Introduction
Linezolid is a unique synthetic antimicrobial agent
of the oxazolidinone class of antibiotics with activity against all
gram-positive microorganisms, including certain mycobacteria and
various gram-negative anaerobes (1–4). This
drug was approved by the Food and Drug Administration in April 2000
for the treatment of serious infections caused by gram-positive
microorganisms, including those with known multidrug resistance,
namely methicillin-resistant Staphylococcus aureus and
vancomycin-resistant Enterococcus faecium (4). In addition, linezolid has been used as
a second-line agent for treating tuberculosis (5–7) and
exhibited no cross-resistance with other anti-tuberculosis drugs
(8–10).
Pharmacokinetic studies of linezolid have been
previously performed (11–13). It has been indicated that linezolid
is used to treat patients with serious infections and in patients
that develop frequent shock (14–17). To
date, it has remained elusive whether the different types of shock
affect the pharmacokinetic parameters of linezolid. In the present
study, a population pharmacokinetic (PPK) analysis was performed to
explore whether the pharmacokinetics of linezolid differ among
patients with different types of shock or patients without shock
and to explore the potential influencing factors.
Methods
Patients and data collection
The data used in the present study were obtained
from clinical routine diagnostic examinations and treatments of
shock patients treated between January 2016 and August 2018 at the
Zhongda Hospital affiliated to Southeast University (Nanjing,
China). The clinical information available from the database was
retrospectively reviewed. The retrospective inclusion criteria were
as follows: Subjects (patients without shock, septic shock,
hemorrhagic shock, neurogenic shock and cardiogenic shock) treated
with linezolid (600 mg linezolid every 12 h, intravenously).
Regarding the different types of shock, the patients were treated
with different therapeutic regimens. In order to avoid the
influence of drug interactions from different therapeutic schemes
on linezolid pharmacokinetics, the following exclusion criteria
were set: Combined use of drugs, which may have affected the
pharmacokinetics of linezolid. Blood concentrations were obtained
from the records of therapeutic drug monitoring. The associated
clinical data were from medical log information. Prior to the
present study, the data (blood parameters and medical log
information) were already available, no organized collection or
biological detection was required, and the information was
retrospectively collated and analyzed. Using the already recorded
and available blood concentrations and medical log information, the
PPK model was built. The present study was approved by the Research
Ethics Committee of the Zhongda Hospital affiliated to the
Southeast University (Nanjing, China). The present study is a
retrospective study and the analysis was approved by the affiliated
ethics committee without any requirement for written informed
consent.
The medical information included the following
parameters: Sex, age, albumin (ALB), globulin (GLB), ALB/GLB (A/G),
alanine transaminase (ALT), aspartate transaminase (AST), serum
creatinine (SCR), urea, total protein (TP), total bile acid (TBA),
total bilibrubin (TBIL), hematocrit (HCT), hemoglobin (HGB), mean
corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin
concentration (MCHC). In addition, the platelet count (PLT) was
measured. Furthermore, the patient status (patients without shock,
septic shock, hemorrhagic shock, neurogenic shock and cardiogenic
shock) was also recorded.
PPK modeling
The data were analysed using the non-linear
mixed-effects model (NONMEM) computer program (version VII; ICON
Development Solutions). A one-compartment model with clearance (CL)
and volumes of distribution (V) was used to describe the
pharmacokinetic parameters of linezolid in the population of the
present study.
Random-effects model
The inter-individual variabilities were evaluated by
the exponential error model, according to the following
equation:
Pi=T(P) × exp (ηi) (a), where
Pi is the individual parameter value, T(P) is the
typical individual parameter value and ηi is the
symmetrical distribution, which includes zero-mean chance variables
with a variance.
The variabilities of the residual error variability
were estimated as follows:
OB=IP × (1+ε1) (b), where OB is the
observation and IP represents the individual predicted
concentration. ε1 represents the symmetrical
distribution, which includes zero-mean chance variables with a
variance.
Covariate model
The following two equations describe the correlation
of the parameters between continuous and categorical covariates,
respectively.
Pi=T(P) ×
(Covi/Covconstant)θ (c)
Pi=T(P) × (1 + θ × Covi) (d), where
Pi is the individual parameter value and T(P) is the
typical individual parameter value. θ is the estimated parameter
and Covi is the covariate of the i-th individual.
Covconstant was fixed at a value similar to the
population median of the covariate.
