Introduction
The bone marrow (BM) is a complex tissue comprised
of multiple subsets of stromal cells. Studies using a number of
different experimental approaches are beginning to unravel the
stromal cell profile in the BM (1).
The BM niche was the first proposed and experimentally defined
niche for stem cells in mammals (2).
Bone marrow stromal cells serve to regulate hematopoiesis in the
hematopoietic BM niche (1–3). Hematopoietic stem cells (HSC) mainly
reside in the BM niche and participate in tissue regeneration under
hematopoietic stress such as transplantation (4). However, under stress condition
including transplantation, these HSCs can lose their capacity for
self-renewal, resulting in stem cell exhaustion. Under these
circumstances, allogenic HSCs have to be engrafted into the BM
niche and subsequently expanded in a process known as
transplantation (5).
The histone deacetylase sirtuin 1 (SIRT1) regulates
a number of cellular responses, including cell proliferation,
apoptosis and inflammatory responses by protein deacetylation
(6–8). In particular, SIRT1 has also been
implicated in stem cell homeostasis (9–12).
Previous studies suggest that SIRT1 serves a role in HSC maturation
and lineage specification as demonstrated by a conditional deletion
approach, where Sirt1 expression was knocked out only in the
hematopoietic system (13,14). However, little is known about the
role of SIRT1 in the BM niche, since SIRT1 was found to be
dispensable for HSC activity due to developmental HSC adaptation in
surviving conventional Sirt1 KO mice in another study
(15). In addition, the SIRT1
activator Resveratrol modulates HSC capacity in the BM in
vivo (16), whereas SRT3025,
another SIRT1 activator, also altered the number of HSCs (17); these observations suggest that SIRT1
is important for HSC maturation. In the present study aim was to
elucidate the role of SIRT1 in the BM niche to provide novel
insight for HSC transplantation.
Materials and methods
Ethics statement and animals
All animals were housed in an air-conditioned room
(22–25°C; 12-h light/dark cycle; 50% humidity) with free access to
food pellets and tap water. Ocn-Cre transgenic mice used in
BM niches (18) and Sirt1
mice (19) have been described
(Fig. S1). Ocn-Cre, floxed
Sirt1 and wild-type C57BL/6 mice were purchased from Jackson
Laboratory and employed for experiments. The Ocn-Cre mice
are widely used to target osteoblasts in the BM niche (18). To investigate the role for HSC
maturation of Sirt1 in the BM niche, such as in osteoblast,
Sirt1floxed/floxed mice were crossed with
Ocn-Cre. A total of 70 mice (female; weight, 15–18 g; age,
8–10 weeks) were used for experiments.
Transplantations of BM cells were performed as
described previously (20). Briefly,
mice were euthanized then tibias and femurs were recovered. Scalpel
was used to cut the ends of bone off and then a syringe with 27G
needle (filled with PBS supplemented with 2% FBS and 1% P/S) was
used to flush into a 50 ml tube covered with 40 µM nylon filter.
Recovered BM cells were lysed in RBC lysis buffer (Lonza Group,
Ltd.) for 5–10 min on ice. Samples were then washed with 10 ml PBS
supplemented with 2% FBS and 1% P/S and centrifuged 5 min at 1,500
rpm. Supernatant was aspirated and the cell pellets were collected
for the experiments.
Control and Sirt1Δ/Δ mice received
lethal doses of irradiation (9.5 Gy, Gammacell 3000) prior to the
transplantation of 200 µl 1×106 BM cells by
retro-orbital injection on the same day as previously described
(21). For serial 5-FU treatments,
5-FU (150 mg/kg) was injected intraperitoneally every seven days
until 100% animal mortality was achieved (22). For the duration of the present study,
the following criteria were applied to define a mouse as moribund,
which would require immediate euthanasia (23,24): i)
Ruffled and/or matted fur; ii) weight loss of >15%; iii)
hypothermia (detected by touching); iv) hunched posture; v) unable
to eat or drink freely; vi) barrel rolling. No animals satisfied
these criteria in the present study.
Reverse transcription-quantitative PCR
(RT-qPCR)
Reverse transcription and quantitative PCR were
performed as previously described (14,25).
