Introduction
Vaginitis is an inflammation of the vagina, most
cases of which are due to bacterial or fungal infections. Common
types of vaginitis are bacterial vaginosis (BV), candida vaginitis
and trichomoniasis; BV is the most frequent type (1–3). BV is
caused by imbalances of the bacterial composition in the vaginal
flora and excessive growth of bacteria on the vaginal epithelium,
which results in inflammation (2).
BV is often diagnosed with a vaginal pH of >4.5, or a Nugent
score of ≥7 (2). Common symptoms of
BV include vaginal itching and pain, foul odor and excessive
vaginal discharge (4). Although BV
is not life-threatening, it creates significant discomfort and may
lead to complications (2). Patients
diagnosed with BV should be treated immediately.
The current standard treatment for BV is the
administration of antibiotics, either locally or systematically
(5). The commonly used antibiotics
for BV treatment include metronidazole, clindamycin and tinidazole,
which have been demonstrated to be effective with cure rates
ranging between 75 and 86% (6–8). Oral
and vaginal treatments are available and demonstrate similar
efficacy (9,10). However, certain side effects have
been noted, such as nausea and stomach discomfort (9). In addition, antibiotic treatments are
associated with the development of antibiotic resistance; high
antibiotic resistance (68–81%) was observed in patients following
metronidazole or clindamycin treatment, which lead to recurrence of
BV (11–13).
Berberine is a natural alkaline product that has a
long history of use in traditional Chinese medicine (14). It had been reported to exhibit
beneficial effects for various diseases such as diabetes mellitus,
kidney damage and cardiovascular disease (15–18). The
underlying mechanisms of the effects of berberine are attributed to
its ability to reduce oxidative stress and apoptosis in cells,
which makes it a promising candidate for treating
inflammation-related diseases or symptoms (15,17). The
aim of this study was to evaluate the effects of Berberine on BV as
a potential alternative approach, especially for patients who have
already developed resistance to antibiotic treatments.
Evaluation of the effects of berberine on reducing
oxidative stress and apoptosis in cells can be achieved by
analyzing the changes in oxidative stress- and apoptosis-related
factors in cells. Superoxide dismutase (SOD) is an enzyme in cells
that catalyzes the conversion of superoxide anion radical
(O2·−) to the less harmful hydrogen peroxide
(H2O2), whereas catalase (CAT) is an
enzymatic antioxidant that converts H2O2 into
water and molecular oxygen (19,20).
Together, SOD and CAT serve important roles in reducing oxidative
stress levels by reducing the amount of reactive oxygen species
(ROS) in cells. Endothelial nitric oxide synthase (eNOS) is an
enzyme that uses L-arginine to produce nitric oxide (NO) (21–24). NO
can also reduce oxidative stress by scavenging ROS and activating
antioxidative enzymes, such as SOD and CAT (25). In addition, malondialdehyde (MDA) is
widely used as a marker for lipid peroxidation in cells (26–28). MDA
is formed when ROS within cells oxidize unsaturated fatty acids;
thus, it can also be used as an indicator for oxidative stress
(28). Apoptosis is mostly mediated
by Bax and the caspase family, whereas cytochrome C is released by
the mitochondria to activate caspase during apoptosis (29,30).
Caspase-3, caspase-12 and the Bax/Bcl2 ratio are commonly used as
measures for apoptosis levels (31–33).
In this study, the changes in SOD, CAT, MDA and
H2O2 were monitored in vaginal discharge from
patients following berberine treatment to evaluate the changes in
oxidative stress levels. Changes in the aforementioned apoptotic
proteins in vaginal epithelial cells following berberine treatment
were also monitored to evaluate the change in apoptosis levels.
Furthermore, in vitro experiments were performed to evaluate
changes in apoptosis and ROS levels in vaginal epithelial cells
with different doses of berberine treatment.
Materials and methods
Patient grouping and treatments
A total of 180 female patients (age, 25–45 years)
diagnosed with BV at Yantai Hospital of Traditional Chinese
Medicine between August 2017 and March 2018 were selected for this
study. The experiment exclusion criteria were as follows: i)
Patients at menstrual bleeding; ii) pregnant patients; iii)
lactating patients; iv) patients who were treated with antibiotics,
hormones or local drug treatments within 14 days; v) patients who
participated in sexual activity within 24 h. Additionally, healthy
individuals undergoing routine vaginal examination and 60 healthy
female individuals (age, 25–45 years) were selected between August
2017 and March 2018 as the control group. Individuals were excluded
from the current study if they were: i) Menstruating; ii) pregnant;
iii) lactating; iv) treated with antibiotics, hormones or local
drug treatments within 14 days; v) participated in sexual activity
within 24 h. Each BV patient was administered berberine treatments
by placing 0.3 g berberine HCl (Northeast Pharmaceutical Group Co.,
Ltd.) at the posterior vaginal fornix daily for 10 days per
treatment for one month. Clinical observation was performed on all
healthy subjects (control) and on patients before treatment (BT)
and after treatment (AT) to monitor and compare the severity of
symptoms. Healthy subjects were only evaluated once. This study was
approved by the Ethics Committee of Yantai Hospital of Traditional
Chinese Medicine. Written informed consent was obtained from all
participants.
Sampling of vaginal discharge and
vaginal epithelial cells
Observe the leucorrhea of the patient. Swabs of
vaginal discharge and virginal epithelial cells were collected at
the posterior vaginal fornix and the cervix from patients with BV
and healthy subjects while the cervix was exposed using a vaginal
speculum. Each swab was dissolved in 1 ml phosphate-buffered saline
(PBS; 0.05 mol/l; pH 7.0) in centrifuge tubes. Samples were
centrifuged at 11,180 × g under refrigeration at 4°C for 20 min and
stored at −80°C until further use.
Cell culture and treatment
Human vaginal epithelial cells VK2/E6E7 (cat. no.
BNCC340628) were purchased from Shanghai Cell Library (http://www.cobioer.com/index.html). Cells were
incubated in DMEM medium (Gibco; Thermo Fisher Scientific, Inc.)
with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA), at
37°C with 5% CO2. Cells were treated with 0, 10, 50 and
100 µg/ml berberine for 24 h prior to the MTT assay.