The potential covariates were sex, age, ALB, GLB,
A/G, ALT, AST, SCR, UREA, TP, TBA, TBIL, PLT, HCT, HGB, MCH and
MCHC levels, as well as the different types of shock. The
stepwise-way set-up covariate model and likelihood ratio were used
to compare the hierarchical models. The alterations in the
objective function values (OFV) were produced by covariate
inclusions and a decrease of OFV to >3.84 (P<0.05) was deemed
as the inclusion standard of the covariates into the basic model
(18,19). Following the establishment of a full
regression model, the assessment was performed by deleting
covariates from each parameter one by one in order to obtain the
final model. An increase in OFV to >6.64 (P<0.01) was
considered as a standard for significant associations in the final
model (18,19).
Model validation
The final model was evaluated using bootstrap, an
internal validation method, which was generated by repeated random
sampling with replacement from the raw database. The process was
performed by the software Wings for NONMEM, which performed 1,000
repetitions with different random sampling. The medians and
percentiles (2.5–97.5) from bootstrap consequences were used for
comparison of these values with those derived from the final model.
Visual inspection of routine diagnostic plots included observations
vs. individual predictions and absolute value of weighted residuals
(iWRES) vs. individual predictions. The prediction-corrected visual
predictive check (VPC) plots were used to assess the predictive
performance of the final model.
Results
Data collection
The data of 37 Chinese patients treated with
linezolid were analyzed, and their demographic and laboratory data
are provided in Table I. The
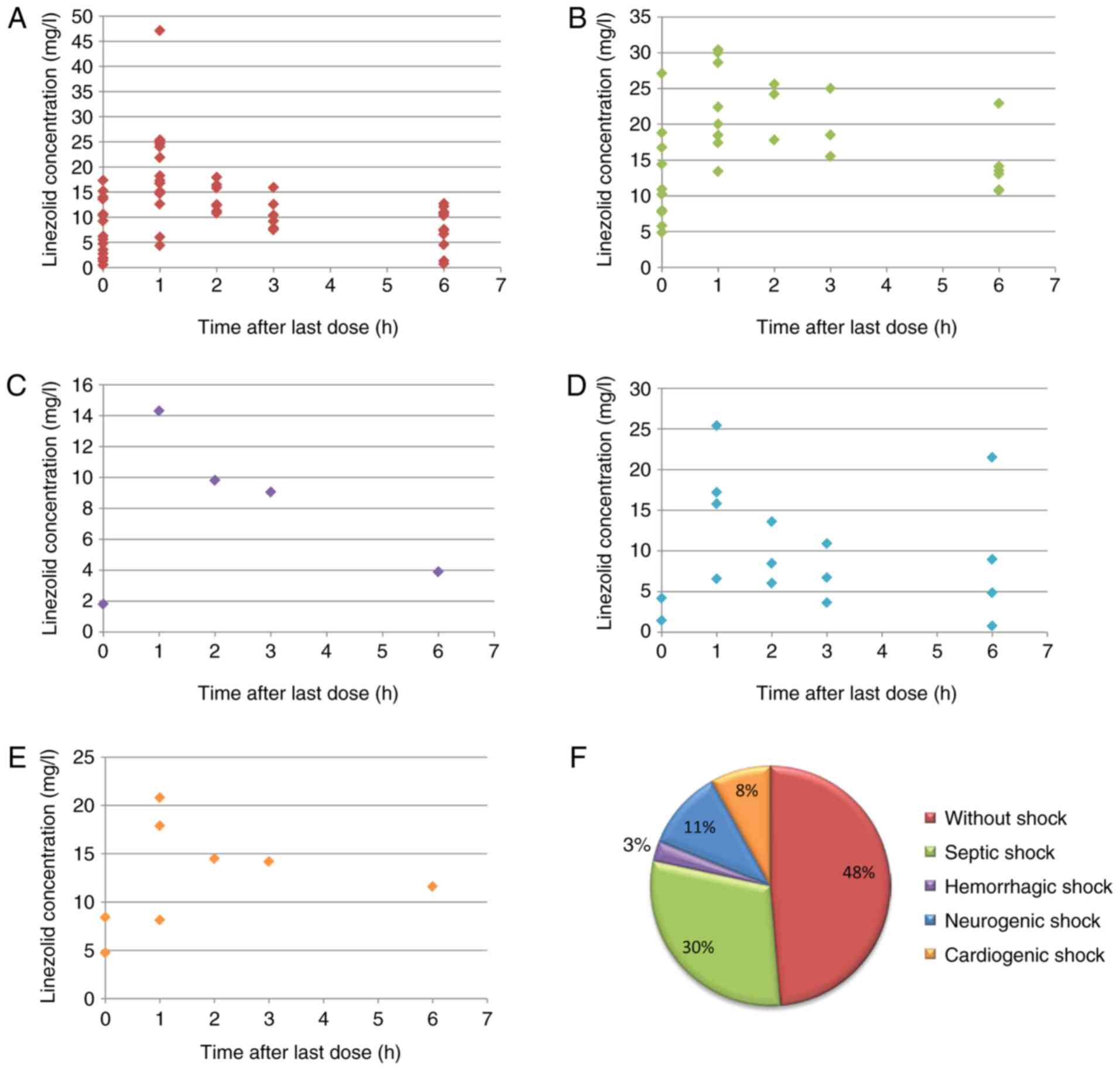
pharmacokinetic profiles of linezolid in patients with different
types of shock are provided in Fig.
