Introduction
Osteosarcoma is histologically defined as abnormal
osteoid arising from malignant spindle tumor cells. This condition
may arise from bone marrow mesenchymal stem cells (BMSC), a type of
pluripotent stem cell, during their differentiation into mature
osteoblasts (1). BMSCs differentiate
into a variety of mature cells, including bone cells (osteoclasts
and osteoblasts), chondrocytes and adipocytes, and possess the
ability to renew, differentiate and be involved in angiogenesis
(2). Due to the unique properties of
BMSCs, including easy isolation, in vitro amplification,
differentiation and immune tolerance (3), this cell type has therapeutic potential
in vascularization and tissue repair. Therefore, BMSCs are widely
used in cell therapy clinical trials (4,5) and
pre-clinical studies (6,7). In addition, the prospect of
applications in alternative therapy is broad. However, abnormal
BMSC differentiation and uncontrolled proliferation may lead to the
development of osteosarcoma (8).
The Notch signaling pathway serves an important role
in the regulation of cell fate during embryonic and postnatal
development and is involved in regulating the differentiation of
adult stem cells (9,10). It is a conserved cell signaling
mechanism found in most multicellular organisms (11). Once a Notch receptor binds with
ligands on the surface of adjacent cells, the intracellular domain
dislocates from the cell membrane, transports into the nucleus and
interacts with downstream molecules to regulate the Notch cascade
(12). The Notch pathway also
mediates intercellular signaling, thus affecting endothelial cell
proliferation, survival and differentiation (13). The Notch signaling pathway serves a
vital role in angiogenesis, and changes in the pathway lead to the
abnormal development of blood vessels (13). Previous studies have reported that
the Notch signaling pathway serves an important role in stem cell
proliferation and angiogenesis (14).
However, whether this pathway is involved in the
malignant transformation of BMSCs remains to be elucidated. The
present study aimed to investigate how the Notch signaling pathway
influences BMSC differentiation and malignant transformation. The
study also aimed to provide a theoretical basis for understanding
the pathophysiologic mechanism of osteosarcoma in detail and
provide a foundation for the eventual incorporation of MSCs into
tissue engineering and clinical application.
Materials and methods
Cell culture
A BMSC cell line was gifted by Professor Yang Xiang
(Human Aging Research Institute, Nanchang University, Nanchang,
China) and cultured in GlutaMAXTM-1 Dulbecco's modified Eagle's
medium (DMEM; cat. no. 1859228; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences) in 5% CO2. at 37°C. The cells
were divided into the following five groups: Control, vector, small
interfering RNA (siRNA)-Notch1, γ-secretase inhibitor (DAPT;
Selleck Chemicals), and siRNA Notch1 + γ-secretase inhibitor
groups. Cells (5×103/well) at 80% confluence were
transfected with 1 µg/ml empty vector (F: 5-CCUACGCCACCAAUUUCGU-3′,
R:5-ACGAAAUUGGUGGCGUAGG-3) or Notch1-siRNA (F:
5-GCACGCGGAUUAAUUUGCA-3′, R: 5-UGCAAAUUAAUCCGCGUGC-3′; GenePharma
Co. Ltd.) using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.). After 6 h, the medium was replaced with
fresh DMEM containing 10% FBS and cultured in a 5% CO2.
incubator at 37°C for 48 h. The cells (5×103/well) were
then cultured in normal medium and treated with 5 µM DAPT for an
additional 24 h. Cell proliferation was confirmed and tumor-related
proteins were detected.
Cell counting Kit-8 (CCK-8) assay
The cells (3×103/ml) were seeded in
96-well plates. Following experimental treatment, 10 µl DMEM with
CCK-8 (cat. no. KGA317; Jiangsu KeyGen Biotech Co., Ltd., Nanjing,
China) was added. The cells were incubated for an additional 4 h in
a CO2. incubator at 37°C and the absorbance at 560 nm
was recorded using a microplate reader (Thermo Fisher Scientific,
Inc.). The optical density (OD) values measured represented cell
viability.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total mRNA was extracted using a TRIzol assay kit
(Baosheng Science & Technology Innovation Co, Ltd.). The mRNA
was transcribed into cDNA using a TakaraReverse Transcription kit
according to the manufacturer's protocol (cat. no. RR037A; Takara
Biotechnology Co., Ltd.). RT-qPCR analysis was performed to detect
the expression level of target genes using SYBR Green. The
amplification reactions were performed with initial denaturation at
95°C for 10 min, followed by 38 cycles of two-step PCR at 95°C for
17 sec and 61°C for 1 min. The levels of Notch1, TGF-β1, c-Myc and
p53 were normalized to those of GAPDH using the 2−ΔΔCq
method (15). The primers were
designed by Sangon Biotech and were as follows: Notch1, forward (F)
5-CGAAGTGGACATTGACGAGT-3′ and Notch1, reverse (R)
5-GGCATAAGCAGAGGTAGGAGT-3′; TGF-β1, F 5-CCTGTCCAAACTAAGGCTCG-3′ and
TGF-β1, R 5-ATGGCGTTGTTGCGGTC-3′; c-Myc, F 5-GCTCGCCCAAATCCTGT-3′
and c-Myc, R 5-TCTTCCTCATCTTCTTGCTCTT-3′; p53, F
5-TGGAGGAGTCACAGTCGGA-3′ and p53, R 5-CCATAGTTGCCCTGGTAAGTT-3′;
GAPDH, F 5-CCTGGAAGATGGTGATGGG-3′ and GAPDH, R
5-GAAGGTCGGAGTCAACGGAT-3′.
