Introduction
The incidence of cardiovascular diseases has been on
the rise annually with the onset of aging. In particular, coronary
heart disease is becoming increasingly common (1,2).
Although percutaneous coronary intervention (PCI) is a commonly
used method to treat atherosclerotic coronary heart disease,
postoperative restenosis occurs in a considerable proportion of
angioplasty patients, leading to a reduction in the success rate of
PCI treatment whilst increasing the risk of new cardiovascular
complications (3). Indeed,
restenosis is an important factor in the long-term outcome of
post-angioplasty surgery (4), with
the incidence of restenosis potentially reaching between 30 and 50%
within 6 months post-surgery without preventive measures (5). Despite developments in novel technology
in combatting restenosis such as drug-eluting angioplasty balloons,
the occurrence of restenosis remains high (6) due to the mechanism of restenosis being
complex and not fully understood. Therefore, understanding the
molecular mechanisms underlying vascular remodeling following
injury is pivotal to the prevention of restenosis after PCI.
There is substantial evidence that aberrant
proliferation of the vascular neointima is central to the
pathophysiology of vascular lumen restenosis (7,8); in
which the phenotypic transformation of vascular smooth muscle cells
(VSMCs) is the main cause. Following transformation, VSMCs display
enhanced proliferative and migratory capabilities (9), but may exhibit different
characteristics depending on the status of their microenvironment
(10). In normal blood vessels,
VSMCs are highly differentiated cells that exhibit a strong
contractile phenotype, and serve to maintain and regulate vascular
tone to stabilize blood pressure (11). However, under pathological
conditions, including atherosclerosis and in-stent restenosis,
VSMCs begin transforming into a more synthetic phenotype,
characterized by high levels of proliferation and migration with a
concomitant reduction in contractility (12,13).
This transition results in intima thickening of the arteries, which
is a common pathogenesis in the formation of multiple vascular
lesions in vivo (14).
Therefore, understanding the changes in the proliferative and
migratory capabilities of VSMCs has important implications for the
prevention and treatment of these diseases.
Platelet-derived growth factors (PDGFs) are strong
mitogens for VSMCs in blood vessels (15). PDGFs function in many vascular
pathophysiological processes, such as atherosclerosis, restenosis
and angiogenesis (16). PDGFs
regulate cell proliferation, cell migration and the production of
inflammatory mediators, to maintain tissue permeability and
hemodynamics, through modulation of several transcription factors
and key molecular signaling pathways (17). PDGF receptor signaling can activate
cell proliferation and migration, protein production or secretion,
and phenotypic modulation of VSMCs (18). Therefore, in the present study,
PDGF-BB, the main isoform of PDGFs, was selected to induce VSMC
dedifferentiation.
Collagen type VI α1 chain (COL6A1) assists in the
synthesis of collagen VI (COL6), which is a component of the
extracellular matrix and forms distinct microfibrillar networks in
the connective tissues of blood vessels and muscles (19,20).
High COL6A1 has been previously associated with hypertension, which
is a main risk factor for cardiovascular diseases (21), whilst another study showed
upregulated COL6A1 expression in the vascular tissues of patients
with atherosclerosis (22). In
addition, COL6A1 was identified as a metastasis-associated protein
using quantitative secretome analysis (23) In light of these reports, it was
therefore hypothesized that COL6A1 may serve important roles in the
synthetic phenotype of VSMCs and the pathogenesis of vascular lumen
restenosis. Therefore, in the present study, a COL6A1 silencing
vector was constructed and transfected into aortic VSMCs to study
the effects of COL6A1 on VSMC proliferation and invasion.
Materials and methods
Cell transfection
T/G Human aortic vascular smooth muscle cells (T/G
HA-VSMC; HA-VSMC thereafter; cat. no. CL-0452; Procell Life Science
& Technology Co., Ltd.) were cultured in Medium 231 (Life
Technologies; Thermo Fisher Scientific, Inc.) supplemented with 5%
smooth muscle growth supplement (SMGS; Thermo Fisher Scientific,
Inc.) at 37°C under 5% CO2. Fresh culture medium was
changed every two days. HA-VSMCs (1×105 cells) were then
transfected with 0.25 µg siRNA specific for COL6A1 (si-COL6A1) or
siRNA-control (EV; both purchased from Shanghai GenePharma Co.,
Ltd.) using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to manufacturer's protocol, the
cells were named si-COL6A1 and EV cells thereafter. The sequences
of si-COL6A1 were: Forward, 5′-CCCACCUGAAGGAGAAUAAUU-3′ and
reverse, 5′-UUAUUCUCCUUCAGGUGGGUU-3′. The sequences of EV were:
Forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. Cells without transfection were
designated as the control group (Cntl). Transfection efficiency was
determined by measuring COL6A1 mRNA and protein levels after 48 h
of transfection.
Cell treatment
Each experiment was designed such that one group of
cells received PDGF-BB and the other group did not. In
PDGF-BB-treated groups, Cntl, EV and si-COL6A1 cells
(5×105) were seeded into a six-well plate for 24 h, then
stimulated with 20 ng/ml platelet-derived growth factor (PDGF-BB;
PeproTech, Inc.) diluted in Medium 231 supplemented 5% SMGS for 12,
24 and 48 h at 37°C.
Cell viability assay
Cell viability was determined using Cell counting
kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.). Cntl, EV and
si-COL6A1 cells were seeded into a 96-well plate at
5×103 cells/well and cultured for 12, 24 or 48 h at 37°C
with or without 20 ng/ml PDGF-BB. Following the addition of 20 µl
CCK-8 reagent/well and a further 1 h incubation, cell viability was
measured by obtaining the optical density values at 450 nm for each
well using a microplate reader (Thermo Fisher Scientific,
Inc.).
Wound healing assay
HA-VSMC migration was measured using wound healing
assay. Cntl, EV and si-COL6A1 cells treated or untreated with 20
ng/ml PDGF-BB were inoculated into a 12-well plate at
1×105 cells/well in Medium 231 supplemented with SMGS,
and gently scratched to form a cell-free area. The cells were then
incubated for 24 h at 37°C. The width of each wound was measured
using an Olympus DSX100 light microscope (Olympus Coporation;
magnification, ×200).