1A-E. The results revealed that the concentration-time relation
from patients with different types of shock or patients without
shock demonstrated certain differences. However, whether the
differences were a result of patient status or other potential
influencing factors requires further study in future research. The
population included 18 patients without shock, 11 septic shock
patients, 1 hemorrhagic shock patient, 4 neurogenic shock patients
and 3 cardiogenic shock patients (Fig.
1F).
 | Table I.Demographic and laboratory data of the
patients (n=37). |
Table I.
Demographic and laboratory data of the
patients (n=37).
| Characteristic | Mean±SD | Median (range) |
|---|
| Sex
(male/female) | 27/10 | / |
| Age (years) | 59.49±16.25 | 62.00
(29.00–89.00) |
| Albumin (g/l) | 32.57±3.40 | 32.40
(25.40–43.80) |
| Globulin (g/l) | 30.50±5.87 | 30.10
(15.40–40.40) |
| Albumin/globulin | 1.12±0.34 | 1.01 (0.67–2.30) |
| Alanine transaminase
(IU/l) | 69.43±85.99 | 48.00
(3.00–525.00) |
| Aspartate
transaminase (IU/l) | 57.89±41.92 | 41.00
(11.00–181.00) |
| Serum creatinine
(µmol/l) | 126.03±113.64 | 85.00
(16.00–499.00) |
| Urea (mmol/l) | 12.03±6.30 | 11.10
(2.40–29.90) |
| Total protein
(g/l) | 62.76±7.00 | 62.80
(45.90–75.10) |
| Total bile acid
(µmol/l) | 7.20±13.06 | 3.80
(1.00–81.50) |
| Total bilibrubin
(µmol/l) | 25.04±65.96 | 11.10
(2.00–414.70) |
| Platelets
(109/l) | 246.54±187.20 | 213.00
(11.00–895.00) |
| Hematocrit (%) | 27.14±5.32 | 26.50
(19.00–44.80) |
| Hemoglobin
(g/l) | 89.84±17.68 | 87.00
(63.00–140.00) |
| Mean corpuscular
hemoglobin (pg) | 29.78±1.93 | 29.90
(25.30–35.00) |
| Mean corpuscular
hemoglobin concentration (g/l) | 321.84±16.45 | 321.00
(277.00–355.00) |
Modeling
All potential covariates were analysed in the
present study, and only the PLT on CL covariate exhibited
statistical significance. The changes in the OFV are presented in
Table II and the final model was
constructed as follows:
 | Table II.Change of objective function value of
covariate analysis. |
Table II.
Change of objective function value of
covariate analysis.
| Model
description | OFV | ΔOFV | P-value |
|---|
| Inclusion step
1 |
|
|
|
| Basic
model | 1080.738 | / | / |
|
Influence of sex on CL | 1075.476 | −5.262 | <0.05 |
|
Influence of age on CL | 1079.979 | −0.759 | >0.05 |
|
Influence of absence of shock
on CL | 1080.620 | −0.118 | >0.05 |
|
Influence of septic shock on
CL | 1078.700 | −2.038 | >0.05 |
|
Influence of hemorrhagic shock
on CL | 1079.133 | −1.605 | >0.05 |
|
Influence of neurogenic shock
on CL | 1080.613 | −0.125 | >0.05 |
|
Influence of cardiogenic shock
on CL | 1080.393 | −0.345 | >0.05 |
|
Influence of ALB on CL | 1078.277 | −2.461 | >0.05 |
|
Influence of GLB on CL | 1080.727 | −0.011 | >0.05 |
|
Influence of A/G on CL | 1080.354 | −0.384 | >0.05 |
|
Influence of ALT on CL | 1080.723 | −0.015 | >0.05 |
|
Influence of AST on CL | 1080.719 | −0.019 | >0.05 |
|
Influence of SCR on CL | 1080.727 | −0.011 | >0.05 |
|
Influence of urea on CL | 1080.508 | −0.230 | >0.05 |
|
Influence of TP on CL | 1080.678 | −0.060 | >0.05 |
|
Influence of TBA on CL | 1077.249 | −3.489 | >0.05 |
|
Influence of TBIL on CL | 1077.598 | −3.140 | >0.05 |
|
Influence of PLT on CL | 1069.637 | −11.101 | <0.05 |
|
Influence of HCT on CL | 1079.774 | −0.964 | >0.05 |
|
Influence of HGB on CL | 1080.241 | −0.497 | >0.05 |
|
Influence of MCH on CL | 1077.168 | −3.570 | >0.05 |
|
Influence of MCHC on CL | 1080.212 | −0.526 | >0.05 |
| Inclusion step
2 |
|
|
|
|
Influence of PLT on CL | 1069.637 | / | / |
|
Influence of PLT and sex on
CL | 1067.456 | −2.181 | >0.05 |
| Elimination |
|
|
|
| Full
model | 1069.637 | / | / |
|
Elimination of PLT on CL | 1080.738 | 11.101 | <0.01 |
CL=θCL × (PLT/200)^θPLT ×
ωCL (e) V=θV × ωV (f), where CL,
V, θCL, θV, θPLT, ωCL
and ωV are the clearance, volume of distribution,
typical value of CL, typical value of V, the coefficient of the
platelet, inter-individual variability of CL and inter-individual
variability of V, respectively. The platelet count was included in
the covariates and the clearance rate of linezolid was increased in
parallel with an increase in the platelet count.
