Introduction
Chronic inducible urticaria (CIndU) is a subgroup of
chronic urticaria, characterized by the presence of wheals (hives),
angioedema, or both, over 6 weeks, triggered by a specific factor
(1). They are divided in physical
urticaria and nonphysical urticaria. The physical urticaria is
induced by exogenous physical triggers: thermal triggers (cold,
heat), solar radiation and mechanichal triggers (friction,
pressure). In nonphysical urticaria (cholinergic, contact or
aquagenic urticaria) the active and passive warming, contact with
some substances or water are required. The recommended treatment
approach in CIndU is the same as that for chronic spontaneous
urticaria (CSU) (1–3). The first line treatment includes
H1-antihistamines in standard doses or four-fold higher doses. Some
patients do not respond even to high level of antihistamines.
Omalizumab, a recombinant humanized monoclonal antibody against
IgE, can be an efficient add-on therapy in recalcitrant CSU
(3). Some case reports and small
case series offer limited data regarding the efficiency of
omalizumab in CIndU.
The study was approved by the Ethics Commitee of
Emergency Clinical Hospital for Children ‘M.S. Curie’ (Bucharest,
Romania), and a signed informed consent was obtained from the
patients or the guardians included in this study.
We present 2 severe cases of CIndU (one with severe
cold urticaria and the other with severe extensive dermographism)
with completely different response to omalizumab.
Case reports
Case 1
A 38-year-old woman presented with a seven months
history of a severe cold urticaria. First symptoms occurred after
swimming in the sea water during her summer holiday. She described
wheals, chills and some dizziness which disappeared after warming.
Her hives were transient, did not burn and did not leave bruising
as they resolved. Shortly thereafter, in addition to the above
symptoms, she began to have episodes of angioedema with shortness
of breath and dysphonia and with severe dizziness after exposure to
cold wind; all these episodes resulted in emergency room visits
where epinephrine was administered with improvement in symptoms.
During winter time, her symptoms were getting worse, with severely
impaired quality of life and with need of interruption of her job
of medical assistant. Her family history revealed a grandfather
with asthma and her mother with Basedow's disease.
Through specific investigations a negative workup
has been shown for mast cell disorders, hereditary angioedema,
autoimmune diseases (including cryoglobulinemic vasculitis),
infections (including hepatitis B and C), and food allergy,
inhalant allergy. She had an intense positive ice cube test result
and an increased level of total serum IgE (828 UI/ml). Diagnosis of
severe cold urticaria (including anaphylaxis) was established.
Initial treatment consisted of high-dose (up to
4-fold) of various H1 antihistamines (AHs) (desloratadine,
bilastine, rupatadine) and H2 blockers (ranitidine) with further
addition of leukotriene receptor antagonist (montelukast). Specific
prophylactic measures have been respected (as much as possible).
Despite this treatment, the response was poor, the patient being
forced to stay at home, with warm clothing and still experienced
some intermittent hives.
We decided to start on monthly omalizumab (300 mg)
injections. At this point, her urticaria activity score for the
preceding 7 days (UAS7) was 42 (Table
I). The benefit came very fast, from the first dose. Within 10
days after starting this medication, her wheals were controlled.