Western blot analysis
Proteins were extracted from the treated cells using
a protein isolation kit (cat. no. 28-9425-44; ReadyPrep; GE
Healthcare Life Sciences) and the protein levels were quantified
using a bicinchoninic acid protein assay kit. The protein (25
µg/lane) was ran on sodium-dodecyl sulfate-polyacrylamide gels
(10%) and then transferred onto nitrocellulose membranes. The
membranes were blocked with 5% skim milk for 2 h at room
temperature. The following primary antibodies were applied for
overnight incubation at 4°C: Anti-TGF-β1 (cat. no. ab92486),
anti-c-Myc (cat. no. ab39688), anti-p53 (cat. no. ab131442),
anti-Notch1 (cat. no. ab65297; all 1:1,000; Abcam) and anti-GAPDH
(cat. no. TA-08; 1:2,000; OriGene Technologies, Inc.). Following
washing with PBST (0.2% Tween-20), the membranes were incubated
with a secondary antibody (cat. no. ab131368; 1:100; Abcam) for 2 h
at room temperature. An enhanced chemiluminescence kit (cat. no.
RJ239676; Thermo Fisher Scientific, Inc.) was used and applied to
the membrane prior to visualization with ChemiDoc™ XRS (Bio-Rad
Laboratories, Inc.). Densitometry was performed using Quantity One
v1.4.6 (Bio-Rad Laboratories, Inc.).
Alizarin red S staining
Osteogenesis was induced using osteogenic
differentiation medium (cat. no. MUCMX-90021, Cyagen Biosciences,
Inc.) 24 h following transfection with Notch1 siRNA or treatment
with γ-secretase inhibitor. Osteogenesis was visualized with
alizarin red staining at 14 and 21 days. Following the removal of
medium, 1X PBS (pH 7.2, without calcium or magnesium) was used to
the wash cells, which were then fixed using 4% polyformaldehyde for
15 min at room temperature. Alizarin red dye was added for staining
of the cells at 37°C for 60 min. Images were subsequently captured
under light microscopy. Grey intensity was analyzed using
ImageProPlus 6.0 (National Institutes of Health). Four fields in
each section were analyzed. The intensity was normalized to that in
the control group.
Measurement of alkaline phosphatase
(AKP) activity
AKP activity was detected 21 days following the
induction of osteogenesis using an AKP assay kit according to the
manufacturer's instructions (cat. no. A059-2, Nanjing Jiancheng
Bioengineering Institute). The AKP content was determined according
to the following formula: AKP activity in culture medium (KU/100
ml)=(OD value-Blank OD value)/(OD value-Blank OD value) × phenol
standard concentration (0.02 mg/ml) ×100 ml × sample dilution prior
to determination.
Statistical analysis
Data are presented as the mean ± SEM. Statistical
significance was assessed by one-way ANOVA with Newman-Keuls
post-test (SPSS 17.0; SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Notch1 inhibition reduces BMSC
viability
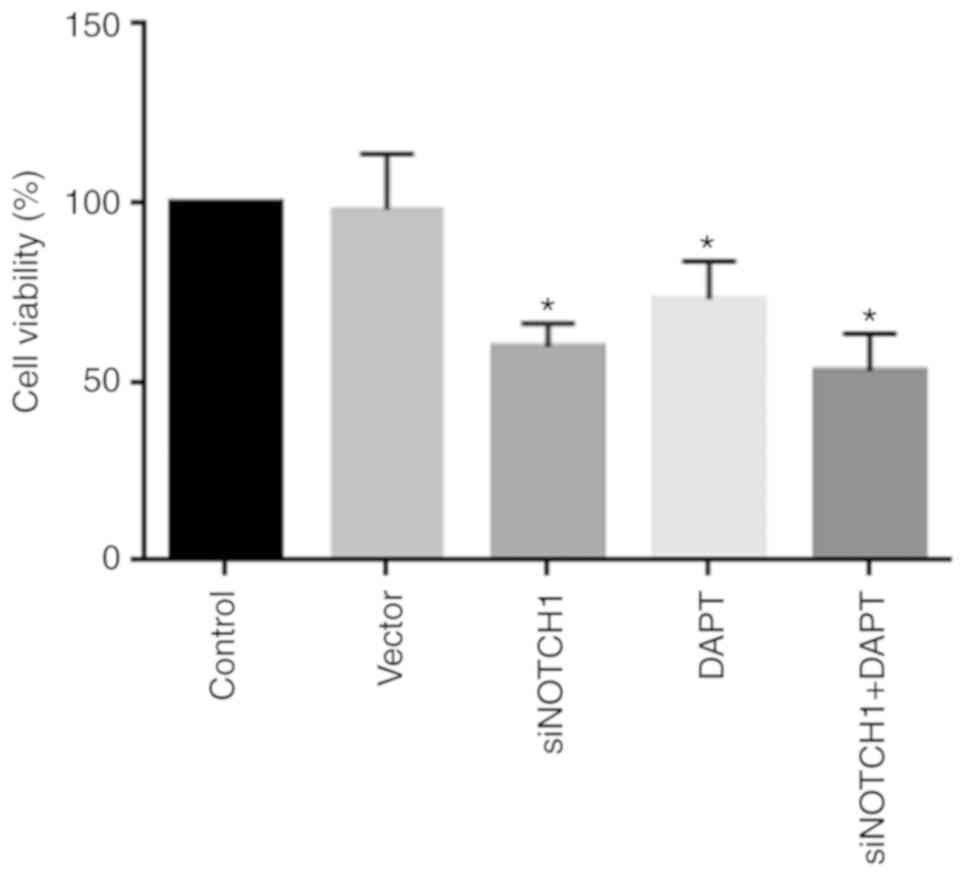
As shown in Fig. 1,
siRNA-Notch1 and γ-secretase inhibitor treatment significantly
reduced MSC viability compared with that in the control
(P<0.05). Of note, combined siRNA-Notch1 and γ-secretase
inhibitor treatment did not reduce cell viability to a greater
extent than single siRNA-Notch1 or γ-secretase inhibitor treatment,
indicating the specific effect of Notch1 inhibition by siRNA-Notch1
or the γ-secretase inhibitor.