Induction of oxidative stress
VK2/E6E7 cells were divided into five groups: i)
Control, samples incubated without FBS in the medium; ii) model,
samples incubated without FBS in DMEM medium for 4 h, followed by
the addition of 400 µM H2O2 for 24 h; iii)
LT, samples incubated in DMEM medium with 10 µg/ml berberine HCl
for 4 h, followed by the addition of 400 µM
H2O2 for 24 h; iv) MT, samples incubated in
EMDM medium with 50 µg/ml berberine HCl for 4 h, followed by the
addition of 400 µM H2O2 for 24 h; and v) HT,
samples incubated in DMEM medium with 100 µg/ml berberine HCl for 4
h, followed by the addition of 400 µM H2O2
for 24 h. Each group consisted of five replicates.
ROS-related factor detection
Spectrophotometry was used for the detection of SOD
(cat. no. BC0170) and CAT (cat. no. BC0200) activity, as well as
levels of MDA (cat. no. BC0020) and H2O2
(cat. no. BC3590), within the vaginal discharge samples. SOD (cat.
no. BC0170), CAT (cat. no. BC0200), MDA (cat. no. BC0020) and
H2O2 (cat. no. BC3590) kits were purchased
from Beijing Solarbio Science & Technology Co., Ltd. and used
following the manufacturers' protocols.
MTT cell viability assay
VK2/E6E7 cells in the logarithmic growth phase were
digested using pancreatic enzymes, and cell density was adjusted to
1×105 cells/ml. The cells were transferred to a 96-well
plate (100 µl/well) and incubated at 37°C with 5% CO2 in
an incubator until ~80% confluence was reached. The cells were
grouped and treated as described above. At 24 h, supernatants were
removed, 20 µl of 5 mg/ml MTT was added to each well and incubated
for 4 h at 37°C. Subsequently, supernatants were removed again and
200 µl DMSO (Sigma-Aldrich; Merck KGaA) was added to each well and
suspended evenly. Absorbance at 490 nm was detected using a
photometric plate reader.
Flow cytometry
VK2/E6E7 cells in the logarithmic phase were
digested using pancreatic enzymes, and cell density was adjusted to
1×105 cells/ml. The cells were transferred to a six-well
plate (1 ml/well) and incubated at 37°C with 5% CO2 in
an incubator until the cells covered the bottom of the wells. The
cells were grouped and treated as described above. Annexin
V-FITC/propidium iodide (PI) Apoptosis Detection kit and Reactive
Oxygen Species Assay kit (cat. nos. CA1020 and CA1410,
respectively; Beijing Solarbio Science & Technology Co., Ltd.)
were used for the detection of apoptosis and ROS levels. For the
detection of apoptosis level, cells from all groups were washed
twice with 4°C sterile PBS and resuspended in 1 ml of 1X binding
buffer to achieve the density of 1×106 cells/ml.
Subsequently, 100 µl of the suspension (1×105 cells) was
added to a tube with 5 µl Annexin V-FITC at room temperature and
agitated gently for 10 min prior to the addition of 5 µl PI for 5
min at room temperature in light-sensitive environment. PBS (500
µl) was added to the tube and mixed evenly, and the mixture was
analysed using a flow cytometer. For the detection of ROS levels,
dichloro-dihydro-fluorescein diacetate (DCFH-DA) was diluted to 10
µmol/l with DMEM medium without FBS (1:1,000). Medium from each
sample was removed and replaced with 1 ml diluted DCFH-DA. Each
sample was incubated at room temperature for 20 min and washed 3
times with medium without FBS to remove excess DCFH-DA outside of
the cells. Cells were dissociated with trypsin and detected using a
flow cytometer (Gallios; Beckman Coulter, Inc.) with Cell Quest 5.1
software (BD Biosciences).
Western blotting
Western blotting was used for the detection of
apoptosis-associated proteins, including Bax, Bcl-2, caspase-3,
caspase-12 and cytochrome C from vaginal epithelial cells obtained
from healthy subjects and patients before and after treatment, and
for the detection of the expression levels of SOD and eNOS in
vitro. Proteins were lysed with RIPA assay lysis buffer
(Beyotime Institute of Biotechnology) and a PierceÔ BCA Protein
Assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.) was used
to measure the total protein concentration according to
manufacturer's instructions. Protein (40 µg/lane) was separated
using 10% SDS-PAGE and transferred onto a PVDF transfer membrane.
The membrane was then blocked in 5% non-fat dry milk for 1 h,
followed by overnight incubation at 4°C with the following primary
antibodies diluted in 5% BSA: Rabbit anti-Bax (cat. no. ab32124),
rabbit anti-Bcl-2 (cat. no. ab32503), rabbit anti-caspase-3 (cat.
no. ab13847), rabbit anti-cytochrome-C (cat. no. ab133504), rabbit
anti-caspase-12 (cat. no. ab62484), rabbit anti-SOD (cat. no.
ab13498) and rabbit anti-eNOS (cat. no. ab76198); all antibodies
were purchased from Abcam and all were diluted to 1:1,000.
Following incubation, the membrane was washed with TBS with 0.1%
Tween-20 (TBST) three times for 10 min and incubated with
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
secondary antibody (1:2,000; cat. no. ab6721; Abcam) at room
temperature for 2 h. The membrane was washed three times with TBST
for 10 min, and the bands were detected using enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.). The resulting
images were processed using ImageJ 1.46r (National Institutes of
Health) for quantitation.
Statistical analysis
SPSS 19.0 software (IBM Corp.) was used for
statistical analysis. All data are presented as mean ± standard
deviation. One-way ANOVA with the least significant difference post
hoc test was used for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Berberine improves clinical symptoms
of patients with BV
Clinical symptoms from all patients before and after
berberine treatment were recorded and summarized (Table I). A total of 95% patients exhibited
yellowish leukorrhea at the beginning of the study, which was
markedly reduced following berberine treatment. The number of
patients with vaginal itching decreased from 88.89 to 36.67%, and
the number of patients with local pain decreased from 92.78 to
32.78% following berberine treatment. Additionally, the number of
patients with yellowish leukorrhea decreased from 95 to 10.56%, and
the number of patients with foul smell decreased from 36.11 to
5.56% following berberine treatment. According to the Nugent scores
(2), all of the 180 patients were
BV-positive (100%) at the beginning of the study; following
berberine treatment, only 13 patients remained positive (7.22%),
which corresponded to a 92.78% cure rate. These results indicated
that berberine treatment significantly improved clinical symptoms
and effectively cured BV.
 | Table I.Clinical symptoms in patients with BV
before and after berberine treatment. |
Table I.