Cell invasion assay
The invasive abilities of HA-VSMCs were measured
using 24-well Transwell® chambers with 8-µm pore filters
(Corning Inc). Cntl, EV and si-COL6A1 cells (5×104
cells) treated or untreated with 20 ng/ml PDGF-BB, diluted in
Medium 231 supplemented with SMGS, were seeded into the
Matrigel® GFR (BD Biosciences)-coated
Transwell® upper chambers, with the coating process at
37°C for 30 min. The lower chambers were filled with Medium 231
supplemented with SMGS. Following 24 h incubation, the Transwell
membranes were stained using 0.1% crystal violet for 30 min at
37°C. The number of invasive cells in random 5 fields was then
calculated from images captured using Olympus DSX100 optical
microscope (Olympus Corporation; magnification, ×200).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from Cntl, EV and si-COL6A1
cells (5×105 cells) treated or untreated with 20 ng/ml
PDGF-BB using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol. cDNA was obtained using
High-capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to manufacturer's
protocol. The mRNA expression levels of factors associated with
metastasis, including fibronectin (FN), matrix metalloproteinase
(MMP)-2, MMP-9 and tissue inhibitor of metalloproteinases 2
(TIMP-2) were then measured using Fast SYBR® Green
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol, in an Applied Biosystems 7300
thermocycler (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The following thermocycling conditions were used: Initial
denaturation at 94°C for 25 sec; 35 cycles of 94°C for 25 sec, 60°C
for 25 sec and 72°C for 30 sec; and final extension at 72°C for 5
min. The quantification was performed using the 2−ΔΔCq
method (24). Β-actin was used as
internal control and the primer sequences were listed in Table I.
 | Table I.The primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
The primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′-3′) |
|---|
| β-actin | Forward:
GTGGACATCCGCAAAGAC |
|
| Reverse:
GAAAGGGTGTAACGCAACT |
| FN | Forward:
ACAACACCGAGGTGACTGAGAC |
|
| Reverse:
GGACACAACGATGCTTCCTGAG |
| MMP-2 | Forward:
CAGCCCTGCAAGTTTCCATT |
|
| Reverse:
GTTGCCCAGGAAAGTGAAGG |
| MMP-9 | Forward:
GAGACTCTACACCCAGGACG |
|
| Reverse:
GAAAGTGAAGGGGAAGACGC |
Western blot analysis
Cntl, EV and si-COL6A1 cells (5×105
cells) treated or untreated with 20 ng/ml PDGF-BB were lysed using
RIPA lysis buffer (Boster Biological Technology) for 20 min, with
the proteins quantified using Bicinchoninic Acid protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). All proteins were
subsequently separated at 10 µg/lane by 15% SDS-PAGE and
transferred onto a PVDF membrane (Millipore; Merck KGaA). The
membranes were blocked using 5% non-fat dry milk in PBS, at 37°C
for 1 h, before being probed with primary antibodies specific for
