Introduction
In some patients with liver injury, the liver
disease proceeds to acute liver failure (ALF), a life-threatening
systemic disorder characterized with severe coagulopathy and
encephalopathy (1). Currently, the
only effective therapy for ALF is liver transplantation (2,3).
Difficulties associated with the development of effective
treatments for ALF may be attributed to the incomplete
understanding of the mechanisms involved in disease
progression.
Intrahepatic microcirculatory disturbance is thought
to play a key role in the progression of ALF (4). Hepatic microcirculatory perfusion
failure is a determinant of liver dysfunction in warm
ischemia-reperfusion (5). Increased
fibrinogen catabolism and low platelet counts in ALF patients were
consistent with the involvement of hepatic hyper-coagulation
(6). Sinusoidal fibrin deposition in
ALF livers in patients and experimental models may support the
presence of intrahepatic coagulopathy, probably associated with
disturbed sinusoidal flow (6–9).
Hemodynamic study in ALF patients showed that blood inflow from
portal vein was mostly excreted directly into hepatic vein,
suggesting impaired parenchymal perfusion (10). Collectively, treatments to improve
the hepatic hyper-coagulation may be useful to attenuate liver
damage in ALF; however, suitable anticoagulant to treat ALF have
not been established (11,12).
Acetaminophen (APAP) is a widely used
analgesic/antipyretic drug with few side effects at therapeutic
doses (13). It is well known that
overdose of APAP causes liver injury via its metabolite
N-acetyl-p-benzoquinone imine (NAPQI) that induces direct
hepatocyte necrosis by oxidative stress and mitochondrial
dysfunction (14–16). N-acetyl cysteine (NAC) is a useful
antidote for APAP induced liver injury (AILI) via replenishing
intracellular glutathione (GSH), however, delayed administration of
NAC diminished its efficacy (17–20). In
addition, hepatic hyper-coagulation seems to be involved in the
pathogenesis of AILI (21).
Tissue-factor (TF) dependent activation of coagulatory system,
elevated concentration of PAI-1, and liver fibrin depositions
suggested the disturbance in local liver perfusion (22).
Thrombomodulin (TM) is a thrombin receptor expressed
on the surface of endothelial cells and plays a crucial role in
regulating the coagulation cascades via anticoagulant activity by
inhibiting thrombin and accelerating activated protein C (APC)
activity (23–25). In addition, TM binds and neutralizes
high-mobility group box 1 (HMGB1) released from necrotic cells,
dampening the inflammatory responses (26–28).
These features of TM have allowed the development of recombinant
soluble human TM alpha (rhTM) as an anticoagulant with low
frequency of hemorrhagic complications (29) and to treat disseminated intravascular
coagulation (DIC) with inflammatory reactions, such as sepsis
(30).
The features of TM tempted us to evaluate its
utility to treat acute liver injury accompanied with hepatic
hyper-coagulation. We administrated rhTM into a mouse model of AILI
and analyzed the efficacy to suppress liver damage.
Materials and methods
Chemicals
APAP was purchased from Sigma (St. Louis, MO). rhTM
was purchased from Asahi Kasei Pharma Co. Ltd. (Tokyo, Japan).
Animals
Eight-week-old male C57BL/6J mice weighing 20–25 g
were obtained from Japan SLC (Shizuoka, Japan). Mice were
maintained under controlled conditions with free access to standard
chow and water. All studies were performed in accordance with the
Guide for the Care and Use of Laboratory Animals (National
Institutes of Health) and approved by the Animal Care Committee of
Kyushu University. Totally 140 mice were used in this study. All
mice were fasted for 16 h before the experiments but allowed water
ad libitum. The weight of the animals at the time of sacrifice was
18–22 g. APAP (200 mg/kg body weight) dissolved in
phosphate-buffered solution (PBS) was injected intraperitoneally.
At the same time as APAP injection, rhTM (20 mg/kg body weight)
dissolved in saline was injected intraperitoneally (TM group). The
dose of rh™ was chosen with reference to the previous reports
(31–34) and our preliminary experiments.
Control animals underwent sham injections with saline (control
group). The mice in control and TM groups were sacrificed at 0, 2,
4, 24 and 48 h (n=10 at each time point/group) in this study. All
amimals were euthanized by sevoflurane at concentrations of 4–5%
for induction and 2–3% for maintenance, as described previously
(35,36). The depth of anesthesia was confirmed
by loss of the postural reaction and righting reflex (the pedal
withdrawal reflex in the forelimbs and hind limbs, the tail pinch
reflex, and the eyelid reflex). Blood samples were drawn from tail
vein or inferior vena cava and the livers were collected.
Approximately 700–1,200 µl of blood was extracted by
exsanguination. A combination of lack of pulse, breathing, corneal
reflex, and presence of rigor morits was used to confirm death. The
blood samples were centrifuged for 15 min at 3,000 rpm (1,500 × g)
at 4°C, and serum samples were collected and stored at −80°C. For
RNA isolation, liver samples were snap-frozen in liquid nitrogen
and stored at −80°C.
To evaluate hepatic glutathione (GSH) contents,
livers were excised to measure GSH contents using Total Glutathione
Quantification kit (Dojindo Molecular Technologies, Kumamoto,
Japan) according to the manufacturer's instructions at 0, 2, and 4
h after APAP injection (n=10 at each time point/group).
Biochemical analyses
Blood samples (200 µl at each time point) were taken
from tail vein or inferior vena cava at 24 and 48 h after the
injection of APAP (n=10 in each group). Serum levels of alanine
aminotransferase (AST), alanine aminotransferase (ALT), fibrin
degradation products (FDP), and HMGB1 were estimated by
Transaminase C-test (Wako Pure Chemical Industry, Osaka, Japan),
FDP-ELISA kit (MyBioSource, San Diego, CA, USA), and HMGB1-ELISA
kit (Shino-test, Japan). Serum levels of Total bilirubin (T.Bil)
and lactate dehydrogenase (LDH) were measured using chemical
analyzer Fuji-Drychem (Fuji Film, Tokyo, Japan). Platelet was
counted using an automated hematology analyzer (Sysmex XE-5000
hematology analyzer; Sysmex, Kobe, Japan) in EDTA-anticoagulated
blood samples. Prothrombin time (PT-INR) measurements were
performed on venous blood sample drawn from the tail vein by a
commercially available point-of-care coagulometer (CoaguChek XS;
Roche, Mannheim, Germany).