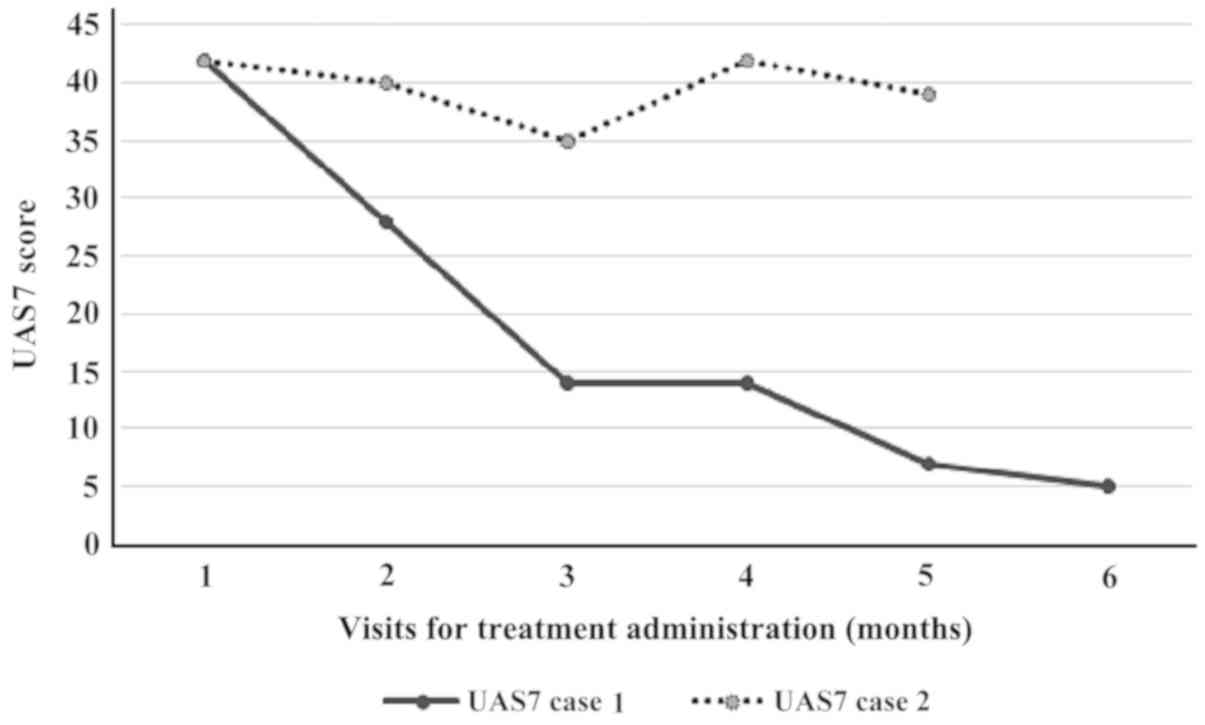
The UAS7 fell to 28 (at first month evaluation) with a progressive
decrease after each course (Fig. 1).
No side effects were noted during her 6 courses of omalizumab. At
the time of this case study, 10 months after the interruption of
the omalizumab, she remains free of severe anaphylactic episodes,
with only some mild hives triggered by specific cold circumstances,
but she is still taking H1 and H2 antihistamines (maximum
doses).
 | Table I.General characteristics of the cases
with physical urticaria. |
Table I.
General characteristics of the cases
with physical urticaria.
| Case | Age (years) | Sex | Duration of disease
(months) | UAS7 at baseline | Type of
urticaria | Total IgE
(UI/ml) | Previous
treatment | Dose of
omalizumab | Duration | Effect |
|---|
| 1 | 38 | F | 7 | 42 | Cold | 828 | 4 AH1, 2 AH2,
LTRA | 300 mg/month | 6 months | Yes |
| 2 | 17 | M | 25 | 42 | Factitia | 348 | 4 AH1, AH2, LTRA,
OCS | 300 mg/month | 5 months | No |
Case 2
The second case we report, is the case of a
17-year-old boy who came for a history of 25 months of severe
symptomatic dermographism (urticaria factitia). The patient's
complaints were severe itchiness and whealing in context of usual
activities at home or at school (dressing, tight clothes, some
physical activities), and in the last year also spontaneous
urticaria added (urticarial plaques from time to time, without an
obvious trigger, without drug-induced or food-induced
exacerbation). In this condition, the patient described decreased
school performance and negative impact on social life. The family
history was negative for any significant disease. Differential
blood count, ESR or CRP and the entire diagnostic workup
(infections, autoimmune disorders, IgE mediated food and inhalant
allergies) were in normal ranges, except for total IgE (348 UI/ml).
The diagnosis of urticaria factitia was supported by the history
and the skin reaction at the typical trigger (itching palpable
wheals within 10 min after light stroking pressure with a wooden
spatula on the volar forearm) (1).
The treatment with various nonsedating antihistamines (bilastine,
desloratadine, rupatadine, levocetirizine) in standard and up to
four-fold higher doses, single or in combination with H2 blocker
(ranitidine) or leukotriene receptor antagonist (montelukast) did
not induce control of urticaria for the patient. Short oral
corticosteroid (OCS) cures were repeatedly needed, followed by a
rapid improvement of episodes of pruritus and induced urticaria.
Considering the severe clinical course, we proposed therapy with
anti-IgE monoclonal antibodies in order to obtain control of the
disease and to reduce or eliminate the need for OCS therapy.