COL6A1 (cat. no. ab151422; 1:1,000), FN (cat. no. ab23750;
1:1,000), MMP-2 (cat. no. ab37150; 1:1,000), MMP-9 (cat. no.
ab73734; 1:1,000), TIMP-2 (cat. no. ab180630; 1:1,000), protein
kinase B (PKB/AKT; cat. no. ab8805; 1:500), phosphorylated (p)-AKT
(p-AKT; cat. no. ab38449; 1:1,000), mammalian target of rapamycin
(mTOR; cat. no. ab2732; 1:2,000), p-mTOR (p-mTOR; cat. no. ab84400;
1:500) and β-actin (cat. no. ab8227; 1:2,000) overnight at 4°C.
β-actin was used as loading control. Next, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (ab6721, 1:5,000) for 2 h at 37°C. All primary and
secondary antibodies were purchased from Abcam. The protein bands
were visualized using enhanced chemiluminescence reagents
(Millipore; Merck KGaA) and quantified using Bio-Rad ChemiDoc™
XRS+ System with Image Lab™ software (version 4.1;
Bio-Rad Laboratories, Inc.,).
Statistical analysis
SPSS 18.0 statistical software (SPSS, Inc.) was used
for statistical analyzes. Five repeats were conducted for each
experiment. Data were presented as the mean ± standard deviation.
One-way ANOVA was followed by Tukey's analysis for further
comparison. P<0.05 was considered to indicate a statistically
significant difference.
Results
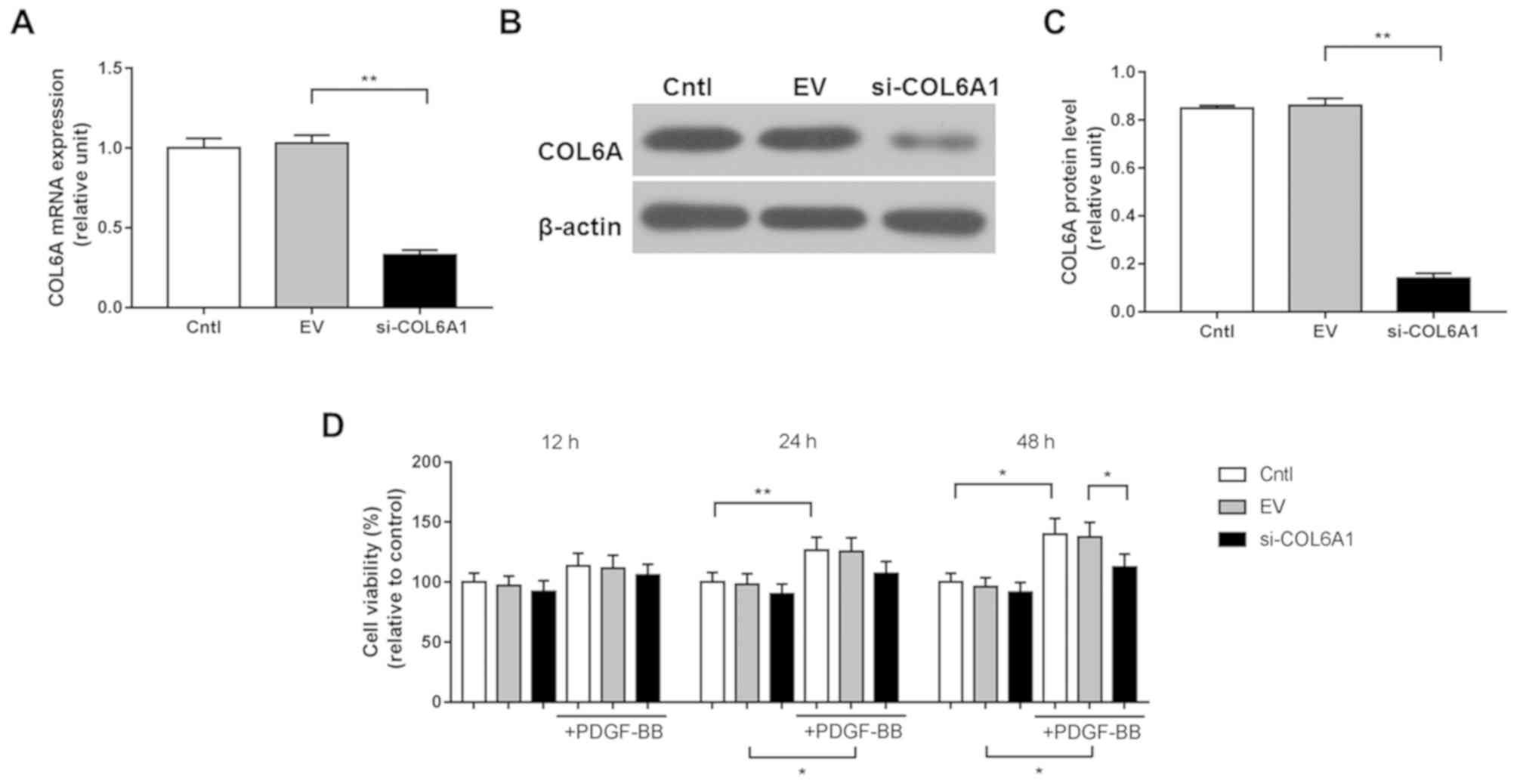
COL6A1 interference inhibits HA-VSMC
viability following PDGF-BB stimulation
HA-VSMCs were transfected with the si-COL6A1
recombinant plasmid before transfection efficiency was measured
using RT-qPCR and western blotting. The mRNA and protein levels of
COL6A1 were significantly downregulated in si-COL6A1 cells compared
with those in the negative control EV group (P<0.01; Fig. 1A-C). HA-VSMCs in the Cntl, EV and
si-COL6A1 groups were subsequently stimulated with PDGF-BB for 12,
24 and 48 h, before cell viability was assessed using CCK-8 assay.
PDGF-BB stimulation significantly increased HA-VSMC viability in
the Cntl and EV groups after 24 and 48 h (P<0.05; Fig. 1D). There was no significant
difference between the si-COL6A1 group in the absence of PDGF-BB
and the si-COL6A1 group in the presence of PDGF-BB, at 24 and 48 h
(Fig. 1D). No significant
differences were observed in cell viability measured between Cntl,
EV and si-COL6A1 groups after 12, 24 or 48 h in the absence of
PDGF-BB treatment. These results demonstrate that PDGF-BB
stimulation improved HA-VSMC viability, which can be negated by
COL6A1 knockdown.
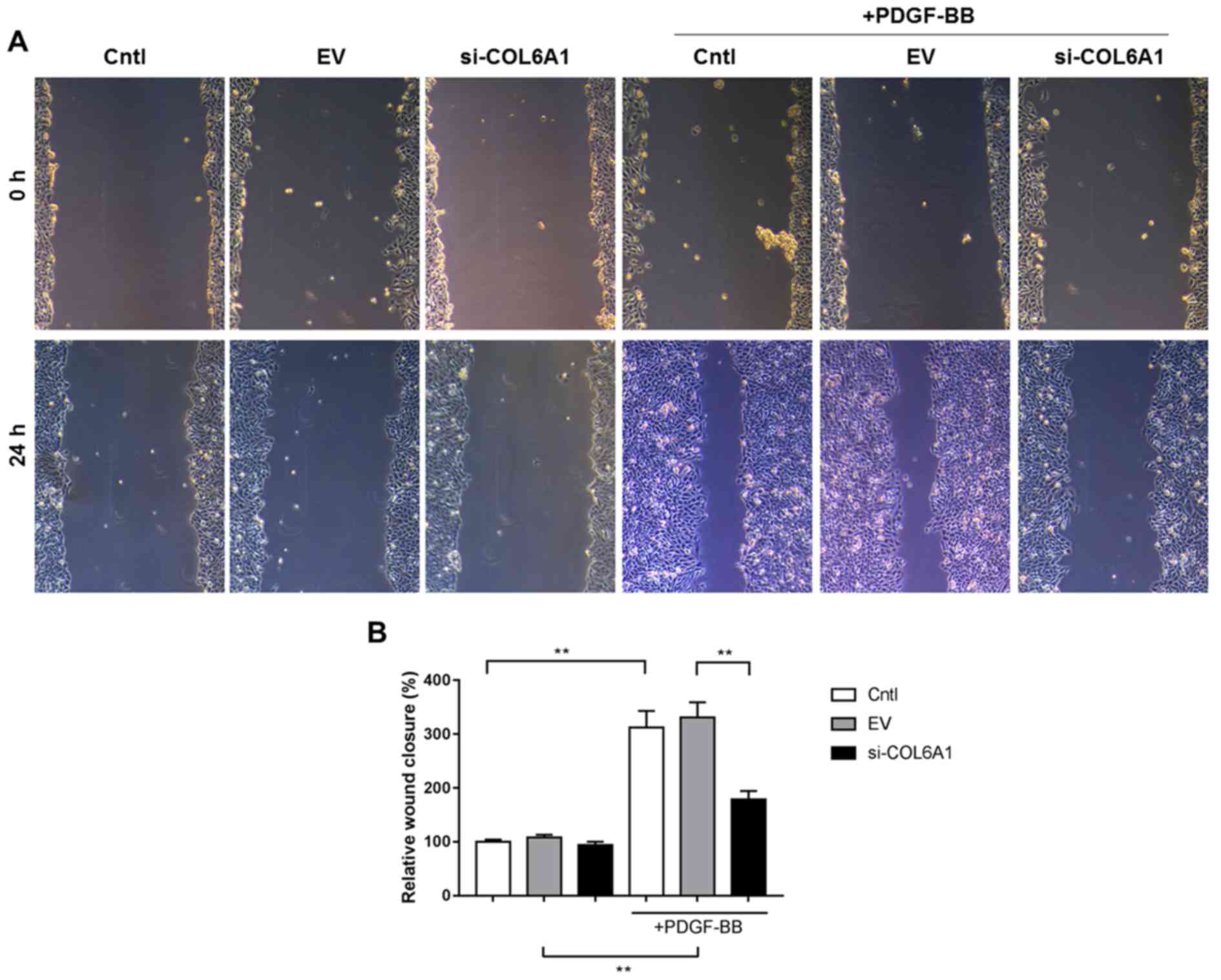
HA-VSMC migration is inhibited by
COL6A1 interference following PDGF-BB stimulation
HA-VSMC migration was assessed using wound healing
assay. PDGF-BB also appeared to have SIGNIFICANTLY increased
HA-VSMC migration si-COL6A1 cell group, albeit not to the same
magnitude as the other two conditions (P<0.01; Fig. 2A and B). No significant differences
were found between Cntl, EV and si-COL6A1 cell migration in the
absence of PDGF-BB stimulation.