Histological examinations
Liver tissue samples were collected at 24 h after
APAP injection, fixed in 10% formalin, and embedded in paraffin
(n=10/group). Sections were stained with hematoxylin and eosin to
assess hepatic damage. Sinusoidal fibrin deposition was detected by
phosphotungstic acid-hematoxylin staining. The F4/80
immunohistochemical staining assays were performed.
Paraffin-embedded tissue sections were deparaffinized and
rehydrated. Antigen retrieval was performed with Proteinase K
(Dako, Carpinteria, CA, USA) treatment. Endogenous peroxidase
activity was blocked for 20 min with 3% hydrogen peroxide
(Sigma-Aldrich, St. Louis, MO, USA). After blocking with diluted
serum from the secondary antibody host for 30 min, the slides were
incubated overnight (4°C) with anti-F4/80 antibody (Bio-Rad,
Hercules, CA, USA; catalog no. MCA497; 1:1,000 dilution). Secondary
anitibody (Histofine Simple Stain Mouse MAX-PO Rat kit; Nichirei
Bioscience, Tokyo, Japan) was applied for 60 min at room
temperature and stained for 1–10 min with diaminobenzidine
tetrahydrochloride (Nichirei Bioscience). The sections were then
counterstained with hematoxylin (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), dehydrated, and mounted. The sections were
visualized under a Keyence BZ-X700 microscope (Keyence, Osaka,
Japan) at different magnifications (×200 magnification, Fig. 1; ×400 magnification, Fig. 2; and ×200 magnification, Fig. 3).
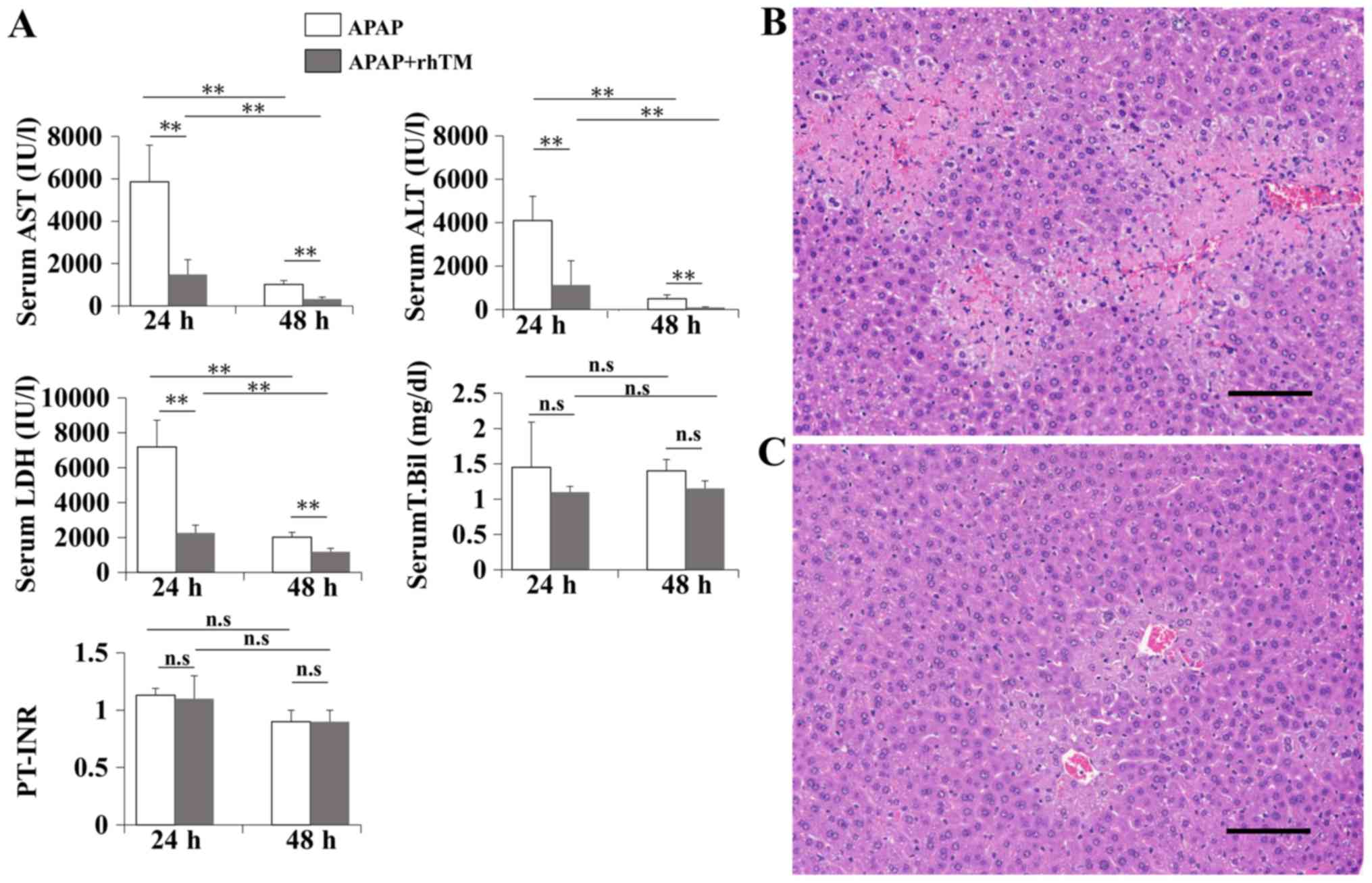 | Figure 1.rhTM suppresses liver damage in a
mouse AILI model. APAP was injected intraperitoneally into
8-week-old C57BL/6 mice. At the same time, rhTM (TM group) or
saline (control group) was injected intraperitoneally. Biochemical
examinations were performed at 24 and 48 h after APAP injection
(n=10 in each group at each time point). Histological examinations
(magnification, ×200) were performed at 24 h after the injection
(n=10 in each group). Scale bar=100 µm. (A) Serum AST, ALT, LDH,
T.Bil and PT-INR levels. Data are expressed as the mean ± SD.