Validation
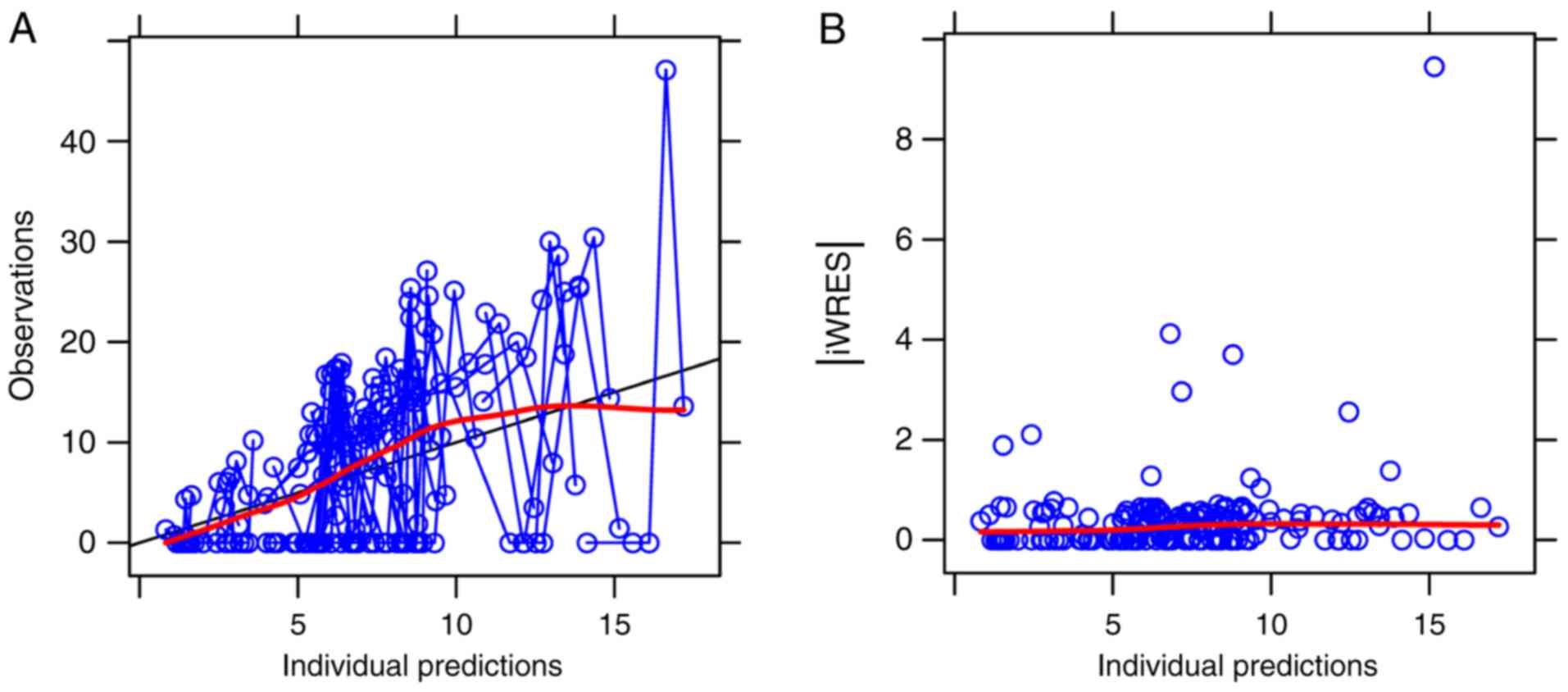
The routine diagnostic plots that were visually
inspected are provided in Fig. 2.