Briefly, total RNAs were extracted from BM cells using QIAGEN
RNeasy-Plus Mini-columns according to manufacturer's protocol
(Qiagen, Inc.). cDNA synthesis were performed following the
protocols of the manufacturer of the kit (Bio-Rad Laboratories,
Inc.). qPCR was performed with SYBR Green Mix (Roche Diagnostics)
and LightCycler 96 instrument (Roche Diagnostics) and data were
normalized to the housekeeping gene GAPDH. The mRNA levels
were measured using the 2−ΔΔCq method of quantification
(26). RT-qPCR was performed using
the following primers: Sirt1 forward,
5′-CTGAAAGTGAGACCAGTAGCA-3′ and reverse, 5′-GATGAGGCAAAGGTTCCCTA-3′
and GAPDH forward, 5′-GCACAGTCAAGGCCGAGAAT-3′ and reverse,
5′-GCCTTCTCCATGGTGGTGAA-3′.
Flow cytometric analysis
Flow cytometry was performed as described previously
(20). Briefly, BM cells were
collected from femurs and tibias of the mice by flushing using
fluorescence-activated cell sorting buffer, which consisted of
phosphate buffered saline containing 2% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences) and 0.1% sodium azide.
Peripheral blood cells were collected from the tail vein. Flow
cytometry was performed using antibodies listed in Table S1. Data acquisition and analysis
were performed with Cell Quest v.3.3 or Diva software v.6.1.3 (BD
Biosciences) and with FlowJo software v.10.3 (FlowJo LLC)
respectively.
Statistical analysis
The statistical significance of differences in the
means between two populations were assessed using two-tailed
unpaired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference for pairwise comparisons
(between indicated genotypes in the same population). The
Kaplan-Meier log-rank test was used to analyze survival data.
Results
Effects of Sirt1 deletion on the BM
niche
SIRT1 is a critical regulator of homeostatic adult
HSCs in mice (13,14). Based on these observations, it is