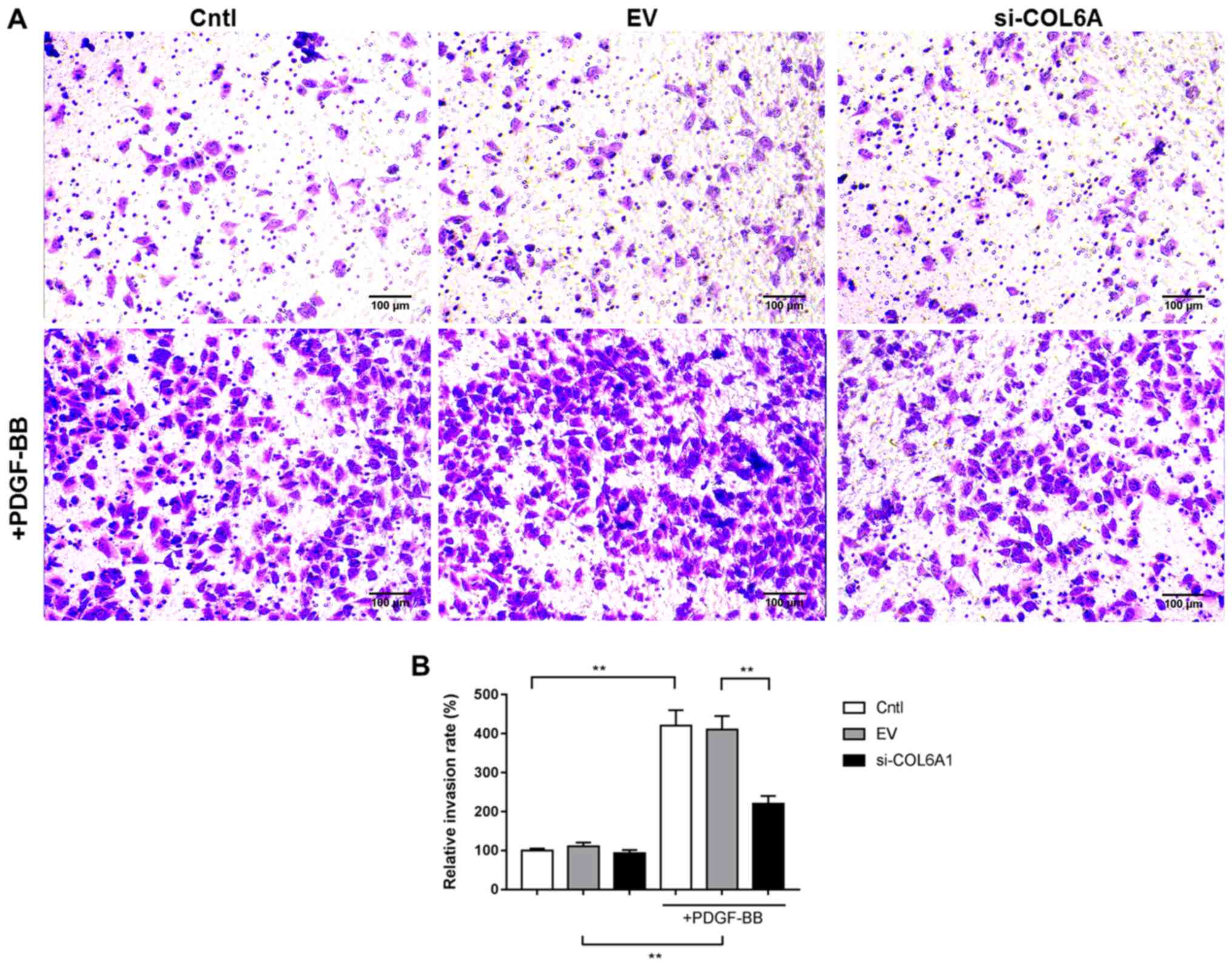
COL6A1 interference inhibits HA-VSMC
invasion following PDGF-BB stimulation
HA-VSMC invasion was measured using Matrigel-coated
Transwell assays. PDGF-BB stimulation significantly increased the
invasive capabilities of Cntl and EV cells, but the extent of
increase in the si-COL6A1 cell group was significantly lower
compared with the corresponding EV group (P<0.01; Fig. 3A and B). In the absence of PDGF-BB,
no significant differences were observed between the invasive
abilities of Cntl, EV and si-COL6A1 cells.
COL6A1 regulates the expression of
factors associated with migration/invasion in HA-VSMCs stimulated
by PDGF-BB
RT-qPCR and western blot analysis were used to
measure the expression levels of factors associated with
migration/invasion in HA-VSMCs following PDGF-BB stimulation.
PDGF-BB treatment significantly upregulated the expression levels
of FN, MMP-2 and MMP-9 mRNA and protein in Cntl and EV cells, but
the scale of this upregulation in si-COL6A1 cells was significantly
lower compared with corresponding EV cells (P<0.05; Fig. 4A-C and E-H). By contrast, PDGF-BB
treatment significantly reduced TIMP-2 expression in Cntl and EV
cells, but the extent of reduction was significantly smaller in
si-COL6A1 cells compared with EV cells. (P<0.01; Fig. 4D, E and I).