They included the following comparison of the variables:
Observations vs. individual predictions and iWRES vs. individual
predictions. The parameter estimates in the final model and
internal validation are provided in Table III. From 1,000 bootstrap runs, 992
runs were minimised with a successful covariance step and were
included in the bootstrap analysis. The bootstrap median values
were approximate to the estimate values in the final model, and the
absolute value of all bias was <6%, indicating that the final
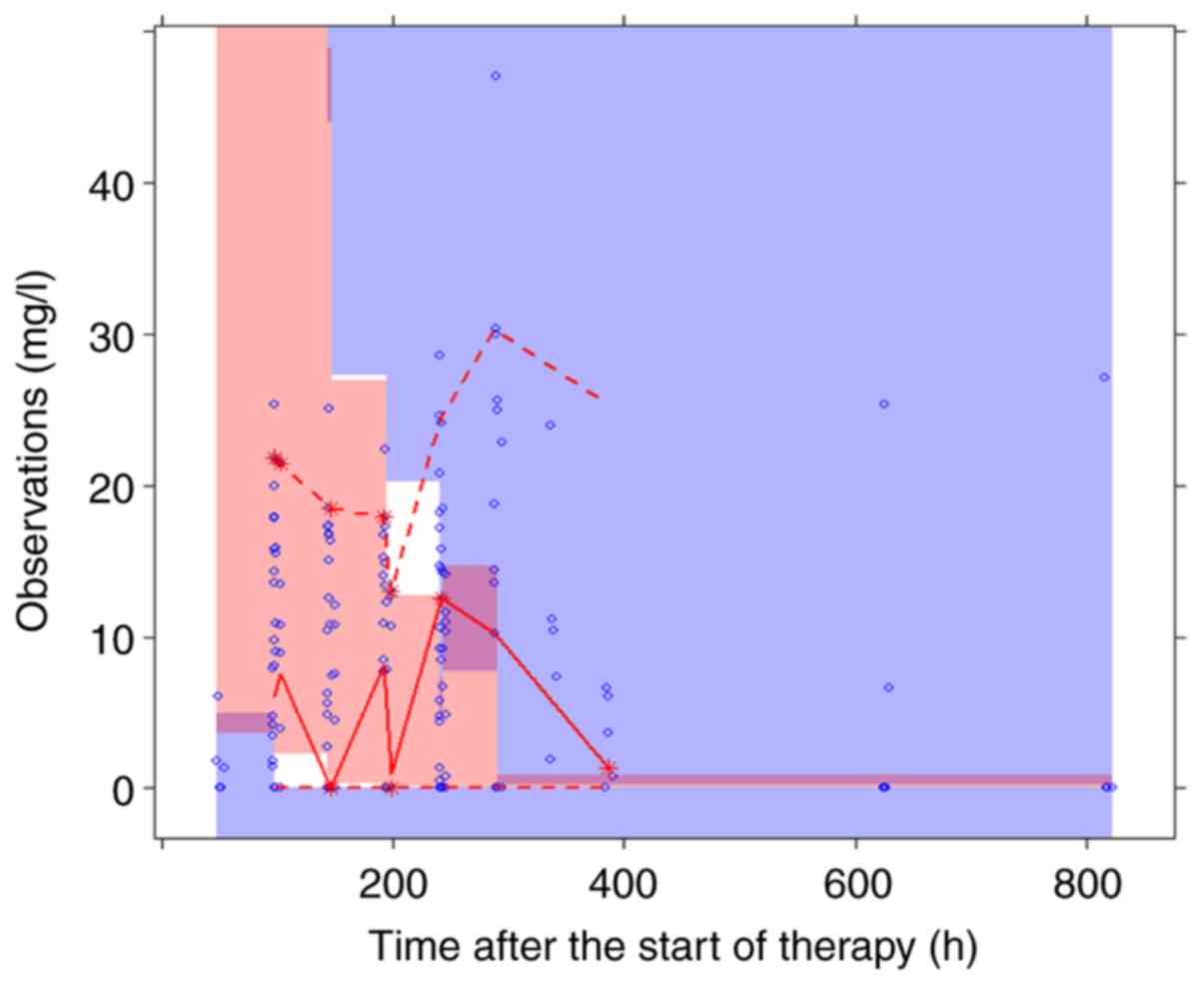
model was dependent on specific parameters. The VPC plots for the
final model are presented in Fig. 3,
revealing that the most frequently observed concentration data were
included in the 95% prediction intervals produced by the simulation
data. Overall, the data suggested that the final model was able to
predict drug concentrations with optimal efficiency.
 | Table III.Parameter estimates of final model
and bootstrap validation. |
Table III.