Clinical symptoms in patients with BV
before and after berberine treatment.
|
| Yellowish
leukorrhea | Foul Smell | Pain | Itching | BV positive |
|---|
|
|
|
|
|
|
|
|---|
| Group | N | % | N | % | N | % | N | % | N | % |
|---|
| Before | 171 | 95.00 | 65 | 36.11 | 167 | 92.78 | 160 | 88.89 | 180 | 100 |
| After | 19 | 10.56 | 10 | 5.56 | 59 | 32.78 | 66 | 36.67 | 13 | 7.22 |
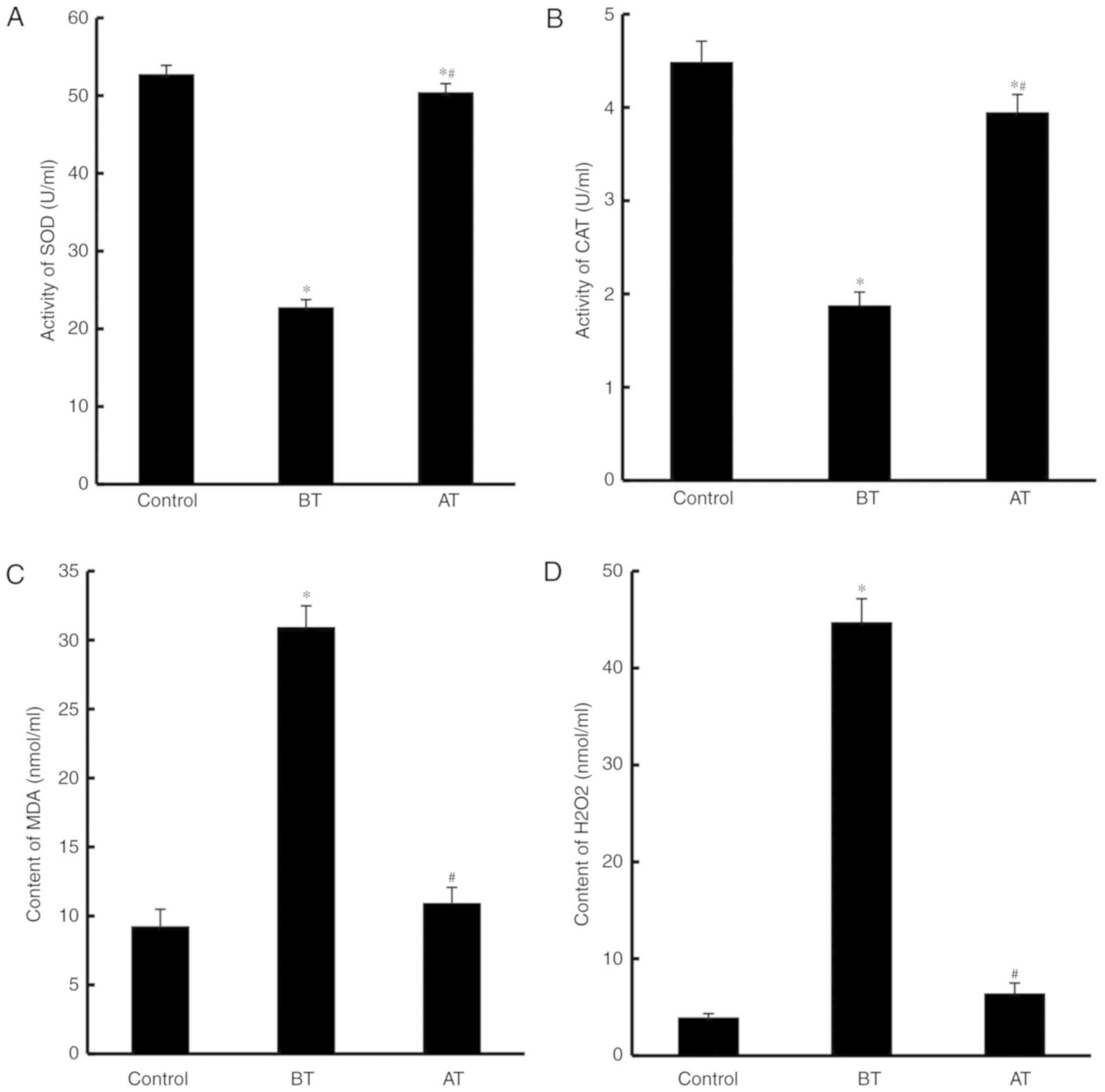
Berberine treatment reduces oxidative
stress in vaginal discharge
SOD and CAT activity, as well as the concentrations
of MDA and H2O2, in the vaginal discharge
from control, BT and AT groups were evaluated. SOD and CAT activity
levels were significantly lower in the BT group compared with the
control group (P<0.05; Fig. 1A and
B), whereas in the AT group, the activity levels were
significantly higher compared with BT (P<0.05; Fig. 1A and B). By contrast, the levels of
MDA and H2O2 were significantly increased in
the BT group compared with the control group (P<0.05); however,
following treatment, these levels decreased significantly compared
with the BT group (P<0.05). These results indicated that
oxidative stress in vaginal discharge may be reduced by treatment
with berberine.
