demographical data were collected: age, sex, age of onset and
duration of psoriasis. Psoriasis Area Severity Index (PASI) score
was calculated based on the lesion aspect and used for assessing
the severity of the disease. It is an objective method that
includes both the severity and the extent of the lesions. It is
calculated at the level of 4 regions: head, trunk, upper limbs, and
lower limbs, each being assigned a certain coefficient and an
extension degree from 0 (without lesions) to 6 (lesions >90% of
the surface). In terms of lesion appearance, for each of the 3
elements: redness, induration and scales, a score of 0 (without
lesions) to 4 (maximum intensity) is given. The maximum score is
72. The PASI score is also used in assessing the effectiveness of
biological treatments. PASI 75 or PASI 90 is the decrease by 75 or
90% of the score from the calculated value before the treatment
administration (12).
Exclusion criteria: psoriatic patients with other
type of psoriasis than plaque and patients with concomitant
systemic or topical treatment.
Two 4 mm punch biopsy specimens were obtained from
the thigh region of each patient with local anaesthesia: one sample
from psoriatic plaque, the other from perilesional skin (up to 2 mm
from the lesion).
Biopsies were fixed in (10%) neutral buffered
formaldehyde (NBF) overnight, following automatic processing and
embedding in paraffin, sectioned at 5–7 µm using a rotary
microtome. The 5-µm sections from the paraffin blocks were prepared
and stained in hematoxylin and eosin for histopathological
examination. For immunohistochemical examination, 3 sections of 7
µm thickness from all paraffin block samples were drawn on
silane-coated slides. The lamellae were incubated and prepared for
each investigated factor. For immunohighlighting of TNF-α, a rabbit
polyclonal anti-human TNF-α antibody (sc-130349; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was used as primary
antibody. For PRL receptor, the cutaneous tissue was incubated with
mouse monoclonal antibodies against human prolactin receptor
(ab2772; Abcam, Cambridge, UK), while for VEGF receptor the samples
were incubated with rabbit monoclonal antibodies against human
VEGFR2 (ab39638; Abcam). The immunohistochemistry protocol was
performed automatically with the Leica Bond-Max system (Leica
Biosystems, Ltd., Newcastle, UK), using a 20-min thermal-mediated
antigenic display. The reaction was highlighted using the Bond
Polymer Refine Detection kit (DS9800; Leica Biosystems Inc.)
detection kit which includes
3,3′-diaminobenzidine-tetrahydrochloride-dihydrate (DAB) as a final
marker molecule. Blocking of the endogenous peroxidase activity was
achieved for 5 min at 37°C using the 3–4% peroxide block reagent
included in the Bond Polymer Refine Detection kit.
Histopathological and immunohistochemical determinations were
carried out in cooperation with the Department of Pathological
Anatomy, University of Agricultural Sciences and Veterinary
Medicine, Faculty of Veterinary Medicine (Cluj-Napoca,
Romania).
Quantification protocol
The immunohistochemically marked preparations,
corresponding to the two types of skin samples, were examined in
each patient. For every slide, 5 microscopic fields were examined
and a semi-quantitative recording was made for both, the number of
positively stained cells (keratinocytes, lymphocytes, endothelial
cells and fibroblasts) and the intensity of the immunostaining was
as follows: the number of positive cells was evaluated with a score
of 0 to 6 (0, no positive cells; 1, 1–5 positive cells; 2, 6–10
positive cells; 3, 11–50 positive cells; 4, 51–100 positive cells;
5, 101–150 positive cells; 6, more than 150 positive cells); the
intensity of immunostaining was graded on a scale from 0 to 3,
where 0, no staining; 1, mild staining; 2, moderate intensity; and
3, maximum intensity. To calculate the overall score for each
analyzed microscopic field, the score for the positive cell number
was multiplied by the corresponding score for intensity of
immunostaining. For each patient, a maximum, a median and a minimum
immunostaining score were then calculated (13,14).
Statistical analysis
For each microscopic field of the two skin samples,
the maximum, the minimum and the average values of the overall
intensity score were calculated. Distribution (normality) of the
scale variables was evaluated and the non-parametric test
(Wilcoxon) was applied to test the difference between the two
tegument samples. Spearman correlation coefficient was performed in
order to evaluate the correlation between the calculated difference
in the two samples (between the maximum, the minimum and the
average scores) and severity of disease (PASI score). Also, the
correlation between TNF-α and other non-normally distributed
variables was assessed by the alike Spearman's rank correlation.
P-value <0.05 was considered to indicate a statistically
significant difference. A manually conducted Bonferroni correction
was used for repetitive comparisons. Analyses were run in SPSS 17.0
(SPSS, Inc., Chicago, IL, USA).
Results
The demographic and clinical data of the psoriasis
patients are presented in Table
I.
 | Table I.Demographic and clinical
characteristics in psoriatic patients. |
Table I.