Notch1 inhibition reduces the
expression of Notch1, TGF-β1 and c-Myc, and promotes the expression
of p53
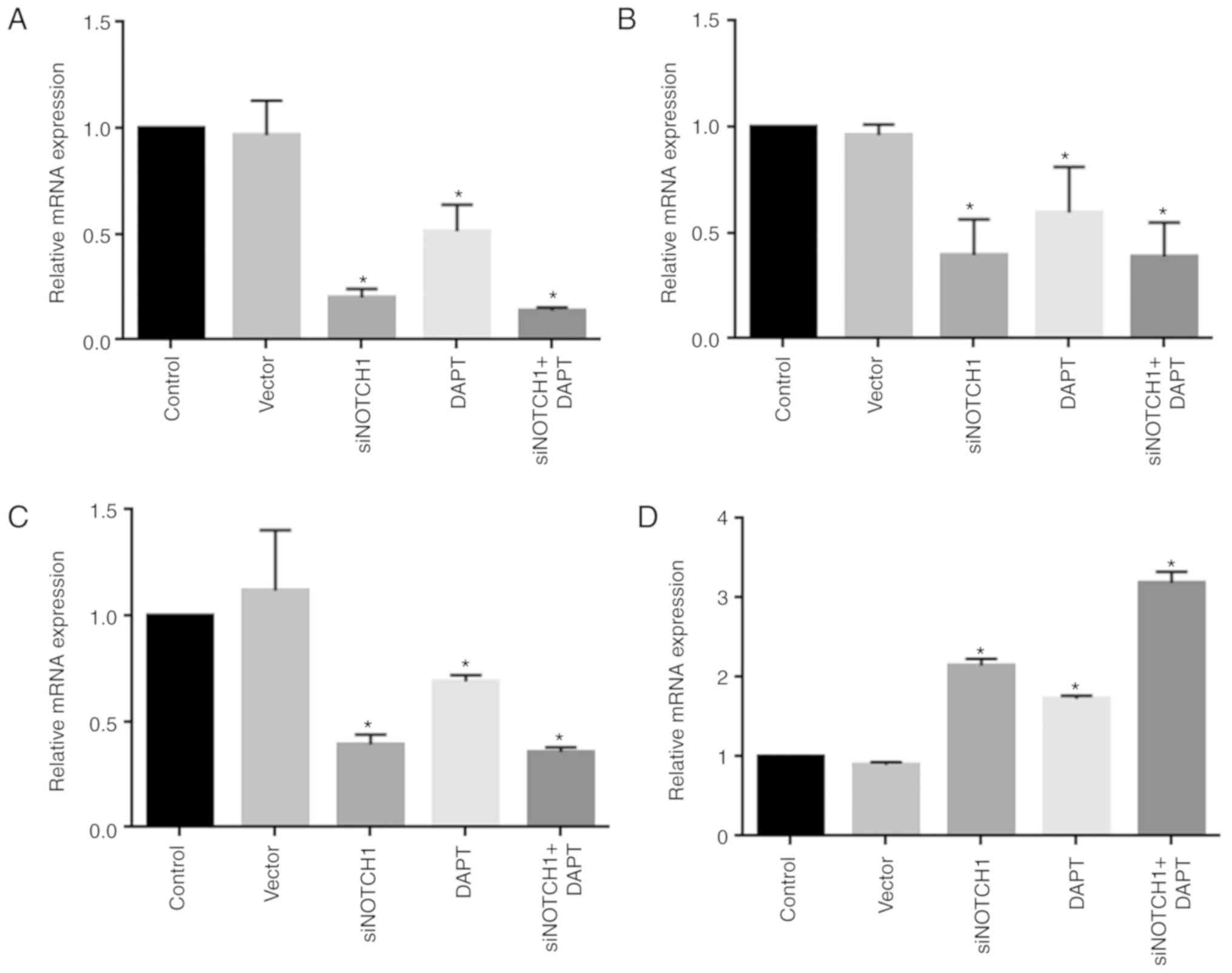
As shown in Fig.