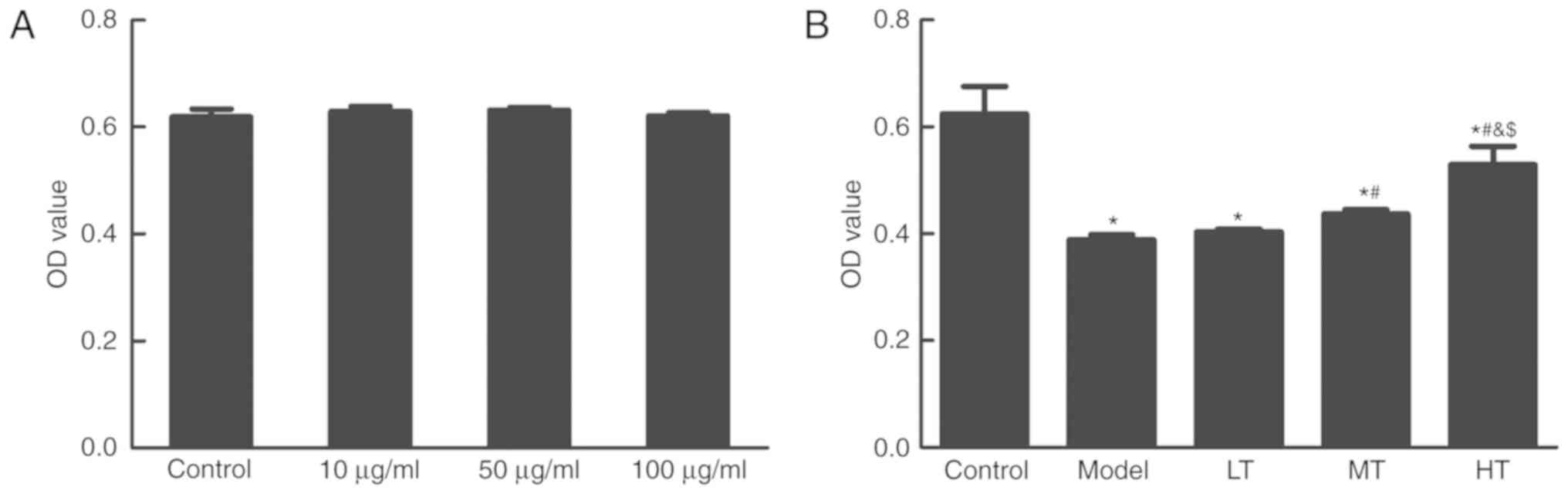
Berberine protects in
H2O2-treated cells against oxidative
damage
The effects of berberine on normal vaginal
epithelial cells were investigated. VK2/E6E7 cells were treated
with 10, 50 and 100 µg/ml berberine for 24 h, and the MTT assay was
used for the evaluation of cell viability. Berberine treatment had
no effect on cell viability (Fig.
2A). To specifically evaluate the effects of berberine on
oxidative damage, the MTT assay was used in
H2O2-treated cells. The results demonstrated
that cell viability was significantly lower in the model group with
H2O2 treatment compared with the control
group (P<0.05; Fig. 2B). The
berberine treatment groups exhibited a significant increase in cell
viability compared with the model group in a dose-dependent manner
(P<0.05; Fig. 2B). Furthermore,
based on the flow cytometry results, ROS levels in the model group
were significantly higher compared with those in the control group,
whereas the berberine-treated groups exhibited significant
decreases in ROS levels compared to the model group (P<0.05;
Fig. 3). These results demonstrated