tempting to speculate that SIRT1 may also serve a role for HSC
maturation in the BM niche. To investigate the role for HSC
maturation of SIRT1 in the BM niche,
Sirt1floxed/floxed mice were crossed with
Ocn-Cre transgenic mice, resulting in
Sirt1Δ/Δ mice. A previous study showed that the
loss of Sirt1 in the hematopoietic system resulted in anemia
and specified lineage decision (myeloid versus lymphoid lineage
specification) (14). However,
Sirt1 deletion in the BM niche demonstrated no differences
in the numbers of white blood cells (WBCs), lymphocytes (LY),
neutrophils (NEU), monocytes (MO), hemoglobin (HGB), and platelets
(PLT) (Fig. 1A). It was also found
that Sirt1Δ/Δ mice did not result in significant
differences compared with wild-type mice in their ability to
produce mature myeloid cells (Mac1+ cells), T cells
(CD3+ cells) and B cells (B220+ cells) in the
peripheral blood (PB) and bone marrow (BM) (Fig. 2 and Fig.
S2). The expression of Sirt1 was decreased in the
CD31−CD45−Ter119− BM niche cells
of Sirt1Δ/Δ mice compared with
Sirt1+/+ mice, which confirmed Sirt1
knockdown (Fig. 1B).
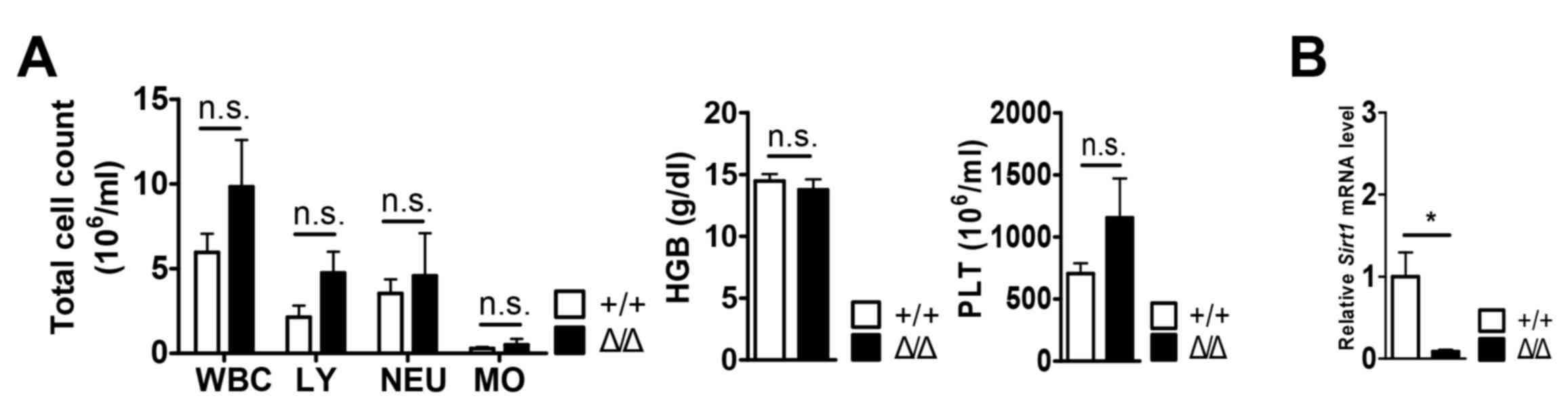 | Figure 1.No changes in white blood cells,
hemoglobin, and platelets counts of Sirt1Δ/Δ
mice. (A) White blood cell counts, lymphocytes, neutrophil,
monocytes, hemoglobin and platelet measurements from mice of the
indicated genotype (n=4–5). (B) Reverse transcription-quantitative
PCR analysis of Sirt1 expression in
CD31−CD45−Ter119−
Sirt1Δ/Δ bone marrow cells (n=3). Data are
presented as mean + SEM. *P<0.05. n.s., not significant; Sirt1,
sirtuin 1; Sirt1Δ/Δ, sirtuin 1 conditional knockout;
WBC; white blood cells; LY, lymphocytes; NEU, neutrophils; MO,
monocytes; HGB, hemoglobin; PLT, platelet; CD, cluster of
differentiation. |
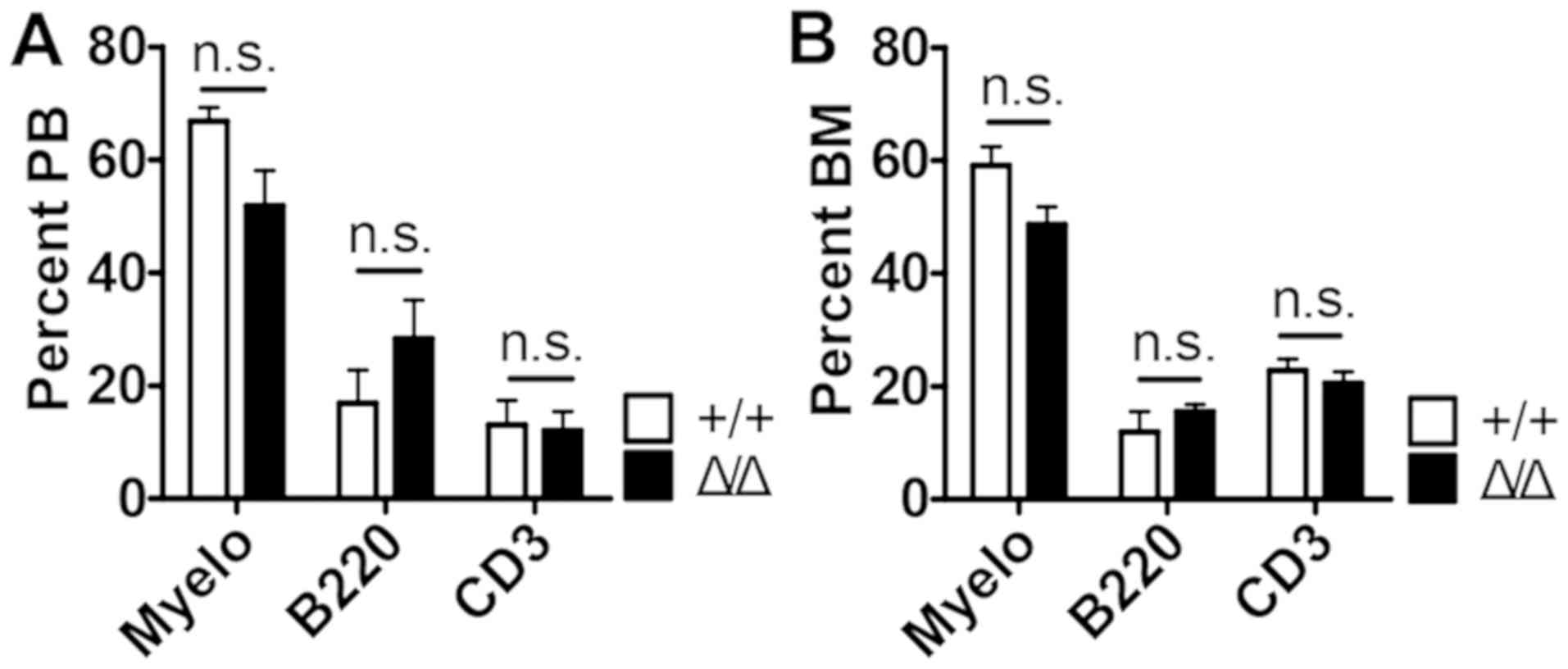 | Figure 2.Sirt1 deletion in the BM niche
is dispensable for maintaining mature hematopoietic lineage cells.