Parameter estimates of final model
and bootstrap validation.
|
|
|
| Bootstrap
(n=992) |
|
|---|
|
|
|
|
|
|
|---|
| Parameter | Estimate | SE | Median | 95% CI | Bias (%) |
|---|
| CL (l/h) | 11.8 | 0.23 | 11.2 | [3.110,
16.625] | −5.085 |
| V (l) | 209 | 0.20 | 197 | [53.850,
308.000] | −5.742 |
|
θPLT | 0.261 | 0.33 | 0.254 | [0.052, 0.425] | −2.682 |
| ωCL | 0.299 | 2.15 | 0.287 | [0.212, 0.359] | −4.013 |
| ωV | 0.299 | 2.15 | 0.287 | [0.211, 0.356] | −4.013 |
| σ1 | 1.020 | 0.08 | 1.015 | [0.864, 1.179] | −0.490 |
Discussion
Linezolid is currently used for the treatment of
severe infections caused by gram-positive microorganisms, including
vancomycin-resistant Enterococcus, vancomycin-sensitive
Enterococcus and Staphylococcus infections (20–22).
Since linezolid has a time-dependent activity, the percentage of
time during which plasma concentrations exceed the minimal
inhibitory concentration (MIC) represents its efficacy.
Furthermore, the area under the concentration-time curve (AUC) over
24 h may be divided by the MIC (AUC0-24/MIC) and used
for evaluating the pharmacokinetics of linezolid (23). A higher success rate was reported
when plasma concentrations remained above the MIC for the entire
dosing interval and when the AUC0-24/MIC values were
between 80 and 120 (24,25). This showcases the importance of
studying the pharmacokinetics of linezolid for evaluating its
efficacy. However, severe infections are frequently accompanied by
shock (14–17), and whether different types of shock
affect the pharmacokinetics of linezolid has remained elusive.
The application of PPK may provide useful
information from limited data of patients. Furthermore, PPK
analysis differentiates between inter-individual and
intra-individual variabilities. Therefore, PPK is more reliable for
confirming the effects of various factors with regard to
pharmacokinetic parameters compared with the traditional use of
pharmacokinetics (18,19,26–33). In
the present study, it was investigated whether the pharmacokinetics
of linezolid are different in patients with different types of
shock or patients without shock and the effects of several
potential confounders on its metabolic profile were assessed. Among
these groups, the population characteristics and biological
features were screened as covariates.
In the present study, the pharmacokinetics of
linezolid in patients with different types of shock or patients
without shock was described by a one-compartment model with the
parameters CL and V. The typical values of CL and V in the final
PPK model were 11.8 l/h and 209 liters, respectively. Plock et
al (34) reported on the
population pharmacokinetics of linezolid of 10 healthy volunteers
and 24 septic patients and the typical value of CL was estimated to
be 11.1 l/h, which was similar to the results obtained in the
present study. Of note, the present study determined that patients
with different types of shock or patients without shock exhibited
no differences in pharmacokinetics, suggesting that patient status
(patients without shock, septic shock, hemorrhagic shock,
neurogenic shock and cardiogenic shock) was not an influencing
factor. However, when the platelet count was included in the
covariates, the clearance rate of linezolid increased with the
increase of the platelet count. Therefore, the data suggested that
adjustment of a patient's linezolid regimen is possible, provided
that the platelet count is measured and the linezolid concentration
values are adjusted accordingly.
The present study has a limitation: The analysis and
patient inclusion were performed at a single center. Therefore,
further multicenter and prospective studies with a larger number of
patients are required.
In conclusion, the pharmacokinetics of linezolid
were not different among patients without shock, as well as
patients with septic shock, hemorrhagic shock, neurogenic shock and
cardiogenic shock. However, the platelet count significantly
influenced the clearance rate of linezolid.
Acknowledgements
Not applicable.