With the consent of the parents, we initiated the
treatement with omalizumab 300 mg/month, starting from an urticaria
activity score for the preceding 7 days (UAS7) of 42 (Table I). Upon a promising improvement after
the first three doses, the activity score of urticaria worsened
again, thus at the end of the 5 doses of treatment, the UAS7 score
was insignificantly modified (Fig.
1) and the patient and his family refused at this point to do
the sixth dose of omalizumab from the schedule. Like in the
previous case, the administration of omalizumab was very well
tolerated, without side effects but the desired improvement of
urticaria was not obtained for this patient. The lack of answer to
recombinant monoclonal antibodies, the fear of adverse effects of
other drugs (cyclosporine), determined the parents of the
adolescent to refuse another option as add-on therapy and they
decided to return to previous treatment schedule (non-sedating
antihistamines, montelukast, OCS at need).
Discussion
The current guidelines on the treatment of urticaria
state that the goal for the treatment of patients has to be
complete symptom control. However, in many patients with physical
urticaria, standard treatment with antihistamines is not sufficient
to reach this goal. Following the recommended treatment algorithm,
updosing of antihistamines should be performed in those patients
with remaining symptoms. With this approach, many physical
urticaria patients can be treated successfully. Nonetheless, there
are some patients who still suffer from severe symptoms, despite
updosing of H1 antihistamines and the addition of other medication
such as leukotriene receptor antagonists and H2 receptor blockers.
For these patients, alternative treatments such as cyclosporine A,
dapsone or omalizumab therapy can be recommended. Omalizumab was
approved by the US Food and Drug Administration and the European
Medicines Agency for CSU. According to EACCI/GA2LEN/EDF/WAO
Guidelines, this drug is recommended as add-on therapy as a third
line treatment for patient unresponsive to high doses of
H1-antihistamines after 2–4 weeks or earlier (2,3). Its
action is to bind free serum IgE and prevent its attachement to the
high-affinity receptor on mast cells and basophils. The effective
doses in CSU are 150–300 mg/month and independent of total serum
IgE level (4 quoted in 3). The good response in cold urticaria
emphasizes the role of IgE in mast-cell activation by cold trigger.
However, omalizumab has proven its effectiveness despite the level
of total serum IgE, possibly because of other unknown effects
(3).
The recommendation of anti-IgE treatment for
patients with recalcitrant physical urticaria is supported by a
recent small clinical trial and a number of case reports (1,5,6). In our first patient, the observed very
rapid improvement of the symptoms during omalizumab treatment
argues in favour of the hypothesis that IgE plays an important role
in the pathogenesis of physical urticarias. The kinetics of the
relief from symptoms for this patient is similar to previously
reported cases of inducible urticarias, as well as of chronic
spontaneous urticaria, and strikingly different from the response
in asthmatic patients which rarely show significant improvement
within the first weeks.
This indicates that in asthma and urticaria,
different pathophysiological mechanisms are responsible for the
respective symptoms. In asthma, tissue remodelling in the lung and
other ‘chronic’ processes are of pathophysiological importance
which take much longer to be reversed. However, there might be
currently unknown effects of omalizumab other than IgE capture,
especially in regard to the inhibition of mast cell
degranulation.
In terms of urticaria factitia, current data show
that the majority of patients benefits from treatment with
nonsedating antihistamines (7). The
off-label use of treatment with omalizumab for the severe cases has
already proven good results and there are data suggesting a better
therapeutical answer to this drug in urticaria factitia compared
with other forms of physical urticaria (6,8). These
encouraging data led us to start this treatment in our second
patient with urticaria factitia. Unfortunately, the therapeutical
response was unsatisfactory.
There are no parameters to select the responders
from non-responders and in the literature the reported cases of
omalizumab treated urticaria factitial, indicated that the patients
with urticaria factitia and normal total IgE before starting the
treatment are those with incomplete response of urticaria symptoms
to omalizumab. In our case the baseline level of total IgE was
increased (348 UI/ml), hence according to previously published
results, we expected to have an improvement of symptoms for our
patient. We could not explain the lack of response to the
omalizumab treatment, so we can only speculate that perhaps
different doses or different interval between the doses would have
induced a better control of symptoms. Although there is no such
recommendation in guidelines, there are authors reporting
empirically use of regimens similar to asthma patients (doses
calculated according to IgE level and weight) or doses between 150
and 300 mg every 2 to 4 weeks, all this cumulated experience
suggesting that the answer of CIndU to omalizumab might require a
dose and administration regimen different from that recommended in
CSU (9).