that berberine may have an antioxidative effect.
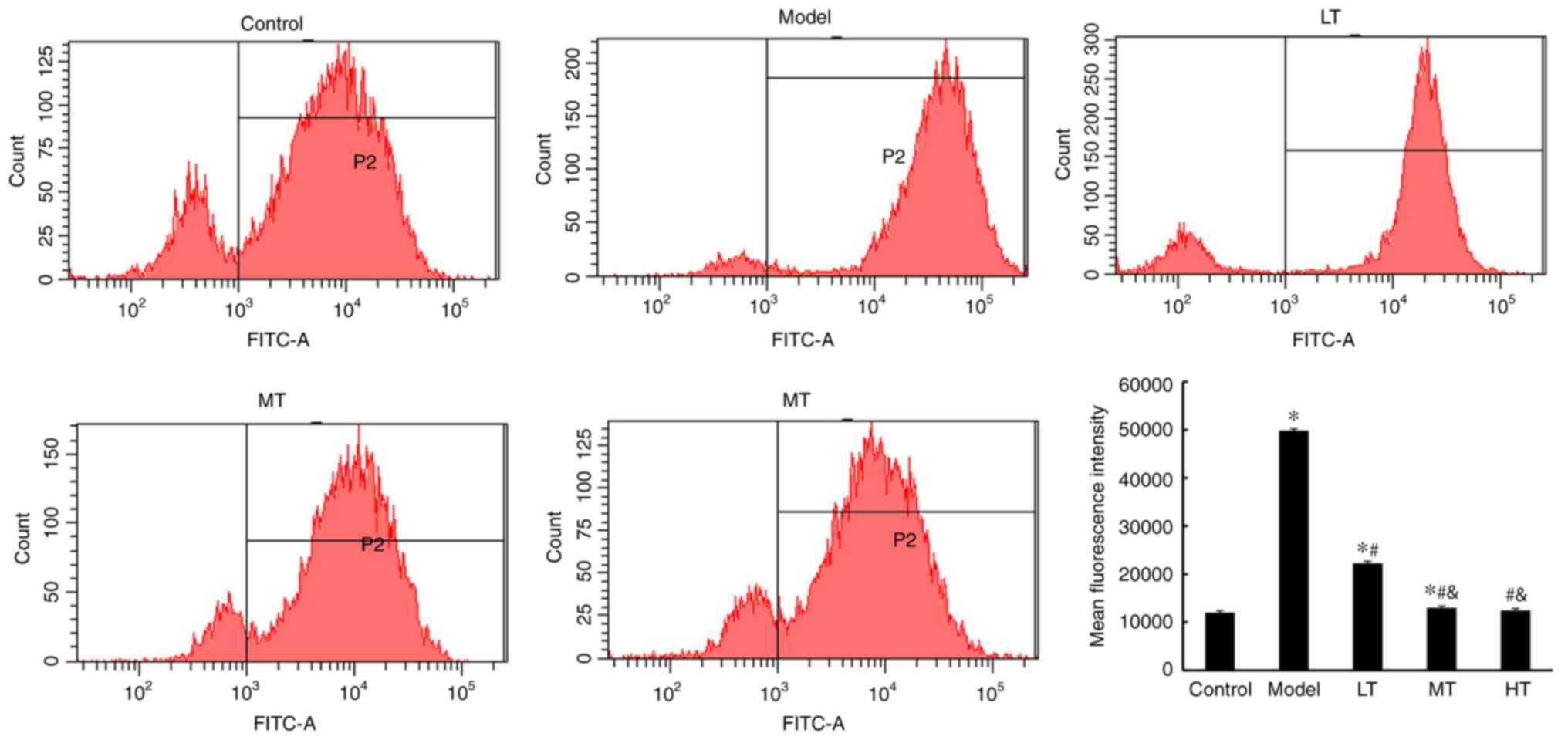
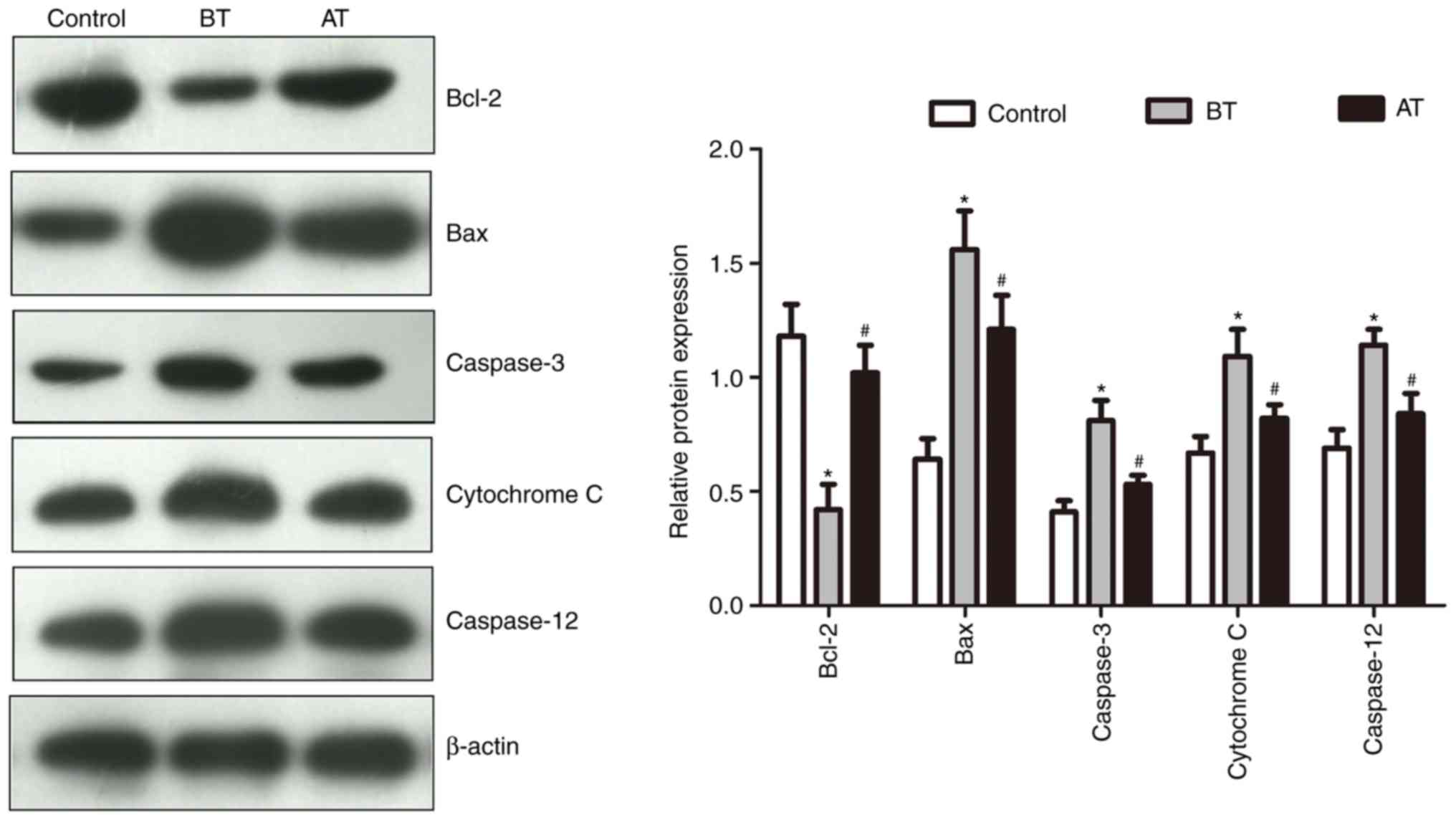
Berberine treatment supresses
apoptosis in vaginal epithelial cells
Bcl2, Bax, caspase-3, cytochrome C and caspase-12
are proteins related to cell apoptosis. The expression levels of
these proteins in the vaginal epithelial cell samples from patients
with BV and healthy subjects are summarized in Fig. 4. The expression levels of Bcl-2 in
the BT group were significantly lower compared with those in the
control group (P<0.05), but exhibited a significant increase in
the AT group compared with the BT group (P<0.05). In addition,
the BT group exhibited significantly increased levels of Bax,
caspase-3, cytochrome C and caspase-12 expression compared with the
control group (P<0.05), whereas the AT group exhibited reduced
levels of these proteins compared with BT (P<0.05). To further
confirm that berberine treatment supressed apoptosis in vaginal
epithelial cells, flow cytometry assay was used; the results
indicated a higher level of apoptosis in the
H2O2-treated model group compared with the
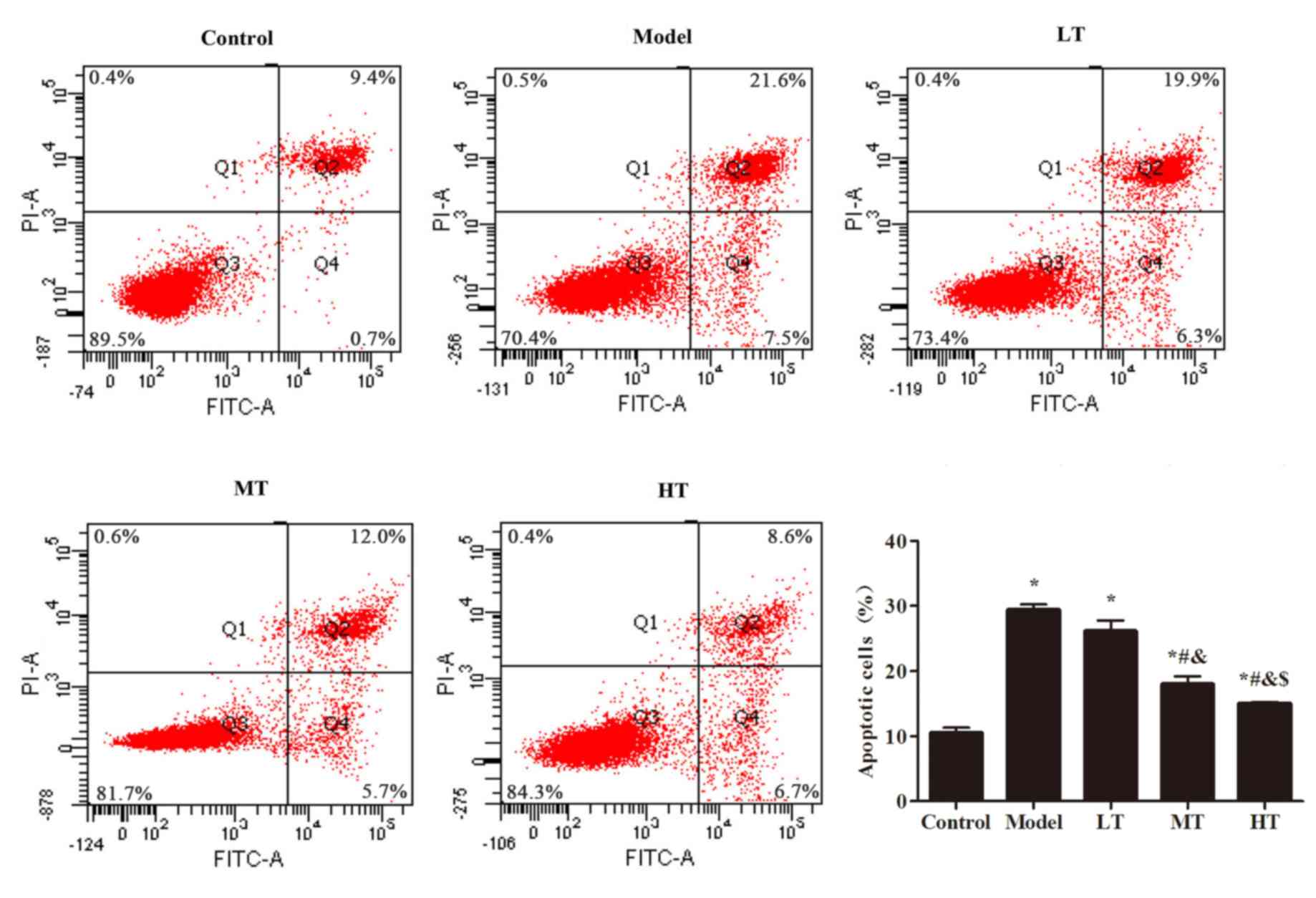