(A and B) The percentages of myeloid, B and T cells from (A) the
peripheral blood, and (B) the bone marrow of
Sirt1Δ/Δ and control mice (n=4–5). Data are
presented as mean + SEM. Mac1, macrophage-1 antigen; n.s., not
significant; Sirt1, sirtuin 1; Sirt1Δ/Δ, sirtuin 1
conditional knockout; Myelo, Myeloid (Mac1+) cells;
B220+ cells, B cells; CD3+ cells, T cells;
PB, peripheral blood; BM, bone marrow. |
Effects of Sirt1 loss on HSPCs
maturation were examined
No differences in the numbers of HSCs
(Lin−Sca1+cKit+CD150+CD48−,
SLAM cells), Lin−Sca1+cKit+ (LSK)
progenitor cells, common myeloid progenitor (CMP) cells
(Lin−Sca1−cKit+CD34+CD16/32−),
granulocyte-macrophage progenitor (GMP) cells
(Lin−Sca1−cKit+CD34+CD16/32+),
megakaryocyte-erythroid progenitor (MEP) cells
(Lin−Sca1−cKit+CD34−CD16/32−)
and common lymphoid progenitor (CMP) cells
(Lin−Sca1lowcKitlowCD127+)
were observed in the Sirt1Δ/Δ mice compared with
control mice (Fig. 3 and Fig. S3).
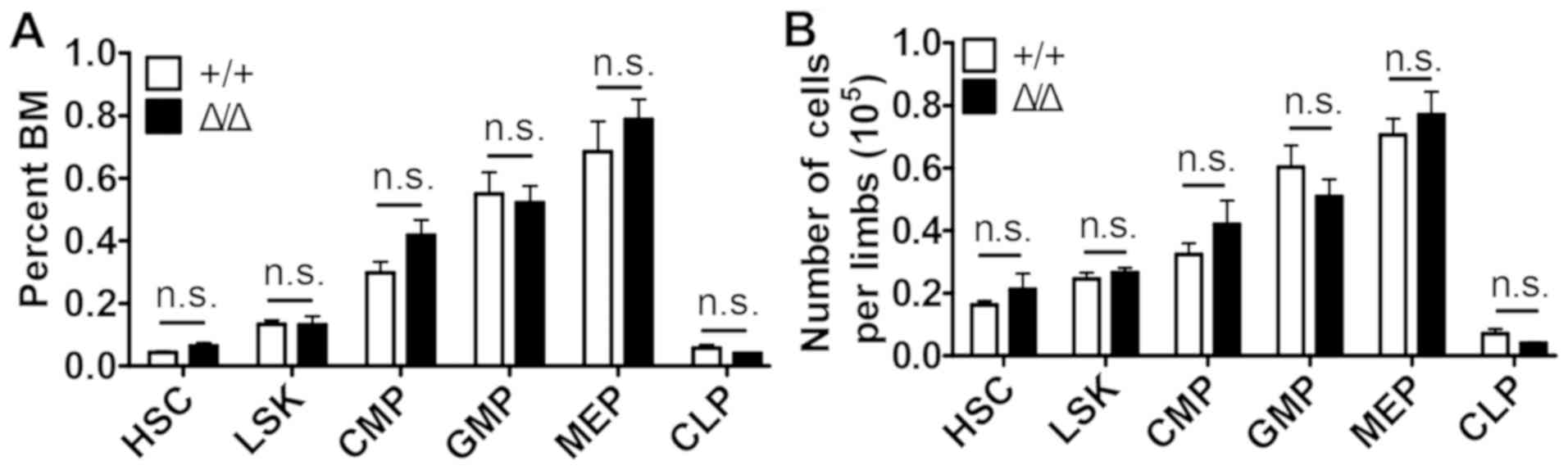 | Figure 3.Sirt1 deletion in the BM niche
is dispensable for maintaining hematopoietic stem and progenitor
cells. (A) The frequencies and (B) number of cells/limb of HSC,
LSK, CMP, GMP, MEP and CLP; from the BM of
Sirt1Δ/Δ and control mice (n=4–5). Data are
presented as mean + SEM. n.s., not significant; Sirt1, sirtuin 1;
Sirt1Δ/Δ, sirtuin 1 conditional knockout; Lin, lineage;
Sca1, spinocerebellar ataxia type 1; cKit, mast/stem cell growth
factor receptor kit; HSC, hematopoietic
(Lin−Sca1+cKit+CD150+CD48−)
stem cells; LSK, Lin−Sca1+cKit+
cells; CMP, common myeloid progenitor
(Lin−Sca1−cKit+CD34+CD16/32−)
cells; GMP, granulocyte-macrophage progenitor
(Lin−Sca1−cKit+CD34+CD16/32+)
cells; MEP, megakaryocyte-erythroid progenitor
(Lin−Sca1−cKit+CD34−CD16/32−)
cells; CLP, common lymphoid progenitor
(Lin−Sca1lowcKitlow
CD127+) cells. BM, bone marrow. |
Sirt1 deletion in the BM niche does
not alter HSC maturation
To assess the role of HSC maturation following
Sirt1 deletion in the BM niche under stress condition,
wild-type BM cells were transplanted into
Sirt1Δ/Δ mice, which had been lethally irradiated
to remove endogenous blood cells (Fig.