Demographic and clinical
characteristics in psoriatic patients.
| Characteristics | Psoriatic patients
(N=19) |
|---|
| Sex |
| Male | 8 |
|
Female | 11 |
| Age (years) | 44.78±16.97 |
| Onset (years) | 34.84±17.88 |
| Duration of disease
(years) | 9.94±7.16 |
| PASI | 20.22±8.12 |
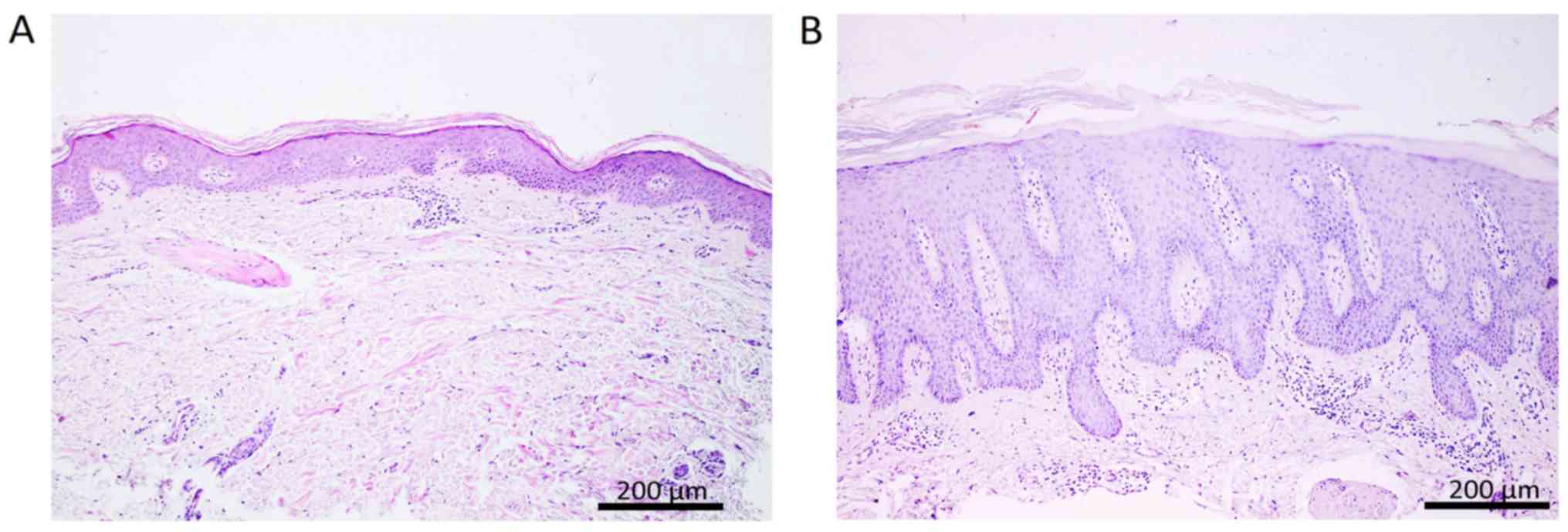
The histopathological examination of the biopsy
samples revealed differences in structure between psoriatic lesion
and perilesional skin, as presented in Fig. 1.
Immunohistochemistry
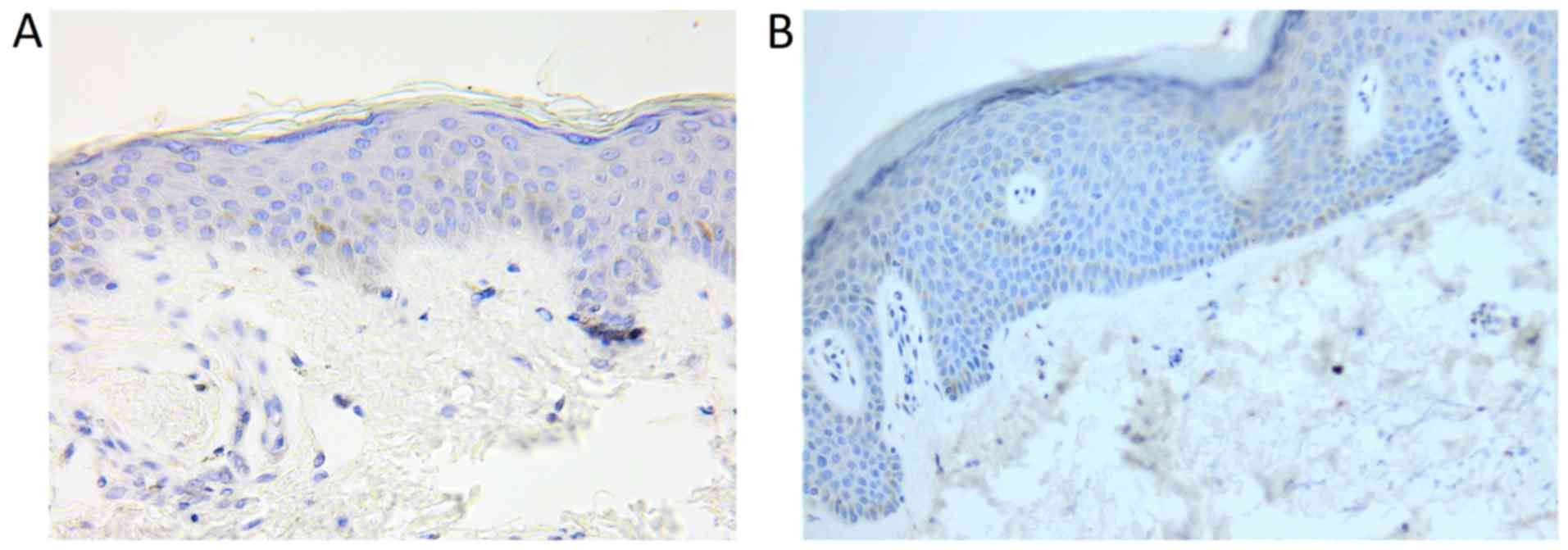
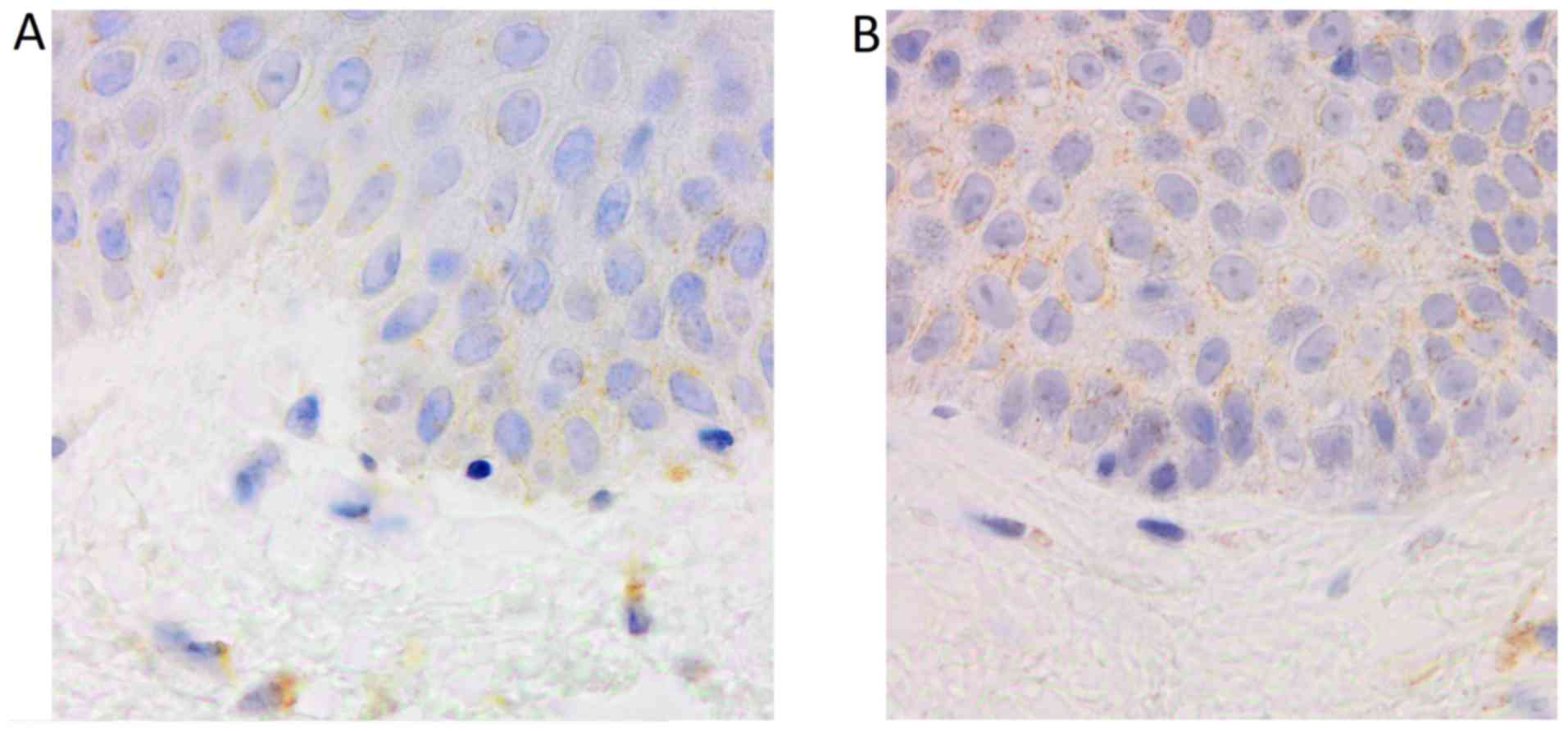