Funding
The current study was supported by AOSAIKANG
pharmaceutical foundation (grant no. A201826), The Young Medical
Talents of Wuxi (grant no. QNRC020), Young Project of Wuxi Health
and Family Planning Research (grant no. Q201706) and the Wuxi
Science and Technology Development Guidance Plan (medical and
health care; grant no. CSZON1744).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and DW conceived and designed the study. XZ
collected the data. DW, XZ and YY built the model. DW wrote the
manuscript. XZ and YY reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of the Zhongda Hospital affiliated to Southeast
University (Nanjing, China). The retrospective analysis was
approved by the ethics committee without the requirement for
written informed consent, since the data were collected without
patient identifiers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ashtekar DR, Costa-Periera R, Shrinivasan
T, Iyyer R, Vishvanathan N and Rittel W: Oxazolidinones, a new
class of synthetic antituberculosis agent. In vitro and in vivo
activities of DuP-721 against Mycobacterium tuberculosis. Diagn
Microbiol Infect Dis. 14:465–471. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jones RN, Johnson DM and Erwin ME: In
vitro antimicrobial activities and spectra of U-100592 and
U-100766, two novel fluorinated oxazolidinones. Antimicrob Agents
Chemother. 40:720–726. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaatz GW and Seo SM: In vitro activities
of oxazolidinone compounds U100592 and U100766 against
Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob
Agents Chemother. 40:799–801. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meagher AK, Forrest A, Rayner CR,
Birmingham MC and Schentag JJ: Population pharmacokinetics of
linezolid in patients treated in a compassionate-use program.
Antimicrob Agents Chemother. 47:548–553. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Condos R, Hadgiangelis N, Leibert E,
Jacquette G, Harkin T and Rom WN: Case series report of a
linezolid-containing regimen for extensively drug-resistant
tuberculosis. Chest. 134:187–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fortun J, Martin-Davila P, Navas E,
Perez-Elias MJ, Cobo J, Tato M, De la Pedrosa EG, Gomez-Mampaso E
and Moreno S: Linezolid for the treatment of multidrug-resistant
tuberculosis. J Antimicrob Chemother. 56:180–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park IN, Hong SB, Oh YM, Kim MN, Lim CM,
Lee SD, Koh Y, Kim WS, Kim DS, Kim WD and Shim TS: Efficacy and
tolerability of daily-half dose linezolid in patients with
intractable multidrug-resistant tuberculosis. J Antimicrob
Chemother. 58:701–704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cynamon MH, Klemens SP, Sharpe CA and
Chase S: Activities of several novel oxazolidinones against
mycobacterium tuberculosis in a murine model. Antimicrob Agents
Chemother. 43:1189–1191. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Molicotti P, Ortu S, Bua A, Cannas S,
Sechi LA and Zanetti S: In vitro efficacy of Linezolid on clinical
strains of Mycobacterium tuberculosis and other mycobacteria. New
Microbiol. 29:275–280. 2006.PubMed/NCBI
|
|
10
|
Sood R, Bhadauriya T, Rao M, Gautam R,
Malhotra S, Barman TK, Upadhyay DJ and Rattan A: Antimycobacterial
activities of oxazolidinones: A review. Infect Disord Drug Targets.
6:343–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keel RA, Schaeftlein A, Kloft C, Pope JS,
Knauft RF, Muhlebach M, Nicolau DP and Kuti JL: Pharmacokinetics of
intravenous and oral linezolid in adults with cystic fibrosis.
Antimicrob Agents Chemother. 55:3393–3398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sazdanovic P, Jankovic SM, Kostic M,
Dimitrijevic A and Stefanovic S: Pharmacokinetics of linezolid in
critically ill patients. Expert Opin Drug Metab Toxicol.
12:595–600. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buerger C, Plock N, Dehghanyar P,
Joukhadar C and Kloft C: Pharmacokinetics of unbound linezolid in
plasma and tissue interstitium of critically ill patients after
multiple dosing using microdialysis. Antimicrob Agents Chemother.
50:2455–2463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calzada Y, Jordan I, Vila-Perez D, Cambra
FJ and Munoz-Almagro C: Pleuropneumonia and septic shock due to
multiresistant Streptococcus pneumoniae serotype 19A treated with
linezolid. An Pediatr (Barc). 81:e22–23. 2014.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Bels D, Garcia-Filoso A, Jeanmaire M,
Preseau T, Miendje Deyi VY and Devriendt J: Successful treatment
with linezolid of septic shock secondary to methicillin-resistant
Staphylococcus aureus arthritis. J Antimicrob Chemother.
55:812–813. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rac H, Bojikian KD, Lucar J and Barber KE:
Successful treatment of necrotizing fasciitis and streptococcal
toxic shock syndrome with the addition of linezolid. Case Rep
Infect Dis. 2017:57207082017.PubMed/NCBI
|
|
17
|
Stevens DL, Wallace RJ, Hamilton SM and
Bryant AE: Successful treatment of staphylococcal toxic shock
syndrome with linezolid: A case report and in vitro evaluation of
the production of toxic shock syndrome toxin type 1 in the presence
of antibiotics. Clin Infect Dis. 42:729–730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang DD, Lu JM, Li Q and Li ZP: Population
pharmacokinetics of tacrolimus in paediatric systemic lupus
erythematosus based on real-world study. J Clin Pharm Ther.