4 and Fig. S4). Compared with
control mice, Sirt1Δ/Δ mice exhibited no
detectable effects on peripheral blood (PB) donor chimerism
(Fig. 4A), bone marrow (BM) donor
chimerism and the numbers of mature hematopoietic cell types up to
16 weeks post-transplantation (Fig.
4B). In addition, Sirt1Δ/Δ mice demonstrated
no differences in the numbers of WBCs, Lys, NEUs, MOs and the
levels of hemoglobin (HGB) four weeks post-transplantation compared
with control mice (Fig. S5).
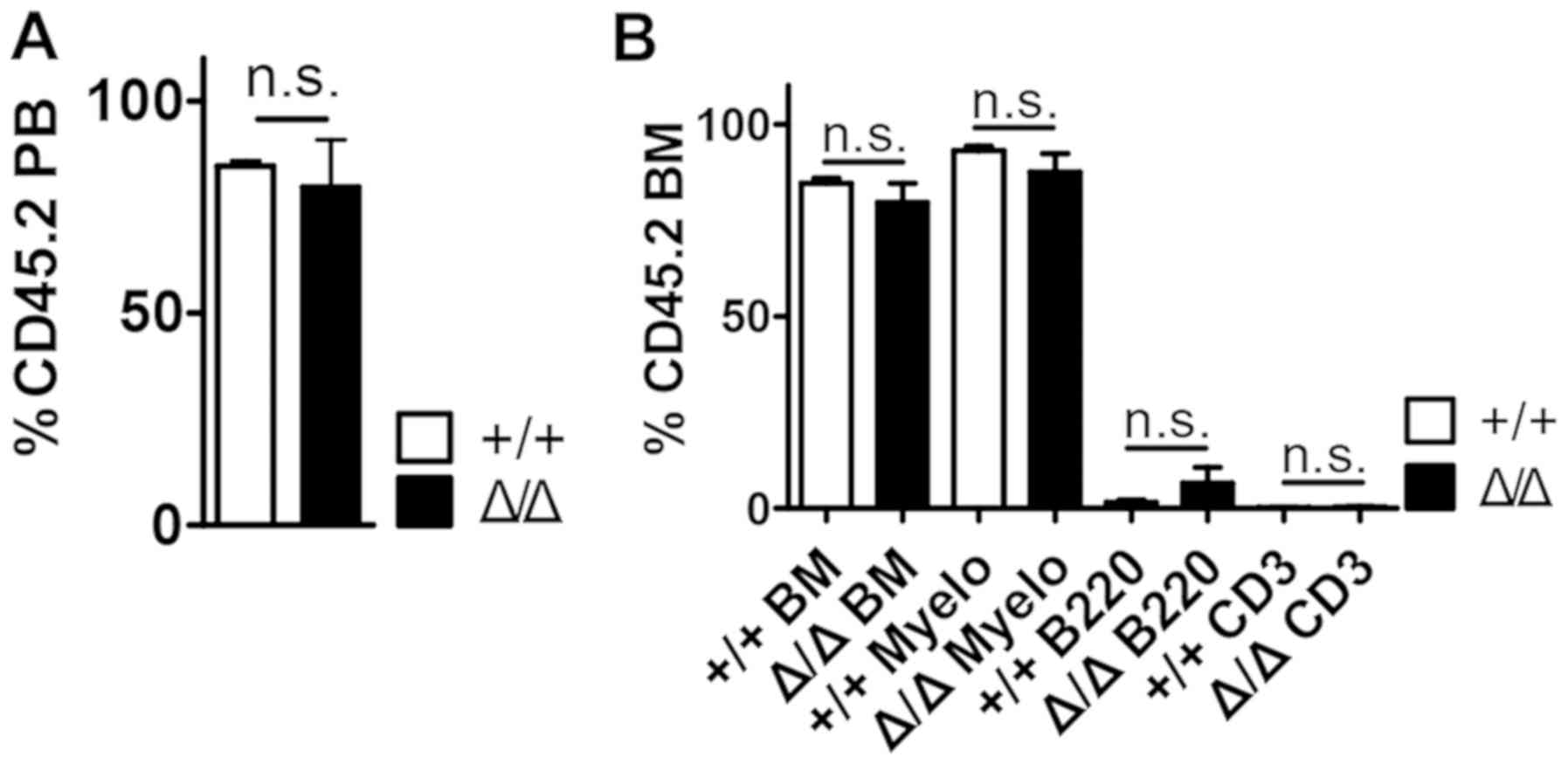 | Figure 4.Hematopoietic stem cells were
preserved in Sirt1Δ/Δ mice under transplantation
stress. (A) Peripheral blood was collected and analyzed for
CD45.2+ chimerism at 4 months post-transplantation
(n=5). (B) The frequencies of myeloid, B and T cells