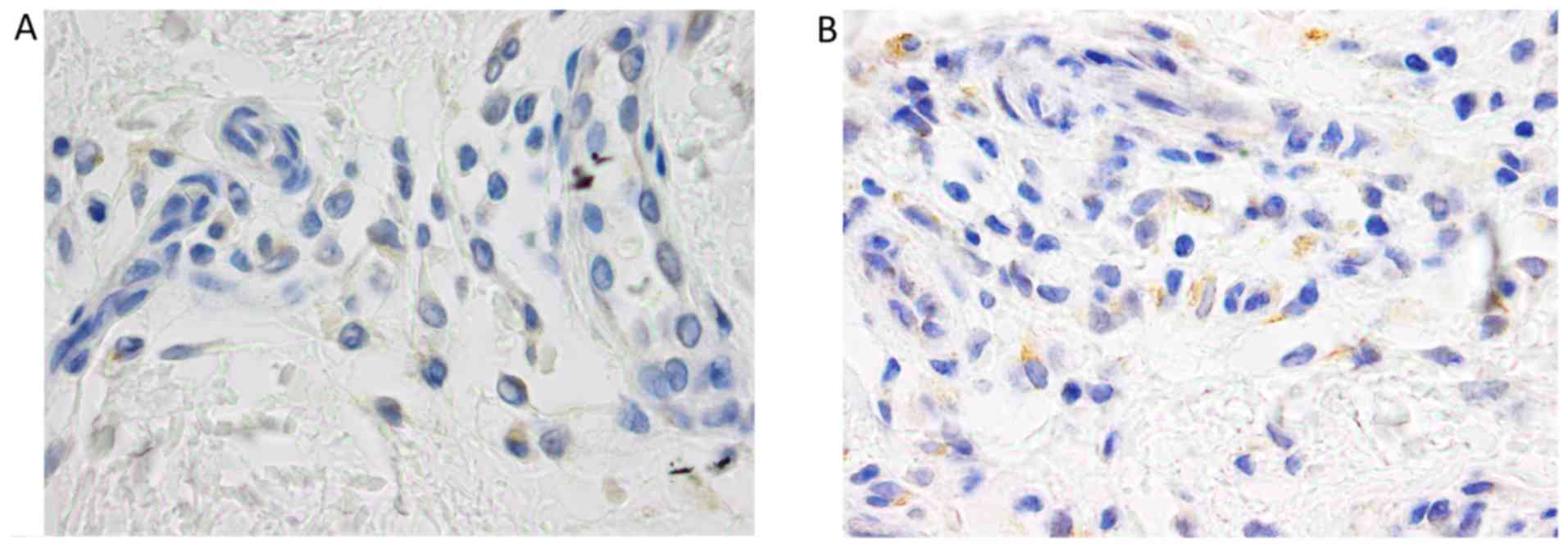
TNF-α
The number of TNF-α positive cells and the intensity
of immunostaining were higher in psoriatic plaque. Also, a
different distribution of these cells was observed between the two
types of skin samples. Thus, the expression of the molecule in
psoriatic plaque was increased in the dermal lymphocytes,
especially perivascular, and in the epidermal keratinocytes.
Regarding the distribution of these cells in the epidermis, for the
perilesional skin sample, TNF-α immunostaining was predominant in
the basal layer keratinocytes, while for psoriatic plaque, all the
keratinocytes layers were positively marked for the molecule: the
expression was generally stronger at the base, with the tendency of
decreasing in the upper layers (Figs.
2, 3 and 4).
VEGFR2 and PRLR
The immunostaining for VEGFR2 and PRLR revealed
positively marked cells in the basal keratinocytes of the epidermis
(VEGFR2) and in the sweat glands or the hair follicle outer sheath
(PRLR). Our results do not reflect significant differences between
the two types of skin samples.
The limited number of positive samples for VEGFR2
and PRLR provided insufficient data for the statistical
analysis.