43:476–483. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vadcharavivad S, Praisuwan S,
Techawathanawanna N, Treyaprasert W and Avihingsanon Y: Population
pharmacokinetics of tacrolimus in Thai kidney transplant patients:
Comparison with similar data from other populations. J Clin Pharm
Ther. 41:310–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sahuquillo Arce JM, Colombo Gainza E, Gil
Brusola A, Ortiz Estevez R, Canton E and Gobernado M: In vitro
activity of linezolid in combination with doxycycline, fosfomycin,
levofloxacin, rifampicin and vancomycin against
methicillin-susceptible Staphylococcus aureus. Rev Esp Quimioter.
19:252–257. 2006.PubMed/NCBI
|
|
21
|
Sweeney MT and Zurenko GE: In vitro
activities of linezolid combined with other antimicrobial agents
against Staphylococci, Enterococci, Pneumococci, and selected
gram-negative organisms. Antimicrob Agents Chemother. 47:1902–1906.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beitdaghar M, Ahmadrajabi R, Karmostaji A
and Saffari F: In vitro activity of linezolid alone and combined
with other antibiotics against clinical enterococcal isolates. Wien
Med Wochenschr. 169:215–221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Craig WA: Basic pharmacodynamics of
antibacterials with clinical applications to the use of
beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North
Am. 17:479–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roger C, Muller L, Wallis SC, Louart B,
Saissi G, Lipman J, Lefrant JY and Roberts JA: Population
pharmacokinetics of linezolid in critically ill patients on renal
replacement therapy: Comparison of equal doses in continuous
venovenous haemofiltration and continuous venovenous
haemodiafiltration. J Antimicrob Chemother. 71:464–470. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rayner CR, Forrest A, Meagher AK,
Birmingham MC and Schentag JJ: Clinical pharmacodynamics of
linezolid in seriously ill patients treated in a compassionate use
programme. Clin Pharmacokinet. 42:1411–1423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang DD, Lu JM, Li YZ, Li Q and Li ZP:
Population pharmacokinetics of sirolimus in pediatric tuberous
sclerosis complex: From real world study. Int J Clin Exp Med.
11:12302–12309. 2018.
|
|
27
|
Chen Y, Wu D, Dong M, Zhu Y, Lu J, Li X,
Chen C and Li Z: Population pharmacokinetics of vancomycin and
AUC-guided dosing in Chinese neonates and young infants. Eur J Clin
Pharmacol. 74:921–930. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gong Y, Chen Y, Li Q and Li Z: Population
pharmacokinetic analysis of digoxin in Chinese neonates and
infants. J Pharmacol Sci. 125:142–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Chen Y, Li Q, Cao D, Shi W, Cao Y,
Wu D, Zhu Y, Wang Y and Chen C: Population pharmacokinetics of
piperacillin/tazobactam in neonates and young infants. Eur J Clin
Pharmacol. 69:1223–1233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andrews LM, Hesselink DA, van Gelder T,
Koch BC, Cornelissen EA, Bruggemann RJ, van Schaik RH, de Wildt SN,
Cransberg K and de Winter BC: A population pharmacokinetic model to
predict the individual starting dose of tacrolimus following
pediatric renal transplantation. Clin Pharmacokinet. 57:475–489.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu ZC, Zhou PJ, Wang XH, Francoise B, Xu
D, Zhang WX and Chen B: Population pharmacokinetics and Bayesian
estimation of mycophenolic acid concentrations in Chinese adult
renal transplant recipients. Acta Pharmacol Sin. 38:1566–1579.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen B, Shi HQ, Liu XX, Zhang WX, Lu JQ,
Xu BM and Chen H: Population pharmacokinetics and Bayesian
estimation of tacrolimus exposure in Chinese liver transplant
patients. J Clin Pharm Ther. 42:679–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chevillard L, Sabo N, Tod M, Labat L,
Chasport C, Chevaleyre C, Thibaut F, Barre J, Azuar J, Questel F,
et al: Population pharmacokinetics of oral baclofen at steady-state
in alcoholic-dependent adult patients. Fundam Clin Pharmacol.
32:239–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Plock N, Buerger C, Joukhadar C, Kljucar S
and Kloft C: Does linezolid inhibit its own metabolism? Population
pharmacokinetics as a tool to explain the observed nonlinearity in
both healthy volunteers and septic patients. Drug Metab Dispos.
35:1816–1823. 2007. View Article : Google Scholar : PubMed/NCBI
|