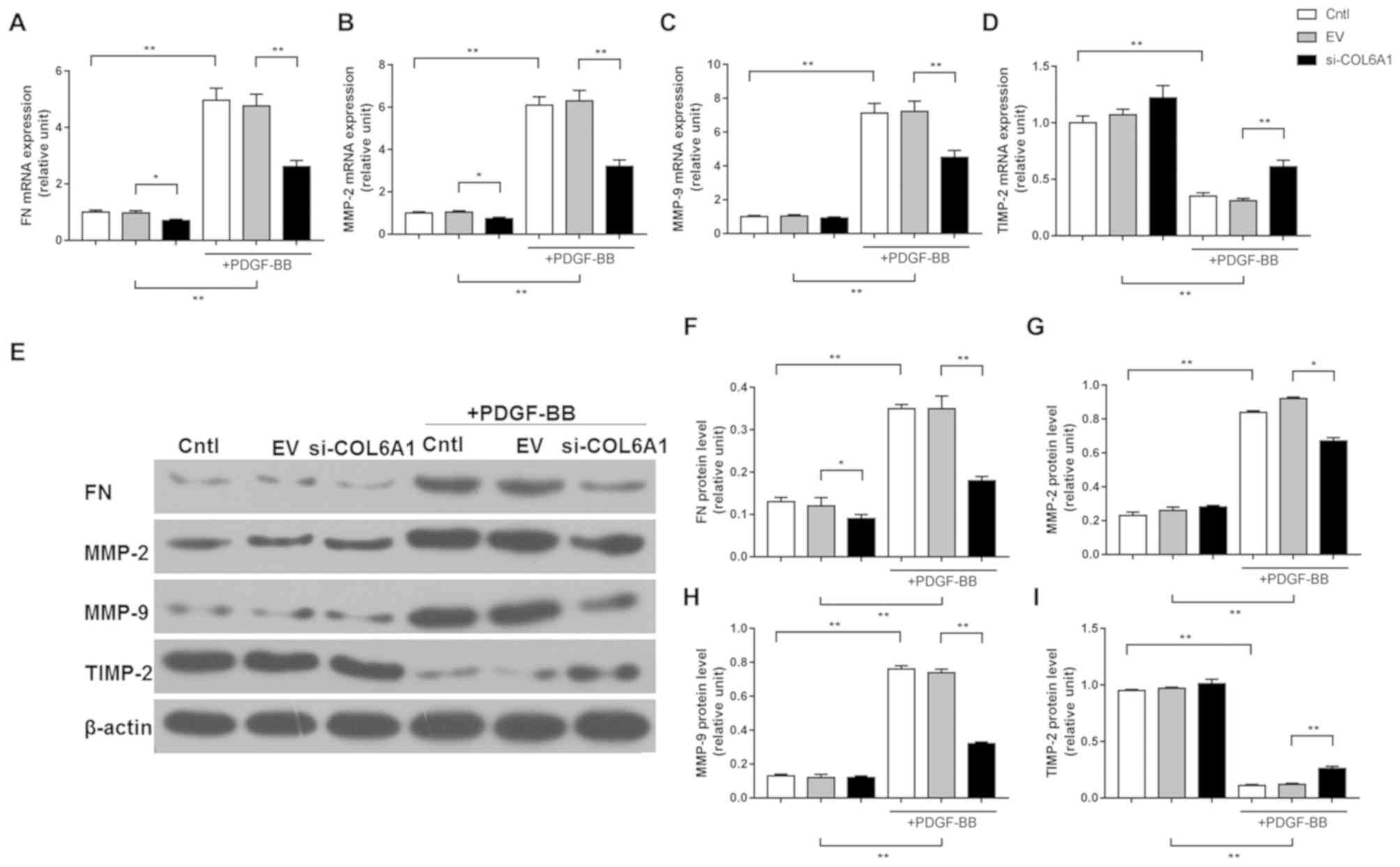 | Figure 4.COL6A1 regulate the expression of
factors associated with migration and invasion in HA-VSMCs
following PDGF-BB stimulation. (A) Reverse
transcription-quantitative PCR was performed to determine the
levels of mRNA expression of FN, (B) MMP-2, (C) MMP-9 and (D)
TIMP-2 after si-COL6A1 transfection and PDGF-BB stimulation. (E-I)
Western blot analysis was performed to determine the protein
expression levels of (F) FN, (G) MMP-2, (H) MMP-9 and (I) TIMP-2
after si-COL6A1 transfection and PDGF-BB stimulation. Each
experiment was repeated five times. *P<0.05, and **P<0.01.
HA-VSMC, human aortic-vascular smooth muscle cells; COL6A1,
collagen type VI α1 chain; PDGF-BB, platelet-derived growth factor;
FN, fibronectin; MMP, matrix metalloproteinase; TIMP-2, tissue
inhibitor of metalloproteinase-2; si, short interfering; Cntl,
control; EV, siRNA-control. |
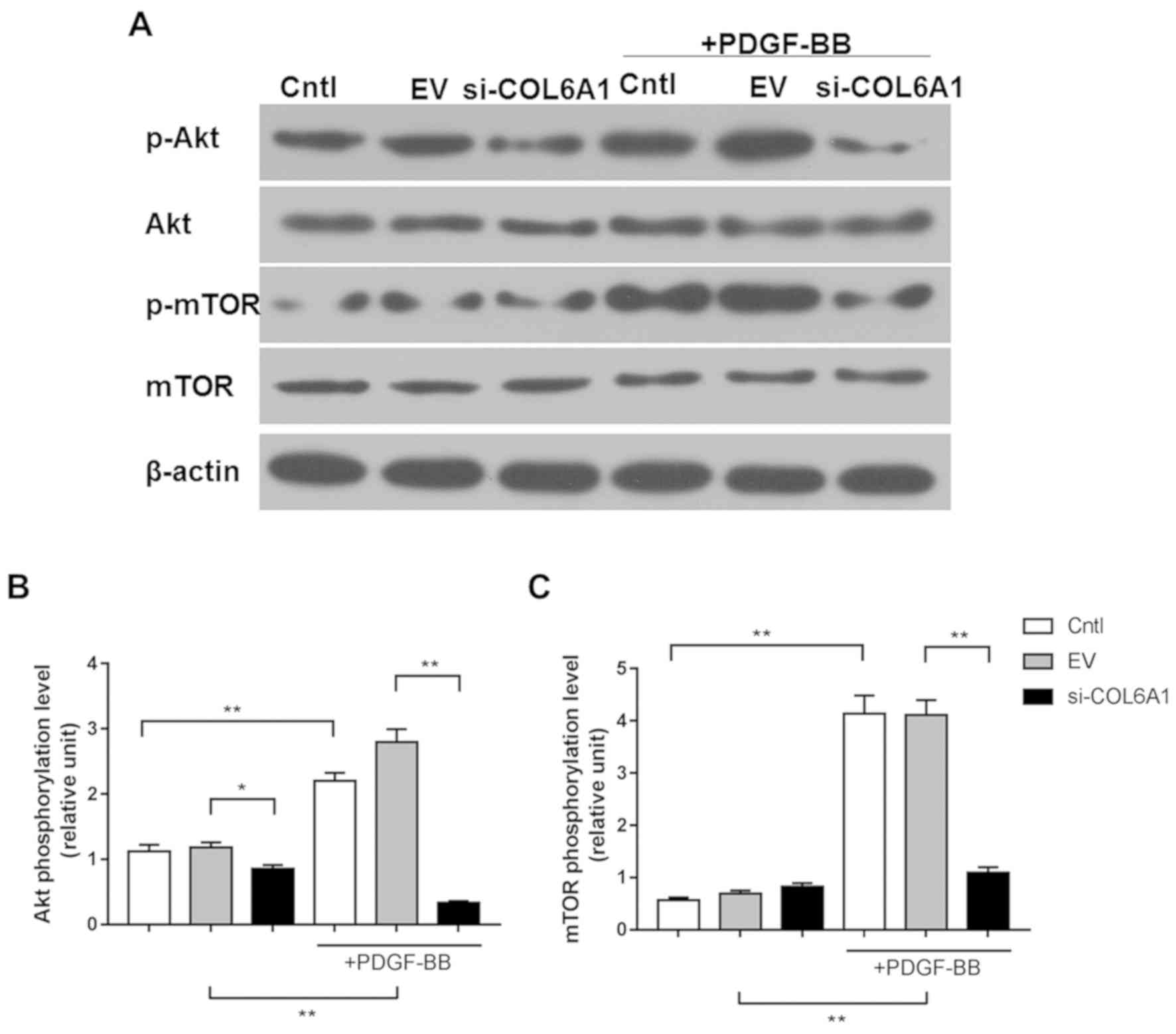
COL6A1 interference inhibits the
AKT-mTOR pathway in HA-VSMCs following PDGF-BB stimulation
AKT and mTOR phosphorylation in Cntl, EV and
si-COL6A1 cells following PDGF-BB treatment were next measured
using western blotting. p-AKT expression was reduced in the
si-COL6A1 group in the absence of PDGF-BB, compared to the other
two groups (P<0.05). Relative AKT and mTOR phosphorylation were
significantly increased in Cntl and EV cells in response to PDGF-BB
stimulation, but not in si-COL6A1 cells (P<0.01; Fig. 5A-C).
Discussion
The phenotypic transformation of VSMCs serves an
important pathophysiological role in neointimal hyperplasia after
vascular injury and luminal stenosis, which is dependent upon
conditions within its microenvironment (25,26).
PDGF-BB is an efficient mitogen stimulator of VSMCs that promote
the dedifferentiation, proliferation and migration of VSMCs during
vascular injury repair (27).
Therefore, PDGF-BB was elected as the agonist in the present study
to facilitate the phenotypic transformation of VSMCs. HA-VSMC
viability, migratory and invasive capabilities were all found to be
promoted by PDGF-BB stimulation.
COL6A1 is a protein associated with metastasis of
cervical cancer (19) and, by
influencing blood pressure, it is also a risk factor for
cardiovascular diseases (28). In
the present study, COL6A1 expression was knocked down in HA-VSMCs
to study the function of COL6A1 on HA-VSMCs following PDGF-BB
stimulation. It was found that cell viability, migratory and
invasive abilities of VSMCs were all significantly potentiated by