(CD3+) in the BM from transplant recipients are shown 4
months post-transplantation (n=5). Data are presented as mean +
SEM. n.s., not significant; Sirt1, sirtuin 1; Sirt1Δ/Δ,
sirtuin 1 conditional knockout; Mac1, macrophage-1 antigen; Myelo,
Myeloid (Mac+) cells; B220, B cells; CD3, T cells; PB,
peripheral blood; BM, bone marrow. |
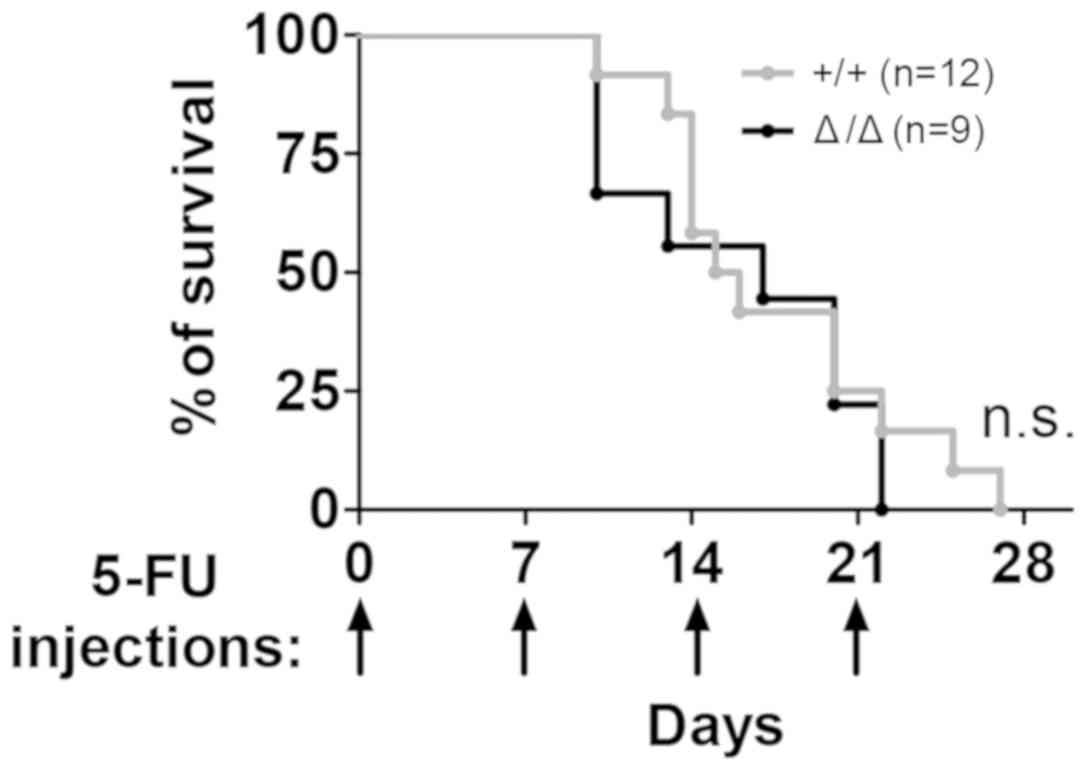
Next the Sirt1Δ/Δ mice were
repeatedly treated with 5-FU weekly to assess their sensitivity to
stress under non-transplantation stress. Exposure of
Sirt1Δ/Δ mice to serial proliferative stress
induced by 5-FU (22) exerted in no
significant differences in animal mortality compared with control
mice, according to the Kaplan-Meier curve (Fig. 5).