*P<0.01. ns, non-significant. Hematoxylin and eosin staining of
liver sections. (B) control group, (C) TM group. rhTM, recombinant
human soluble thrombomodulin alpha; AILI, acetaminophen induced
liver disease; APAP, acetaminophen; AST, alanine aminotransferase;
ALT, alanine aminotransferase; LDH, lactate dehydrogenase; T.Bil,
total bilirubin; PT-INR, prothrombin time. |
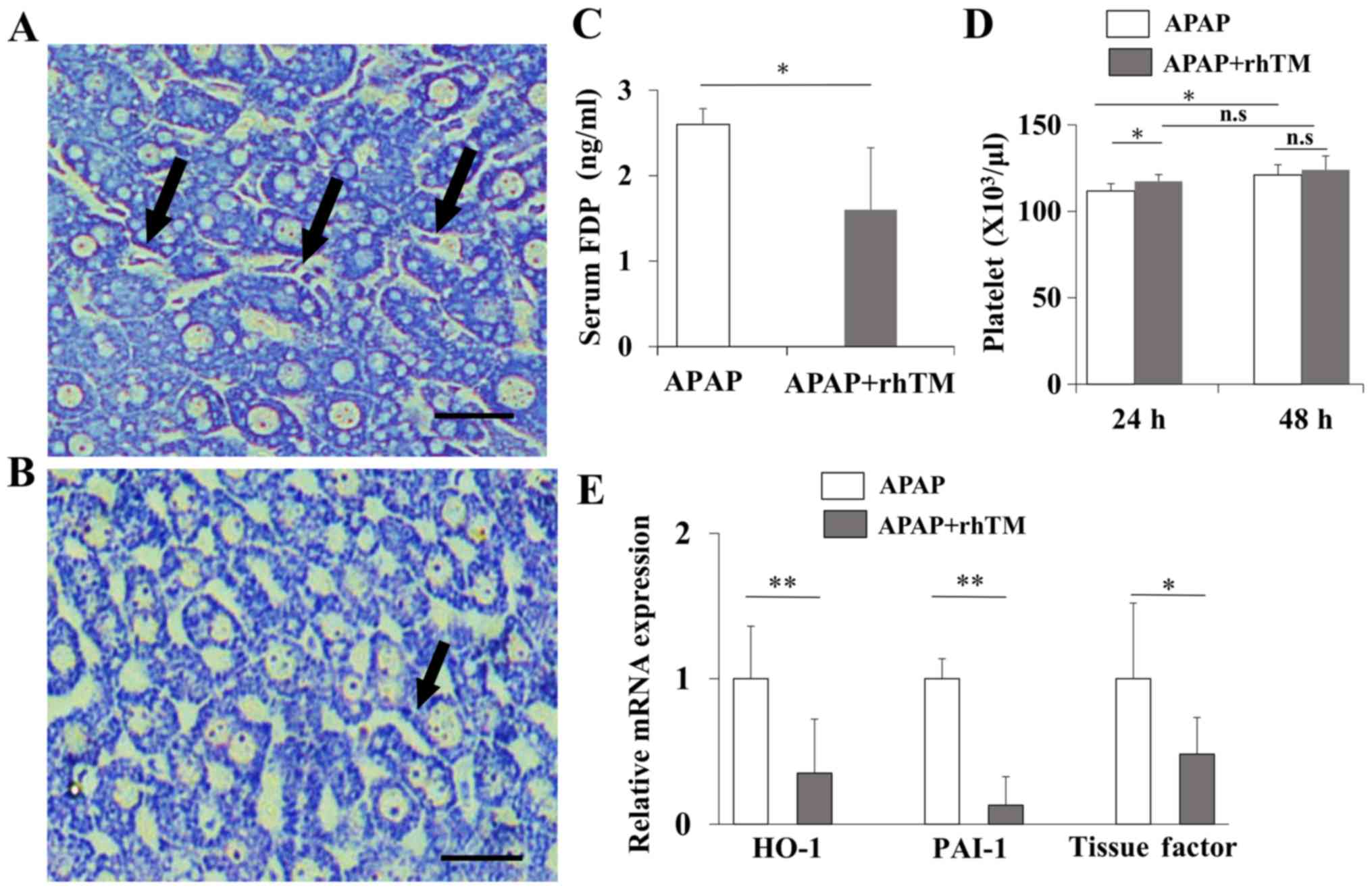 | Figure 2.rhTM improves intrahepatic
coagulopathy. Phosphotungstic acid-hematoxylin staining was
performed to detect the sinusoidal fibrin deposition induced by
sinusoidal coagulopathy (magnification, ×400). (A) Control group.
(B) TM group. The arrows indicate fibrin depositions in sinusoids.
Scale bar=25 µm). (C) Serum FDP levels were measured at 24 h after
APAP injection. (D) Platelet counts were measured at 24 and 48 h
after APAP injection (n=10 in each group at each time point). Data
are expressed as the mean ± SD (n=10 in each group). (E) Hepatic
expression levels of HO-1, PAI-1 and TF were quantified by RT-qPCR.
Data are expressed as the mean ± SEM (n=10 in each group).
*P<0.05, **P<0.01. ns, non-significant. rhTM, recombinant
human soluble thrombomodulin alpha; FDP, fibrin degradation
products; APAP, acetaminophen; HO-1, heme oxygenase-1; PAI-1,
plasminogen activator inhibitor type 1; TF, tissue factor. |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from liver tissue was prepared with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA was
synthesized by GeneAmp RNA PCR (Applied Biosystems; Thermo Fisher
Scientific, Inc.). RT-qPCR was performed using SYBR-Green on the
ABI 7500 real-time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The PCR reaction was carried out with a
denaturation step at 95°C for 30 sec, then 40 cycles at 95°C for 5
sec and finally at 60°C for 34 sec. To control for variations in
the reactions, all data were normalized to GAPDH expression.
Relative expression was presented using the 2−ΔΔCq
method (37). The primer sequences
are listed in Table I.
 | Table I.qPCR primer sequence. |
Table I.
qPCR primer sequence.
| Gene | Primer sequences
(5′-3′) |
|---|
| TNFα | F:
TATGGCTCAGGGTCCAACTC |
|
| R:
CTCCCTTTGCAGAACTCAGG |
| IL6 | F:
AGTTGCCTTCTTGGGACTGA |
|
| R:
TCCACGATTTCCCAGAGAAC |
| IFNγ | F:
ACTGGCAAAAGGATGGTGAC |
|
| R:
TGAGCTCATTGAATGCTTGG |
| HO-1 | F:
ACGCATATACCCGCTACCTG |
|
| R:
AAGGCGGTCTTAGCCTCTTC |
| PAI-1 | F:
TCTGGGAAAGGGTTCACTTTACC |
|
| R:
GACACGCCATAGGGAGAGAAG |
| TF | F:
TGCTTCTCGACCACAGACAC |
|
| R:
TAAAAACTTTGGGGCGTTTG |
Statistical analysis
Data were analyzed using JMP Pro Version 11
statistical software (SAS Institute, Inc. Cary, NC, USA). The
values of serum biomarkers (AST, ALT, LDH, T.Bil, FDP and HMGB1)
and PT-INR were expressed as the means and standard deviation (SD).