Unfortunately, there is not enough evidence in
patient with CIndU, especially in AHs resistant cases and there are
no criteria to identify the patients who would not profit from
omalizumab treatment.
Clinical trials with larger patient numbers should
be performed to further investigate the value of omalizumab, the
individualized treatment dosages and administration intervals, and
the biomarkers for future treatment algorithms in urticaria
factitia and other forms of physical urticaria (9).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RSB contributed to the conception and design of the
study. CGD was responsible for the conception of the study and
contributed to drafting the manuscript. ECB contributed to the
acquisition of the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Commitee of the
Clinical Emergency Hospital for Children ‘M.S. Curie’ (Bucharest,
Romania), and signed informed consent was obtained from the
patients or the guardians included in this study.
Patient consent for publication
Signed informed consent was obtained from the
patients or the guardians included in this study.
Competing interests
RSB and CGD declare that they have no conflicts of
interest. ECB has participated in trials sponsored by Novartis.
Glossary
Abbreviations
Abbreviations:
|
CSU
|
chronic spontaneous urticaria
|
|
CIndU
|
chronic inducible urticaria
|
|
AHs
|
H1 antihistamines
|
|
OCS
|
oral corticosteroids
|
|
LTRA
|
leukotriene receptor antagonist
|
References
|
1
|
Magerl M, Altrichter S, Borzova E,
Giménez-Arnau A, Grattan CEH, Lawlor F, Mathelier-Fusade P,
Meshkova RY, Zuberbier T, Metz M, et al: The definition, diagnostic
testing, and management of chronic inducible urticarias - The
EAACI/GA(2) LEN/EDF/UNEV consensus recommendations 2016 update and
revision. Allergy. 71:780–802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuberbier T, Aberer W, Asero R,
Bindslev-Jensen C, Brzoza Z, Canonica GW, Church MK, Ensina LF,
Giménez-Arnau A, Godse K, et al European Academy of Allergy
Clinical Immunology, Global Allergy Asthma European and Network
European Dermatology Forum; World Allergy Organization, : The
EAACI/GA(2) LEN/EDF/WAO Guideline for the definition,
classification, diagnosis, and management of urticaria: The 2013
revision and update. Allergy. 69:868–887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dressler C, Rosumeck S, Werner RN, Magerl
M, Metz M, Maurer M, Nast A and Zuberbier T: Executive summary of
the methods report for ‘The EAACI/GA2 LEN/EDF/WAO
Guideline for the Definition, Classification, Diagnosis and
Management of Urticaria. The 2017 Revision and Update’. Allergy.
73:1145–1146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saini S, Rosen KE, Hsieh HJ, Wong DA,
Conner E, Kaplan A, Spector S and Maurer M: A randomized,
placebo-controlled, dose-ranging study of single-dose omalizumab in
patients with H1-antihistamine-refractory chronic idiopathic
urticaria. J Allergy Clin Immunol. 128:567–573.e1. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maurer M, Metz M, Brehler R, Hillen U,
Jakob T, Mahler V, Pföhler C, Staubach P, Treudler R, Wedi B, et
al: Omalizumab treatment in patients with chronic inducible
urticaria: A systematic review of published evidence. J Allergy
Clin Immunol. 141:638–649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chicharro P, Rodríguez P and de Argila D:
Omalizumab in the treatment of chronic inducible urticaria. Actas
Dermosifiliogr. 108:423–431. 2017.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krause K, Ardelean E, Kessler B, Magerl M,
Metz M, Siebenhaar F, Weller K, Worm M, Zuberbier T and Maurer M:
Antihistamine-resistant urticaria factitia successfully treated
with anti-immunoglobulin E therapy. Allergy. 65:1494–1495. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Metz M, Ohanyan T, Church MK and Maurer M:
Omalizumab is an effective and rapidly acting therapy in
difficult-to-treat chronic urticaria: A retrospective clinical
analysis. J Dermatol Sci. 73:57–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uysal P, Eller E, Mortz CG and
Bindslev-Jensen C: An algorithm for treating chronic urticaria with
omalizumab: Dose interval should be individualized. J Allergy Clin
Immunol. 133:914–915. 2014. View Article : Google Scholar : PubMed/NCBI
|