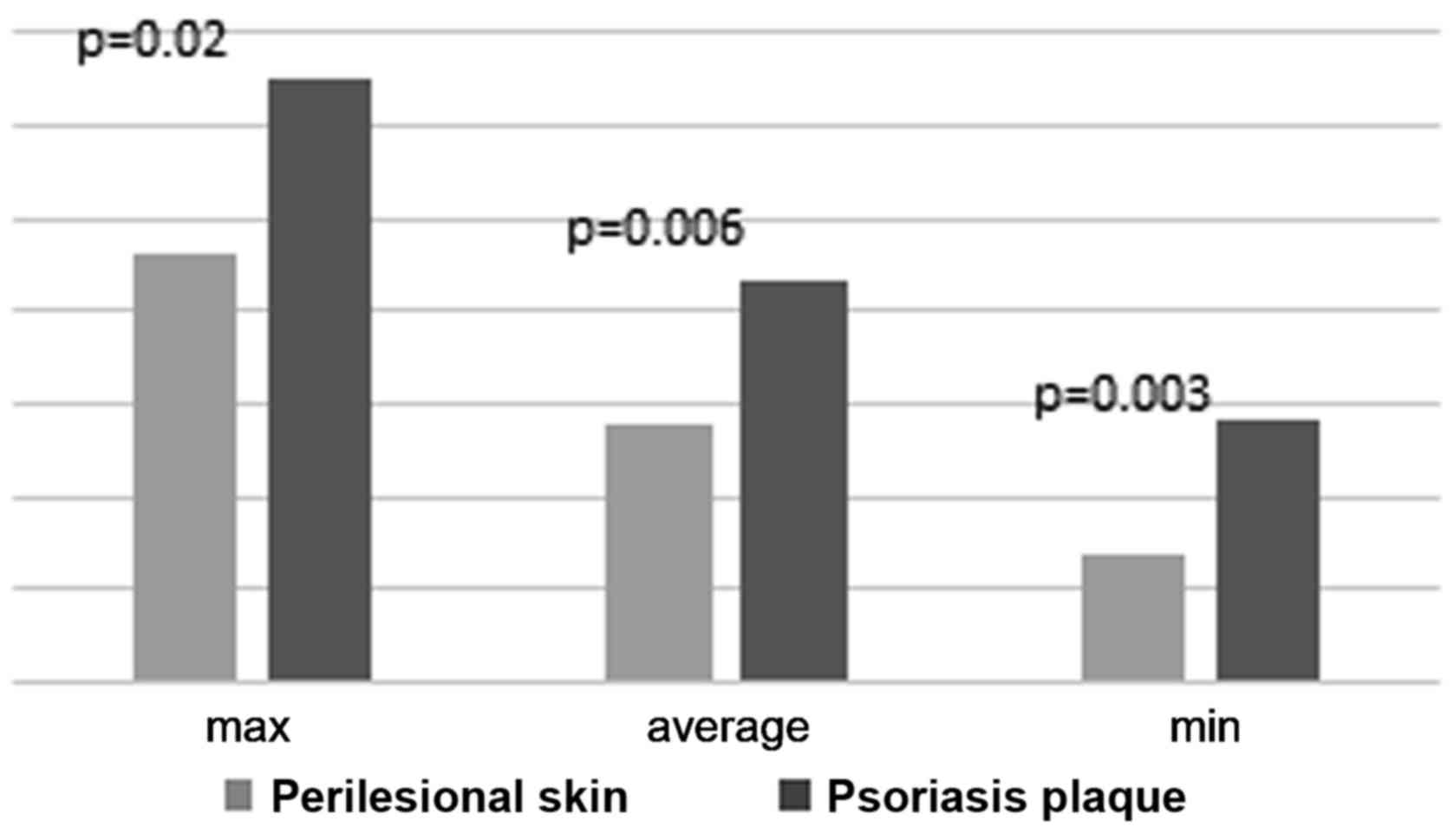
The statistical analysis of TNF-α calculated scores
revealed a significant difference between the intensity of the
immunostaining in the two types of skin samples (Fig. 5).
When testing the correlation of the differences
between the maximum, the minimum and the average scores from the
two skin samples with the overall PASI score (reflecting the
severity of disease), no significant statistical data were
obtained. However, in patients with a moderate form of psoriasis,
PASI score under 15, a positive significant correlation was found
(Table II).
 | Table II.PASI correlation with differences
between intensity scores for TNF-α in examined specimens. |
Table II.
PASI correlation with differences
between intensity scores for TNF-α in examined specimens.
|
| Dif max | Dif average | Dif min |
|---|
|
|
|
|
|
|---|
| Parameters | r | P-value | r | P-value | r | P-value |
|---|
| Overall PASI | 0.225 | 0.335 | 0.363 | 0.127 | 0.225 | 0.335 |
| PASI <15 | 0.902 | 0.014a | 0.983 |
<0.001a | 0.902 | 0.014a |
| Female PASI | 0.581 | 0.061 | 0.543 | 0.085 | 0.581 | 0.061 |
| Male PASI | −0.268 | 0.520 | −0.119 | 0.779 | −0.268 | 0.520 |
| Onset >40
PASI | 0.954 | 0.001a | 0.786 | 0.036a | 0.954 | 0.001a |
Regarding the sex of the patients, the difference
between the maximum, the average and the minimum intensity of
immunostaining for TNF-α in perilesional skin vs. psoriatic plaque
was positively correlated with the disease severity only in women,
at a significance threshold level of 10% (0.1>P>0.05). No
correlation was found in men. The abve parameters were positively
correlated with the PASI score only in patients with the onset of
disease over the age of 40 (Table
II).