The results of hepatic mRNA expression were expressed as standard
error of the means (SEM). Significant differences between two
groups were assessed using the Mann-Whitney U-test. The differnces
of means among multiple groups were analyzed by using one-way ANOVA
and Tukey's post hoc test. A P-value <0.05 indicated statistical
significance.
Results
rhTM attenuates APAP induced
hepatotoxicity in mice
In preliminary experiment, we used 500 mg/kg of APAP
to induce AILI, but rhTM did not affect liver damages (data not
shown). Then we reduced APAP to 200 mg/kg and carried out the
following experiments. Elevated serum liver transaminases in the
control group indicated successful induction of AILI.
Administration of rhTM significantly decreased serum AST, ALT, and
LDH compared to those in the control group and this suppressive
effect of rhTM on liver damage continued for 48 h (Fig. 1A). We also evaluated serum levels of
T.Bil and PT-INR. The value remained in mostly normal range and
rhTM did not significantly affect the both levels of T.Bil (control
group vs TM group: 1.4±0.6 vs. 1.1±0.1 mg/dl, P=0.37) and PT-INR
(control group vs. TM group: 1.2±0.1 vs. 1.1±0.2 mg/dl, P=0.82) at
24 h after APAP injection (Fig. 1A).
T.Bil and PT-INR are recognized as useful markers to speculate the
prognosis of liver failure patients (38). However, elevations of theses factors
have not been obviously shown in APAP-induced ALF model mice
(39,40), suggesting that the experimental doses
of APAP may not disrupt these values. Histological examination
showed extensive hepatocellular necrosis and hemorrhage with modest
infiltration of inflammatory cells in the control group (Fig. 1B) and rhTM markedly reduced the
necrotic area and diminished the intralobular hemorrhage at 24 h
after APAP injection (Fig. 1C).
Previously, rhTM has been reported to exert its anticoagulant and
anti-inflammatory activity at a dose of 1–200 mg/kg (32–35). In
preliminary experiments, we evaluated the effects of rhTM on
APAP-induced liver injury at a dose of 10 mg/ml, however, rhTM in
this dose did not show significant suppression of liver damages
(data not shown). Then we tried rhTM at a dose of 20 mg/ml and
significant improving effects of rhTM in this dose allowed us to
continue to investigate this study. It is unclear why higher dose
was required in this study, however, we speculate that the
difference of animal models may affect the appropriate dose for
treatment.
rhTM improves intrahepatic
coagulopathy
Diffuse distribution of sinusoidal fibrin
depositions was observed in control liver and rhTM treatment mostly
extinguished the depositions (Fig. 2A
and B). Then we evaluated serum FDP levels. In ALF patients,
serum FDP was occasionally found elevated and considered to be
useful to speculate the extent of complicated coagulopathy
(8). As shown in Fig. 2C, rhTM significantly reduced serum
FDP compared with control group at 24 h after APAP injection.
Hemostatic alterations in liver disease with intrahepatic
coagulopathy occasionally reduces the platelet counts (41). rhTM improved platelet counts at 24 h
after APAP injection in TM group (117.3±4.0 vs. control group:
111.7±4.3×104/µl, P<0.05 Fig. 2D). In addition, rhTM treatment
significantly reduced the hepatic expressions of heme oxygenase-1
(HO-1), plasminogen activator inhibitor type 1 (PAI-1) and tissue
factor (TF), suggesting that rhTM suppressed the further
exacerbation of hepatic hyper-coagulation (Fig. 2E).
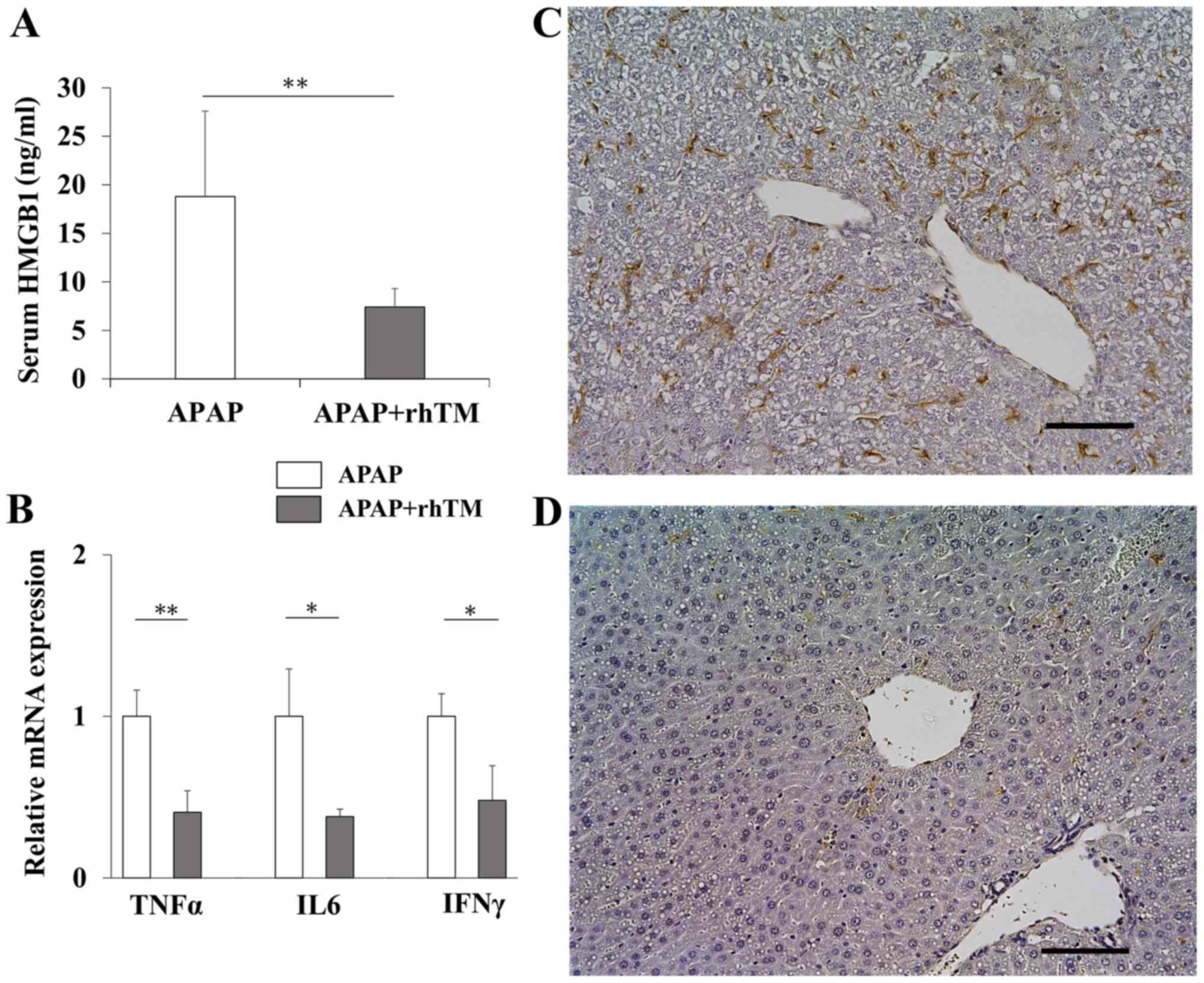
rhTM treatment suppresses inflammatory
reactions
As shown in Fig. 3A,
serum HMGB1 in the TM group was significantly lower than those in
the control group. To evaluate the induction of proinflammatory
activity, we quantified mRNA expressions of TNFα, IL6, and IFNγ.