2A-D, treatment with siRNA Notch1 and the γ-secretase inhibitor
significantly reduced the expression of Notch1, TGF-β1 and c-Myc,
and promoted the expression of p53 at the mRNA level, respectively,
compared with levels in the control (P<0.05). Combined treatment
with siRNA-Notch1 and the γ-secretase inhibitor did not alter gene
expression to a greater extent than single siRNA Notch1 or
γ-secretase inhibitor treatment.
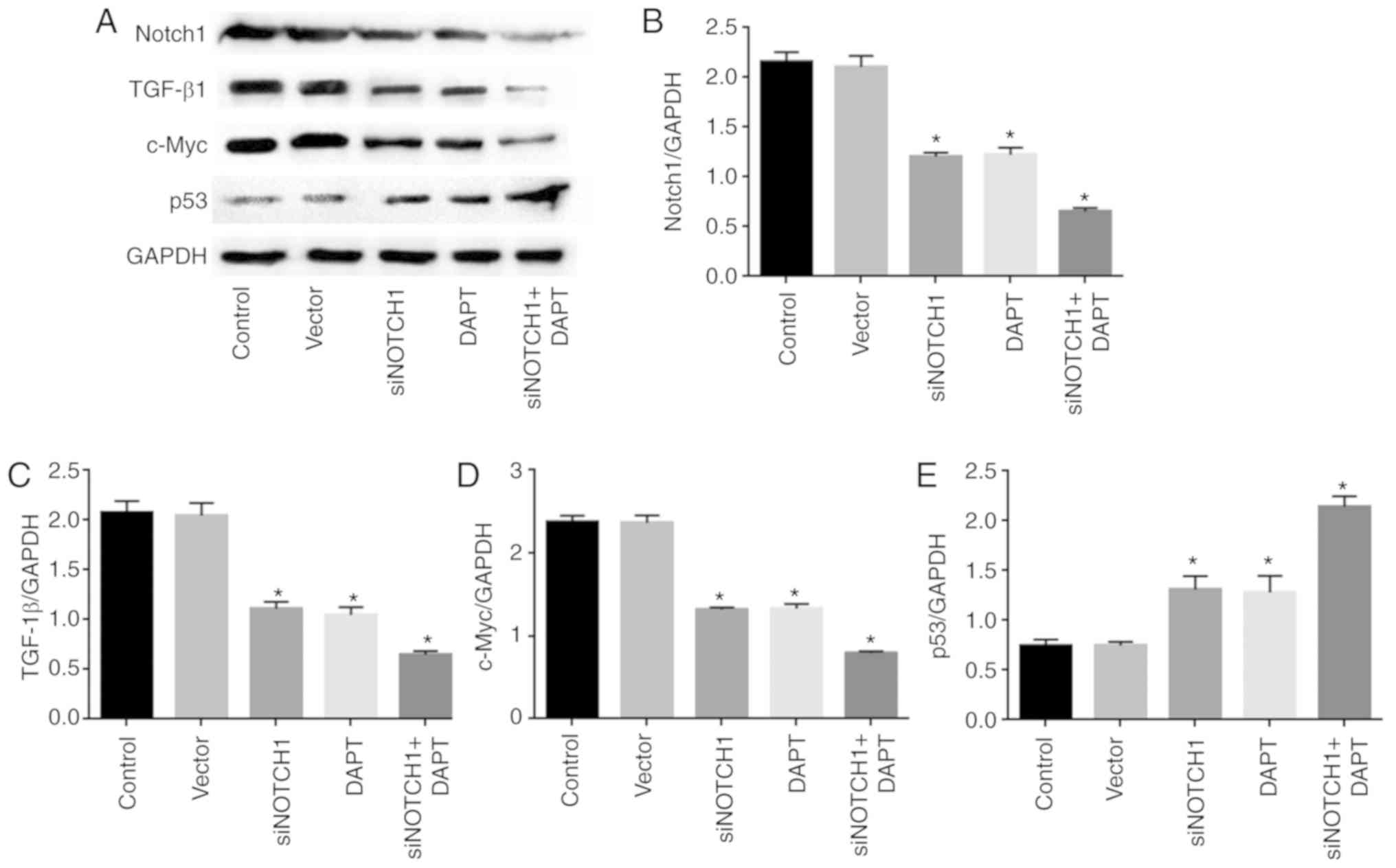
Consistently, siRNA Notch1 and γ-secretase inhibitor
treatment significantly reduced the expression of Notch1, TGF-β1
and c-Myc, and promoted the expression of p53 at the protein level,
compared with levels in the control (P<0.05). Combined
siRNA-Notch1 and γ-secretase inhibitor treatment did not alter gene
expression to a greater extent than single siRNA-Notch1 or
γ-secretase inhibitor treatment (Fig.
3A-E).