In perilesional skin compared to psoriatic plaque,
the difference between the maximum, the average and the minimum
intensity score of immunostaining was neither correlated with the
duration of disease nor with the patients' age (Table III).
 | Table III.Correlation of calculated scores for
TNF-α with age, age at onset and duration of disease. |
Table III.
Correlation of calculated scores for
TNF-α with age, age at onset and duration of disease.
|
| Dif max | Dif average | Dif min |
|---|
|
|
|
|
|
|---|
| Parameters | r | P-value | r | P-value | r | P-value |
|---|
| Age (years) | −0.150 | 0.539 | −0.088 | 0.720 | −0.150 | 0.539 |
| Age at onset
(years) | 0.81 | 0.743 | 0.044 | 0.859 | 0.81 | 0.743 |
| Duration of disease
(years) | 0.114 | 0.743 | −0.46 | 0.853 | 0.114 | 0.743 |
Based on a 1.59 difference in immunostaining score
between psoriasis plaque and perilesional skin, for a confidence
interval of 95% (estimating threshold for α-type error), a minimum
of 80% power of tests (estimating threshold for β-type error), we
calculated a minimum required sample size of 70 patients to be
included in the final study.
Discussion
Tumor necrosis factor-α represents a major mediator
in the pathogenesis of psoriasis, inducing effects ranging from
inflammation to apoptosis. As proved by several studies, TNF-α
levels seem to be elevated both locally and in the serum (15–19). The
results of this study are based on the semi-quantitative assessment
of positive cells and suggest that the differences between plaque
psoriasis and perilesional skin are significant (P<0.05). We
have detected an increased expression of TNF-α in psoriatic skin,
an aspect consistent with literature data. Johansen et al
(17) have described, by using
quantitative methods, a higher level in psoriasis plaque, compared
to normal skin. The study conducted by Kristensen et al
revealed a more important immunostaining of psoriasis plaque, over
unaffected skin (20,21). The same authors described a different
distribution of immunostaining in the two skin samples: in
perilesional skin in the keratinocytes of the basal layer, while in
psoriatic lesion a more intense staining was observed, with the
presence of positively marked cells also in the superficial layers.
Our results confirm the same distribution, but positively-marked
cells (lymphocytes) were also found in the superficial dermis,
especially perivascular. We also obtained a higher score for
immunostaining in areas with significant acanthosis.
Moorchung et al (16) revealed an inverse correlation between
the immunostaining for TNF-α and the degree of epidermal
hyperplasia. In literature, a positive correlation was mentioned
between the presence of positive TNF-α cells in psoriasis plaque
and the severity of the disease, but further details were not
provided (22). The results of this
study, based on the differences in the maximum, mean and minimum
immunostaining scores between the two types of skin samples,
indicate a positive correlation of the TNF-α staining with the
clinical severity score in patients with the moderate form of
psoriasis (PASI <15). Thus, for lower PASI values, we found a
relatively uniform immunostaining in the two areas. The more the
clinical aspect of the disease is significant, the better the
immunostaining is marked in the psoriasis plaque. In this study the
correlation was not preserved in severe forms of psoriasis. A
possible explanation is that the age of the plaque may affect
immunostaining, but a study with a larger sample size could confirm
this hypothesis.
We have also verified the correlation between the
duration of the disease and the differences in the maximum, mean
and minimum immunostaining scores between the two types of skin
samples. A significant positive correlation was found in patients
with onset over the age of 40, classified as type II psoriasis
patients. Literature data were not available for
immunohistochemical studies, but we found immunocytochemistry
studies that mention a higher density of CD4+
lymphocytes in the psoriasis plaque of the patients with onset over
the age of 40, as compared to patients with an earlier onset
(23). Sidhom et al (22) reported, the expression of TNF-α was
increased in patients with a long history of psoriasis. This
correlation was tested on our study data, but no statistically
significant result was found.
Besides the TNF-α involvement in psoriasis
pathogenesis, newly discovered factors also play a key role. It is
still under question if in psoriasis patients, the skin first
changes at the epidermal or dermal level, due to the fact that
vascular proliferation has an important role in the pathogenesis of
the disease, leading to epidermal changes (24,25). An
increased level of VEGF, mediator of angiogenesis, has been found
in the blood and skin of patients with psoriasis vulgaris (20,26).
Increased expression of VEGF and VEGF receptors was revealed in
plaque psoriasis as compared to unaffected skin (27). In the present study, only in two
cases, positive cells were identified in the keratinocyte cells of
the basal layer. Thus, it was not possible to define a pattern of
immunostaining and establish a correlation with the immunostaining
for TNF-α.
A possible role of prolactin has been brought to the
forefront as its expression was proven in plaque psoriasis, but not
in uninvolved skin (11).