PDGF-BB treatment, which were partially negated by COL6A1
silencing. RT-qPCR and western blot analysis were subsequently
performed to investigate the downstream effects of COL6A1 knockdown
on PDGF-BB-stimulated VSMCs, specifically the expression of FN,
MMP-2, MMP-9 and TIMP-2, classical factors associated with
migration and invasion (29).
PDGF-BB promoted FN, MMP-2 and MMP-9 expression whilst
downregulating TIMP-2 expression; all of which were partially
reversed by COL6A1 knockdown.
FN is an important component of the extracellular
matrix which is upregulated in renal cell carcinoma cells (30). Non-enzymatic glycation interferes
with FN-integrin interactions in VSMC and glycation of FN shifts
the nature of cellular adhesion from integrin- to receptor for
advanced glycation end products-dependent mechanisms (31). MMPs are proteolytic enzymes that
require calcium, zinc and other metal ions as cofactors (32,33).
MMP-9 was reported to be associated with cell proliferation and
migration of VSMCs (34). In
particular, MMP-2 and MMP-9 serve important roles in the
proliferation and migration of VSMCs (35). During VSMC proliferation and
migration, the outer membrane of VSMCs interacts with the
extracellular matrix to release MMPs for subsequent degradation
(36). In contrast, TIMP-2 is a key
inhibitor of MMPs and suppresses cell migration/invasion by
inhibiting the function of MMPs (37). In the present study, the reduction in
FN, MMP-2 and MMP-9 expression, in conjunction with the increase in
TIMP-2 expression were caused by COL6A1 knockdown in VSMCs.
The PI3K/AKT signaling pathway commonly serves roles
in a number of physiological processes, including cell
proliferation, migration, invasion and angiogenesis (38,39).
Importantly, it is also the central signaling component of PDGF-BB
induced invasion in VSMCs. PDGF-BB-induced cell growth and
migration of human airway smooth muscle (40), retinal pigment epithelial (41) and endothelial progenitor cells
(42) were all closely associated
with the PI3K/AKT pathway. The activation of mTOR has also been
reported in the PDGF-BB-induced proliferation and migration of
VSMCs (43–45). In the present study, the
phosphorylation levels of AKT and mTOR were significantly increased
by PDGF-BB treatment in VSMCs, consistent with data from the
previous studies aforementioned. The silencing of COL6A1 potently
reversed PDGF-BB-induced activation of AKT and mTOR in VSMCs. These
observations suggest that COL6A1 knockdown inhibited VSMC
viability, migration and invasion by suppressing Akt/mTOR
activation.