rhTM treatment significantly reduced the expressions of all these
genes (Fig. 3B). We also estimated
hepatic macrophage accumulation by F4/80 immunostaining. In APAP
hepatotoxicity, resident hepatic macrophage, Kupffer cell, and
bone-marrow derived monocytes are activated to aggravate
inflammation and monocyte derived macrophage (MoMF) is involved in
the resolution of inflammation (42,43). As
shown in Fig. 3C, F4/80
immunostaining showed the abundant hepatic infiltration of
macrophages in control group, but mostly disappeared in TM group
(Fig. 3D).
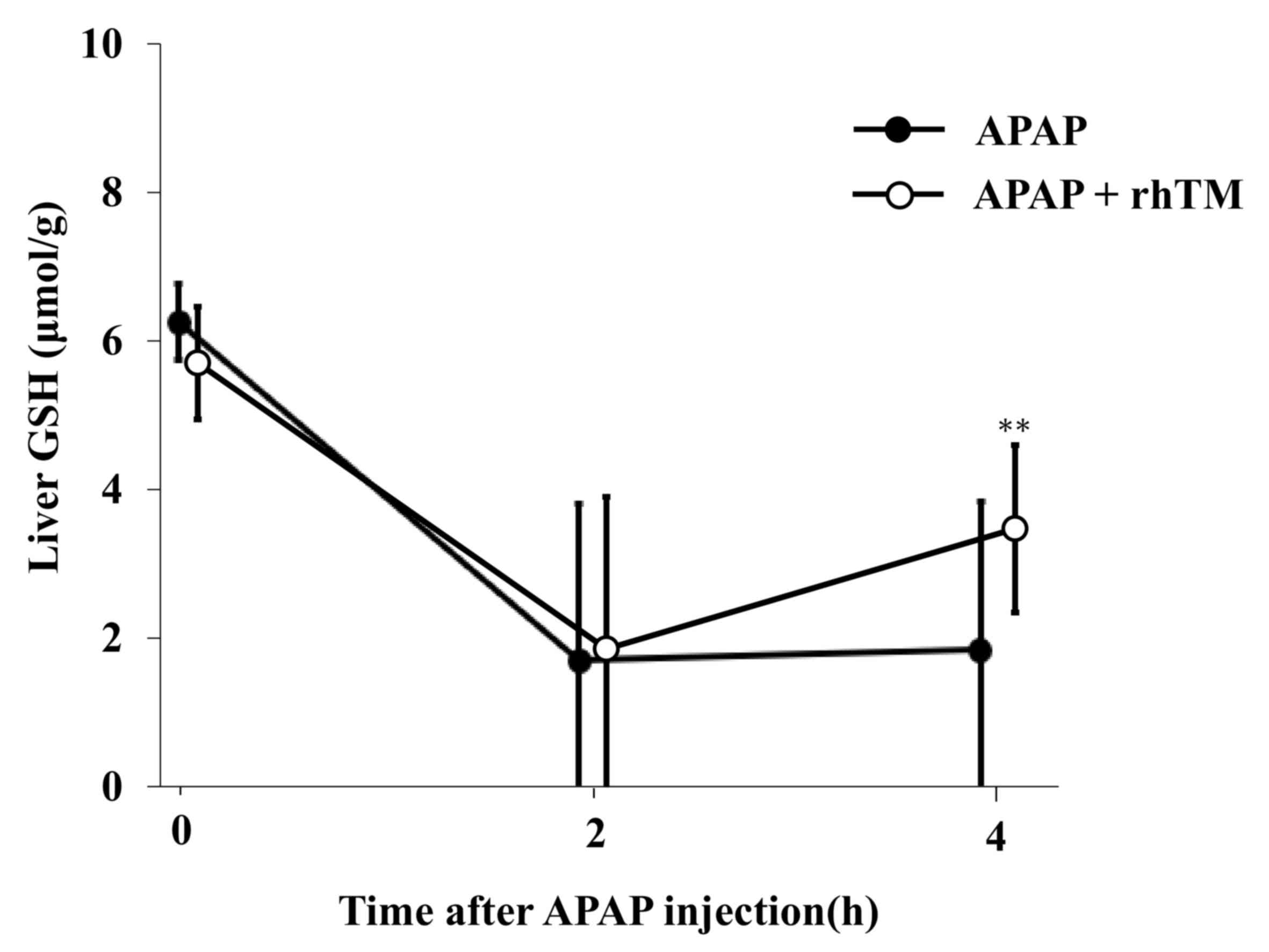
Temporal changes in liver GSH
contents
The APAP metabolite NAPQI induces hepatic GSH
depletion, resulting in hepatocyte necrosis (25). To evaluate the effect of rhTM on the
direct hepatotoxicity, we evaluated hepatic GSH contents. At 2 h
after APAP injection, hepatic GSH levels were equally decreased in
both groups (Fig. 4). At 4 h after
APAP injection, GSH contents in the TM group rose again with a
significant difference compared to those in the control group.