Furthermore, a higher level of the hormone was found in the serum
of psoriasis patients, as compared to control group (28–30). In
the present study, the PRL receptor was highlighted at the level of
the sweat gland cells and external hair follicles, both in
perilesional skin and in psoriatic plaque, but the limited number
of positive samples for PRLR provided insufficient data for
statistical analysis. Kanda et al (11) mentioned a prolactin expression that
was highlighted in the keratinocytes of the basal and suprabasal
layer in psoriasis plaque, but not in normal skin. Also, a slight
mild positivity for PRLR has been observed in the sweat glands.
In conclusion, results of this study confirmed the
essential role of TNF-α in the pathogenesis of psoriasis vulgaris.
The increased presence of TNF-α in the skin was in direct
correlation with histopathological changes and the lesion severity.
No significant differences were found for VEGFR2 and PRLR. However,
we are aware of the limitations in our study. We have presented the
data obtained from samples collected from 19 patients. Calculations
of sample size returned a minimum required sample size of 70
patients for acceptable levels of α-type and β-type errors.
Therefore, collected data in this pilot study will continue to be
completed up to the necessary patient count that allows statistical
significance to be obtained.
Acknowledgements
Not applicable.
Funding
The authors wish to acknowledge financial support
from the Romanian National Authority for Scientific Research and
Innovation, CNCS-UEFISCDI, project no. PN-III-P2-2.1-BG-2016-0446
(Bucharest, Romania).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
IIM was responsible for the conception, design,
acquisition, analysis and interpretation of the data; the drafting
and writing of the manuscript, and revising it for intellectual
content. FAT contributed to the conception, design, histopathologic
analysis and interpretation, analysis and interpretation of the
data, and the drafting of the manuscript. TM was involved in the
conception, acquisition, analysis and interpretation of the data;
statistical analysis, the drafting and revising of the manuscript.
EMJ was responsible for the conception, design, acquisition,
analysis and interpretation of the data, and the drafting of the
manuscript. MSO and ADP contributed to the acquisition and analysis
of the data, the drafting and revising of the manuscript. RIO was
responsible for the conception and design of the experiments,
analysis and interpretation of the data, the drafting of the
manuscript and revising it for intellectual content. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
‘Iuliu Haţieganu’ University of Medicine and Pharmacy
(300/28.07.2014; Cluj-Napoca, Romania), and all the patients signed
an informed consent prior to enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parisi R, Symmons DP, Griffiths CE and
Ashcroft DM; Identification, Management of Psoriasis and Associated
ComorbidiTy (IMPACT) project team, : Global epidemiology of
psoriasis: A systematic review of incidence and prevalence. J
Invest Dermatol. 133:377–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI
|
|
3
|
Deng Y, Chang C and Lu Q: The inflammatory
response in psoriasis: A comprehensive review. Clin Rev Allergy
Immunol. 50:377–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boda D, Negrei C, Nicolescu F and Balalau
C: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
5
|
Raţiu MP, Purcărea I, Popa F, Purcărea VL,
Purcărea TV, Lupuleasa D and Boda D: Escaping the economic turn
down through performing employees, creative leaders and growth
driver capabilities in the Romanian pharmaceutical industry.
Farmacia. 59:119–130. 2011.
|
|
6
|
Negrei C, Caruntu C, Ginghina O, Burcea
Dragomiroiu GT, Toderescu CD and Boda D: Qualitative and
quantitative determination of methotrexate polyglutamates in
erythrocytes by high performance liquid chromatography. Rev Chim.
66:607–610. 2015.
|
|
7
|
Negrei C, Ginghină O, Căruntu C, Burcea
Dragomiroiu GT, Jinescu G and Boda D: Investigation relevance of
methotrexate polyglutamates in biological systems by high
performance liquid chromatography. Rev Chim. 66:766–768. 2015.
|
|
8
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015. View Article : Google Scholar
|
|
9
|
Kanda N, Hau CS, Tada Y and Watanabe S:
Prolactin may promote the development of psoriasis: Reawakened
issue. J Clin Exp Dermatol Res. 4:10001982013.
|
|
10
|
Foitzik K, Langan EA and Paus R: Prolactin
and the skin: A dermatological perspective on an ancient
pleiotropic peptide hormone. J Invest Dermatol. 129:1071–1087.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanda N, Shibata S, Tada Y, Nashiro K,
Tamaki K and Watanabe S: Prolactin enhances basal and IL-17-induced
CCL20 production by human keratinocytes. Eur J Immunol.
39:996–1006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Olteanu R, Constantin MM, Zota A,
Dorobantu D, Constantin T, Serban ED, Balanescu P, Mihele D and
Gheuca-Solovastru L: Original clinical experience and approach to
treatment study with interleukine 12/23 inhibitor in
moderate-to-severe psoriasis patients. Farmacia. 64:918–921.