control group (P<0.05; Fig. 5).
Among the berberine-treated groups, apoptosis levels in the MT and
HT groups were significantly lower compared with the model group
(P<0.05; Fig. 5).
Berberine reduces the levels of
oxidative stress and apoptosis markers
Expression levels of SOD and eNOS in the model group
were significantly lower compared with those in the control group
(P<0.05; Fig. 6). The Bax/Bcl2
ratio was significantly higher in the model group compared with
that in the control group, which indicated a higher apoptotic rate.
The Bax/Bcl2 ratio decreased significantly in the berberine-treated
groups in a dose-dependent manner (P<0.05). Similarly,
caspase-3, cytochrome C and caspase-12 exhibited significant
increases in expression levels in the model group compared with the
control group (P<0.05), whereas the berberine-treated groups
exhibited decreased levels of expression of these proteins compared
with the model group in a dose-dependent manner (P<0.05).
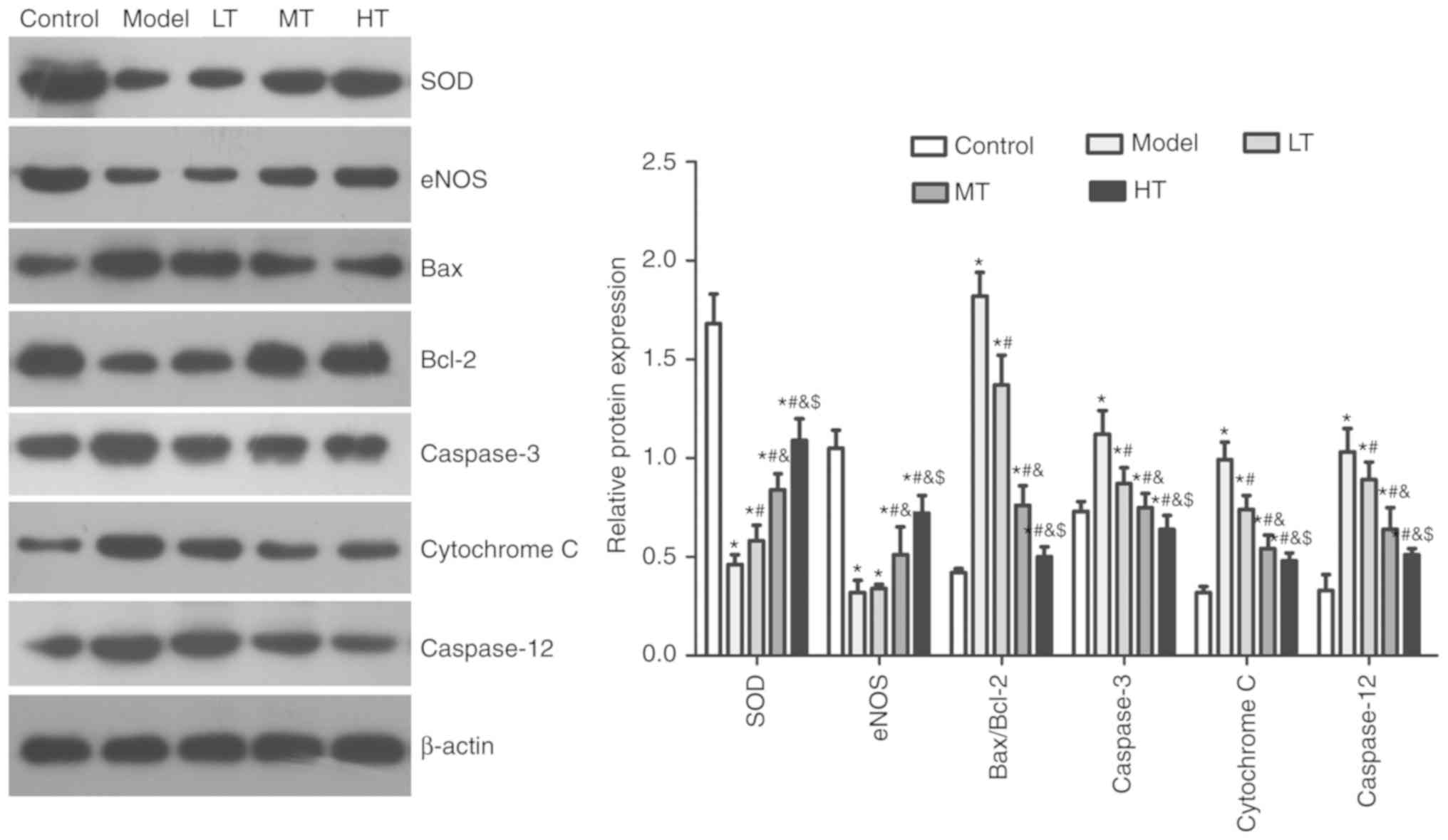 | Figure 6.Berberine affects oxidative stress
and apoptosis marker expression in an oxidative stress model.