Notch1 inhibition accelerates
osteogenic differentiation
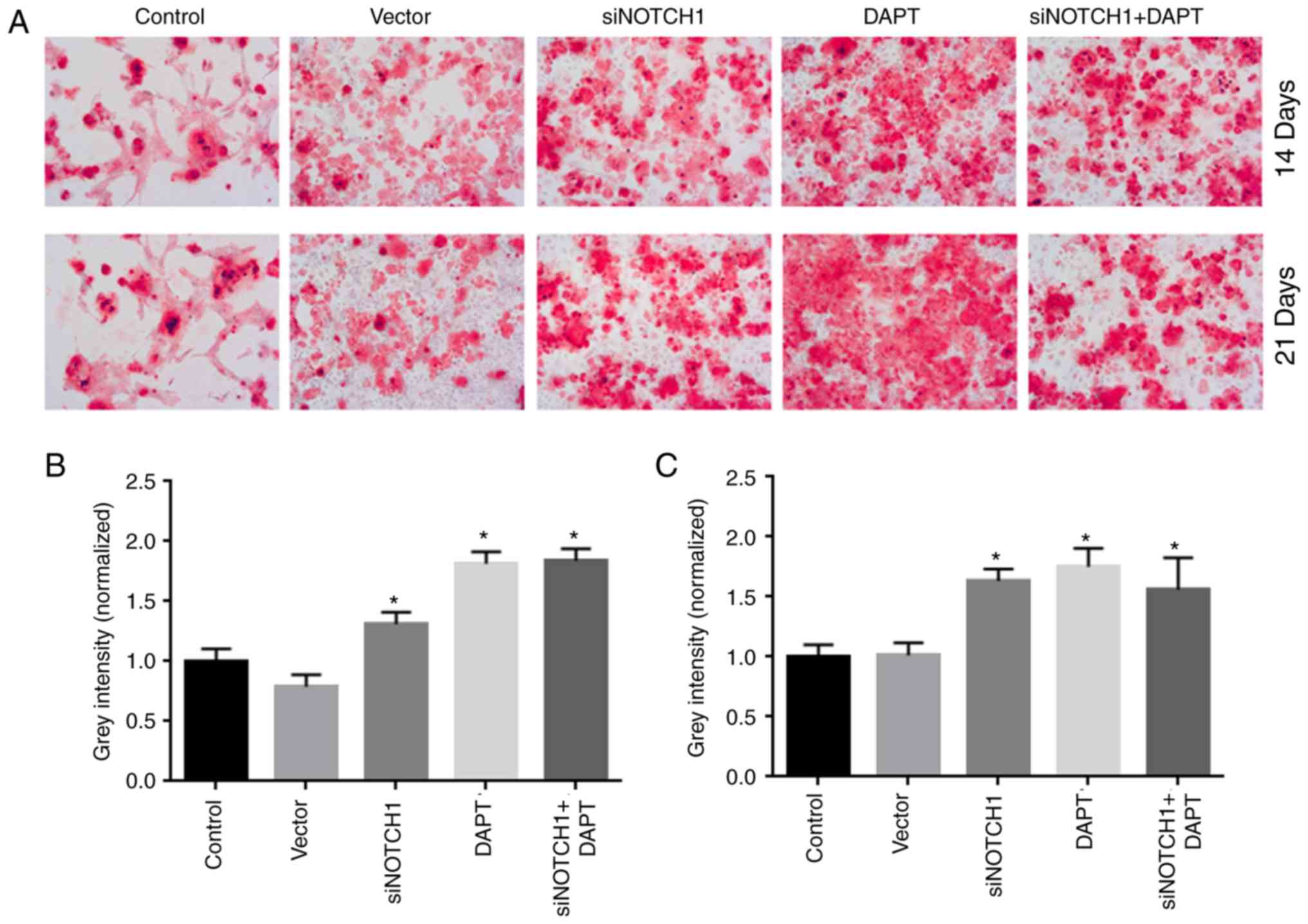
As shown in Fig. 4A,
siRNA Notch1 and γ-secretase inhibitor treatment significantly
accelerated osteogenic differentiation at days 14 and 21,
respectively, compared with that in the control group (P<0.05).
Combined siRNA-Notch1 and γ-secretase inhibitor treatment did not
promote osteogenic differentiation to a greater extent than single
siRNA-Notch1 or γ-secretase inhibitor treatment.
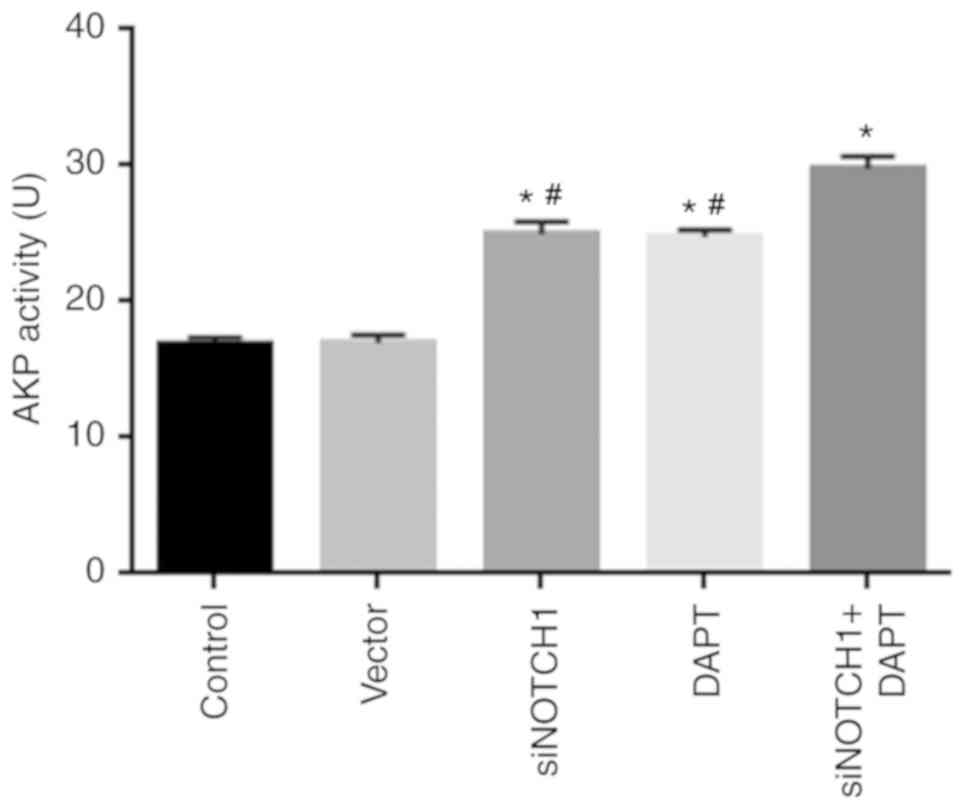
Notch1 inhibition promotes AKP
activity
As shown in Fig. 5,
siRNA-Notch1 and γ-secretase inhibitor treatment significantly
promoted AKP activity, respectively, compared with that in the
control (P<0.05). Combined siRNA-Notch1 and γ-secretase
inhibitor treatment further promoted osteogenic differentiation
compared with that following treatment with either siRNA-Notch1 or
γ-secretase inhibitor treatment alone.