In conclusion, the silencing of COL6A1 inhibited
cell viability, migration and invasion of PDGF-BB-stimulated VSMCs
by suppressing the expression of MMPs and Akt/mTOR activation. This
suggest that COL6A1 may be a potential therapeutic target in the
treatment of cardiovascular diseases, but this needs to be
investigated further.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author upon reasonable
request.
Authors' contributions
ZC and QW performed PCR and western blotting assays.
CY performed the remaining assays. JD conceived and designed the
study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tian Y, Deng P, Li B, Wang J, Li J, Huang
Y and Zheng Y: Treatment models of cardiac rehabilitation in
patients with coronary heart disease and related factors affecting
patient compliance. Rev Cardiovasc Med. 20:27–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khamis RY, Ammari T and Mikhail GW: Gender
differences in coronary heart disease. Heart. 102:1142–1149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orme RC, Parker WAE, Thomas MR, Judge HM,
Baster K, Sumaya W, Morgan KP, McMellon HC, Richardson JD, Grech
ED, et al: Study of two dose regimens of ticagrelor compared with
clopidogrel in patients undergoing percutaneous coronary
intervention for stable coronary artery disease (STEEL-PCI).
Circulation. Jun 21–2018.(Epub ahead of print) doi:
10.1161/CIRCULATIONAHA.118.034790. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee MS, Cheng RK, Kandzari DE and Kirtane
AJ: Long-term outcomes of heart transplantation recipients with
transplant coronary artery disease who develop in-stent restenosis
after percutaneous coronary intervention. Am J Cardiol.
109:1729–1732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prasad K: Do statins have a role in
reduction/prevention of post-PCI restenosis? Cardiovasc Ther.
31:12–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang CY, Fang HY, Chen CJ, Yang CH, Wu CJ
and Lee WC: Comparison of clinical outcomes after drug-eluting
balloon and drug-eluting stent use for in-stent restenosis related
acute myocardial infarction: A retrospective study. PeerJ.
6:e46462018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee KJ, Park SH, Lee JY, Joo HC, Jang EH,
Youn YN and Ryu W: Perivascular biodegradable microneedle cuff for
reduction of neointima formation after vascular injury. J Control
Release. 192:174–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishimura S, Furuhashi M, Mita T, Fuseya T,
Watanabe Y, Hoshina K, Kokubu N, Inoue K, Yoshida H and Miura T:
Reduction of endoplasmic reticulum stress inhibits neointima
formation after vascular injury. Sci Rep. 4:69432014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guan H, Gao L, Zhu L, Yan L, Fu M, Chen C,
Dong X, Wang L, Huang K and Jiang H: Apigenin attenuates neointima
formation via suppression of vascular smooth muscle cell phenotypic
transformation. J Cell Biochem. 113:1198–1207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Chen J, Xu C, Yang J, Guo Q, Hu Q
and Jiang H: Resveratrol inhibits phenotypic switching of
neointimal vascular smooth muscle cells after balloon injury
through blockade of Notch pathway. J Cardiovasc Pharmacol.
63:233–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang L, Dai F, Liu Y, Yu X, Huang C, Wang
Y and Yao W: RhoA/ROCK signaling regulates smooth muscle phenotypic
modulation and vascular remodeling via the JNK pathway and vimentin
cytoskeleton. Pharmacol Res. 133:201–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang F, Chen Q, He S, Yang M, Maguire EM,
An W, Afzal TA, Luong LA, Zhang L and Xiao Q: miR-22 is a novel
mediator of vascular smooth muscle cell phenotypic modulation and
neointima formation. Circulation. 137:1824–1841. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hasanov Z, Ruckdeschel T, König C, Mogler
C, Kapel SS, Korn C, Spegg C, Eichwald V, Wieland M, Appak S and
Augustin HG: Endosialin promotes atherosclerosis through phenotypic
remodeling of vascular smooth muscle cells. Arterioscler Thromb
Vasc Biol. 37:495–505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi N, Li CX, Cui XB, Tomarev SI and Chen
SY: Olfactomedin 2 regulates smooth muscle phenotypic modulation
and vascular remodeling through mediating runt-related
transcription factor 2 binding to serum response factor.
Arterioscler Thromb Vasc Biol. 37:446–454. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang JC, Li GY, Wang B, Han SX, Sun X,
Jiang YN, Shen YW, Zhou C, Feng J, Lu SY, et al: Metformin inhibits
metastatic breast cancer progression and improves chemosensitivity
by inducing vessel normalization via PDGF-B downregulation. J Exp
Clin Cancer Res. 38:2352019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hellström M, Kalén M, Lindahl P, Abramsson
A and Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of
vascular smooth muscle cells and pericytes during embryonic blood
vessel formation in the mouse. Development. 126:3047–3055.
1999.PubMed/NCBI
|
|
17
|
Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao
T, Miyazaki H and Iwao H: Role of JNK, p38, and ERK in
platelet-derived growth factor-induced vascular proliferation,
migration, and gene expression. Arterioscler Thromb Vasc Biol.
23:795–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satoh K, Kikuchi N, Kurosawa R and
Shimokawa H: PDE1C negatively regulates growth factor receptor
degradation and promotes VSMC proliferation. Circ Res.
116:1098–1100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou T, Tong C, Kazobinka G, Zhang W, Huang
X, Huang Y and Zhang Y: Expression of COL6A1 predicts prognosis in
cervical cancer patients. Am J Transl Res. 8:2838–2844.
2016.PubMed/NCBI
|
|
20
|
Wan F, Wang H, Shen Y, Zhang H, Shi G, Zhu
Y, Dai B and Ye D: Upregulation of COL6A1 is predictive of poor
prognosis in clear cell renal cell carcinoma patients. Oncotarget.
6:27378–27387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nandakumar P, Lee D, Richard MA,
Tekola-Ayele F, Tayo BO, Ware E, Sung YJ, Salako B, Ogunniyi A, Gu
CC, et al: Rare coding variants associated with blood pressure
variation in 15 914 individuals of African ancestry. J Hypertens.
35:1381–1389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sleptsov AA, Nazarenko MS, Lebedev IN,
Skriabin NA, Frolov AV, Popov VA, Barbarash LS and Puzyrev VP:
Somatic genome variations in vascular tissues and peripheral blood
leukocytes in patients with atherosclerosis. Genetika. 50:986–995.