Discussion
In the present study, it was demonstrated that
Sirt1 deletion in the BM niche (Sirt1Δ/Δ)
was dispensable for maintaining mature hematopoietic lineage cells
and HSC maturation. Additionally, HSCs were preserved in
Sirt1Δ/Δ under transplantation stress. Also,
Sirt1Δ/Δ mice did not respond differently to
5-fluorouracil (5-FU)-induced stress compared with control mice.
Collectively, these findings suggested that Sirt1 deletion
in the BM niche is dispensable for maturation of HSC.
First, a previous study showed that Sirt1
deletion in the HSCs resulted in anemia, a significantly decreased
number of lymphocytes and increased numbers of neutrophils,
monocytes and eosinophils (16).
SIRT1 is essential for the myeloid versus lymphoid lineage
specification in the hematopoietic blood cells (14). However, in the present study it was
found that Sirt1 deletion in the BM niche lead to normal
production of mature blood cells and lineage distribution within BM
cells. In addition, no differences were observed between
Sirt1Δ/Δ mice and wild-type mice in their ability
to produce mature cells in the PB and BM.
A previous study showed that HSC expanded in
response to the loss of Sirt1 in the hematopoietic
compartment (13), suggesting that
SIRT1 was dispensable for HSC activity due to the developmental
adaptation of HSCs (15). The
question on the role of SIRT1 in HSC maturation has long been
debated. However, the present study found that Sirt1
deletion in the BM niche demonstrated pools of HSPC populations
that were comparable to those of controls.
Finally, previous studies showed that SIRT1 is
essential for HSC maturation under stress conditions, including
resveratrol treatment and Fanconi anemia, SIRT1 activators enhanced
HSPC capacity of the BM in vivo and increased the number of
HSCs (Lin−Sca1+cKit+ cells and
Lin−Sca1+cKit+CD150+CD48−
cells) (16,17). However, in the present study it was
found that Sirt1Δ/Δ deletion in the BM niche did
not perturb HSC maturation under transplantation stress or in the
presence of 5-FU, unlike Sirt1 deletion in the HSCs
(13,14). Collectively, SIRT1 serves a
dispensable role in HSC maturation in the BM niche, but not in the
hematopoietic compartment. The findings of the present study
contributed to better understanding of the molecular biology
involved in the BM niche and hematopoiesis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Korea
government (MSIT) (grant no. NRF-2018R1C1B6001290) and the
Convergence Medical Institute of Technology R&D project (grant
no. CMIT2019-03), Pusan National University Hospital.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SMP, DHSh, JYK, DYP, DHSo, YHK, SMK, JHK, SSB, KK,
CDKi, CDKa and DL contributed to the design of the study. SMP, JYK
and CMH acquired and analyzed the data. SMP and DL drafted the
manuscript. DHSh, JYK, DYP, DHSo, YHK, SMK, JHK, SSB, KK, CDKi,
CDKa and DL revised and edited the manuscript. DL acquired funding
and resources. All authors read and approved the final version of
the manuscript.
Ethical approval and consent to
participate
The study was approved by the Ethics Committee of
Pusan National University School of Medicine, Republic of
Korea.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schofield R: The relationship between the
spleen colony-forming cell and the haemopoietic stem cell. Blood
Cells. 4:7–25. 1978.PubMed/NCBI
|
|
2
|
Calvi LM, Adams GB, Weibrecht KW, Weber
JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P,
Bringhurst FR, et al: Osteoblastic cells regulate the
haematopoietic stem cell niche. Nature. 425:841–846. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li L and Xie T: Stem cell niche: Structure
and function. Annu Rev Cell Dev Biol. 21:605–631. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trumpp A, Essers M and Wilson A: Awakening
dormant haematopoietic stem cells. Nat Rev Immunol. 10:201–209.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thomas ED: Bone marrow transplantation
from the personal viewpoint. Int J Hematol. 81:89–93. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lavu S, Boss O, Elliott PJ and Lambert PD:
Sirtuins-novel therapeutic targets to treat age-associated
diseases. Nat Rev Drug Discov. 7:841–853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SY, Lee SW, Kim HY, Lee SY, Lee WS,
Hong KW and Kim CD: SIRT1 inhibits differentiation of monocytes to
macrophages: Amelioration of synovial inflammation in rheumatoid
arthritis. J Mol Med (Berl). 94:921–931. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park SY, Lee SW, Lee SY, Hong KW, Bae SS,
Kim K and Kim CD: SIRT1/adenosine monophosphate-activated protein
kinase α signaling enhances macrophage polarization to an
anti-inflammatory phenotype in rheumatoid arthritis. Front Immunol.