Discussion
The mechanism of APAP induced hepatotoxity involves
a oxidative stress and mitochondrial dysfunction via its metabolite
NAPQI (13,15,25). In
addition, recent studies indicated that APAP hepatotoxity is
accompanied by inflammation response (44,45).
Mediators released from necrotic hepatocyte stimulate Kupffer cell
and sinusoidal endothelial cell. These mediators activate hepatic
hyper-coagulation including fibrin deposition and exacerbate liver
injury in AILI (22). In clinical
cases, the characteristic finding in patients with AILI is the
distinct elevation of serum lactate dehydrogenase similar to
hypoxic hepatitis, suggesting that the liver could be in hypoxic
state associated with hepatic microcirculatory perfusion failure
(46).
Here, we showed that rhTM ameliorated APAP induced
liver damage in mice. rhTM suppressed serum ALT elevation and
reduced liver cell necrosis with decreased sinusoidal fibrin
deposition, probably induced by preserved liver perfusion.
In preliminary experiment, we used 500 mg/kg of APAP
to induce hepatotoxicity and rhTM did not fully suppress liver
damages. Then we reduced APAP into 200 mg/kg and rhTM significantly
improved liver damages. We speculate that the inefficiency of rhTM
on mice injected with 500 mg/kg of APAP might be attributable to
the strong direct hepatocyte necrosis proceeded independently of
hepatic hyper-coagulation and inflammation. The involvement of
inflammatory cell activations has been reported in AILI (43), liver injury induced by high-dose APAP
(400 mg/kg) could not be attenuated in TNF/lymphotoxin-alpha knock
out mice (47). The inefficiency of
blunting TNF signaling suggests that the inflammatory reaction
seems to be unnecessary in cell death in high-dose APAP. Treating
hepatic hyper-coagulation seems ineffective to suppress liver cell
necrosis in high-dose APAP induced liver damage.
In this study, treatment with rhTM was accompanied
with reduced sinusoidal fibrin deposition. Not only a result of
hemostatic disturbance, fibrin deposition may be a causal player in
acute liver injury (48). Fibrin
could be deposited as intravascular microthrombi, leading to
obstruct local liver perfusion, resulting in the secondary enhanced
liver injury. Thus, anticoagulants have been tried to diminish
tissue damages in various types of liver injury (11,49–51). In
AILI, pretreatment of heparin significantly attenuated liver injury
with diminished fibrin deposition (22). Collectively, we speculate that rhTM
might suppress liver damage via preserved liver perfusion by the
improvement in hepatic hyper-coagulation. The changes in liver GSH
contents may support this idea. We observed that liver GSH in the
TM group reduced equally at 2 h after APAP injection but rose again
with a significant difference at 4 h compared to those in the
control group. The reduction of GSH in the early stage suggests
that rhTM could not interfere the production of harmful metabolite.
Restoration of GSH contents at 4 h suggests that the preserved
liver perfusion might supply GSH to injured hepatocytes, preventing
further expansion of necrosis.
The role of rhTM in the prevention of secondary
liver damage is also suggested by the downregulations of hepatic
hyper-coagulation and inflammation related genes. HO-1 has been
shown as an important component of antioxidant defense in APAP
hepatotoxicity (52). PAI-1 and TF
released from damaged cells exacerbate coagulopathy by inhibiting
the anticoagulative cascades (53–55).
Thus the downregulations of PAI-1 and TF suggest that the
preservation of liver perfusion by rhTM improved the redox state in
diseased liver, thereby prevented secondary coagulopathy induced by
damaged tissue. In similar fashion, preserved liver perfusion by
rhTM likely reduced the release of damage-associated molecular
patterns (DAMPs), followed by the downregulations of
proinflammatory cytokines, resulting in the prevention of secondary
damages.
In the present study, the administration of rhTM
effectively suppressed liver damage in the APAP-induced ALF model,
probably by improving the intrahepatic coagulopathy. Because the
improving effects of rhTM on liver damage could be observed in
low-dose APAP intoxication, the utility of rhTM in AILI might be
limited. Treatment with NAC should be considered primarily,
however, rhTM might be useful to support the treatment in cases
with hepatic hyper-coagulation. Also, this study has a limitation
because the improving effects of rhTM on liver injury were shown
when rhTM was injected at the same time of APAP intoxication, which
unlikely happens in clinical situations. In addition, as an
anticoagulant, rhTM is known to increase the risk of bleeding. We
did not experience hemorrhagic complications in this study,
however, intensive attention for such adverse effects is necessary
in the cases with ALF. The involvement of anti-inflammatory
activity of rhTM in the suppression of AILI is still unclear,
further study would be helpful to understand the role of rhTM in
the suppression of AILI.
Acknowledgements
The authors would like to acknowledge the technical
assistance from The Research Support Center, Kyushu University
Graduate School of Medical Sciences. The authors would also like to
thank J. Ludovic Croxford, from the Edanz Group (www.edanzediting.com/ac) for editing a draft of
this manuscript.
Funding
This study was supported in part by the Takeda
Science Foundation.
Availability of data and materials
The dataset used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AK, MKo, MKa and YO designed the study. AK performed
experiments. HS, AY, TO, KI, MKu and YM assisted experiments and
data analyses. AK wrote the initial draft of the manuscript. MKo,
MKa and YO contributed to analysis and interpretation of data. MKo,
MKa and YO assisted in the preparation of the manuscript and
critically reviewed the manuscript. All authors approved the final
version of the manuscript and agreed to be accountable for all
aspects of the work.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Guide for the Care and Use of Laboratory Animals (National
Institutes of Health) and approved by the Animal Care Committee of
Kyushu University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fujiwara K, Mochida S, Matsui A, Nakayama
N, Nagoshi S and Toda G; Intractable Liver Diseases Study Group of
Japan, : Fulminant hepatitis and late onset hepatic failure in
Japan. Hepatol Res. 38:646–657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Starzl TE, Iwatsuki S, Van Thiel DH,
Gartner JC, Zitelli BJ, Malatack JJ, Schade RR, Shaw BW Jr, Hakala
TR, Rosenthal JT and Porter KA: Evolution of liver transplantation.