2014.(In Russian). PubMed/NCBI
|
|
23
|
Chiu KH, Chang YH, Wu YS, Lee SH and Liao
PC: Quantitative secretome analysis reveals that COL6A1 is a
metastasis-associated protein using stacking gel-aided purification
combined with iTRAQ labeling. J Proteome Res. 10:1110–1125. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang MJ, Zhou Y, Chen L, Wang YQ, Wang X,
Pi Y, Gao CY, Li JC and Zhang LL: An overview of potential
molecular mechanisms involved in VSMC phenotypic modulation.
Histochem Cell Biol. 145:119–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fellows BD, Ghobrial N, Mappus E, Hargett
A, Bolding M, Dean D and Mefford OT: In vitro studies of
heparin-coated magnetic nanoparticles for use in the treatment of
neointimal hyperplasia. Nanomedicine. 14:1191–1200. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen S, Liu B, Kong D, Li S, Li C, Wang H
and Sun Y: Atorvastatin calcium inhibits phenotypic modulation of
PDGF-BB-induced VSMCs via down-regulation the Akt signaling
pathway. PLoS One. 10:e01225772015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sato T, Takano R, Tokunaka K, Saiga K,
Tomura A, Sugihara H, Hayashi T, Imamura Y and Morita M: Type VI
collagen α1 chain polypeptide in non-triple helical form is an
alternative gene product of COL6A1. J Biochem. 164:173–181. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prado AF, Pernomian L, Azevedo A, Costa
RAP, Rizzi E, Ramos J, Paes Leme AF, Bendhack LM, Tanus-Santos JE
and Gerlach RF: Matrix metalloproteinase-2-induced epidermal growth
factor receptor transactivation impairs redox balance in vascular
smooth muscle cells and facilitates vascular contraction. Redox
Biol. 18:181–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ou YC, Li JR, Wang JD, Chang CY, Wu CC,
Chen WY, Kuan YH, Liao SL, Lu HC and Chen CJ: Fibronectin promotes
cell growth and migration in human renal cell carcinoma cells. Int
J Mol Sci. 20:E27922019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dhar S, Sun Z, Meininger GA and Hill MA:
Nonenzymatic glycation interferes with fibronectin-integrin
interactions in vascular smooth muscle cells. Microcirculation.
24:2017.doi: 10.1111/micc.12347. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hunter MC, O'Hagan KL, Kenyon A, Dhanani
KC, Prinsloo E and Edkins AL: Hsp90 binds directly to fibronectin
(FN) and inhibition reduces the extracellular fibronectin matrix in
breast cancer cells. PLoS One. 9:e868422014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang M, Fan Z, Wang F, Tian ZH, Ma B, Dong
B, Li Z, Zhang M and Zhao W: BMP-2 enhances the migration and
proliferation of hypoxia-induced VSMCs via actin cytoskeleton, CD44
and matrix metalloproteinase linkage. Exp Cell Res. 368:248–257.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin J, Xia W, Wu M, Zhang Y, Huang S,
Zhang A and Jia Z: Inhibition of mitochondrial complex I activity
attenuates neointimal hyperplasia by inhibiting smooth muscle cell
proliferation and migration. Chem Biol Interact. 304:73–82. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song IS, Jeong YJ, Park JH, Shim S and
Jang SW: Chebulinic acid inhibits smooth muscle cell migration by
suppressing PDGF-Rβ phosphorylation and inhibiting matrix
metalloproteinase-2 expression. Sci Rep. 7:117972017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seo KW, Lee SJ, Ye BH, Kim YW, Bae SS and
Kim CD: Mechanical stretch enhances the expression and activity of
osteopontin and MMP-2 via the Akt1/AP-1 pathways in VSMC. J Mol
Cell Cardiol. 85:13–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smiljanic K, Obradovic M, Jovanovic A,
Djordjevic J, Dobutovic B, Jevremovic D, Marche P and Isenovic ER:
Thrombin stimulates VSMC proliferation through an EGFR-dependent
pathway: Involvement of MMP-2. Mol Cell Biochem. 396:147–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu Q, Lin X, Ding L, Zeng Y, Pang D,
Ouyang N, Xiang Y and Yao H: ARHGAP42 promotes cell migration and
invasion involving PI3K/Akt signaling pathway in nasopharyngeal
carcinoma. Cancer Med. 7:3862–3874. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han R, Gu S, Zhang Y, Luo A, Jing X, Zhao
L, Zhao X and Zhang L: Estrogen promotes progression of
hormone-dependent breast cancer through CCL2-CCR2 axis by
upregulation of Twist via PI3K/AKT/NF-kappaB signaling. Sci Rep.
8:95752018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou H, Wu Q, Wei L and Peng S:
Paeoniflorin inhibits PDGFBBinduced human airway smooth muscle cell
growth and migration. Mol Med Rep. 17:2660–2664. 2018.PubMed/NCBI
|
|
41
|
Chan CM, Chang HH, Wang VC, Huang CL and
Hung CF: Inhibitory effects of resveratrol on PDGF-BB-induced
retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and
MAPK pathways. PLoS One. 8:e568192013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang H, Yin Y, Li W, Zhao X, Yu Y, Zhu J,
Qin Z, Wang Q, Wang K, Lu W, et al: Over-expression of PDGFR-β
promotes PDGF-induced proliferation, migration, and angiogenesis of
EPCs through PI3K/Akt signaling pathway. PLoS One. 7:e305032012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cidad P, Miguel-Velado E, Ruiz-McDavitt C,
Alonso E, Jiménez-Pérez L, Asuaje A, Carmona Y, García-Arribas D,
López J, Marroquín Y, et al: Kv1.3 channels modulate human vascular
smooth muscle cells proliferation independently of mTOR signaling
pathway. Pflugers Arch. 467:1711–1722. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu QB, Wan MY, Wang PY, Zhang CX, Xu DY,
Liao X and Sun HJ: Chicoric acid prevents PDGF-BB-induced VSMC
dedifferentiation, proliferation and migration by suppressing
ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biol. 14:656–668.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan S, Lin H, Luo H, Gao F, Meng L, Zhou
C, Jiang C, Guo Y, Ji Z, Chi J and Guo H: Folic acid inhibits
dedifferentiation of PDGF-BB-induced vascular smooth muscle cells
by suppressing mTOR/P70S6K signaling. Am J Transl Res. 9:1307–1316.
2017.PubMed/NCBI
|