8:11352017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han MK, Song EK, Guo Y, Ou X, Mantel C and
Broxmeyer HE: SIRT1 regulates apoptosis and nanog expression in
mouse embryonic stem cells by controlling p53 subcellular
localization. Cell Stem Cell. 2:241–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oberdoerffer P, Michan S, McVay M,
Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner
A, Loerch P, et al: SIRT1 redistribution on chromatin promotes
genomic stability but alters gene expression during aging. Cell.
135:907–918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang RH, Sengupta K, Li C, Kim HS, Cao L,
Xiao C, Kim S, Xu X, Zheng Y, Chilton B, et al: Impaired DNA damage
response, genome instability, and tumorigenesis in SIRT1 mutant
mice. Cancer Cell. 14:312–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ou X, Chae HD, Wang RH, Shelley WC, Cooper
S, Taylor T, Kim YJ, Deng CX, Yoder MC and Broxmeyer HE: SIRT1
deficiency compromises mouse embryonic stem cell hematopoietic
differentiation, and embryonic and adult hematopoiesis in the
mouse. Blood. 117:440–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh SK, Williams CA, Klarmann K, Burkett
SS, Keller JR and Oberdoerffer P: Sirt1 ablation promotes
stress-induced loss of epigenetic and genomic hematopoietic stem
and progenitor cell maintenance. J Exp Med. 210:987–1001. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rimmele P, Bigarella CL, Liang R, Izac B,
Dieguez-Gonzalez R, Barbet G, Donovan M, Brugnara C, Blander JM,
Sinclair DA and Ghaffari S: Aging-like phenotype and defective
lineage specification in SIRT1-deleted hematopoietic stem and
progenitor cells. Stem Cell Reports. 3:44–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leko V, Varnum-Finney B, Li H, Gu Y,
Flowers D, Nourigat C, Bernstein ID and Bedalov A: SIRT1 is
dispensable for function of hematopoietic stem cells in adult mice.
Blood. 119:1856–1860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rimmele P, Lofek-Czubek S and Ghaffari S:
Resveratrol increases the bone marrow hematopoietic stem and
progenitor cell capacity. Am J Hematol. 89:E235–E238. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang QS, Deater M, Schubert K,
Marquez-Loza L, Pelz C, Sinclair DA and Grompe M: The Sirt1
activator SRT3025 expands hematopoietic stem and progenitor cells
and improves hematopoiesis in fanconi anemia mice. Stem Cell Res.
15:130–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Xuan S, Bouxsein ML, von Stechow
D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis
A and Clemens TL: Osteoblast-specific knockout of the insulin-like
growth factor (IGF) receptor gene reveals an essential role of IGF
signaling in bone matrix mineralization. J Biol Chem.
277:44005–44012. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Rajendran GK, Liu N, Ware C, Rubin
BP and Gu Y: SirT1 modulates the estrogen-insulin-like growth
factor-1 signaling for postnatal development of mammary gland in
mice. Breast Cancer Res. 9:R12007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee DH, Kim TS, Lee D and Lim DS:
Mammalian sterile 20 kinase 1 and 2 are important regulators of
hematopoietic stem cells in stress condition. Sci Rep. 8:9422018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deveza L, Ortinau L, Lei K and Park D:
Comparative analysis of gene expression identifies distinct
molecular signatures of bone marrow- and periosteal-skeletal
stem/progenitor cells. PLoS One. 13:e01909092018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goncalves KA, Silberstein L, Li S, Severe
N, Hu MG, Yang H, Scadden DT and Hu GF: Angiogenin promotes
hematopoietic regeneration by dichotomously regulating quiescence
of stem and progenitor cells. Cell. 166:894–906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thrall KD, Mahendra S, Jackson MK, Jackson
W 3rd, Farese AM and MacVittie TJ: A comparative dose-response
relationship between sexes for mortality and morbidity of
radiation-induced lung injury in the rhesus macaque. Health Phys.
116:354–365. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edin NF, Altaner C, Altanerova V, Ebbesen
P and Pettersen EO: Low-dose-rate irradiation for 1 hour induces
protection against lethal radiation doses but does not affect life
span of DBA/2 mice. Dose Response. 14:15593258166739012016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu J, Bao J, Kim M, Yuan S, Tang C, Zheng
H, Mastick GS, Xu C and Yan W: Two miRNA clusters, miR-34b/c and
miR-449, are essential for normal brain development, motile
ciliogenesis, and spermatogenesis. Proc Natl Acad Sci U S A.
111:E2851–E2857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|