Effects of low-, medium- and high-dose berberine on apoptosis
marker expression in H2O2-treated vaginal
epithelial cells were determined western blotting. *P<0.05 vs.
control, #P<0.05 vs. model, &P<0.05
vs. LT, $P<0.05 vs. MT. Model, cells treated with
H2O2; LT, low-dose berberine treatment; MD,
medium-dose berberine treatment; HT, high-dose berberine treatment;
SOD, superoxide dismutase; eNOS, endothelial nitric oxide
synthase. |
Discussion
SOD and CAT are important antioxidative enzymes that
are responsible for reducing oxygen species that are chemically
active such as O2·−, hydroxyl radical and
H2O2. In the present study, patients with BV
exhibited a higher level of oxidative stress in vaginal discharge,
based on the supressed levels of SOD and CAT activities and the
significantly higher levels of H2O2 and MDA
compared with healthy subjects. Following berberine treatment,
activity levels of SOD and CAT were significantly increased, which
resulted in a reduction of the level of H2O2
and possibly other ROS. Reduced levels of ROS also lead to less
lipid peroxidation as exhibited by reduced MDA level following
treatment. Taken together, these findings suggest that berberine
may be effective in reducing oxidative stress in vaginal discharge
from patients with BV. This is similar to the results from a
previous study on other diseases such as diabetes mellitus, in
which antioxidative effects of berberine were also described
(15).
The Bcl-2 family of intracellular proteins regulates
programmed cell death by activating the caspase family (34). Bax and Bcl-2 are integral membrane
proteins on the mitochondrial membrane (30). Bcl-2 is an inhibitor of apoptosis,
whereas Bax promotes cell death (30). The Bax/Bcl2 ratio was significantly
higher in vaginal epithelial cells in patients prior to treatment
when compared with that in healthy subjects, indicating a higher
level of apoptosis. A previous study has suggested that Bcl-2 is
responsible for maintaining the integrity of the mitochondrial
membrane, whereas Bax contributes to the formation of channels for
the release of cytochrome C from within the mitochondria, which
activates the caspase cascade for cell death (34). In the present study, berberine
treatment was able to lower the Bax/Bcl2 ratio significantly, which
in turn lowered the levels of the apoptosis-associated proteins
caspase-3, caspase-12 and cytochrome C.
Results from the in vitro experiment in the
present study using human vaginal epithelial cells further
supported the antiapoptotic effects of berberine. Similar to the
in vivo study, berberine-treated cells exhibited a
significantly lower Bax/Bcl2 ratio, which reduced the release of
cytochrome C from the mitochondria and the activation of the
caspase family. In addition, the levels of SOD and eNOS were
significantly higher in the treated groups compared with the model
group, suggesting less oxidative stress and better protection
against oxidative damage for cells. The treatment groups exhibited
lower levels of ROS and apoptosis and increased cell viability
compared with the model group. These differences increased with
higher doses of berberine. These results further supported the
antioxidative and antiapoptotic effects of berberine.
Marked improvements were observed in all documented
clinical symptoms of 180 patients with BV following berberine
treatment. These improvements included lower incidence of yellowish
leukorrhea, vaginal itching, vaginal pain, coloration and smell
compared with that prior to the treatment. Therefore, by reducing
the level of oxidative stress and programmed cell death, berberine
treatment may be effective in improving the clinical symptoms of
patients with BV. The 92.82% BV cure rate based on the Nugent score
suggested a higher efficacy of berberine treatment compared with
the current standard antibiotic treatments (75–86%) (6). Although berberine treatment raises no
concerns about antibiotic resistance, further clinical studies are
needed to examine BV recurrence following treatment and to
determine the optimal dosage for possible shorter treatment
periods. In addition, further in vitro studies are required
to investigate the detailed mechanisms of the effects of berberine,
such as its effects on gene expression, and to provide insight for
possible treatment options for other types of vaginitis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Traditional
Chinese Medicine Science and Technology Development Plan Project of
Shandong Province (grant no. 2017-368).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
WW designed the experiments. XM and JD performed the
experiments, analyzed all data and were major contributors in
writing the manuscript. XC and QC performed a part of the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Ethics
Committee of Yantai Hospital of Traditional Chinese Medicine. All
procedures performed in studies involving human participants were
in accordance with the ethical standards of Yantai Hospital of
Traditional Chinese Medicine and with the 1964 Declaration of
Helsinki and its later amendments or comparable ethical standards.
Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nyirjesy P: Management of persistent
vaginitis. Obstet Gynecol. 124:1135–1146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atashili J, Poole C, Ndumbe PM, Adimora AA
and Smith JS: Bacterial vaginosis and HIV acquisition: A
meta-analysis of published studies. AIDS. 22:1493–1501. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brocklehurst P, Gordon A, Heatley E and
Milan SJ: Antibiotics for treating bacterial vaginosis in
pregnancy. Cochrane Database Syst Rev CD000262. 2013. View Article : Google Scholar
|
|
4
|
Peters BM, Yano J, Noverr MC and Fidel PL
Jr: Candida vaginitis: When opportunism knocks, the host responds.
PLoS Pathog. 10:e10039652014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donders GG, Zodzika J and Rezeberga D:
Treatment of bacterial vaginosis: What we have and what we miss.
Expert Opin Pharmacother. 15:645–657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Voorspoels J, Casteels M, Remon JP and
Temmerman M: Local treatment of bacterial vaginosis with a
bioadhesive metronidazole tablet. Eur J Obstet Gynecol Reprod Biol.