Discussion
The present study demonstrated that Notch1
inhibition by siRNA or a specific inhibitor reduced MSC viability,
as evidenced by the decreased expression of TGF-β1 and c-Myc and
increased expression of p53, but it promoted osteogenic
differentiation.
Osteosarcoma is a condition likely arising from
cancerous stem cells. Gibbs et al (16) first isolated stem cell-like tumor
cells from an osteosarcoma. These cells were found to express
embryonic stem cell markers (Oct 3/4 and Nanog) and surface
molecules of MSCs (Stro-1, CD105 and CD44). Osteosarcoma stem cells
are considered to be homologous to MSCs. Based on the common
pathological manifestations of osteosarcoma, in addition to
previous findings of malignant MSCs and osteoid cells (17,18), it
has been suggested that the condition likely originates from adult
stem cells (16–18). In addition, MSCs and tumor stem cells
have several similar characteristics, including the capacity of
unlimited proliferation and differentiation, a common molecular
diffusion mechanism, and telomerase activity. The microenvironment
is important for MSC differentiation and regulation. These cells
are also involved in the formation and maintenance of the
microenvironment, which is vital for effective networking between
tumor cells. MSC homing to the tumor mass also promotes tumor
growth and metastasis (19,20). For the aforementioned reasons, MSCs
can be considered as potential cancer therapy targets.
The Notch signaling pathway is a conserved signaling
mechanism that is widely distributed among most multicellular
organisms. This pathway has been reported to exert a major
influence on the proliferation of mouse embryonic fibroblasts in
the process of bone morphogenetic protein-9-induced osteogenic
differentiation (21). In a previous
study, the overexpression of Notch1 improved the proliferation,
migration and survival of MSCs induced by cigarette smoke extract
(22). However, the effects of
Notch1 on the proliferation of MSCs under normal conditions were
not evaluated. In the present study, it was demonstrated that
Notch1 inhibition reduced BMSC proliferation, suggesting that
Notch1 is a potential oncogene for BMSC transformation into
osteosarcoma.
TGF-β1, a multifunctional cytokine (23,24), is
involved in the differentiation, survival and growth of a variety
of cell types. TGF-β1 promotes the differentiation of bone MSCs
in vitro (25) and has been
reported to serve an important role in the development of the mouse
myocardium via the induction of mouse embryonic stem cell
differentiation into cardiac myocytes (26). This cytokine is also involved in
SMAD2 and SMAD3 signaling pathways and the induction of neural
crest stem cell differentiation into functional smooth muscle cells
(27). TGF-β1 also induces
cardiomyocyte hypertrophy via SMAD4/SMAD3 protein complex
interactions and the alteration of downstream gene functions
(28). The present study found that
Notch1 inhibition reduced the expression of TGF-β1 at the mRNA and
protein levels. As TGF-β1 can promote tumorigenesis in this setting
(29–31), a reduction in TGF-β1 by Notch1
inhibition further underscored the oncogenic activity of
Notch1.
c-Myc is one of the three closely related
transcription factor family genes, and Myc genes are upregulated in
various types of human cancer. The upregulated expression of c-Myc
promotes cell proliferation and cell growth, the synthesis of
ribosomes and proteins, energy production (i.e. glycolysis) and
anabolism (32,33). The present study found that
siRNA-Notch1 and γ-secretase inhibitor treatment reduced the
expression of TGF-β1 and c-Myc and increased the expression of p53.
Excessive expression of c-Myc reduces the effect of growth factors
and promotes cell proliferation. It also downregulates c-Myc and
may arrest cells at the G1 phase, thus slowing the rate of cell
growth (34).
Once osteoblasts are differentiated, they secrete
extracellular matrix proteins and control the mineralization of
bone matrix (35). AKP,
characteristically expressed in osteoblasts, serves a key role in
calcification in vitro. In an alkaline buffer, AKP catalyzes
the hydrolysis of a-monophosphate to produce α-naphthol, which is
coupled to a stable diazonium salt to produce insoluble azo dyes.