Hepatology. 2:614–636. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polson J and Lee WM; American Association
for the Study of Liver Disease, : AASLD position paper: The
management of acute liver failure. Hepatology. 41:1179–1197. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mochida S and Fujiwara K: Symposium on
clinical aspects in hepatitis virus infection. 2. Recent advances
in acute and fulminant hepatitis in Japan. Intern Med. 40:175–177.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vollmar B, Glasz J, Leiderer R, Post S and
Menger MD: Hepatic microcirculatory perfusion failure is a
determinant of liver dysfunction in warm ischemia-reperfusion. Am J
Pathol. 145:1421–1431. 1994.PubMed/NCBI
|
|
6
|
Hillenbrand P, Parbhoo SP, Jedrychowski A
and Sherlock S: Significance of intravascular coagulation and
fibrinolysis in acute hepatic failure. Gut. 15:83–88. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kotoh K, Kato M, Kohjima M, Tanaka M,
Miyazaki M, Nakamura K, Enjoji M, Nakamuta M and Takayanagi R:
Lactate dehydrogenase production in hepatocytes is increased at an
early stage of acute liver failure. Exp Ther Med. 2:195–199. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirata K, Ogata I, Ohta Y and Fujiwara K:
Hepatic sinusoidal cell destruction in the development of
intravascular coagulation in acute liver failure of rats. J Pathol.
158:157–165. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mochida S, Arai M, Ohno A, Yamanobe F,
Ishikawa K, Matsui A, Maruyama I, Kato H and Fujiwara K: Deranged
blood coagulation equilibrium as a factor of massive liver necrosis
following endotoxin administration in partially hepatectomized
rats. Hepatology. 29:1532–1540. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyazaki M, Kato M, Tanaka K, Tanaka M,
Takao S, Kohjima M, Enjoji M, Nakamuta M, Kotoh K and Takayanagi R:
Contrast-enhanced ultrasonography using Sonazoid to evaluate
changes in hepatic hemodynamics in acute liver injury. J
Gastroenterol Hepatol. 26:1749–1756. 2100. View Article : Google Scholar
|
|
11
|
Miyazaki M, Kato M, Tanaka K, Tanaka M,
Takao S, Kohjima M, Ito T, Enjoji M, Nakamuta M, Kotoh K and
Takayanagi R: Antithrombin III injection via the portal vein
suppresses liver damage. World J Gastroenterol. 18:1884–1891. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujiwara K, Okita K, Akamatsu K, Abe H,
Tameda Y, Sakai T, Inoue N, Kanai K, Aoki N and Oka H: Antithrombin
III concentrate in the treatment of fulminant hepatic failure.
Gastroenterol Jpn. 23:423–427. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Graham GG, Scott KF and Day RO:
Tolerability of paracetamol. Drug Saf. 28:227–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C and Martin BC: Trends in emergency
department visits attributable to acetaminophen overdoses in the
United States: 1993–2007. Pharmacoepidemiol Drug Saf. 20:810–818.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaeschke H and McGill MR: Cytochrome
P450-derived versus mitochondrial oxidant stress in acetaminophen
hepatotoxicity. Toxicol Lett. 235:216–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han D, Dara L, Win S, Than TA, Yuan L,
Abbasi SQ, Liu ZX and Kaplowitz N: Regulation of drug-induced liver
injury by signal transduction pathways: Critical role of
mitochondria. Trends Pharmacol Sci. 34:243–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
James LP, McCullough SS, Lamps LW and
Hinson JA: Effect of N-acetylcysteine on acetaminophen toxicity in
mice: Relationship to reactive nitrogen and cytokine formation.
Toxicol Sci. 75:458–467. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smilkstein MJ, Knapp GL, Kulig KW and
Rumack BH: Efficacy of oral N-acetylcysteine in the treatment of
acetaminophen overdose. Analysis of the national multicenter study
(1976 to 1985). N Engl J Med. 319:1557–1562. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koch DG, Speiser JL, Durkalski V, Fontana
RJ, Davern T, McGuire B, Stravitz RT, Larson AM, Liou I, Fix O, et
al: The natural history of severe acute liver injury. Am J
Gastroenterol. 112:1389–1396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michael Brown J, Ball JG, Wright MS, Van
Meter S and Valentovic MA: Novel protective mechanisms for
S-adenosyl-L-methionine against acetaminophen hepatotoxicity:
Improvement of key antioxidant enzymatic function. Toxicol Lett.
212:320–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
James LP, Wells E, Beard RH and Farrar HC:
Predictors of outcome after acetaminophen poisoning in children and
adolescents. J Pediatr. 140:522–526. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ganey PE, Luyendyk JP, Newport SW, Eagle
TM, Maddox JF, Mackman N and Roth RA: Role of the coagulation
system in acetaminophen-induced hepatotoxicity in mice. Hepatology.
46:1177–1186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Esmon CT: The interactions between
inflammation and coagulation. Br J Haematol. 131:417–430. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ikezoe T: Thrombomodulin/activated protein
C system in septic disseminated intravascular coagulation. J
Intensive Care. 3:12015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du K, Ramachandran A and Jaeschke H:
Oxidative stress during acetaminophen hepatotoxicity: Sources,
pathophysiological role and therapeutic potential. Redox Biol.
10:148–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen R, Hou W, Zhang Q, Kang R, Fan XG and
Tang D: Emerging role of high-mobility group box 1 (HMGB1) in liver
diseases. Mol Med. 19:357–366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okamoto T, Tanigami H, Suzuki K and
Shimaoka M: Thrombomodulin: A bifunctional modulator of
inflammation and coagulation in sepsis. Crit Care Res Pract.
2012:6145452012.PubMed/NCBI
|
|
28
|
Li YH, Kuo CH, Shi GY and Wu HL: The role
of thrombomodulin lectin-like domain in inflammation. J Biomed Sci.