105:64–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paavonen J, Mangioni C, Martin MA and
Wajszczuk CP: Vaginal clindamycin and oral metronidazole for
bacterial vaginosis: A randomized trial. Obstet Gynecol.
96:256–260. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livengood CH 3rd, Ferris DG, Wiesenfeld
HC, Hillier SL, Soper DE, Nyirjesy P, Marrazzo J, Chatwani A, Fine
P, Sobel J, et al: Effectiveness of two tinidazole regimens in
treatment of bacterial vaginosis: A randomized controlled trial.
Obstet Gynecol. 110:302–309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brandt M, Abels C, May T, Lohmann K,
Schmidts-Winkler I and Hoyme UB: Intravaginally applied
metronidazole is as effective as orally applied in the treatment of
bacterial vaginosis, but exhibits significantly less side effects.
Eur J Obstet Gynecol Reprod Biol. 141:158–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanson JM, McGregor JA, Hillier SL,
Eschenbach DA, Kreutner AK, Galask RP and Martens M: Metronidazole
for bacterial vaginosis. A comparison of vaginal gel vs. oral
therapy. J Reprod Med. 45:889–896. 2000.PubMed/NCBI
|
|
11
|
Nagaraja P: Antibiotic resistance of
Gardnerella vaginalis in recurrent bacterial vaginosis. Indian J
Med Microbiol. 26:155–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bahar H, Torun MM, Oçer F and Kocazeybek
B: Mobiluncus species in gynaecological and obstetric infections:
Antimicrobial resistance and prevalence in a Turkish population.
Int J Antimicrob Agents. 25:268–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beigi RH, Austin MN, Meyn LA, Krohn MA and
Hillier SL: Antimicrobial resistance associated with the treatment
of bacterial vaginosis. Am J Obstet Gynecol. 191:1124–1129. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abd El-Wahab AE, Ghareeb DA, Sarhan EE,
Abu-Serie MM and El Demellawy MA: In vitro biological assessment of
Berberis vulgaris and its active constituent, berberine:
Antioxidants, anti-acetylcholinesterase, anti-diabetic and
anticancer effects. BMC Complement Altern Med. 13:2182013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Geng YN, Jiang JD and Kong WJ:
Antioxidant and anti-inflammatory activities of berberine in the
treatment of diabetes mellitus. Evid Based Complement Alternat Med.
2014:2892642014.PubMed/NCBI
|
|
16
|
Dong H, Zhao Y, Zhao L and Lu F: The
effects of berberine on blood lipids: A systemic review and
meta-analysis of randomized controlled trials. Planta Med.
79:437–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Domitrović R, Cvijanović O, Pernjak-Pugel
E, Skoda M, Mikelić L and Crnčević-Orlić Z: Berberine exerts
nephroprotective effect against cisplatin-induced kidney damage
through inhibition of oxidative/nitrosative stress, inflammation,
autophagy and apoptosis. Food Chem Toxicol. 62:397–406. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao J, Kong W and Jiang J: Learning from
berberine: Treating chronic diseases through multiple targets. Sci
China Life Sci. 58:854–859. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Duan X, Yang J, Beeching JR and
Zhang P: Enhanced reactive oxygen species scavenging by
overproduction of superoxide dismutase and catalase delays
postharvest physiological deterioration of cassava storage roots.
Plant Physiol. 161:1517–1528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iwase T, Tajima A, Sugimoto S, Okuda K,
Hironaka I, Kamata Y, Takada K and Mizunoe Y: A simple assay for
measuring catalase activity: A visual approach. Sci Rep.
3:30812013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
King AL, Polhemus DJ, Bhushan S, Otsuka H,
Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, et
al: Hydrogen sulfide cytoprotective signaling is endothelial nitric
oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA.
111:3182–3187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rochette L, Lorin J, Zeller M, Guilland
JC, Lorgis L, Cottin Y and Vergely C: Nitric oxide synthase
inhibition and oxidative stress in cardiovascular diseases:
Possible therapeutic targets? Pharmacol Ther. 140:239–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mao K, Chen S, Chen M, Ma Y, Wang Y, Huang
B, He Z, Zeng Y, Hu Y, Sun S, et al: Nitric oxide suppresses NLRP3
inflammasome activation and protects against LPS-induced septic
shock. Cell Res. 23:201–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cortese-Krott MM and Kelm M: Endothelial
nitric oxide synthase in red blood cells: Key to a new erythrocrine
function? Redox Biol. 2:251–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gill SS, Hasanuzzaman M, Nahar K, Macovei
A and Tuteja N: Importance of nitric oxide in cadmium stress
tolerance in crop plants. Plant Physiol Biochem. 63:254–261. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahmad A, Singhal U, Hossain MM, Islam N
and Rizvi I: The role of the endogenous antioxidant enzymes and
malondialdehyde in essential hypertension. J Clin Diagn Res.
7:987–990. 2013.PubMed/NCBI
|
|
28
|
Kwiecien S, Jasnos K, Magierowski M,
Sliwowski Z, Pajdo R, Brzozowski B, Mach T, Wojcik D and Brzozowski
T: Lipid peroxidation, reactive oxygen species and antioxidative
factors in the pathogenesis of gastric mucosal lesions and
mechanism of protection against oxidative stress-induced gastric
injury. J Physiol Pharmacol. 65:613–622. 2014.PubMed/NCBI
|
|
29
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia G, Wang Q, Wang R, Deng D, Xue L, Shao
N, Zhang Y, Xia X, Zhi F and Yang Y: Tubeimoside-1 induces glioma
apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome
C/Caspase-3 pathway. Onco Targets Ther. 8:303–311. 2015.PubMed/NCBI
|
|
31
|
Hoshyar R, Bathaie SZ and Sadeghizadeh M:
Crocin triggers the apoptosis through increasing the Bax/Bcl-2
ratio and caspase activation in human gastric adenocarcinoma, AGS,
cells. DNA Cell Biol. 32:50–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim ME, Ha TK, Yoon JH and Lee JS:
Myricetin induces cell death of human colon cancer cells via
BAX/BCL2-dependent pathway. Anticancer Res. 34:701–706.
2014.PubMed/NCBI
|
|
33
|
Shirali S, Aghaei M, Shabani M, Fathi M,
Sohrabi M and Moeinifard M: Adenosine induces cell cycle arrest and
apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian
cancer cell line OVCAR-3. Tumour Biol. 34:1085–1095. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|