In the present study, the results of alizarin red staining and AKP
activity assays indicated that the inhibition of Notch1 reduced
BMSC proliferation and promoted osteogenic differentiation. These
results indicate that the Notch signaling pathway was inhibited
during BMSC differentiation, suggesting that Notch signaling has a
negative effect on BMSC osteogenic differentiation (35). In the present study, Notch1
inhibition was performed by knocking out Notch1 at the gene level
and by using γ-secretase inhibitors. No difference between the
γ-secretase inhibitor and siRNA Notch1 was observed regarding cell
viability and the molecular changes of different proteins. In
addition, the combination of γ-secretase inhibitor and siRNA Notch1
did not further promote the effects. These data implicate the
specific effects of Notch1 inhibition by siRNA-Notch1 or the
γ-secretase inhibitor.
There were several limitations in the present study.
Firstly, a CCK assay was used to detect cell viability, which
explained cell proliferation to an extent. However, the
tumorigenesis of BMSCs can be assessed by other methods, such as
adding proliferating cell nuclear antigen as an indicator.
Secondly, further experiments are required to confirm that the
development of osteosarcoma is related to Notch1. Whether Notch1
activation correlates with the development of osteosarcoma could be
confirmed in the future. Finally, in vivo data of
tumorigenesis may provide further evidence.
In conclusion, Notch1 inhibition reduced BMSC
proliferation and promoted the osteogenic differentiation of this
cell type. The results of the present study outline a novel
potential therapeutic target in the prevention of BMSC
tumorigenesis and provides a foundation for future clinical
applications.
Acknowledgements
Not applicable.
Funding
This study was supported by NSFC (81000676,
81660364), the Special Research Fund for Doctoral Disciplines
(grant no. 20093601120005) and Jiangxi Natural Science Foundation
(grant no. 2012ZBAB205004).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and LZ performed the experiments and analyzed the
data. LZ designed the study and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ren T, Piperdi S, Koirala P, Park A, Zhang
W, Ivenitsky D, Zhang Y, Villanueva-Siles E, Hawkins DS, Roth M and
Gorlick R: CD49b inhibits osteogenic differentiation and plays an
important role in osteosarcoma progression. Oncotarget.
8:87848–87859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nombela-Arrieta C, Ritz J and Silberstein
LE: The elusive nature and function of mesenchymal stem cells. Nat
Rev Mol Cell Biol. 12:126–131. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams AR and Hare JM: Mesenchymal stem
cells: Biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morancho A, Ma F, Barcelo V, Giralt D,
Montaner J and Rosell A: Impaired vascular remodeling after
endothelial progenitor cell transplantation in MMP9-deficient mice
suffering cortical cerebral ischemia. J Cereb Blood Flow Metab.
35:1547–1551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wallner C, Abraham S, Wagner JM, Harati K,
Ismer B, Kessler L, Zöllner H, Lehnhardt M and Behr B: Local
application of isogenic adipose-derived stem cells restores bone
healing capacity in a type 2 diabetes model. Stem Cell Transl Med.
5:836–844. 2016. View Article : Google Scholar
|
|
6
|
Gupta PK, Chullikana A, Parakh R, Desai S,
Das A, Gottipamula S, Krishnamurthy S, Anthony N, Pherwani A and
Majumdar AS: A double blind randomized placebo controlled phase
I/II study assessing the safety and efficacy of allogeneic bone
marrow derived mesenchymal stem cell in critical limb ischemia. J
Transl Med. 11:1432013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lara-Hernandez R, Lozano-Vilardell P,
Blanes P, Torreguitart-Mirada N, Galmes A and Besalduch J: Safety
and efficacy of therapeutic angiogenesis as a novel treatment in
patients with critical limb ischemia. Ann Vasc Surg. 24:287–294.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He Y, Zhu W, Shin MH, Gary J, Liu C,
Dubois W, Hoover SB, Jiang S, Marrogi E, Mock B, et al: cFOS-SOX9
axis reprograms bone marrow-derived mesenchymal stem cells into
chondroblastic osteosarcoma. Stem Cell Rep. 8:1630–1644. 2017.
View Article : Google Scholar
|
|
9
|
Yuan TM and Yu HM: Notch signaling: Key
role in intrauterine infection/inflammation, embryonic development,
and white matter damage? J Neurosci Res. 88:461–468.
2010.PubMed/NCBI
|
|
10
|
Reddy BV, Rauskolb C and Irvine KD:
Influence of fat-hippo and notch signaling on the proliferation and
differentiation of Drosophila optic neuroepithelia. Development.
137:2397–2408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bray SJ: Notch signalling in context. Nat
Rev Mol Cell Biol. 17:722–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colombo M, Galletti S, Garavelli S,
Platonova N, Paoli A, Basile A, Taiana E, Neri A and Chiaramonte R:
Notch signaling deregulation in multiple myeloma: A rational
molecular target. Oncotarget. 6:26826–26840. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cristofaro B and Emanueli C: Possible
novel targets for therapeutic angiogenesis. Curr Opin Pharmacol.