2016.
|
|
13
|
Sunnemark D, Ulfgren AK, Örn A and Harris
RA: Cytokine production in hearts of Trypanosoma
cruzi-infected CBA mice: Do cytokine patterns in chronic stage
reflect the establishment of myocardial pathology? Scand J Immunol.
44:421–429. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Charles CA, Romanelli P, Martinez ZB, Ma
F, Roberts B and Kirsner RS: Tumor necrosis factor-alfa in
nonhealing venous leg ulcers. J Am Acad Dermatol. 60:951–955. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ettehadi P, Greaves MW, Wallach D, Aderka
D and Camp RD: Elevated tumour necrosis factor-α (TNF-α) biological
activity in psoriatic skin lesions. Clin Exp Immunol. 96:146–151.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moorchung N, Vasudevan B, Mani NS and
Verma R: Expression of tumor necrosis factor-α and nuclear
factor-kappaB/RelA and the pathogenesis of psoriasis. Indian J
Pathol Microbiol. 57:205–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johansen C, Funding AT, Otkjaer K,
Kragballe K, Jensen UB, Madsen M, Binderup L, Skak-Nielsen T,
Fjording MS and Iversen L: Protein expression of TNF-alpha in
psoriatic skin is regulated at a posttranscriptional level by
MAPK-activated protein kinase 2. J Immunol. 176:1431–1438. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sereflican B, Goksugur N, Bugdayci G,
Polat M and Haydar Parlak A: Serum visfatin, adiponectin, and tumor
necrosis factor alpha (TNF-α) levels in patients with psoriasis and
their correlation with disease severity. Acta Dermatovenerol Croat.
24:13–19. 2016.PubMed/NCBI
|
|
19
|
Olteanu R, Zota A and Constantin M:
Biosimilars: An update on clinical trials (review of published and
ongoing studies). Acta Dermatovenerol Croat. 25:57–66.
2017.PubMed/NCBI
|
|
20
|
Zhu JW, Wu XJ, Lu ZF, Luo D, Cai SQ and
Zheng M: Role of VEGF receptors in normal and psoriatic human
keratinocytes: Evidence from irradiation with different UV sources.
PLoS One. 8:e554632013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kristensen M, Chu CQ, Eedy DJ, Feldmann M,
Brennan FM and Breathnach SM: Localization of tumour necrosis
factor-alpha (TNF-alpha) and its receptors in normal and psoriatic
skin: Epidermal cells express the 55-kD but not the 75-kD TNF
receptor. Clin Exp Immunol. 94:354–362. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sidhom E, Pilmane M and Kisis J: Local
antimicrobial, protease and cytokine defense systems in psoriatic
skin. Indian J Dermatol Venereol Leprol. 82:284–291. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Theodorakopoulou E, Yiu ZZ, Bundy C,
Chularojanamontri L, Gittins M, Jamieson LA, Motta L, Warren RB and
Griffiths CE: Early- and late-onset psoriasis: A cross-sectional
clinical and immunocytochemical investigation. Br J Dermatol.
175:1038–1044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Campanati A, Goteri G, Simonetti O,
Ganzetti G, Giuliodori K, Giuliano A, Sabato S, Stramazzotti D,
Gulini E, Dusi D, et al: Angiogenesis in psoriatic skin and its
modifications after administration of etanercept:
Videocapillaroscopic, histological and immunohistochemical
evaluation. Int J Immunopathol Pharmacol. 22:371–377. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Negrei C, Arsene AL, Toderescu CD, Boda D
and Ilie M: Acitretin treatment in psoriazis may influence the cell
membrane fluidity. Farmacia. 60:767–771. 2012.
|
|
26
|
Simonetti O, Lucarini G, Goteri G, Zizzi
A, Biagini G, Lo Muzio L and Offidani A: VEGF is likely a key
factor in the link between inflammation and angiogenesis in
psoriasis: Results of an immunohistochemical study. Int J
Immunopathol Pharmacol. 19:751–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Flisiak I, Zaniewski P, Rogalska M,
Myśliwiec H, Jaroszewicz J and Chodynicka B: Effect of psoriasis
activity on VEGF and its soluble receptors concentrations in serum
and plaque scales. Cytokine. 52:225–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keen MA and Hassan I: Serum prolactin
levels in psoriasis and its association with disease activity: A
case-control study. Indian J Dermatol. 59:562–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kato AM, Gheida SF and El-Bendary AS:
Serum level of prolactin in psoriatic patients. EDOJ. 2:12012.
|
|
30
|
Dilmé-Carreras E, Martín-Ezquerra G,
Sánchez-Regaña M and Umbert-Millet P: Serum prolactin levels in
psoriasis and correlation with cutaneous disease activity. Clin Exp
Dermatol. 36:29–32. 2011. View Article : Google Scholar
|