19:342012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikezoe T: Pathogenesis of disseminated
intravascular coagulation in patients with acute promyelocytic
leukemia, and its treatment using recombinant human soluble
thrombomodulin. Int J Hematol. 100:27–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eguchi Y, Gando S, Ishikura H, Saitoh D,
Mimuro J, Takahashi H, Kitajima I, Tsuji H, Matsushita T, Tsujita
R, et al: Post-marketing surveillance data of thrombomodulin alfa:
Sub-analysis in patients with sepsis-induced disseminated
intravascular coagulation. J Intensive Care. 2:302014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Osumi W, Jin D, Imai Y, Tashiro K, Li ZL,
Otsuki Y, Maemura K, Komeda K, Hirokawa F, Hayashi M, et al:
Recombinant human soluble thrombomodulin improved
lipopolysaccharide/d-galactosamine-induced acute liver failure in
mice. J Pharmacol Sci. 129:233–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hagiwara S, Iwasaka H, Matsumoto S,
Hasegawa A, Yasuda N and Noguchi T: In vivo and in vitro effects of
the anticoagulant, thrombomodulin, on the inflammatory response in
rodent models. Shock. 33:282–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakamura K, Hatano E, Miyagawa-Hayashino
A, Okuno M, Koyama Y, Narita M, Seo S, Taura K and Uemoto S:
Soluble thrombomodulin attenuates sinusoidal obstruction syndrome
in rat through suppression of high mobility group box 1. Liver Int.
34:1473–1487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawasaki T, Okamoto K, Kawasaki C and Sata
T: Thrombomodulin improved liver injury, coagulopathy, and
mortality in an experimental heatstroke model in mice. Anesth
Analg. 118:956–963. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Soto-Montenegro ML, Vicente-Rodríguez M,
Pérez-García C, Gramage E, Desco M and Herradón G: Functional
neuroimaging of amphetamine-induced striatal neurotoxicity in the
pleiotrophin knockout mouse model. Neurosci Lett. 591:132–137.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mortensen MB, Nilsson L, Larsen TG,
Espeseth E, Bek M, Bjorklund MM, Hagensen MK, Wolff A, Gunnersen S,
Füchtbauer EM, et al: Prior renovascular hypertension does not
predispose to atherosclerosis in mice. Atherosclerosis.
249:157–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zaman MB, Hoti E, Qasim A, Maguire D,
McCormick PA, Hegarty JE, Geoghegan JG and Traynor O: MELD score as
a prognostic model for listing acute liver failure patients for
liver transplantation. Transplant Proc. 38:2097–2098. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mahrous Abdel Basset Ibrahim, Farooq Ahmed
Wani and Shaik Rahiman: Hepatoprotective effect of olive oil and
camel milk on acetaminophen-induced liver toxicity in mice. Int J
Med Sci Public Health. 6:186–194. 2017. View Article : Google Scholar
|
|
40
|
Saini SP, Zhang B, Niu Y, Jiang M, Gao J,
Zhai Y, Hoon Lee J, Uppal H, Tian H, Tortorici MA, et al:
Activation of liver X receptor increases acetaminophen clearance
and prevents its toxicity in mice. Hepatology. 54:2208–2217.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lisman T and Porte RJ: Rebalanced
hemostasis in patients with liver disease: Evidence and clinical
consequences. Blood. 116:878–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Choi DY, Ban JO, Kim SC and Hong JT: CCR5
knockout mice with C57BL6 background are resistant to
acetaminophen-mediated hepatotoxicity due to decreased macrophages
migration into the liver. Arch Toxicol. 89:211–220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krenkel O, Mossanen JC and Tacke F: Immune
mechanisms in acetaminophen-induced acute liver failure.
Hepatobiliary Surg Nutr. 3:331–343. 2014.PubMed/NCBI
|
|
44
|
Williams CD, Farhood A and Jaeschke H:
Role of caspase-1 and interleukin-1beta in acetaminophen-induced
hepatic inflammation and liver injury. Toxicol Appl Pharmacol.
247:169–178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maddox JF, Amuzie CJ, Li M, Newport SW,
Sparkenbaugh E, Cuff CF, Pestka JJ, Cantor GH, Roth RA and Ganey
PE: Bacterial- and viral-induced inflammation increases sensitivity
to acetaminophen hepatotoxicity. J Toxicol Environ Health A.
73:58–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cassidy WM and Reynolds TB: Serum lactic
dehydrogenase in the differential diagnosis of acute hepatocellular
injury. J Clin Gastroenterol. 19:118–121. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Boess F, Bopst M, Althaus R, Polsky S,
Cohen SD, Eugster HP and Boelsterli UA: Acetaminophen
hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene
knockout mice. Hepatology. 27:1021–1029. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kopec AK and Luyendyk JP: Role of fibrin
(ogen) in progression of liver disease: Guilt by association? Semin
Thromb Hemost. 42:397–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weerasinghe SV, Moons DS, Altshuler PJ,
Shah YM and Omary MB: Fibrinogen-γ proteolysis and solubility
dynamics during apoptotic mouse liver injury: Heparin prevents and
treats liver damage. Hepatology. 53:1323–1332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nagato M, Okamoto K, Abe Y, Higure A and
Yamaguchi K: Recombinant human soluble thrombomodulin decreases the
plasma high-mobility group box-1 protein levels, whereas improving
the acute liver injury and survival rates in experimental
endotoxemia. Crit Care Med. 37:2181–2186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fujiwara K, Ogata I, Ohta Y, Hirata K, Oka
Y, Yamada S, Sato Y, Masaki N and Oka H: Intravascular coagulation
in acute liver failure in rats and its treatment with antithrombin
III. Gut. 29:1103–1108. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chiu H, Brittingham JA and Laskin DL:
Differential induction of heme oxygenase-1 in macrophages and
hepatocytes during acetaminophen-induced hepatotoxicity in the rat:
Effects of hemin and biliverdin. Toxicol Appl Pharmacol.
181:106–115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
van der Poll T, Büller HR, ten Cate H,
Wortel CH, Bauer KA, van Deventer SJ, Hack CE, Sauerwein HP,
Rosenberg RD and ten Cate JW: Activation of coagulation after
administration of tumor necrosis factor to normal subjects. N Engl
J Med. 322:1622–1627. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sawdey MS and Loskutoff DJ: Regulation of
murine type 1 plasminogen activator inhibitor gene expression in
vivo. J Clin Investig. 88:1346–1353. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li W, Chen W, Xie M, Huang H, Su H, Han H,
Zhang D, Zhang Y, Yang X and Xu W: Fasudil inhibits tissue factor
and plasminogen activator inhibitor-1 secretion by peripheral blood
mononuclear cells in CAPD patients. Ren Fail. 38:1359–1363. 2016.
View Article : Google Scholar : PubMed/NCBI
|