9:102–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang T, Zhu L, Gao W, Gong M, Ren J, Yao
H, Wang K and Shi D: Coculture of endothelial progenitor cells and
mesenchymal stem cells enhanced their proliferation and
angiogenesis through PDGF and Notch signaling. FEBS Open Bio.
7:1722–1736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gibbs C, Kukekov VG, Reith JD, Tchigrinova
O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN and Steindler
DA: Stem-like cells in bone sarcomas: Implications for
tumorigenesis. Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berman SD, Calo E, Landman AS, Danielian
PS, Miller ES, West JC, Fonhoue BD, Caron A, Bronson R, Bouxsein
ML, et al: Metastatic osteosarcoma induced by inactivation of Rb
and p53 in the osteoblast lineage. Proc Natl Acad Sci USA.
105:11851–11856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tataria M, Quarto N, Longaker MT and
Sylvester KG: Absence of the p53 tumor suppressor gene promotes
osteogenesis in mesenchymal stem cells. J Pediatr Surg. 41:624–632.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Z, Wang S and Zhao RC: The roles of
mesenchymal stem cells in tumor inflammatory microenvironment. J
Hematol Oncol. 7:142014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hill BS, Pelagalli A, Passaro N and
Zannetti A: Tumor-educated mesenchymal stem cells promote
pro-metastatic phenotype. Oncotarget. 8:73296–73311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao J, Wei Y, Lian J, Yang L, Zhang X, Xie
J, Liu Q, Luo J, He B and Tang M: Notch signaling pathway promotes
osteogenic differentiation of mesenchymal stem cells by enhancing
BMP9/Smad signaling. Int J Mol Med. 40:378–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng Y, Gu W, Zhang G, Li X and Guo X:
Activation of Notch1 signaling alleviates dysfunction of bone
marrow-derived mesenchymal stem cells induced by cigarette smoke
extract. Int J Chron Obstruct Pulmon Dis. 12:3133–3147. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li TS, Hayashi M, Ito H, Furutani A,
Murata T, Matsuzaki M and Hamano K: Regeneration of infarcted
myocardium by intramyocardial implantation of ex vivo transforming
growth factor-beta-preprogrammed bone marrow stem cells.
Circulation. 111:2438–2445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Powell S, Brunette E, Lebkowski J
and Mandalam R: Expansion of human embryonic stem cells in defined
serum-free medium devoid of animal-derived products. Biotechnol
Bioeng. 91:688–698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gwak SJ, Bhang SH, Yang HS, Kim SS, Lee
DH, Lee SH and Kim BS: In vitro cardiomyogenic differentiation of
adipose-derived stromal cells using transforming growth
factor-beta1. Cell Biochem Funct. 27:148–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akhurst RJ, Lehnert SA, Faissner A and
Duffie E: TGF beta in murine morphogenetic processes: The early
embryo and cardiogenesis. Development. 108:645–656. 1990.PubMed/NCBI
|
|
27
|
Chen S and Lechleider RJ: Transforming
growth factor-beta-induced differentiation of smooth muscle from a
neural crest stem cell line. Circ Res. 94:1195–1202. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mueller MB, Fischer M, Zellner J, Berner
A, Dienstknecht T, Prantl L, Kujat R, Nerlich M, Tuan RS and Angele
P: Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of
TGF-beta isoforms and chondrogenic conditioning. Cells Tissues
Organs. 192:158–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu ML, Wang XY and Chen WM: TGF-β1
upregulates the expression of lncRNA UCA1 and its downstream HXK2
to promote the growth of hepatocellular carcinoma. Eur Rev Med
Pharmacol Sci. 22:4846–4854. 2018.PubMed/NCBI
|
|
30
|
Viloria ME, Bravo J, Carrero Y and
Mosquera JA: In situ expressions of protein 16
(p16CDKN2A)) and transforming growth factor beta-1 in
patients with cervical intraepithelial neoplasia and cervical
cancer. Eur J Obstet Gynecol Reprod Biol. 228:303–307. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui M, Chang Y, Du W, Liu S, Qi J, Luo R
and Luo S: Upregulation of lncRNA-ATB by transforming growth factor
beta1 (TGF-β1) promotes migration and invasion of papillary thyroid
carcinoma cells. Med Sci Monit. 24:5152–5158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gallant P: Myc/Max/Mad in invertebrates:
The evolution of the Max network. Curr Top Microbiol Immunol.
302:235–253. 2006.PubMed/NCBI
|
|
33
|
Lin TP, Li J, Li Q, Li X, Liu C, Zeng N,
Huang JM, Chu GC, Lin CH, Zhau HE, et al: R1 Regulates prostate
tumor growth and progression by transcriptional suppression of the
E3 Ligase HUWE1 to Stabilize c-Myc. Mol Cancer Res. 16:1940–1951.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou X, Wen Y, Tian Y, He M, Ke X, Huang
Z, He Y, Liu L, Scharf A, Lu M, et al: Hsp90alpha-dependent
B-Claf-1 promotes hepatocellular carcinoma proliferation by
regulating c-MYC mRNA stability. Hepatology. 2018.
|
|
35
|
Zanotti S, Smerdel-Ramoya A, Stadmeyer L,
Durant D, Radtke F and Canalis E: Notch inhibits osteoblast
differentiation and causes osteopenia. Endocrinology.
149:3890–3899. 2008. View Article : Google Scholar : PubMed/NCBI
|